Abstract
In this study, we have characterized reconstitution of the natural killer (NK) cell repertoire after haploidentical CD34+ selected hematopoietic stem cell transplantation (HSCT) for high-risk hematologic malignancies. Analysis focused on alloreactive single-KIR+ NK cells, which reportedly are potent antileukemic effectors. One month after HSCT, CD56bright/CD56dim NK-cell subsets showed inverted ratio and phenotypic features. CD25 and CD117 down-regulation on CD56bright, and NKG2A and CD62L up-regulation on CD56dim, suggest sequential CD56bright-to-CD56dim NK-cell maturation in vivo. Consistently, the functional potential of these maturation intermediates against leukemic blasts was impaired. Mature receptor repertoire reconstitution took at least 3 months. Importantly, at this time point, supposedly alloreactive, single-KIR+ NK cells were not yet fully functional. Frequency of these cells was highly variable, independently from predicted NK alloreactivity, and below 1% of NK cells in 3 of 6 alloreactive patients studied. In line with these observations, no clinical benefit of predicted NK alloreactivity was observed in the total cohort of 56 patients. Our findings unravel the kinetics, and limits, of NK-cell differentiation from purified haploidentical hematopoietic stem cells in vivo, and suggest that NK-cell antileukemic potential could be best exploited by infusion of mature single-KIR+ NK cells selected from an alloreactive donor.
Introduction
Hematopoietic stem cell transplantation (HSCT) from one-human leukocyte antigen (-HLA)–matched (haploidentical) family members is a promising therapeutic strategy for the cure of hematologic malignancies in patients lacking a fully HLA-matched donor.1-4 In most common protocols, a CD34+ purified hematopoietic stem cell (HSC) graft is infused after a highly immunosuppressive conditioning regimen, to prevent occurrence of acute graft-versus-host disease (aGVHD) and graft rejection. Natural killer (NK) cells are the first lymphocyte subset to reconstitute in vivo from HSCs, representing the only lymphocyte population potentially able to control leukemic relapse in the months preceding T-cell reconstitution.3,5-8
One HLA-haplotype disparity between HSC donor and recipient in 30% to 60% of transplantations determines NK alloreactivity.9 This condition, according to the ligand-ligand model,10-12 can be predicted whenever NK cells from a donor carrying a defined KIR ligand (ie, an HLA molecule recognized by a given killer cell immunoglobulin–like receptor) are challenged with target cells lacking that KIR ligand. Alloreactivity is therefore mediated by single-KIR+ NK cells not encountering any inhibitory signal from self-HLA molecules on host target cells. NK clones with patient-specific alloreactivity have been isolated from the peripheral blood of patients in the first 3 to 4 months after haploidentical HSCT.10,13
In retrospective analyses of a large series of haploidentical HSCTs, predicted donor-versus-host NK alloreactivity has been associated with a decrease in the incidence of leukemia relapse, ultimately improving overall survival (OS).3,10,13,14 However, subsequent studies failed to confirm the protective effect of NK alloreactivity on leukemia relapse after haploidentical HSCT.15,16 In particular, Nguyen et al15 provided evidence for a role of CD94:NKG2A, the inhibitory receptor specific for HLA-E, in limiting the antileukemic effect of NK cells that reconstitute at an early stage after haploidentical HSCT.
Little is currently known about the reconstitution of single-KIR+, CD94:NKG2A− NK cells, which are expected to be the effectors of NK alloreactivity. Their presence in patients with expected alloreactivity following haploidentical HSCT has been demonstrated.13 However, evaluation of their recovery kinetics, frequency, and functionality in relation to predicted NK alloreactivity is still lacking.
The study of single-KIR+ NK cells is also important to investigate how NK-cell tolerance is achieved. A longstanding hypothesis stated that all circulating NK cells carry at least one KIR able to recognize self-HLA molecules (the “at least one” model).12,17,18 More recent data proposed that single-KIR+ NK cells specific for non–self-HLA molecules are present but hyporesponsive, and that interaction between KIRs and their respective ligands is necessary to endow the NK cell with effector function toward their targets (the “licensing” model).19-22 HLA-mismatched HSCT is an ideal context in which to study whether such education takes place, and whether it is dictated by hematopoietic (donor) or nonhematopoietic (host) cells.
Here, we report on the reconstitution of the NK-cell repertoire, in particular of single-KIR+ NK cells, after CD34+ selected haploidentical HSCT for high-risk hematologic malignancies, and its relevance in determining clinical outcome in patients receiving T-cell add-backs after a myeloablative chemotherapy-based conditioning.
Methods
Patient characteristics and transplantation procedure
A total of 56 haploidentical HSCTs were considered in our analysis, as summarized in Table 1. Donor-versus-host NK alloreactivity, predicted according to the previously described ligand-ligand model,10 was used as preferential selection criteria for donors, and in 27 (48.2%) of 56 transplantations an NK-alloreactive donor was chosen. Donor peripheral blood (PB) HSCs were mobilized with granulocyte colony-stimulating factor (G-CSF) at the dose of 12 to 15 μg/kg per day. CD34+ cells were selected using the CliniMacs one-step procedure (Miltenyi Biotech, Bergisch Gladbach, Germany). Final graft product contained a median of 11.3 × 106 CD34+ cells/kg (range, 4.7-19.5 × 104 CD3+ cells/kg), extensively T cell–depleted (median, 1.07 × 104 CD3+ cells/kg; range, 0.29-3.2 × 104 CD3+ cells/kg). Fifty-three patients received a fully myeloablative conditioning regimen, including antithymocyte globulin (ATG) Fresenius at the total dose of 25 mg/kg. Three patients underwent a second salvage HSCT after rejection of the first transplant, receiving an immune-modulating conditioning, including thymoglobulin (6 mg/kg total dose). In consideration of the extensive T-cell depletion, patients did not receive any posttransplantation pharmacological GVHD prophylaxis. All patients with fitting clinical conditions and no sign of spontaneous T-cell reconstitution (n = 35) received donor T-cell add-backs starting from day +30 after HSCT (median, day +42; range, days +14-130) at the median total dose of 10 × 106 CD3+ cells/kg (range, 0.1-85.4 × 106 CD3+ cells/kg). Nineteen patients received a single infusion, 8 received 2 infusions, and the remaining 8 received 3 or more infusions. T-cell add-backs were in all cases GMP-manipulated to control or reduce their GVHD potential by, respectively, transduction with the HSV-Tk suicide gene (n = 29; TK007 protocol; Bonini et al23 ) or by coculture with donor cells in presence of rhIL-10 (n = 6; ALT-TEN protocol; Battaglia et al24 ). No aGVHD prophylactic treatment was administered following T-cell add-backs. Seven patients who developed GVHD needed to receive immunosuppressive treatment by use of steroids (n = 2), mycophenolate (n = 1), combined steroids and cyclosporin A (n = 2), combined steroid and mycophenolate (n = 1), or all 3 drugs (n = 1). No phenotypic or functional analysis from these patients was performed during or after the treatment, and they were censored from statistical analysis of clinical outcome at the beginning of the therapy, to exclude any pharmacological confounding effect on the evaluation of the role of NK-cell alloreactivity in determining clinical outcome.
