Abstract
Imatinib mesylate (IM, Gleevec) has largely supplanted allogeneic hematopoietic cell transplantation (HCT) as first line therapy for chronic myeloid leukemia (CML). Nevertheless, many people with CML eventually undergo HCT, raising the question of whether prior IM therapy impacts HCT success. Data from the Center for International Blood and Marrow Transplant Research on 409 subjects treated with IM before HCT (IM+) and 900 subjects who did not receive IM before HCT (IM−) were analyzed. Among patients in first chronic phase, IM therapy before HCT was associated with better survival but no statistically significant differences in treatment-related mortality, relapse, and leukemia-free survival. Better HLA-matched donors, use of bone marrow, and transplantation within one year of diagnosis were also associated with better survival. A matched-pairs analysis was performed and confirmed a higher survival rate among first chronic phase patients receiving IM. Among patients transplanted with advanced CML, use of IM before HCT was not associated with treatment-related mortality, relapse, leukemia-free survival, or survival. Acute graft-versus-host disease rates were similar between IM+ and IM− groups regardless of leukemia phase. These results should be reassuring to patients receiving IM before HCT.
Introduction
Chronic myeloid leukemia (CML) used to be the leading indication for allogeneic hematopoietic cell transplantation (HCT) from unrelated donors, reflecting poor options for prolonged disease control with available medical therapy.1 However, initial reports of frequent cytogenetic responses with imatinib mesylate (IM, Gleevec or Glivec, Novartis, East Hanover, NJ) were published in 1999, and follow-up of patients treated only with IM indicates that rates of complete cytogenetic and major molecular responses are high and durable, whereas drug toxicity is low.2 The U.S. Food and Drug Administration approved IM in May 2001, and its widespread availability has changed CML treatment. Major position statements now recommend allogeneic HCT only after a trial of IM,3-5 and there has been a dramatic decrease in the number of allogeneic transplantation procedures for CML.6 Almost all persons with CML in economically advantaged countries will be treated with IM before considering HCT.7,8
Because detectable CML tends to reappear if IM is stopped, IM therapy cannot be considered curative at this time, leading to the general recommendation that IM be continued indefinitely.4 Median follow-up in the large IM studies is approximately 6 years.2,9 Ablative allogeneic HCT is the curative therapy with the longest track record for CML. Data from large studies report 5-year disease-free survival rates of 60% to 80%10 and 20-year survival rates of 40%.11 A potential risk is that the widespread and prolonged use of IM will compromise the excellent outcomes obtained with allogeneic transplantation. Poorer long-term outcomes could result from direct drug toxicity,12 compromised organ function,13 immune dysfunction,14 selection of resistant or more aggressive clones before transplantation, a delay in transplantation beyond one year from diagnosis, more advanced disease at the time of transplantation, or inability (because of age, inability to find a donor, limited insurance coverage, etc) to undergo transplantation once disease progresses.
Several retrospective studies have suggested no increase in treatment-related mortality if patients receive IM before allogeneic HCT.15-20 However, most of these studies reported smaller numbers of patients with heterogeneous characteristics, and the populations were high risk so that subtle alterations in morbidity and mortality from IM may have not been detected.
The resources and volume of cases reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) provide a means to overcome some of these limitations. Therefore, we analyzed observational data to assess whether exposure to IM before allogeneic HCT is associated with higher or lower treatment-related mortality, relapse, and overall survival compared with patients not exposed to IM before transplantation.
Methods
Data sources
The National Marrow Donor Program (NMDP) provides unrelated donor stem cells to facilitate transplantations throughout the world and maintains a longitudinal outcomes database on the procedures it facilitates. A formal affiliation of the research division of the NMDP, the International Bone Marrow Transplant Registry, and the Autologous Blood and Marrow Transplant Registry led to establishment of the CIBMTR in 2004. The CIBMTR is a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic HCTs to the Statistical Center at the Medical College of Wisconsin in Milwaukee or the NMDP Coordinating Center in Minneapolis. Approximately two thirds of all active transplantation centers worldwide report data to the registry. The registry database includes information on 40% to 45% of all patients who have received an allogeneic transplantation since 1970, with annual updates. The CIBMTR collects data at 2 levels: registration and research. Registration data include disease type, age, sex, pretransplantation performance status, disease stage and chemotherapy-responsiveness, date of diagnosis, donor and graft type (bone marrow- and/or blood-derived stem cells), high-dose conditioning regimen, posttransplantation engraftment, disease recurrence and survival, development of a new malignancy, and cause of death. Requests for data on disease recurrence or death for registered patients are at 6-month intervals. All CIBMTR centers contribute registration data on all patients. Research data are collected on subsets of registered patients selected using a weighted randomization scheme, including comprehensive pretransplantation and posttransplantation clinical information. Compliance is assessed by periodic audits, and accuracy of data is ensured by computerized record checks, physician review of submitted data, and on-site audits. Observational studies conducted by the CIBMTR are done with a waiver of informed consent obtained in accordance with the Declaration of Helsinki and in compliance with Health Insurance Portability Accountability Act regulations as determined by the Institutional Review Board and Privacy Officer of Medical College of Wisconsin.
Patients
Eligible subjects were patients undergoing first allogeneic HCT for CML between 1999 and 2004, reported to the CIBMTR. Syngeneic twin or cord blood recipients were excluded. Furthermore, to minimize potential bias, only transplantation centers providing data on 80% or more of patients receiving IM and also transplanting patients who did not receive pretransplantation IM during the study period were included.
Data collection
In addition to standard CIBMTR forms, teams completed a 3-page supplementary data form on subjects who received IM before HCT. A total of 476 of 702 requested forms were completed (67.8%). Comparison of patients for whom supplementary data were and were not available showed form completion was associated with cytomegalovirus (CMV)–negative donor and recipient serologic status, white race, traditional ablative preparative regimens, and later-stage disease. However, forms completion was most predicted by transplantation center, with centers either submitting all or none of their supplementary forms, suggesting that forms completion is primarily a center characteristic (data not shown).
Data were derived from both registration forms (which collect abbreviated information) and full research forms. Some details, such as posttransplantation organ toxicities, are requested only on the full research forms.
Definitions
Early phase disease was considered first chronic phase, regardless of duration. Advanced disease phase included accelerated phase, second or later chronic phase, and blast crisis according to CIBMTR criteria. Patients receiving IM before HCT were designated IM+, whereas patients not receiving IM were IM−. Treatment-related mortality (TRM) was defined as death resulting from any cause while CML was in remission. Acute graft-versus-host disease (GVHD) grades II to IV and extensive chronic GVHD were classified according to CIBMTR criteria. Relapse of CML was defined by hematologic or cytogenetic evidence of disease. Data on molecular evidence of relapsed disease were not available. Leukemia-free survival was considered the time to death or relapse, whereas overall survival was calculated from the day of graft infusion. The European Blood and Marrow Transplantation (EBMT) risk score was calculated based on donor type, disease stage, patient age, donor-recipient sex match, and interval between diagnosis and HCT.21
Statistical analysis
Patient-, disease-, and transplantation-related factors were compared between groups using the χ2 test for categorical variables and the Wilcoxon 2-sample test for continuous variables. The product-limit estimator proposed by Kaplan-Meier was used to estimate the median and range of the follow-up time. The probabilities of overall survival and leukemia-free survival for all patients were calculated using the Kaplan-Meier estimator, with the variance estimated by Greenwood's formula. Patients were censored at date of last known follow-up. Cumulative incidence estimates were calculated for other endpoints to account for competing risks.22,23 Data were analyzed using the Cox proportional hazards regression model with a multivariate analysis performed to identify clinical variables that were associated with particular outcomes. Potential interactions between significant covariates were assessed and were not present. Because of multiple testing, a P value less than .01 was considered statistically significant.
All multivariate analyses were adjusted for statistically significant covariates using stepwise forward-backward selection. Potential covariates included patient age, sex and race, Karnofsky performance status, time from diagnosis to HCT, donor type, donor-recipient sex match and CMV serologic status, human leukocyte antigen (HLA) matching grade, type of conditioning regimen, graft source, year of transplantation, and GVHD prophylaxis regimen. The imatinib exposure variable (IM+ vs IM−) was included in all models, which included both populations.
Separate analyses were conducted for patients transplanted in first chronic phase and those with more advanced disease. In a separate subgroup analysis, factors associated with survival and TRM were assessed among patients in the IM+ group. Potential predictors included reason for proceeding to HCT, best response to IM before HCT, duration of IM treatment, and interval between IM discontinuation and HCT, in addition to the other potential clinical predictors listed in the preceding paragraph. Adequate details about cytogenetic and molecular burden of disease just before transplantation were not available.
Because of concern about possible selection bias in the types of patients undergoing HCT over the course of the study, a matched pairs analysis was also conducted. IM+ and IM− patients were matched based on time from diagnosis to transplantation (± 3 months), degree of HLA matching, graft type, and sex matching. For the first chronic phase group, the matched pairs analysis included 143 IM+ and 236 IM− patients. For the advanced disease group, this analysis included 216 IM+ and 216 IM− patients.
Results
Patient characteristics
The final dataset included information from 82 teams reporting 409 patients receiving IM before HCT (IM+) and 900 patients who did not receive IM before HCT (IM−).
Table 1 shows the characteristics of 185 IM+ subjects and 675 IM− controls transplanted in first chronic phase. IM+ subjects had a longer time from diagnosis to transplantation and a higher percentage of unrelated donors, reduced intensity conditioning, and peripheral blood. They were transplanted more recently and had higher EBMT scores. There were no differences in age, sex, sex match, race, Karnofsky performance status, and donor-recipient CMV serologic status. Hematologic control at time of HCT was better in the IM+ group as shown by the statistically decreased white blood counts, platelet counts, and percentage of blasts. Details of IM administration and response in the subset who received IM before HCT are presented in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Approximately half (48%) of the IM+ group received less than 400 mg/day IM. This is not because of pediatric patients, as 86% of patients in this category were more than 18 years old. Most (90%) of the IM+ group had at least a hematologic response before HCT with 9% achieving molecular response before transplantation. The majority (n = 97, 54%) planned to proceed to HCT regardless of response to IM, whereas 17 (9%) underwent HCT because of IM intolerance or IM therapy failure (n = 66, 37%). The median duration of IM therapy was 9 months (range, 0.2-55 months) with 90% receiving IM for less than 24 months. Approximately half (n = 94, 51%) received IM within 2 weeks of HCT. Twenty-nine patients (16%) received IM after HCT, primarily for persistent disease or relapse.