Patient characteristics
| Characteristic . | No. . |
|---|---|
| All patients (M/F) | 56 (28/28) |
| Median age, y (range) | 48 (17-66) |
| Diagnosis | |
| Acute myeloid leukemia | 39 |
| Myelodysplastic syndromes | 4 |
| Chronic myeloid leukemia | 1 |
| Biphenotypic acute leukemia | 1 |
| Acute lymphoblastic leukemia | 3 |
| Non-Hodgkin lymphoma | 3 |
| Hodgkin disease | 4 |
| Multiple myeloma | 1 |
| Status at transplantation | |
| CR1 | 14 |
| CR2 | 11 |
| CR3 or more | 3 |
| Refractory–Persistence | 15 |
| Relapse | 13 |
| Conditioning regimen* | |
| Mel-Th-Flu-ATG | 30 |
| Treo-Flu-ATG-Rit-TBI | 19 |
| Flu-Cy-Thy | 3 |
| Th-Flu-ATG-TBI | 2 |
| Treo-Flu-Alem | 1 |
| Th-Flu-ATG-Rit-TBI | 1 |
| Median CD34+ cells in graft, × 106/kg (range) | 11.1 (4.7-19.5) |
| Median CD3+ cells in graft, ×104/kg (range) | 1.07 (0.29-3.2) |
| Donor lymphocyte add-back (range) | 35 |
| Median total T-cell dose, ×106/kg | 10 (0.1-85.4) |
| Median day of first add-back infusion | 42 (14-130) |
| Protocol of add-back cell manipulation (TK007/ALT-TEN) | 29/6 |
| Characteristic . | No. . |
|---|---|
| All patients (M/F) | 56 (28/28) |
| Median age, y (range) | 48 (17-66) |
| Diagnosis | |
| Acute myeloid leukemia | 39 |
| Myelodysplastic syndromes | 4 |
| Chronic myeloid leukemia | 1 |
| Biphenotypic acute leukemia | 1 |
| Acute lymphoblastic leukemia | 3 |
| Non-Hodgkin lymphoma | 3 |
| Hodgkin disease | 4 |
| Multiple myeloma | 1 |
| Status at transplantation | |
| CR1 | 14 |
| CR2 | 11 |
| CR3 or more | 3 |
| Refractory–Persistence | 15 |
| Relapse | 13 |
| Conditioning regimen* | |
| Mel-Th-Flu-ATG | 30 |
| Treo-Flu-ATG-Rit-TBI | 19 |
| Flu-Cy-Thy | 3 |
| Th-Flu-ATG-TBI | 2 |
| Treo-Flu-Alem | 1 |
| Th-Flu-ATG-Rit-TBI | 1 |
| Median CD34+ cells in graft, × 106/kg (range) | 11.1 (4.7-19.5) |
| Median CD3+ cells in graft, ×104/kg (range) | 1.07 (0.29-3.2) |
| Donor lymphocyte add-back (range) | 35 |
| Median total T-cell dose, ×106/kg | 10 (0.1-85.4) |
| Median day of first add-back infusion | 42 (14-130) |
| Protocol of add-back cell manipulation (TK007/ALT-TEN) | 29/6 |
Conditioning regimens were as follows: Mel-Th-Flu-ATG: melphalan 140 mg/m2, thiotepa 13 mg/kg, fludarabine 200 mg/m2, ATG-Fresenius 25 mg/kg; Treo-Flu-ATG-Rit-TBI: treosulphan 42 g/m2, fludarabine 150 mg/m2, ATG-Fresenius 25 mg/kg, rituximab 500 mg, TBI 200 cGy; Flu-Cy-Thy: fludarabine 200mg/m2, cyclophosphamide 100 mg/kg, thymoglobulin 6 mg/kg; Th-Flu-ATG-TBI: thiotepa 10 mg/kg, fludarabine 200 mg/m2, ATG-Fresenius 25 mg/kg, TBI 600 cGY; Treo-Flu-Alem: treosulphan 42 g/m2, fludarabine 150 mg/m2, alemtuzumab 90 mg; Th-Flu-ATG-Rit-TBI: thiotepa 10 mg/kg, fludarabine 150 mg/m2, ATG-Fresenius 25 mg/kg, rituximab 700 mg/m2, TBI 200 cGY.
In addition, 4, 2, and 5 patients who, respectively, underwent matched unrelated donor (MUD), HLA-identical sibling, and autologous HSCT for hematologic malignancies were studied. All of these patients received an unmanipulated HSC graft after fully myeloablative conditioning regimen, which, in the case of MUD HSCT, included ATG-Fresenius. aGVHD prophylaxis after MUD and HLA-identical sibling HSCT was performed by administration of cyclosporin A and short-course methotrexate.
Collection of peripheral blood samples
Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep Ficoll gradient separation (Axis-Shield PoCAS, Oslo, Norway) from PB samples from 31 patients who underwent haploidentical HSCT, 6 patients who underwent HLA-matched HSCT, 5 patients who underwent autologous HSCT, and 31 healthy donors (18 of whom were the actual HSC donors). For patients who underwent haploidentical HSCT, samples were collected at different time points during follow-up, as shown in Figure 3. Phenotypic analysis of NK cells taken 30 days after HSCT was performed on 22 of the patients who underwent haploidentical HSCT; cytotoxic assay on 7; γ-INF release assay on 6; and single-KIR+ NK-cell analysis on 11 (8 of which were studied for single-KIR2DL1+ and 7 for single-KIR2DL3+ NK cells). The number of patients studied for the time course of NK-cell repertoire reconstitution are shown in Figure 3. All participants gave informed consent according to protocols approved by the San Raffaele ethical committee and in accordance with the Declaration of Helsinki.
HLA and KIR typing
Six-HLA locus molecular genomic typing of patients and donors was performed by polymerase chain reaction (PCR)–based standard methods, at a level of resolution sufficient for establishment of genotypic haploidentity by family descent or of phenotypic haploidentity.25 In particular, the methods used for typing of the HLA-B and -C locus genes allowed for univocal discrimination between the Bw4/Bw6 epitopes as well as the S77N80/N77K80 motifs associated with the 3 main KIR ligand groups.
Low-resolution genomic typing of 16 KIR genes (KIR2DL1-5, KIR2DS1-5, KIR3DL1-3, KIR3DS1, KIR2DP1, KIR3DP1) was performed according to a protocol adapted from Crum et al,26 or by a commercial SSP KIR typing kit (PEL-Freez, Brown Deer, WI).
Cell cultures
The HLA class I–deficient human 721.221 Epstein-Barr virus–transformed B-lymphoblastoid cell line (BLCL) has been described.27
PBMCs cryopreserved at diagnosis from 6 patients with more than 80% circulating acute myeloid leukemia (AML) blasts were cultured in X-VIVO medium (Cambrex Bio Science, Verviers, Belgium) with 5% human serum for at least 48 hours before use for in in vitro studies.
NK-cell purification and culture
NK cells were purified by negative immunobead selection for CD3+, CD19+, and CD14+ cells, as previously described27 ; final CD56+CD3− purity was always superior to 80% (mean, 85.39%; range, 82.12%-94.19%), with negligible CD3+ T-cell contamination (mean, 1.93%; range, 0.53%-5.02%). Samples from patients at 30 days after haploidentical HSCT invariably had a proportion of NK cells superior to 80%, and were used unselected.
Before testing, all samples used for functional studies were kept overnight in IMDM 10% FCS in the presence of 100 IU/mL recombinant human interleukin-2 (rhIL-2; Chiron, Emeryville, CA).
51Cr and IFN-γ release assays
Cytotoxicity was assessed in a standard 4-hour 51Cr release assay, as previously described.28 For mAb inhibition of cytotoxicity, effector cells were incubated for 30 minutes at 37°C with relevant or control mAbs at the final concentration of 20 μg/mL, prior to addition of target cells.
IFN-γ release was measured by standard enzyme-linked immunosorbent assay (ELISA; BD Biosciences, Mountain View, CA) after 24-hour incubation of 3 × 104 NK cells/well in 96-well round-bottom plates, at 1:1 ratio with target cells, in medium containing 100 IU/mL rhIL-2. Where relevant, mAbs were added to the culture at the final concentration of 20 μg/mL.
Antibodies and flow cytometric analysis
Monoclonal antibodies (mAbs) clone EB6B/anti-KIR2DL1/KIR2DS1, GL183/anti-KIR2DL2/KIR2DL3/KIR2DS2, Z27/anti-KIR3DL1, Z199/anti-NKG2A, Z25/anti-NKp30, Z231/anti-NKp44, BAB281/anti-NKp46, ON72/anti-NKG2D, UCHT1/anti-CD3, N901/anti-CD56 (Beckman-Coulter-Immunotech, Brea, CA), B73.1/anti-CD16, 2A3/anti-CD25, AG184/anti-CD132, DREG-56/anti-CD62L, 104D2/anti-CD117, and H4A3/anti-CD107a (BD Biosciences) were used for flow cytometric assays, with appropriate isotype controls. For 51Cr and IFN-γ release assays, purified anti-NKG2A mAb Z199 and the control anti–nerve growth factor receptor (NGFR) antibody 20.429 were used. Analyses were performed with FACS Calibur or FACS Canto II instruments (BD Biosciences), and results analyzed with the FCS Express 3 program (De Novo Software, Thornhill, ON).
CD107a mobilization assay
NK-cell degranulation was evaluated in a CD107a flow cytometric assay, according to a protocol adapted from Alter et al.30 Briefly, triplicates of 2 × 105 PBMCs were plated in IMDM medium containing 10% FCS, rhIL-2 1200 IU/mL, and anti-CD107a mAb 20 μL/mL, in 96-well round-bottom plates, in the presence or absence of 2 × 104 721.221 target cells at 37°C. After 3 hours, monensin A (Sigma-Aldrich, St Louis, MO) was added to the culture at the final concentration of 30 μg/mL. After 3 additional hours of incubation, cells were washed and extracellular staining for CD3, CD56, KIR2DL1/2DS1, KIR2DL2/2DL3/2DS2, KIR3DL1, and NKG2A was performed.