Characteristics of patients who underwent allogeneic hematopoietic cell transplantation for the treatment of chronic myeloid leukemia from 1999-2004, reported to the CIBMTR
| Variable . | n eval . | First chronic phase . | n eval . | Advanced phase . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Imatinib n (%) . | n eval . | No imatinib n (%) . | P* . | Imatinib n (%) . | n eval . | No imatinib n (%) . | P . | |||
| No. of patients | 185 | 675 | 224 | 225 | ||||||
| No. of centers | 63 | 71 | 56 | 59 | ||||||
| Age, y, median (range) | 185 | 38 (3-66) | 675 | 37 (2-66) | .86 | 224 | 42 (12-70) | 225 | 40 (7-70) | .04 |
| Age at transplantation, y | 185 | 675 | .44 | 224 | 225 | .37 | ||||
| ≤ 30 | 62 (34) | 202 (30) | 39 (17) | 46 (20) | ||||||
| 31-40 | 42 (23) | 193 (29) | 53 (24) | 65 (29) | ||||||
| 41-50 | 50 (27) | 177 (26) | 66 (29) | 58 (26) | ||||||
| > 50 | 31 (17) | 103 (15) | 66 (29) | 56 (25) | ||||||
| Male sex | 185 | 103 (56) | 675 | 389 (58) | .63 | 224 | 142 (63) | 225 | 138 (61) | .65 |
| White race | 185 | 152 (82) | 675 | 506 (75) | .04 | 224 | 173 (77) | 225 | 172 (76) | .84 |
| Karnofsky score at transplantation | 162 | 663 | .21 | 201 | 214 | .34 | ||||
| ≤ 80% | 12 (7) | 71 (11) | 66 (33) | 61 (29) | ||||||
| > 80% | 150 (93) | 592 (89) | 135 (67) | 153 (71) | ||||||
| Time from diagnosis to transplant, mo, median (range) | 185 | 13 (2-107) | 675 | 9 (1-126) | < .001 | 224 | 22 (1-142) | 225 | 13 (2-180) | < .001 |
| Time from diagnosis to transplantation | 185 | 675 | < .001 | 224 | 225 | < .001 | ||||
| ≤ 12 mo | 75 (41) | 457 (68) | 69 (31) | 107 (48) | ||||||
| > 12 mo | 110 (59) | 218 (32) | 155 (69) | 118 (52) | ||||||
| EBMT score | 185 | 675 | .001 | < .001 | ||||||
| 0-1 | 18 (10) | 134 (20) | 0 | 2 (1) | ||||||
| 2 | 54 (29) | 227 (34) | 2 (1) | 8 (4) | ||||||
| 3 | 67 (36) | 207 (30) | 24 (11) | 35 (15) | ||||||
| 4 | 41 (22) | 95 (14) | 48 (21) | 73 (32) | ||||||
| ≥ 5 | 5 (3) | 12 (2) | 150 (67) | 107 (48) | ||||||
| Disease stage pretransplantation | 185 | 675 | 1.0 | 224 | 225 | .65 | ||||
| First chronic phase | 185 (100) | 675 (100) | ||||||||
| Accelerated phase | 0 (0) | 0 (0) | 91 (41) | 94 (42) | ||||||
| ≥ Second chronic phase | 0 (0) | 0 (0) | 96 (43) | 88 (39) | ||||||
| Blast phase | 0 (0) | 0 (0) | 37 (17) | 43 (19) | ||||||
| Hemoglobin, g/dL | 180 | 13 (8-17) | 665 | 13 (0-18) | .52 | 173 | 11 (0-15) | 167 | 11 (0-15) | .07 |
| Platelets, 109/L | 181 | 220 (33-1568) | 559 | 264 (0-2684) | < .001 | 188 | 172 (0-1570) | 186 | 217 (12-2115) | .02 |
| White blood count, 109/L | 183 | 6 (1-141) | 662 | 9 (0-504) | < .001 | 218 | 5 (0.1-100) | 214 | 8 (0.3-165) | < .001 |
| Blasts, % | 156 | 0 (0-8) | 651 | 0 (0-22) | .0003 | 194 | 0 (0-86) | 183 | 0 (0-90) | .04 |
| Donor/recipient sex match | 185 | 675 | .85 | 224 | 225 | .91 | ||||
| Male-male | 68 (37) | 242 (36) | 93 (42) | 86 (38) | ||||||
| Male-female | 43 (23) | 154 (23) | 44 (20) | 46 (20) | ||||||
| Female-male | 35 (19) | 147 (22) | 49 (22) | 52 (23) | ||||||
| Female-female | 39 (21) | 132 (20) | 38 (17) | 41 (18) | ||||||
| Donor | 185 | 675 | < .001 | 224 | 225 | .01 | ||||
| HLA-identical sibling | 52 (28) | 290 (43) | 47 (21) | 77 (34) | ||||||
| Related | 4 (2) | 18 (3) | 7 (3) | 6 (3) | ||||||
| Unrelated | 129 (70) | 367 (54) | 170 (76) | 142 (63) | ||||||
| Donor-recipient HLA match | 185 | 675 | < .001 | 224 | 225 | < .001 | ||||
| Matched sibling | 52 (28) | 290 (43) | 47 (21) | 77 (34) | ||||||
| Well matched | 85 (46) | 135 (20) | 93 (42) | 43 (19) | ||||||
| Partially matched | 26 (14) | 135 (20) | 50 (22) | 57 (26) | ||||||
| Mismatch | 13 (7) | 82 (12) | 18 (8) | 25 (11) | ||||||
| Unknown | 9 (5) | 33 (5) | 16 (7) | 23 (10) | ||||||
| Donor-recipient cytomegalovirus status | 172 | 619 | .06 | 211 | 204 | .03 | ||||
| +/+ | 43 (25) | 211 (34) | 65 (31) | 79 (39) | ||||||
| +/− | 19 (11) | 82 (13) | 20 (9) | 31 (15) | ||||||
| −/+ | 41 (24) | 133 (21) | 65 (31) | 43 (21) | ||||||
| −/− | 69 (40) | 193 (31) | 61 (29) | 51 (25) | ||||||
| Conditioning regimen | 185 | 675 | < .001 | 224 | 225 | .71 | ||||
| Myeloablative | 143 (77) | 599 (89) | 175 (78) | 179 (80) | ||||||
| Nonmyeloablative | 42 (23) | 76 (11) | 49 (22) | 46 (20) | ||||||
| Total body irradiation | 185 | 675 | .01 | 224 | 225 | .17 | ||||
| Yes | 72 (39) | 334 (49) | 104 (46) | 119 (53) | ||||||
| No | 113 (61) | 341 (51) | 120 (54) | 106 (47) | ||||||
| Graft type | 185 | 675 | < .001 | 224 | 225 | < .001 | ||||
| Bone marrow | 100 (54) | 481 (71) | 86 (38) | 148 (66) | ||||||
| Peripheral blood | 85 (46) | 194 (29) | 138 (62) | 77 (34) | ||||||
| Year of transplantation | 185 | 675 | < .001 | 224 | 225 | < .001 | ||||
| 1999-2000 | 3 (2) | 472 (70) | 12 (5) | 141 (63) | ||||||
| 2001-2002 | 51 (28) | 163 (24) | 88 (39) | 47 (21) | ||||||
| 2003-2004 | 131 (71) | 40 (6) | 124 (55) | 37 (16) | ||||||
| GVHD prophylaxis | 185 | 675 | < .001 | 224 | 225 | < .001 | ||||
| MTX + CsA ± others | 91 (49) | 446 (66) | 104 (46) | 132 (59) | ||||||
| FK506 + MTX ± others | 55 (30) | 75 (11) | 71 (32) | 31 (14) | ||||||
| CsA ± others | 18 (10) | 75 (11) | 27 (12) | 32 (14) | ||||||
| FK506 ± others | 9 (5) | 10 (1) | 10 (4) | 6 (3) | ||||||
| T-cell depletion | 8 (4) | 51 (8) | 4 (2) | 15 (7) | ||||||
| Other† | 1 (1) | 7 (1) | 4 (2) | 5 (2) | ||||||
| None | 3 (2) | 11 (2) | 4 (2) | 4 (2) | ||||||
| Median follow-up of survivors, mo | 134 | 32 (8-86) | 421 | 69 (3-96) | 81 | 36 (13-73) | 75 | 59 (3-95) | ||
| Variable . | n eval . | First chronic phase . | n eval . | Advanced phase . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Imatinib n (%) . | n eval . | No imatinib n (%) . | P* . | Imatinib n (%) . | n eval . | No imatinib n (%) . | P . | |||
| No. of patients | 185 | 675 | 224 | 225 | ||||||
| No. of centers | 63 | 71 | 56 | 59 | ||||||
| Age, y, median (range) | 185 | 38 (3-66) | 675 | 37 (2-66) | .86 | 224 | 42 (12-70) | 225 | 40 (7-70) | .04 |
| Age at transplantation, y | 185 | 675 | .44 | 224 | 225 | .37 | ||||
| ≤ 30 | 62 (34) | 202 (30) | 39 (17) | 46 (20) | ||||||
| 31-40 | 42 (23) | 193 (29) | 53 (24) | 65 (29) | ||||||
| 41-50 | 50 (27) | 177 (26) | 66 (29) | 58 (26) | ||||||
| > 50 | 31 (17) | 103 (15) | 66 (29) | 56 (25) | ||||||
| Male sex | 185 | 103 (56) | 675 | 389 (58) | .63 | 224 | 142 (63) | 225 | 138 (61) | .65 |
| White race | 185 | 152 (82) | 675 | 506 (75) | .04 | 224 | 173 (77) | 225 | 172 (76) | .84 |
| Karnofsky score at transplantation | 162 | 663 | .21 | 201 | 214 | .34 | ||||
| ≤ 80% | 12 (7) | 71 (11) | 66 (33) | 61 (29) | ||||||
| > 80% | 150 (93) | 592 (89) | 135 (67) | 153 (71) | ||||||
| Time from diagnosis to transplant, mo, median (range) | 185 | 13 (2-107) | 675 | 9 (1-126) | < .001 | 224 | 22 (1-142) | 225 | 13 (2-180) | < .001 |
| Time from diagnosis to transplantation | 185 | 675 | < .001 | 224 | 225 | < .001 | ||||
| ≤ 12 mo | 75 (41) | 457 (68) | 69 (31) | 107 (48) | ||||||
| > 12 mo | 110 (59) | 218 (32) | 155 (69) | 118 (52) | ||||||
| EBMT score | 185 | 675 | .001 | < .001 | ||||||
| 0-1 | 18 (10) | 134 (20) | 0 | 2 (1) | ||||||
| 2 | 54 (29) | 227 (34) | 2 (1) | 8 (4) | ||||||
| 3 | 67 (36) | 207 (30) | 24 (11) | 35 (15) | ||||||
| 4 | 41 (22) | 95 (14) | 48 (21) | 73 (32) | ||||||
| ≥ 5 | 5 (3) | 12 (2) | 150 (67) | 107 (48) | ||||||
| Disease stage pretransplantation | 185 | 675 | 1.0 | 224 | 225 | .65 | ||||