Statistical analysis
Since all the comparisons were carried over small groups, percentages of expression of NK receptors were compared using a nonparametric approach: when more than 2 groups were compared, a heterogeneity Kruskal-Wallis test was followed (when giving a significant result) by a post hoc analysis comparing groups of interest. For each hypothesis tested, when multiple variables were compared between groups, a correction for multiple testing using the false discovery rate (FDR) approach was applied, to keep the probability of false-positive results within 5% for each hypothesis tested.31 Paired measures over the same subjects or between donors and recipients were compared using a Wilcoxon test.
A random effect model accounting for repeated measures over the same subjects and using all the available information was used to study the decrease over time in the percentage of cells; this model was applied after a log-odds transform of these percentages: log-odds (p) = ln(p/100 − p), to normalize their distribution. A time × group interaction term was included in the model to assess differences in time trends between groups.
Correlations were analyzed using the Spearman rank correlation coefficient.
Graft rejection, death before engraftment, and aGVHD were analyzed using the Fisher exact χ2 test. The cumulative incidence of transplant-related mortality (TRM) and relapses accounting for competing risks were estimated using the library “cmprsk” in the R statistical package (University of Auckland, Auckland, New Zealand; http://www.r-project.org). The statistical significance was assessed using the Gray test32 implemented in the same library. Patients who received an immunosuppressive treatment to control GVHD (n = 7) were censored at the beginning of the therapy. Multivariate analysis adjusting for relevant covariates was carried out using a Cox model.
Results
NK cells that reconstitute at an early stage in vivo from CD34+ HSCs display distinct phenotypic characteristics intrinsic to their maturation
To investigate the role of HLA-matching status and transplantation regimen on NK cell reconstitution after HSCT, we studied NK cells arising at day 30 in 22 patients who underwent haploidentical transplantation, and compared them with their counterparts arising after HLA-matched (n = 6) and autologous (n = 5) HSCT.
In line with previous observations,6,7,33 NK cells are significantly expanded 30 days after haploidentical (mean, 86%; standard error [SE], 3.3%; P < .001), HLA-matched (36% ± 7.6%, P < .001), and autologous (32% ± 9.2%; P = .013) HSCT, in comparison with healthy controls (16% ± 1.3%). NK cells that reconstitute at an early stage in all 3 transplantation types were significantly enriched for CD56bright cells compared with healthy donors (P < .001; data not shown).
The phenotypic features of NK cells at 1 month after all 3 types of HSCT were similar, and showed several differences from those of healthy donor NK cells, both in the CD56bright and CD56dim subsets (Figure 1). In particular, patient CD56bright cells expressed lower amounts of CD25 and CD117, both expressed since the stage of NK-cell precursors,34,35 than their counterparts in healthy donors (P = .02 and P = .03, respectively). Patient CD56dim cells were invariably NKG2A+CD62L+ and showed diminished expression of CD16 compared with those of healthy donors (P < .001, P = .02, and P = .006, respectively), thus bearing a close resemblance to CD56bright cells.
NK cells that reconstitute at an early stage in vivo from CD34+ HSCs display distinct phenotypic characteristics intrinsic to their maturation. CD56bright (left panels) and CD56dim (right panels) NK-cell subsets of healthy blood donors (n = 31;  ) and of patients at day 30 after haploidentical (n = 22; ■), HLA-matched (related, n = 2, and unrelated, n = 4; □), and autologous (n = 5; ▧) HSCT were analyzed separately for expression of several NK-cell subset-specific receptors. From top to bottom: (A) inhibitory receptors (KIRs and NKG2A), (B) activating receptors (NKp30, NKp44, NKp46, NKG2D), (C) well-characterized markers of NK-cell functions: CD16 for antibody-dependent-cellular cytotoxicity (ADCC); CD25 and CD132, respectively, the α and γ chain of the IL-2 receptor; CD62L for lymph node homing; and CD117 (c-kit), which is expressed by hematopoietic progenitors, as well as by differentiating and mature CD56bright NK cells.34,35 Shown are the average percentages of expression and SE. Both donors and patients with a “null” KIR3DL1 phenotype were not considered in the analysis of KIR3DL1 expression. Asterisks indicate significant posthoc comparisons of patients at day 30 after HSCT from haploidentical donors after correction for multiple comparisons (false discovery rate; see “Statistical analysis” for details).
) and of patients at day 30 after haploidentical (n = 22; ■), HLA-matched (related, n = 2, and unrelated, n = 4; □), and autologous (n = 5; ▧) HSCT were analyzed separately for expression of several NK-cell subset-specific receptors. From top to bottom: (A) inhibitory receptors (KIRs and NKG2A), (B) activating receptors (NKp30, NKp44, NKp46, NKG2D), (C) well-characterized markers of NK-cell functions: CD16 for antibody-dependent-cellular cytotoxicity (ADCC); CD25 and CD132, respectively, the α and γ chain of the IL-2 receptor; CD62L for lymph node homing; and CD117 (c-kit), which is expressed by hematopoietic progenitors, as well as by differentiating and mature CD56bright NK cells.34,35 Shown are the average percentages of expression and SE. Both donors and patients with a “null” KIR3DL1 phenotype were not considered in the analysis of KIR3DL1 expression. Asterisks indicate significant posthoc comparisons of patients at day 30 after HSCT from haploidentical donors after correction for multiple comparisons (false discovery rate; see “Statistical analysis” for details).
NK cells that reconstitute at an early stage in vivo from CD34+ HSCs display distinct phenotypic characteristics intrinsic to their maturation. CD56bright (left panels) and CD56dim (right panels) NK-cell subsets of healthy blood donors (n = 31;  ) and of patients at day 30 after haploidentical (n = 22; ■), HLA-matched (related, n = 2, and unrelated, n = 4; □), and autologous (n = 5; ▧) HSCT were analyzed separately for expression of several NK-cell subset-specific receptors. From top to bottom: (A) inhibitory receptors (KIRs and NKG2A), (B) activating receptors (NKp30, NKp44, NKp46, NKG2D), (C) well-characterized markers of NK-cell functions: CD16 for antibody-dependent-cellular cytotoxicity (ADCC); CD25 and CD132, respectively, the α and γ chain of the IL-2 receptor; CD62L for lymph node homing; and CD117 (c-kit), which is expressed by hematopoietic progenitors, as well as by differentiating and mature CD56bright NK cells.34,35 Shown are the average percentages of expression and SE. Both donors and patients with a “null” KIR3DL1 phenotype were not considered in the analysis of KIR3DL1 expression. Asterisks indicate significant posthoc comparisons of patients at day 30 after HSCT from haploidentical donors after correction for multiple comparisons (false discovery rate; see “Statistical analysis” for details).
) and of patients at day 30 after haploidentical (n = 22; ■), HLA-matched (related, n = 2, and unrelated, n = 4; □), and autologous (n = 5; ▧) HSCT were analyzed separately for expression of several NK-cell subset-specific receptors. From top to bottom: (A) inhibitory receptors (KIRs and NKG2A), (B) activating receptors (NKp30, NKp44, NKp46, NKG2D), (C) well-characterized markers of NK-cell functions: CD16 for antibody-dependent-cellular cytotoxicity (ADCC); CD25 and CD132, respectively, the α and γ chain of the IL-2 receptor; CD62L for lymph node homing; and CD117 (c-kit), which is expressed by hematopoietic progenitors, as well as by differentiating and mature CD56bright NK cells.34,35 Shown are the average percentages of expression and SE. Both donors and patients with a “null” KIR3DL1 phenotype were not considered in the analysis of KIR3DL1 expression. Asterisks indicate significant posthoc comparisons of patients at day 30 after HSCT from haploidentical donors after correction for multiple comparisons (false discovery rate; see “Statistical analysis” for details).
NK cells that reconstitute at an early stage in vivo from CD34+ HSCs have impaired functional activity against leukemic targets
In line with previous reports,15 96% of NK cells at day 30 after haploidentical HSCT were positive for CD94:NKG2A, the inhibitory receptor for HLA-E (Figure 1A), and the supposedly alloreactive KIR+NKG2A− subpopulation of NK cells was not detected (data not shown).