| First chronic phase | 185 (100) | 675 (100) | ||||||||
| Accelerated phase | 0 (0) | 0 (0) | 91 (41) | 94 (42) | ||||||
| ≥ Second chronic phase | 0 (0) | 0 (0) | 96 (43) | 88 (39) | ||||||
| Blast phase | 0 (0) | 0 (0) | 37 (17) | 43 (19) | ||||||
| Hemoglobin, g/dL | 180 | 13 (8-17) | 665 | 13 (0-18) | .52 | 173 | 11 (0-15) | 167 | 11 (0-15) | .07 |
| Platelets, 109/L | 181 | 220 (33-1568) | 559 | 264 (0-2684) | < .001 | 188 | 172 (0-1570) | 186 | 217 (12-2115) | .02 |
| White blood count, 109/L | 183 | 6 (1-141) | 662 | 9 (0-504) | < .001 | 218 | 5 (0.1-100) | 214 | 8 (0.3-165) | < .001 |
| Blasts, % | 156 | 0 (0-8) | 651 | 0 (0-22) | .0003 | 194 | 0 (0-86) | 183 | 0 (0-90) | .04 |
| Donor/recipient sex match | 185 | 675 | .85 | 224 | 225 | .91 | ||||
| Male-male | 68 (37) | 242 (36) | 93 (42) | 86 (38) | ||||||
| Male-female | 43 (23) | 154 (23) | 44 (20) | 46 (20) | ||||||
| Female-male | 35 (19) | 147 (22) | 49 (22) | 52 (23) | ||||||
| Female-female | 39 (21) | 132 (20) | 38 (17) | 41 (18) | ||||||
| Donor | 185 | 675 | < .001 | 224 | 225 | .01 | ||||
| HLA-identical sibling | 52 (28) | 290 (43) | 47 (21) | 77 (34) | ||||||
| Related | 4 (2) | 18 (3) | 7 (3) | 6 (3) | ||||||
| Unrelated | 129 (70) | 367 (54) | 170 (76) | 142 (63) | ||||||
| Donor-recipient HLA match | 185 | 675 | < .001 | 224 | 225 | < .001 | ||||
| Matched sibling | 52 (28) | 290 (43) | 47 (21) | 77 (34) | ||||||
| Well matched | 85 (46) | 135 (20) | 93 (42) | 43 (19) | ||||||
| Partially matched | 26 (14) | 135 (20) | 50 (22) | 57 (26) | ||||||
| Mismatch | 13 (7) | 82 (12) | 18 (8) | 25 (11) | ||||||
| Unknown | 9 (5) | 33 (5) | 16 (7) | 23 (10) | ||||||
| Donor-recipient cytomegalovirus status | 172 | 619 | .06 | 211 | 204 | .03 | ||||
| +/+ | 43 (25) | 211 (34) | 65 (31) | 79 (39) | ||||||
| +/− | 19 (11) | 82 (13) | 20 (9) | 31 (15) | ||||||
| −/+ | 41 (24) | 133 (21) | 65 (31) | 43 (21) | ||||||
| −/− | 69 (40) | 193 (31) | 61 (29) | 51 (25) | ||||||
| Conditioning regimen | 185 | 675 | < .001 | 224 | 225 | .71 | ||||
| Myeloablative | 143 (77) | 599 (89) | 175 (78) | 179 (80) | ||||||
| Nonmyeloablative | 42 (23) | 76 (11) | 49 (22) | 46 (20) | ||||||
| Total body irradiation | 185 | 675 | .01 | 224 | 225 | .17 | ||||
| Yes | 72 (39) | 334 (49) | 104 (46) | 119 (53) | ||||||
| No | 113 (61) | 341 (51) | 120 (54) | 106 (47) | ||||||
| Graft type | 185 | 675 | < .001 | 224 | 225 | < .001 | ||||
| Bone marrow | 100 (54) | 481 (71) | 86 (38) | 148 (66) | ||||||
| Peripheral blood | 85 (46) | 194 (29) | 138 (62) | 77 (34) | ||||||
| Year of transplantation | 185 | 675 | < .001 | 224 | 225 | < .001 | ||||
| 1999-2000 | 3 (2) | 472 (70) | 12 (5) | 141 (63) | ||||||
| 2001-2002 | 51 (28) | 163 (24) | 88 (39) | 47 (21) | ||||||
| 2003-2004 | 131 (71) | 40 (6) | 124 (55) | 37 (16) | ||||||
| GVHD prophylaxis | 185 | 675 | < .001 | 224 | 225 | < .001 | ||||
| MTX + CsA ± others | 91 (49) | 446 (66) | 104 (46) | 132 (59) | ||||||
| FK506 + MTX ± others | 55 (30) | 75 (11) | 71 (32) | 31 (14) | ||||||
| CsA ± others | 18 (10) | 75 (11) | 27 (12) | 32 (14) | ||||||
| FK506 ± others | 9 (5) | 10 (1) | 10 (4) | 6 (3) | ||||||
| T-cell depletion | 8 (4) | 51 (8) | 4 (2) | 15 (7) | ||||||
| Other† | 1 (1) | 7 (1) | 4 (2) | 5 (2) | ||||||
| None | 3 (2) | 11 (2) | 4 (2) | 4 (2) | ||||||
| Median follow-up of survivors, mo | 134 | 32 (8-86) | 421 | 69 (3-96) | 81 | 36 (13-73) | 75 | 59 (3-95) | ||
Patient-related variables include number, age, age at transplantation, sex, race, and Karnovsky score at transplantation. Disease-related variables include time from diagnosis to transplantation, EBMT score, and disease stage (pretransplantation). All remaining variables are transplantation-related.
CIBMTR indicates Center for International Blood and Marrow Transplant Research; EBMT, European Blood and Marrow Transplantation; HLA, human leukocyte antigen; GVHD, graft-versus-host disease; MTX, methotrexate; CsA, cyclosporine; FK506, tacrolimus; and HCT, hematopoietic cell transplantation.
Chi-square test for discrete covariates; Kruskal-Wallis test for continuous covariates.
Other GVHD prophylaxis (n = 17); MTX (n = 5); Tacrolimus + MTX (n = 4); Cortice Steroid + MTX (n = 3); Cellcept + MTX (n = 2); Rapamycin + MTX (n = 2); Cyclophosphamide (n = 1).
Table 1 shows the characteristics of 224 IM+ subjects and 225 IM− controls with advanced disease. IM+ subjects had a longer time from diagnosis to transplantation, higher percentage of unrelated donors, and were more probably to receive peripheral blood. They were transplanted more recently and had higher EBMT scores. However, the proportions with accelerated phase, second chronic phase, and blast crisis were similar between IM+ and IM− patients (P = .65). Hematologic control was better in the IM+ group at the time of HCT with lower white blood counts, platelet counts, and percentage of blasts. Most (84%) of the IM+ group had at least a hematologic response before HCT with 6% achieving molecular response. The majority (n = 114, 51%) proceeded to HCT because of IM therapy failure, although 95 (43%) underwent planned HCT. The median duration of IM therapy was 8 months (range, 1-60 months) with 90% receiving IM for less than 30 months. Approximately half (n = 117, 52%) received IM within 2 weeks of HCT (Table S1).
Outcomes among early-stage patients
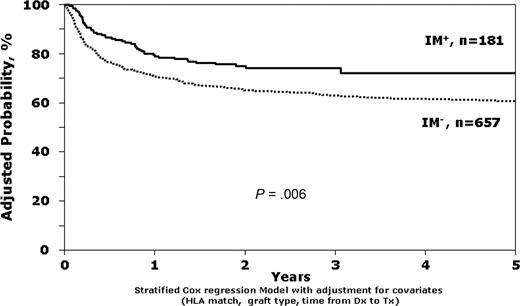
The unadjusted survival estimates for IM+ and IM− patients in first chronic phase were 79% versus 74% at 1 year (P = .08) and 72% versus 65% at 3 years (P = .07). In both the multivariate models adjusted for clinical characteristics (relative risk (RR) = 0.63; 95% confidence interval [CI], 0.46-0.88; P = .006; Table 2; Figure 1) and the matched pairs analysis (RR = 0.48; 95% CI, 0.31-0.75; P = .001; Table 3), exposure to IM before HCT was associated with better survival. Other factors statistically associated with better survival were better donor-recipient HLA matching, use of bone marrow instead of peripheral blood, and transplantation within the first year after diagnosis instead of beyond the first year. However, leukemia-free survival (LFS) was not significantly different between IM+ and IM− patients (RR = 0.89; 95% CI, 0.68-1.15, P = .36), perhaps explained by a trend toward lower TRM (RR = 0.70; 95% CI, 0.49-0.98; P = .04) and higher relapse (RR = 1.54; 95% CI, 1.02-2.35; P = .04) in the IM+ group (Table 4). Univariate analyses for overall survival and TRM for patients in first chronic phase are available in Table S2.
Multivariate analysis comparing survival among patients who underwent an allogeneic transplantation for the treatment of first chronic phase chronic myeloid leukemia from 1999 to 2004
| Variable . | n . | Relative risk of death (95% CI) . | P . |
|---|---|---|---|
| Main effect | |||
| No imatinib | 657 | 1.00* | |
| Imatinib | 181 | 0.63 (0.46-0.88) | .006† |
| Other significant covariates | |||
| Donor-recipient HLA match | < .001‡ | ||
| Matched sibling | 327 | 1.00* | |
| Well matched | 217 | 1.53 (1.10-2.13) | .011 |
| Partially matched | 160 | 1.90 (1.36-2.66) | < .001 |
| Mismatch | 92 | 2.57 (1.78-3.71) | < .001 |
| Unknown | 42 | 1.75 (1.02-3.00) | .042 |
| Graft type | |||
| BM | 570 | 1.00 | |
| PB | 268 | 1.51 (1.16-1.96) | .002† |
| Time from diagnosis to transplantation | |||
| ≤ 12 mo | 516 | 1.00 | |
| > 12 mo | 322 | 1.46 (1.15-1.85) | .002† |
| Variable . | n . | Relative risk of death (95% CI) . | P . |
|---|---|---|---|
| Main effect | |||
| No imatinib | 657 | 1.00* | |
| Imatinib | 181 | 0.63 (0.46-0.88) | .006† |
| Other significant covariates | |||
| Donor-recipient HLA match | < .001‡ | ||
| Matched sibling | 327 | 1.00* | |
| Well matched | 217 | 1.53 (1.10-2.13) | .011 |
| Partially matched | 160 | 1.90 (1.36-2.66) | < .001 |
| Mismatch | 92 | 2.57 (1.78-3.71) | < .001 |
| Unknown | 42 | 1.75 (1.02-3.00) | .042 |
| Graft type | |||
| BM | 570 | 1.00 | |
| PB | 268 | 1.51 (1.16-1.96) | .002† |
| Time from diagnosis to transplantation | |||
| ≤ 12 mo | 516 | 1.00 | |
| > 12 mo | 322 | 1.46 (1.15-1.85) | .002† |
CI indicates confidence interval.