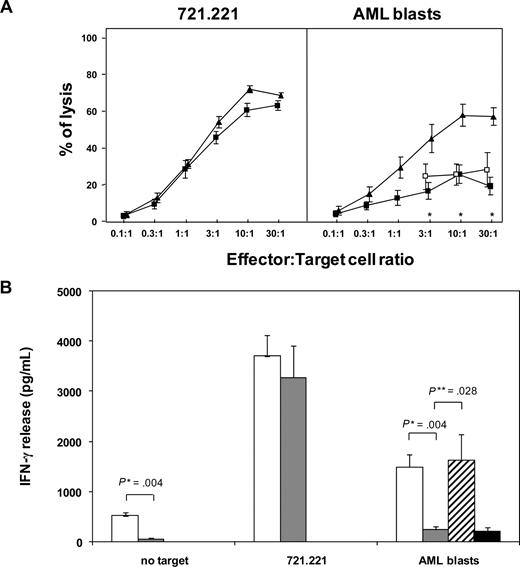
Therefore, at this time point after a short-course IL-2 activation we analyzed functional activity of the total population of patient NK cells. Concordant with previous observations,15,36 patient NK cells efficiently lysed the HLA class I–negative 721.221 BLCL. In contrast, they showed negligible levels of cytotoxicity against fresh AML blasts (at 30:1 effector:target ratio: 19% ± 4.6%), compared with healthy donor NK cells (57% ± 4.7%; P < .05). Contrary to what was reported by others,15 lysis of AML blasts by patient NK cells could not be restored by masking the inhibitory receptor NKG2A (Figure 2A). The cytotoxicity defect was apparently not due to an impaired response to IL-2, since it was evident also when patient and donor NK cells were compared without prior IL-2 activation, and since short-course IL-2 pretreatment consistently enhanced lysis of the 721.221 cell line (data not shown).
NK cells that reconstitute at an early stage in vivo from CD34+ HSCs have impaired functional activity against leukemic targets. (A) NK cells from 7 patients at 30 days after haploidentical HSCT (■) or from 5 healthy blood donors (▴) were tested in a standard 4-hour 51Cr release assay against the 721.221 BLCL (left panel) or against fresh allogeneic AML blasts (right panel) after overnight activation with 100 IU/mL IL-2. Patient NK cells were also tested against AML blasts in the presence of 20 μg/mL anti-NKG2A mAb (□). Shown are the average values with SE. Asterisks indicate effector-target ratios where differences between donors and patients after haploidentical HSCT were statistically significant. (B) NK cells from 6 patients at 30 days after haploidentical HSCT ( ) or from 5 healthy blood donors (□) were tested by standard ELISA for IFN-γ release after 24-hour incubation in response to medium containing 100 IU/mL rhIL-2 alone, the 721.221 BLCL, or fresh allogeneic AML blasts. Patient NK cells were also tested against AML blasts in the presence of 20 μg/mL anti-NKG2A mAb (
) or from 5 healthy blood donors (□) were tested by standard ELISA for IFN-γ release after 24-hour incubation in response to medium containing 100 IU/mL rhIL-2 alone, the 721.221 BLCL, or fresh allogeneic AML blasts. Patient NK cells were also tested against AML blasts in the presence of 20 μg/mL anti-NKG2A mAb ( ) or isotype control anti-NGFR mAb (■). Shown are the average values with SE. *P value from a Mann-Whitney U test; **P value from a Wilcoxon test for paired data.
) or isotype control anti-NGFR mAb (■). Shown are the average values with SE. *P value from a Mann-Whitney U test; **P value from a Wilcoxon test for paired data.
NK cells that reconstitute at an early stage in vivo from CD34+ HSCs have impaired functional activity against leukemic targets. (A) NK cells from 7 patients at 30 days after haploidentical HSCT (■) or from 5 healthy blood donors (▴) were tested in a standard 4-hour 51Cr release assay against the 721.221 BLCL (left panel) or against fresh allogeneic AML blasts (right panel) after overnight activation with 100 IU/mL IL-2. Patient NK cells were also tested against AML blasts in the presence of 20 μg/mL anti-NKG2A mAb (□). Shown are the average values with SE. Asterisks indicate effector-target ratios where differences between donors and patients after haploidentical HSCT were statistically significant. (B) NK cells from 6 patients at 30 days after haploidentical HSCT ( ) or from 5 healthy blood donors (□) were tested by standard ELISA for IFN-γ release after 24-hour incubation in response to medium containing 100 IU/mL rhIL-2 alone, the 721.221 BLCL, or fresh allogeneic AML blasts. Patient NK cells were also tested against AML blasts in the presence of 20 μg/mL anti-NKG2A mAb (
) or from 5 healthy blood donors (□) were tested by standard ELISA for IFN-γ release after 24-hour incubation in response to medium containing 100 IU/mL rhIL-2 alone, the 721.221 BLCL, or fresh allogeneic AML blasts. Patient NK cells were also tested against AML blasts in the presence of 20 μg/mL anti-NKG2A mAb ( ) or isotype control anti-NGFR mAb (■). Shown are the average values with SE. *P value from a Mann-Whitney U test; **P value from a Wilcoxon test for paired data.
) or isotype control anti-NGFR mAb (■). Shown are the average values with SE. *P value from a Mann-Whitney U test; **P value from a Wilcoxon test for paired data.
Likewise, IL-2–pretreated patient and donor NK cells released similar amounts of IFN-γ in response to the 721.221 BLCL, whereas the IFN-γ response of patient NK cells to allogeneic AML blasts was impaired (IFN-γ release from patient NK cells: 228 ± 65 pg/mL, compared with 1482 ± 388 pg/mL in donors; P = .004). Interestingly, addition of anti-NKG2A mAb was able to fully restore their cytokine release in response to allogeneic AML blasts (1639 ± 506 pg/mL), suggesting the presence of different effector mechanisms leading to cytotoxicity or cytokine release in these cells (Figure 2B).
NK-cell receptor repertoire reconstitution kinetics after haploidentical HSCT
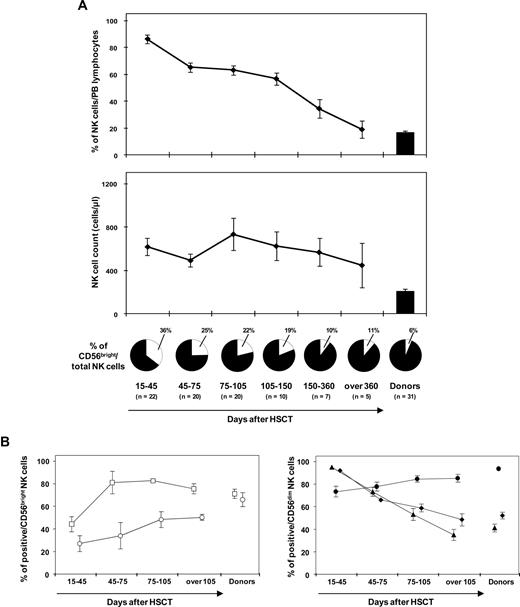
We next evaluated whether and when NK cells reconstituting after haploidentical HSCT recover a mature phenotype. The percentage of circulating NK cells steadily decreased after day 30 after HSCT, starting from day 60 (mean, 65%; SE, 3.5%; P < .001, random effect model), and became comparable to that of healthy donors after approximately 1 year after HSCT (mean, 19%; SE, 6.6%; P = .99; Figure 3A top panel). Absolute NK-cell counts remained fairly stable but always higher than the respective donors for the entire period of observation, with considerable variability among patients (Figure 3A bottom panel). Recovery of a CD56bright/CD56dim ratio comparable to healthy controls was achieved 5 months following HSCT (Figure 3A pies below the panels), with a significant decrease of CD56bright NK cells starting from day 60 (mean, 25%; SE, 3.7%; P = .02). Recovery of a repertoire superimposable to that of healthy blood donors for those receptors that were significantly deregulated at day 30 after HSCT (Figure 1) was observed in all patients and took a minimum of 3 months (Figure 3B).
Reconstitution of a mature, donor-type NK-cell repertoire after haploidentical HSCT takes at minimum 3 months. (A) Mean percentages, with SE, of PB CD56+CD3− cells over total circulating lymphocytes (top panel), absolute NK cell counts (bottom panel), and percentage of CD56bright NK cells within the total NK cell pool (pies below the panel) over time after haploidentical HSCT. (B) Recovery of cell surface markers for which statistically significant differences were observed in CD56bright (left panel) or CD56dim (right panel) at day 30 after haploidentical HSCT, compared with healthy blood donors (see asterisks in Figure 1). Mean percentages of expression, with SE, of CD25 (○), CD117 (□), NKG2A (▴), CD62L (♦), and CD16 (●) were analyzed at different time points after haploidentical HSCT in the relevant NK-cell subsets.