Reference group.
One degree of freedom.
Four degrees of freedom.
Matched pair analysis comparing survival among patients who underwent allogeneic HSCT for first chronic phase chronic myeloid leukemia from 1999 to 2004
| Variable . | n . | Relative risk of death (95% CI) . | P . |
|---|---|---|---|
| Main effect | |||
| No imatinib | 236 | 1.00* | |
| Imatinib | 143 | 0.48 (0.31-0.75) | .001† |
| Variable . | n . | Relative risk of death (95% CI) . | P . |
|---|---|---|---|
| Main effect | |||
| No imatinib | 236 | 1.00* | |
| Imatinib | 143 | 0.48 (0.31-0.75) | .001† |
Matched variables include (1) time from diagnosis to transplant plus or minus 3 months, (2) HLA match, (3) graft type, and (4) sex match versus other. There are 143 imatinib cases for which matched controls were identified.
CI indicates confidence interval.
Reference group.
One degree of freedom.
Multivariate analyses of leukemia-free survival, treatment-related mortality, and relapse in patients transplanted for first chronic phase
| Variable . | n . | Relative risk of death (95% CI) . | P . |
|---|---|---|---|
| Leukemia-free survival | |||
| Main effect | |||
| No imatinib | 657 | 1.00* | |
| Imatinib | 181 | 0.89 (0.68- 1.15) | .36† |
| Other significant covariates | |||
| GVHD prophylaxis | < .001‡ | ||
| MTX + CsA ± others | 537 | 1.00* | |
| T-cell depletion | 59 | 1.96 (1.40-2.76) | < .001 |
| FK506 + MTX ± others | 130 | 1.15 (0.86-1.55) | .340 |
| FK506 ± others | 19 | 2.60 (1.45-4.67) | .001 |
| CsA ± others | 93 | 1.58 (1.15-2.15) | .004 |
| Time from diagnosis to transplantation | |||
| ≤ 12 mo | 516 | 1.00 | |
| > 12 mo | 322 | 1.56 (1.27-1.93) | < .001† |
| Treatment-related mortality | |||
| Main effect | |||
| No imatinib | 657 | 1.00* | |
| Imatinib | 181 | 0.70 (0.49- 0.98) | .040† |
| Other significant covariates | |||
| Donor-recipient HLA match | < .001‡ | ||
| Matched sibling | 327 | 1.00* | |
| Well matched | 217 | 1.36 (0.96-1.93) | .080 |
| Partially matched | 160 | 1.74 (1.24-2.44) | .001 |
| Mismatch | 92 | 2.40 (1.65-3.47) | < .001 |
| Unknown | 42 | 1.78 (1.01-3.16) | .048 |
| Time from diagnosis to transplantation | |||
| ≤ 12 mo | 516 | 1.00 | |
| > 12 mo | 322 | 1.54 (1.20-1.99) | < .001† |
| Relapse§ | |||
| Main effect | |||
| No imatinib | 657 | 1.00* | |
| Imatinib | 181 | 1.54 (1.02-2.35) | .041† |
| Other significant covariates | |||
| GVHD prophylaxis | .004‡ | ||
| MTX + CsA ± others | 537 | 1.00* | |
| T-cell depletion | 59 | 2.67 (1.50-4.76) | < .001 |
| FK506 + MTX ± others | 130 | 1.08 (0.63-1.85) | .790 |
| FK506 ± others | 19 | 2.66 (1.10-6.46) | .031 |
| CsA ± others | 93 | 1.66 (0.94-2.95) | .083 |
| Variable . | n . | Relative risk of death (95% CI) . | P . |
|---|---|---|---|
| Leukemia-free survival | |||
| Main effect | |||
| No imatinib | 657 | 1.00* | |
| Imatinib | 181 | 0.89 (0.68- 1.15) | .36† |
| Other significant covariates | |||
| GVHD prophylaxis | < .001‡ | ||
| MTX + CsA ± others | 537 | 1.00* | |
| T-cell depletion | 59 | 1.96 (1.40-2.76) | < .001 |
| FK506 + MTX ± others | 130 | 1.15 (0.86-1.55) | .340 |
| FK506 ± others | 19 | 2.60 (1.45-4.67) | .001 |
| CsA ± others | 93 | 1.58 (1.15-2.15) | .004 |
| Time from diagnosis to transplantation | |||
| ≤ 12 mo | 516 | 1.00 | |
| > 12 mo | 322 | 1.56 (1.27-1.93) | < .001† |
| Treatment-related mortality | |||
| Main effect | |||
| No imatinib | 657 | 1.00* | |
| Imatinib | 181 | 0.70 (0.49- 0.98) | .040† |
| Other significant covariates | |||
| Donor-recipient HLA match | < .001‡ | ||
| Matched sibling | 327 | 1.00* | |
| Well matched | 217 | 1.36 (0.96-1.93) | .080 |
| Partially matched | 160 | 1.74 (1.24-2.44) | .001 |
| Mismatch | 92 | 2.40 (1.65-3.47) | < .001 |
| Unknown | 42 | 1.78 (1.01-3.16) | .048 |
| Time from diagnosis to transplantation | |||
| ≤ 12 mo | 516 | 1.00 | |
| > 12 mo | 322 | 1.54 (1.20-1.99) | < .001† |
| Relapse§ | |||
| Main effect | |||
| No imatinib | 657 | 1.00* | |
| Imatinib | 181 | 1.54 (1.02-2.35) | .041† |
| Other significant covariates | |||
| GVHD prophylaxis | .004‡ | ||
| MTX + CsA ± others | 537 | 1.00* | |
| T-cell depletion | 59 | 2.67 (1.50-4.76) | < .001 |
| FK506 + MTX ± others | 130 | 1.08 (0.63-1.85) | .790 |
| FK506 ± others | 19 | 2.66 (1.10-6.46) | .031 |
| CsA ± others | 93 | 1.66 (0.94-2.95) | .083 |
CI indicates confidence interval.