Reconstitution of a mature, donor-type NK-cell repertoire after haploidentical HSCT takes at minimum 3 months. (A) Mean percentages, with SE, of PB CD56+CD3− cells over total circulating lymphocytes (top panel), absolute NK cell counts (bottom panel), and percentage of CD56bright NK cells within the total NK cell pool (pies below the panel) over time after haploidentical HSCT. (B) Recovery of cell surface markers for which statistically significant differences were observed in CD56bright (left panel) or CD56dim (right panel) at day 30 after haploidentical HSCT, compared with healthy blood donors (see asterisks in Figure 1). Mean percentages of expression, with SE, of CD25 (○), CD117 (□), NKG2A (▴), CD62L (♦), and CD16 (●) were analyzed at different time points after haploidentical HSCT in the relevant NK-cell subsets.
Recovery kinetics and functional activity of single-KIR+ NK cells after haploidentical HSCT
Alloreactive donor NK cells, defined by their expression of a single-KIR whose ligand is missing in the patient and by their lack of CD94:NKG2A, have been cloned from the PB of patients in the first 3 to 4 months after haploidentical HSCT, but are difficult to isolate at later time points.10,13 However, as already shown by others,15 we could not detect single-KIR+/NKG2A− NK cells by day 60 after HSCT (data not shown), mainly due to high expression of CD94:NKG2A in NK cells that reconstitute at an early stage. We therefore addressed frequency and activity of single-KIR+ NK cells as soon as they became detectable in patients (ie, from day 75 after HSCT).
To this end, from our patient series we selected 11 donor-recipient pairs in which the donor by KIR genotyping was KIR2DL1+KIR2DS1− (n = 8; Table 2) and/or KIR2DL3+KIR2DL2−KIR2DS2− (n = 7; Table 3), thus allowing for specific detection of KIR2DL1 or KIR2DL3 on the NK-cell surface by use of the mAb EB6 and GL183, respectively. Single-KIR+ NK cells were quantified by flow cytometry as the subset of NK cells expressing the relevant inhibitory KIR but neither of the other KIRs nor CD94:NKG2A. Patients between days 75 and 156 after HSCT were studied comparatively with their donors, and 5 additional analyses were performed at 2 years after transplantation.
Characteristics of patients studied for single-KIR 2DL1+ NK cells
| UPN no. . | 13 . | 25 . | 37 . | 3 . | 17 . | 18 . | 23 . | 55 . |
|---|---|---|---|---|---|---|---|---|
| Predicted NK alloreactivity | yes | yes | yes | no | no | no | no | no |
| KIR 2DL1 ligand status, don/pat | +/− | +/− | +/− | +/+ | +/+ | +/+ | +/+ | +/+ |
| Diagnosis | AML | AML | AML | AML | AML | ALL | AML | MDS |
| Status at transplantation | Rel | CR2 | CR2 | Ref/per | CR1 | CR2 | CR1 | CR1 |
| Graft composition | ||||||||
| CD34+ cell dose, ×106/kg | 15.5 | 12.4 | 12.6 | 9.2 | 10.9 | 19.5 | 12.7 | 10.3 |
| CD3+ cell dose, ×104/kg | 0.9 | 1.0 | 1.0 | 0.3 | 1.2 | 0.8 | 1.2 | 1.1 |
| Day of KIR2DL1-single–positive NK cell analysis | 94 | 75 | 91 | 95 | 97 | 93 | 150 | 90 |
| No. of T-cell add-backs received at analysis | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 1 |
| Day of T-cell add-backs | 44, 72 | 25 | 56, 84 | − | 47 | 43, 72 | 45 | 34 |
| Total T-cell add-back dose received at analysis | 7 | 10 | 10 | − | 1 | 11 | 10 | 1 |
| UPN no. . | 13 . | 25 . | 37 . | 3 . | 17 . | 18 . | 23 . | 55 . |
|---|---|---|---|---|---|---|---|---|
| Predicted NK alloreactivity | yes | yes | yes | no | no | no | no | no |
| KIR 2DL1 ligand status, don/pat | +/− | +/− | +/− | +/+ | +/+ | +/+ | +/+ | +/+ |
| Diagnosis | AML | AML | AML | AML | AML | ALL | AML | MDS |
| Status at transplantation | Rel | CR2 | CR2 | Ref/per | CR1 | CR2 | CR1 | CR1 |
| Graft composition | ||||||||
| CD34+ cell dose, ×106/kg | 15.5 | 12.4 | 12.6 | 9.2 | 10.9 | 19.5 | 12.7 | 10.3 |
| CD3+ cell dose, ×104/kg | 0.9 | 1.0 | 1.0 | 0.3 | 1.2 | 0.8 | 1.2 | 1.1 |
| Day of KIR2DL1-single–positive NK cell analysis | 94 | 75 | 91 | 95 | 97 | 93 | 150 | 90 |
| No. of T-cell add-backs received at analysis | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 1 |
| Day of T-cell add-backs | 44, 72 | 25 | 56, 84 | − | 47 | 43, 72 | 45 | 34 |
| Total T-cell add-back dose received at analysis | 7 | 10 | 10 | − | 1 | 11 | 10 | 1 |
MDS indicates myelodysplastic syndrome; don, donor; pat, patient; and −, not applicable.
At analysis, all patients were in complete remission, did not have GVHD, and were not on immunosuppressive therapy.
Characteristics of patients studied for single-KIR 2DL3+ NK cells
| UPN no. . | 10 . | 18 . | 54 . | 3 . | 16 . | 17 . | 55 . |
|---|---|---|---|---|---|---|---|
| Predicted NK alloreactivity | yes | yes | yes | no | no | no | no |
| KIR 2DL3 ligand status, don/pat | +/− | +/− | +/− | +/+ | −/+ | +/+ | −/− |
| Diagnosis | AML | ALL | AML | AML | AML | AML | MDS |
| Status at transplantation | CR1 | CR2 | CR2 | Ref/per | Rel | CR1 | CR1 |
| Graft composition | |||||||
| CD34+ cell dose, ×106/kg | 7.0 | 19.5 | 12.1 | 9.2 | 12.3 | 10.9 | 10.3 |
| CD3+ cell dose, ×104/kg | 0.4 | 0.8 | 1.2 | 0.3 | 1.0 | 1.2 | 1.1 |
| Day of KIR2DL3-single positive NK-cell analysis | 156 | 105 | 86 | 151 | 75 | 97 | 115 |
| No. of T-cell add-backs received at analysis | 1 | 2 | 1 | 2 | 2 | 1 | 1 |
| Day of T-cell add-backs | 98 | 43, 72 | 34 | 104, 143 | 37, 75 | 47 | 34 |
| Total T-cell add-back dose received at analysis | 10 | 11 | 0.1 | 11 | 11 | 1 | 1 |
| Disease status at analysis | CR | CR | CR | Relapse | CR | CR | CR |
| UPN no. . | 10 . | 18 . | 54 . | 3 . | 16 . | 17 . | 55 . |
|---|---|---|---|---|---|---|---|
| Predicted NK alloreactivity | yes | yes | yes | no | no | no | no |
| KIR 2DL3 ligand status, don/pat | +/− | +/− | +/− | +/+ | −/+ | +/+ | −/− |
| Diagnosis | AML | ALL | AML | AML | AML | AML | MDS |
| Status at transplantation | CR1 | CR2 | CR2 | Ref/per | Rel | CR1 | CR1 |
| Graft composition | |||||||
| CD34+ cell dose, ×106/kg | 7.0 | 19.5 | 12.1 | 9.2 | 12.3 | 10.9 | 10.3 |
| CD3+ cell dose, ×104/kg | 0.4 | 0.8 | 1.2 | 0.3 | 1.0 | 1.2 | 1.1 |
| Day of KIR2DL3-single positive NK-cell analysis | 156 | 105 | 86 | 151 | 75 | 97 | 115 |
| No. of T-cell add-backs received at analysis | 1 | 2 | 1 | 2 | 2 | 1 | 1 |
| Day of T-cell add-backs | 98 | 43, 72 | 34 | 104, 143 | 37, 75 | 47 | 34 |
| Total T-cell add-back dose received at analysis | 10 | 11 | 0.1 | 11 | 11 | 1 | 1 |
| Disease status at analysis | CR | CR | CR | Relapse | CR | CR | CR |
At analysis, no patient had GVHD or was on immunosuppressive therapy.
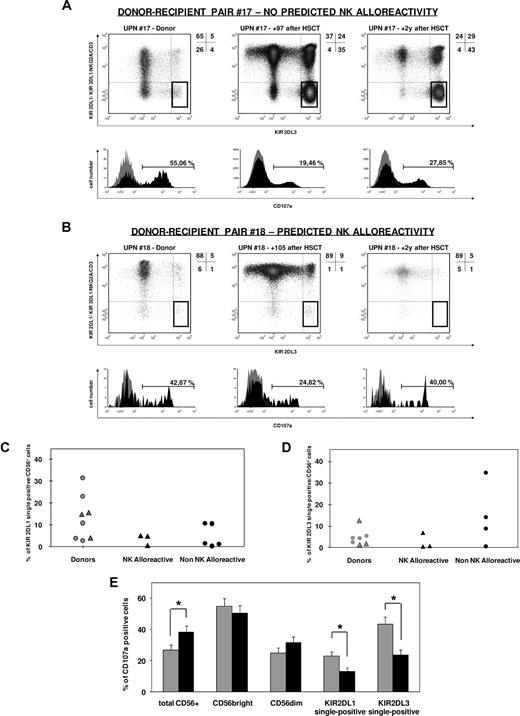
As shown in Figure 4A-D, single-KIR+ NK cells were present in both the donor and in the recipient after transplantation, with variable frequency that did not show any apparent correlation with the presence or absence of donor NK alloreactivity. Importantly, neither single-KIR2DL1+ nor single-KIR2DL3+ NK cells were expanded in any of the patients from the NK alloreactive group (Figure 4B-D). In line with previous reports,37,38 total KIR2DL1+ NK cells showed a correlation in frequency between patients and donor (r = 0.71, P = .047) that was not significant for total KIR2DL3+ NK cells (r = 0.38, P = .40). Interestingly, no such correlation was observed for single-KIR2DL1+ or single-KIR2DL3+ NK cells (r = 0.31, P = .46 and r = 0.10, P = .82, respectively).
Single-KIR+ NK cells after haploidentical HSCT are highly variable in frequency and hyporesponsive, independently from predicted NK alloreactivity. (A,B) CD107a mobilization of single-KIR2DL3+ NK cells in response to the 721.221 BLCL in patients at 75 to 150 days or more than 2 years after haploidentical HSCT, and their HSC donors, was analyzed as described in “CD107a mobilization assay.” Donors carried the KIR2DL3 gene but not KIR2DL2 or KIR2DS2, thus allowing for specific detection of KIR2DL3 by use of the mAb GL183. Out of a first gate on CD56+ lymphocytes, a subsequent gate was selected for cells positive for KIR2DL3 and negative for CD3, NKG2A, KIR2DL1/2DS1, and KIR3DL1 (single-KIR2DL3+ NK cells, highlighted gates in each density plot). CD107a mobilization by this subset of cells was assessed after a 6-hour incubation with high-dose rhIL-2 in the presence (black profile in histograms) or in the absence (gray profile in histograms) of the 721.221 HLA class I–deficient BLCL. Shown are one representative nonalloreactive (A) and one alloreactive (B) donor-recipient pairs. Frequency of KIR2DL1 (C) or KIR2DL3 (D) single-positive NK cells in donors (gray symbols) and in patients at 3 to 5 months following haploidentical HSCT (black symbols), in the presence or absence of predicted NK alloreactivity (triangles and circles, respectively). (E) Mean percentage of CD107a mobilization, with SE, by total CD56+, CD56bright, CD56dim, single-KIR2DL1+, or single-KIR2DL3+ NK cells in patients at days 75 to 150 after haploidentical HSCT ( ) or in their donors (
) or in their donors ( ). Asterisks indicate a P value of .05 or less from a Wilcoxon test for paired data.
). Asterisks indicate a P value of .05 or less from a Wilcoxon test for paired data.
Single-KIR+ NK cells after haploidentical HSCT are highly variable in frequency and hyporesponsive, independently from predicted NK alloreactivity. (A,B) CD107a mobilization of single-KIR2DL3+ NK cells in response to the 721.221 BLCL in patients at 75 to 150 days or more than 2 years after haploidentical HSCT, and their HSC donors, was analyzed as described in “CD107a mobilization assay.” Donors carried the KIR2DL3 gene but not KIR2DL2 or KIR2DS2, thus allowing for specific detection of KIR2DL3 by use of the mAb GL183. Out of a first gate on CD56+ lymphocytes, a subsequent gate was selected for cells positive for KIR2DL3 and negative for CD3, NKG2A, KIR2DL1/2DS1, and KIR3DL1 (single-KIR2DL3+ NK cells, highlighted gates in each density plot). CD107a mobilization by this subset of cells was assessed after a 6-hour incubation with high-dose rhIL-2 in the presence (black profile in histograms) or in the absence (gray profile in histograms) of the 721.221 HLA class I–deficient BLCL. Shown are one representative nonalloreactive (A) and one alloreactive (B) donor-recipient pairs. Frequency of KIR2DL1 (C) or KIR2DL3 (D) single-positive NK cells in donors (gray symbols) and in patients at 3 to 5 months following haploidentical HSCT (black symbols), in the presence or absence of predicted NK alloreactivity (triangles and circles, respectively). (E) Mean percentage of CD107a mobilization, with SE, by total CD56+, CD56bright, CD56dim, single-KIR2DL1+, or single-KIR2DL3+ NK cells in patients at days 75 to 150 after haploidentical HSCT ( ) or in their donors (
) or in their donors ( ). Asterisks indicate a P value of .05 or less from a Wilcoxon test for paired data.
). Asterisks indicate a P value of .05 or less from a Wilcoxon test for paired data.
Importantly, the functional reactivity of single-KIR2DL1+ and single-KIR2DL3+ NK cells was invariably impaired in 15 independent analyses performed in 11 patients at 3 to 5 months after transplantation. In particular, an average of 13% plus or minus 2.1% single-KIR2DL1+ NK cells responded to the 721.221 BLCL compared with 23% plus or minus 2.4% in the donors (P = .01); and an average of 24% plus or minus 3.2% single-KIR2DL3+ NK cells, compared with 43% plus or minus 4.7% in the donors (P = .02; Figure 4A,B,E). Functional activity was partly recovered in 5 analyses of patients at 2 years after transplantation (Figure 4A,B and data not shown). Interestingly, hyporesponsiveness of patient NK cells in the first months after transplantation was limited to the single-KIR+ NK subset, but it was not observed in the analysis of CD56bright/dim subsets at the same time point (P = n.s.), and total CD56+ cell activity was even significantly higher in patient cells (P = .02, Figure 4E) compared with the donors.
Role of KIR ligands in determining frequency and functionality of reconstituting KIR-positive cells
The donor-recipient pairs described in the previous paragraph were used to analyze the role of interactions between KIRs and their ligands in determining the frequency and activity of single-KIR+ NK cells.
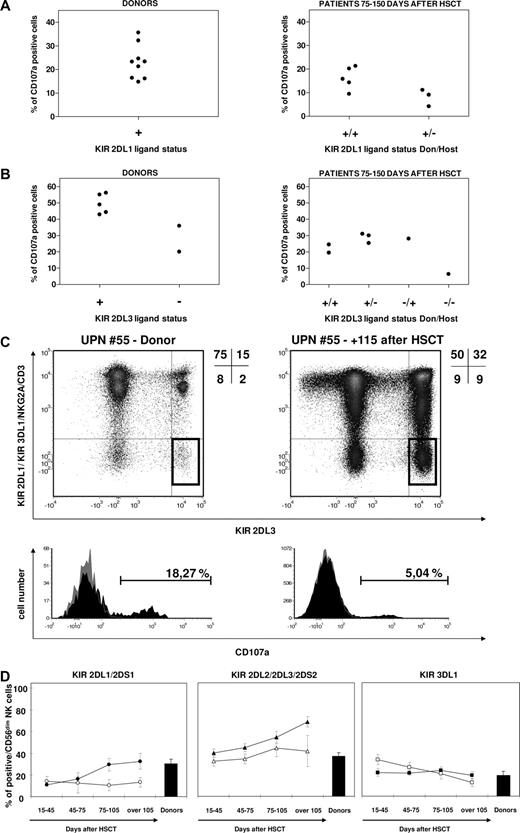
Partly because NK alloreactivity (ie, donor positive and patient negative for the KIR ligand) was used as criteria for preferential donor selection in this study, only 2 donors negative for the KIR2DL3 HLA-C group 1 (HLA-C1) ligand and none negative for the KIR2DL1 HLA-C group 2 (HLA-C2) ligand were present in our cohort (Figure 5A,B). In accordance with recently published observations,19,22 single-KIR2DL3+ NK cells were present in HSC donors missing the HLA-C1 ligand, but were hyporesponsive when challenged with 721.221 cells, compared with their counterparts in donors carrying the ligand (20% and 35% in the 2 HLA-C1 ligand–negative donors against 49% ± 2.7% in the 5 HLA-C1 ligand–positive donors; Figure 5A-C). In the only informative pair in which both donor and recipient were negative for the KIR2DL3 ligand, at 120 days after haploidentical HSCT single-KIR2DL3+ NK cells were present in the patient and were even expanded compared with the donor (9% vs 2% of circulating CD56+ cells), but had negligible reactivity against 721.221 cells (Figure 5B,C) compared with their counterparts in the donor or in other studied patients. In the latter, activity of single-KIR2DL3+ NK cells was similar regardless of the different donor-recipient KIR2DL3 ligand combinations (Figure 5A,B), but always lower compared with the donors.
KIR ligands play a role in determining frequency and functionality of reconstituting KIR-positive cells. CD107a mobilization in response to the 721.221 BLCL by KIR2DL1 (A) and KIR2DL3 (B) single-positive NK cells from donors (left panels) and patients 75 to 150 days after haploidentical HSCT (right panels), subgrouped according to KIR ligand status. (C) Frequency (highlighted gates in dot plots) and CD107a mobilization in response to the 721.221 BLCL (histograms) of single-KIR2DL3+ NK cells in the donor (left) and in the patient 4 months after haploidentical HSCT (right) in the single donor-recipient pair in which both were negative for KIR2DL3 HLA-C1 ligand. (D) Mean percentages, with SE, of total KIR expression over time in CD56dim NK cells after haploidentical HSCT in patients carrying (filled symbols) or lacking (open symbols) the HLA-ligand for the group of KIRs under analysis. Both donors and patients with a null KIR3DL1 phenotype were excluded from the analysis of KIR3DL1 expression recovery.
KIR ligands play a role in determining frequency and functionality of reconstituting KIR-positive cells. CD107a mobilization in response to the 721.221 BLCL by KIR2DL1 (A) and KIR2DL3 (B) single-positive NK cells from donors (left panels) and patients 75 to 150 days after haploidentical HSCT (right panels), subgrouped according to KIR ligand status. (C) Frequency (highlighted gates in dot plots) and CD107a mobilization in response to the 721.221 BLCL (histograms) of single-KIR2DL3+ NK cells in the donor (left) and in the patient 4 months after haploidentical HSCT (right) in the single donor-recipient pair in which both were negative for KIR2DL3 HLA-C1 ligand. (D) Mean percentages, with SE, of total KIR expression over time in CD56dim NK cells after haploidentical HSCT in patients carrying (filled symbols) or lacking (open symbols) the HLA-ligand for the group of KIRs under analysis. Both donors and patients with a null KIR3DL1 phenotype were excluded from the analysis of KIR3DL1 expression recovery.
HLA-C ligands also appeared to have some modulating effect on the total number of NK cells expressing the KIR2DL1/2DS1 and KIR2DL2/2DL3/2DS3 groups in the 22 patients analyzed. Higher frequencies of total KIR2DL2/2DL3/2DS3+ and total KIR2DL1/2DS1+ NK cells were observed in patients carrying the relevant HLA-C ligands, compared with those lacking the ligands, although these differences did not reach statistical significance (P = .13 for KIR2DL1/2DS1+ and P = .07 for KIR2DL2/2DL3/2DS3+). In contrast, no apparent influence in the presence or absence of HLA-Bw4 in the patients was observed on the recovery of total KIR3DL1+ NK cells (Figure 5D).
The ligand-ligand model does not predict clinically relevant NK alloreactivity in our series of haploidentical HSCTs
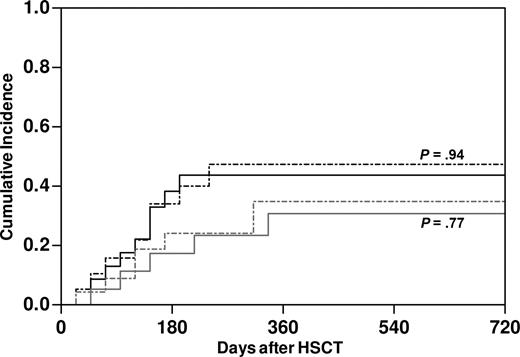
The impact on clinical outcome of NK alloreactivity, as predicted by the ligand-ligand model,10-12 was assessed in retrospective analysis of 56 haploidentical transplantations performed for high-risk malignancies in our center. Of note, our protocol differed from those adopted in previous studies,3,10,13,14 both in the administration of T-cell add-backs in the early posttransplantation phase to 35 of the patients and in the conditioning regimens used (Table 1). In the total cohort, predicted NK alloreactivity did not have any impact on immune rejection or death before engraftment (Table 4). Thus, we assessed its role on major clinical end points in the 43 patients informative for HSC engraftment. Within this group, 67% of patients suffered from AML, and 56% were in complete remission at the time of transplantation. Both the competitive risks of disease relapse and TRM were strikingly similar between patients with (n = 23) or without (n = 20) predicted NK alloreactivity (Figure 6). This observation held true also when possibly relevant factors were considered in multivariate analysis, namely underlying disease, disease status at transplantation, conditioning regimen, and infusion of T-cell add-backs. Furthermore, predicted NK alloreactivity did not affect occurrence of aGVHD nor overall survival (OS) in this cohort (data not shown).
Clinical outcome and predicted NK alloreactivity
| . | All, no. (%) . | NK alloreactivity, no. (%) . | No NK alloreactivity, no. (%) . | P . |
|---|---|---|---|---|
| All patients | 56 | 27 | 29 | |
| Graft rejection | 6 (10.7) | 2 (7.4) | 4 (13.8) | .73 |
| Death before engraftment | 7 (12.5) | 2 (7.4) | 5 (17.2) | .48 |
| Informative patients for engraftment | 43 | 23 | 20 | |
| Disease relapse | 13 (30.2) | 8 (34.8) | 5 (25) | .77* |
| Transplant-related mortality | 17 (39.5) | 9 (39.1) | 8 (40) | .94* |
| aGvHD | 11 (25.6) | 8 (34.8) | 3 (15) | .18 |
| Overall grade I | 2 (4.6) | 1 (4.3) | 1 (5) | |
| Overall grade II | 7 (16.3) | 5 (21.7) | 2 (10) | |
| Overall grade III | 2 (4.6) | 2 (8.7) | 0 | |
| Overall grade IV | 0 | 0 | 0 | |
| T-cell reconstitution at day 120 after HSCT† | 20 (46.5) | 10 (43.5) | 10 (50) | .94 |
| . | All, no. (%) . | NK alloreactivity, no. (%) . | No NK alloreactivity, no. (%) . | P . |
|---|---|---|---|---|
| All patients | 56 | 27 | 29 | |
| Graft rejection | 6 (10.7) | 2 (7.4) | 4 (13.8) | .73 |
| Death before engraftment | 7 (12.5) | 2 (7.4) | 5 (17.2) | .48 |
| Informative patients for engraftment | 43 | 23 | 20 | |
| Disease relapse | 13 (30.2) | 8 (34.8) | 5 (25) | .77* |
| Transplant-related mortality | 17 (39.5) | 9 (39.1) | 8 (40) | .94* |
| aGvHD | 11 (25.6) | 8 (34.8) | 3 (15) | .18 |
| Overall grade I | 2 (4.6) | 1 (4.3) | 1 (5) | |
| Overall grade II | 7 (16.3) | 5 (21.7) | 2 (10) | |
| Overall grade III | 2 (4.6) | 2 (8.7) | 0 | |
| Overall grade IV | 0 | 0 | 0 | |
| T-cell reconstitution at day 120 after HSCT† | 20 (46.5) | 10 (43.5) | 10 (50) | .94 |
P value estimated by a competing risk analysis.
Defined as CD3+ cell count 100/μL or more by day 120.
No effect of predicted donor NK alloreactivity on the clinical outcome of haploidentical HSCT in our patient series. Cumulative incidence of relapse of underlying disease (gray lines) and TRM (black lines) in patients with (dashed lines, n = 23) and without (full line, n = 20) predicted NK alloreactivity. P values were estimated by a competing risk analysis.
No effect of predicted donor NK alloreactivity on the clinical outcome of haploidentical HSCT in our patient series. Cumulative incidence of relapse of underlying disease (gray lines) and TRM (black lines) in patients with (dashed lines, n = 23) and without (full line, n = 20) predicted NK alloreactivity. P values were estimated by a competing risk analysis.
Discussion
In this report, we have addressed NK cell reconstitution in patients undergoing highly purified CD34+ HSCT for high-risk hematologic malignancies, and its correlation with clinical outcome of this treatment in a cohort of 56 patients, the largest analyzed since the Perugia studies.3,10,13,14 Of note, most of our patients received a myeloablative chemotherapy-based conditioning and GMP-manipulated donor T-cell add-backs after transplantation to promote immune reconstitution.
Our studies demonstrate that NK cells that reconstitute at an early stage in vivo from CD34+ HSCs present distinctive phenotypic features that are likely to reflect physiologic NK cell maturation stages (Figure 1). Early arousal of CD56bright cells followed by CD56dim cell recovery after HSCT supports reported evidence for CD56bright-to-CD56dim NK cell maturation,34,39-41 and receptor profiling suggests that differentiation takes place through sequential discrete CD56brightCD25−CD117− and CD56dimCD16lowCD62L+NKG2A+ stages. We show that the process of maturation after haploidentical HSCT takes at least 3 months (Figure 3A,B), and that fine-tuning of KIR and CD94:NKG2A expression on developing NK cells is an even slower process that occurs over several months after HSCT.
Currently, no definitive notion supports the ability of NK cells to proliferate specifically in response to their targets, so quantification of the relevant effectors in vivo is important to evaluate their overall efficiency. In our analyses, the frequency of single-KIR+ NK cells, mediators of NK alloreactivity after HSCT into patients lacking the relevant ligand, was negligible until day 75 after haploidentical HSCT and highly variable thereafter (Figure 4). Of note, none of the 6 patients with predicted NK alloreactivity studied for KIR2DL1 or KIR2DL3 showed a relative expansion of the relevant single-KIR+ cells compared with their donors, and in 3 of these patients, the single-KIR+ subset accounted for less than 1% of NK cells.
Apart from their number, single-KIR+ NK cells that arise early from CD34+ HSCs have not yet acquired full functional competence. In fact, single-KIR+ NK cells from 11 patients in the first months after transplantation had significantly lower functional activity against 721.221 targets compared with their counterparts in the respective stem cell donors, both in the single-KIR2DL1+ and the single-KIR2DL3+ subset (Figure 4). Recovery of functional activity comparable to donor levels in 5 analyses of patients studied at more than 2 years after HSCT suggests that acquisition of effector function is eventually achieved but is a slow process that lags behind the recovery of phenotypic maturity.
Our data show that functional impairment at this time point is limited to the single-KIR+ subset (Figure 4E). Considering that most NK cells express a combination of different inhibitory receptors,12,18 our observations are consistent with recently published data22 demonstrating that cells carrying multiple inhibitory receptors acquire functional competence more efficiently and sooner than those relying on the interaction of one single receptor with its ligand.
Single-KIR+ NK cells arising in patients after haploidentical HSCT are an ideal model to study the mechanisms shaping the NK cell repertoire to avoid autoimmune aggression. In the only KIR ligand–negative patient who received a transplant from a KIR ligand–negative donor, single-KIR+ NK cells were markedly hyporesponsive compared with their counterparts in other patients who underwent transplantation (Figure 5B,C). Unfortunately, given the rarity of informative donor-recipient pairs that need to be selected on the basis of KIR ligand negativity in patient and donor as well as of the absence in the donor of confounding genes encoding for activating KIRs, we were so far not able to extend this type of analysis to additional patients. Although preliminary, our findings suggest that acquisition of functional activity by NK cells is governed by a process of licensing by the presence of KIR ligands, also active after transplantation; and that licensing takes place irrespective of whether the ligand is expressed by hematopoietic (donor) or nonhematopoietic (host) cells. In any case, it is possible, and in full concordance with our data on KIR recovery in the total NK cell population (Figure 5D), that the process of repertoire shaping may subsequently be regulated by additional mechanisms to limit the frequency of unlicensed cells by deletion or by induction of inhibitory receptors recognizing self-ligands.
It should be noted that single-KIR+ NK cells were analyzed ex vivo for most patients after at least one donor T-cell add-back (Table 2). This might have had an impact on their functional activity, since a cross-talk between T cells, NK cells, and antigen-presenting cells (APCs) has been shown to govern the biology of these cell subsets.42,43 On the other hand, protocols of haploidentical HSCT are being more and more widely modified to include T-cell add-backs to overcome the important infectious transplantation complications associated with stringent T-cell depletion, and this must be taken into account when studying NK cell reconstitution and its clinical relevance in these transplantations. Importantly, none of our patients received immunosuppressive treatment at the time of or prior to ex vivo functional analysis of single-KIR+ NK cells, ruling out the possibility that impaired function of these cells might be related to interference by pharmacological treatment.
Ultimately, predicted donor-recipient KIR ligand incompatibility had no impact on the overall satisfactory clinical outcome observed in the large, although partly heterogeneous, patient series analyzed in this study (Table 4; Figure 6), not even in the 16 patients with AML who underwent transplantation in complete remission, a condition in which predicted NK alloreactivity has been reported to be more relevant3,10,13,14 (data not shown). Several considerations can be made to link these clinical observations with our biologic findings ex vivo. The considerable time lapse that is necessary for full CD56dim effector NK cell maturation from CD34+ HSCs suggests that NK cells circulating during the first 2 months after HSCT provide no efficient antileukemic immunity in this important period for minimal residual disease control. Even later, frequency of single-KIR+ NK cells is highly variable among patients, a confounding factor not considered in the algorithm for alloreactivity prediction, suggesting that quantitative analyses should be performed also in other retrospective studies to identify the number of alloreactive NK cells sufficient to play a relevant clinical role. The conditioning regimens adopted for our patients, although myeloablative, were mostly chemotherapy based rather than irradiation based, and the relevance on NK cell biology of this difference from the Perugia protocol should be addressed in future studies. Finally, as discussed above, T-cell add-backs given to most of our patients starting from day 30 after HSCT, although unlikely to be relevant in determining the phenotypic and functional defects observed in the first 2 months after HSCT, might have subsequently delayed NK cell reconstitution, or masked less pronounced effects related to NK alloreactivity on clinical outcome.44,45
Taken together, our data shed new light on NK cell differentiation after haploidentical HSCT. Reconstitution of single-KIR+ alloreactive NK cells from CD34+ hematopoietic stem cells is hampered by several features intrinsic to NK cell physiologic maturation, and even when alloreactive NK cells arise, it is unpredictable how many and how functional they will be. In view of these considerations, we suggest to favor the use of mature donor-derived single-KIR+ alloreactive NK cells for adoptive immunotherapy of high-risk leukemia, either after immunophenotypic selection from total NK cells or as NK cell clones. Feasibility of immunotherapy with mature NK cells in the haploidentical setting has been proven,46-48 and even transfer of unselected mature alloreactive NK cells can provide the patient with an important clinical benefit.44,49 Fine-tuning of appropriate methods for selection and expansion of mature single-KIR+ alloreactive NK cells is warranted to fully exploit NK alloreactivity in new protocols for adoptive immunotherapy following transplantation, for control of minimal residual disease limiting the risk of concomitant aGVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Thorsten Graef and Dr Achim Klaus Moesta for critical reading of the paper.
This study was supported by grants from the Italian Ministry of Health (Rome, Italy; grants no. 166 and 122) and the Associazione Italiana per la Ricerca sul Cancro (AIRC, Milano, Italy).
Authorship
Contribution: L.V. designed and performed research, and wrote the paper; B.F. performed experimental tests, and discussed and critically read the paper; M.P.S. and R.C. performed statistical analyses; E.Z. supervised experimental assays; S.D.T. and D.M. performed and provided counseling for KIR genotyping; B.M. performed HLA and KIR typing; S.K.P., A.B., S.R., M.G.R., and C. Bordignon provided advice and participated in general discussion; A.P. supervised flow cytometry; M.T.L.S., M.B., R.B., and J.P. clinically managed patients; C. Bonini and F.C. counseled experimental setup and participated in critical discussion; and K.F. supervised experimental setup, evaluated data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katharina Fleischhauer, San Raffaele Telethon Institute for Gene Therapy (HSR-TIGET), Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) H San Raffaele, via Olgettina 60, 20132, Milano, Italy; e-mail: fleischhauer.katharina@hsr.it.