Reference group.
One degree of freedom.
Four degrees of freedom.
Stratified on conditioning regimen because of nonproportional hazards.
Stratified Cox regression model comparing survival of IM+ versus IM− patients, adjusted for HLA match, interval from diagnosis to transplantation, and donor-recipient sex match.
Stratified Cox regression model comparing survival of IM+ versus IM− patients, adjusted for HLA match, interval from diagnosis to transplantation, and donor-recipient sex match.
Cumulative rates of acute and chronic GVHD were similar in univariate analyses and confirmed in multivariate models. Specifically, grades II to IV acute GVHD rates were 43% (IM+) and 42% (IM−; P = .94). Extensive chronic GVHD occurred in 55% of IM+ and 53% of IM− patients by 3 years (P = .62).
To further explore the observed association between IM before HCT and better survival, separate models excluded 264 patients less than 30 years old or 118 recipients of reduced intensity conditioning regimens. Results were consistent with the full dataset despite exclusion of these groups. We also evaluated whether the best dichotomy for time from diagnosis to transplantation was still 12 months. Model fitting suggested a slightly more optimal cut-point of 15 to 16 months, but this change would not affect the other parameters in the model so the standard cutoff of 12 months was used in subsequent analyses.
We could not evaluate organ-specific toxicities after HCT. Evaluation of listed causes of death did not reveal any obvious differences between IM+ and IM− patients (data not shown).
Outcomes among advanced-disease patients
In patients with advanced CML, the unadjusted survival estimates for IM+ and IM− patients were 48% for both at 1 year and 36% versus 34%, P = .61 at 3 years. The multivariate models did not identify differences in survival, LFS, TRM, or relapse according to whether IM was given before HCT (Table 5). Univariate rates of grades II to IV acute GVHD were similar, 49% versus 48%, P = .78 for IM+ and IM− patients, respectively, confirmed in the multivariate analysis (RR = 0.89; 95% CI, 0.70-1.14; P = .37). Unadjusted rates of chronic GVHD were higher in IM+ patients at both 1 year (52% vs 41%, P = .02) and 3 years (54% vs 42%, P < .001), but not in the multivariate time to event analysis (RR = 1.31; 95% CI, 0.98-1.75; P = .07).
Multivariate analyses of survival, leukemia-free survival, treatment-related mortality, and relapse in patients undergoing transplantation for advanced phase chronic myeloid leukemia
| Variable . | n . | Relative risk of death (95% CI) . | P . |
|---|---|---|---|
| Survival | |||
| Main effect | |||
| No imatinib | 216 | 1.00* | |
| Imatinib | 216 | 0.93 (0.74-1.18) | .570† |
| Leukemia-free survival | |||
| Main effect | |||
| No imatinib | 216 | 1.00* | |
| Imatinib | 216 | 1.05 (0.84-1.32) | .670† |
| Treatment-related mortality | |||
| Main effect | |||
| No imatinib | 216 | 1.00* | |
| Imatinib | 216 | 0.87 (0.64-1.18) | .350† |
| Other significant covariates | |||
| Karnofsky score at transplantation | .007‡ | ||
| > 80% | 276 | 1.00* | |
| ≤ 80% | 122 | 1.67 (1.21-2.31) | .002 |
| Unknown | 34 | 1.08 (0.60-1.94) | .790 |
| Relapse | |||
| Main effect | |||
| No imatinib | 216 | 1.00* | |
| Imatinib | 216 | 1.35 (0.95-1.89) | .090† |
| Variable . | n . | Relative risk of death (95% CI) . | P . |
|---|---|---|---|
| Survival | |||
| Main effect | |||
| No imatinib | 216 | 1.00* | |
| Imatinib | 216 | 0.93 (0.74-1.18) | .570† |
| Leukemia-free survival | |||
| Main effect | |||
| No imatinib | 216 | 1.00* | |
| Imatinib | 216 | 1.05 (0.84-1.32) | .670† |
| Treatment-related mortality | |||
| Main effect | |||
| No imatinib | 216 | 1.00* | |
| Imatinib | 216 | 0.87 (0.64-1.18) | .350† |
| Other significant covariates | |||
| Karnofsky score at transplantation | .007‡ | ||
| > 80% | 276 | 1.00* | |
| ≤ 80% | 122 | 1.67 (1.21-2.31) | .002 |
| Unknown | 34 | 1.08 (0.60-1.94) | .790 |
| Relapse | |||
| Main effect | |||
| No imatinib | 216 | 1.00* | |
| Imatinib | 216 | 1.35 (0.95-1.89) | .090† |
Reference group.
One degree of freedom.
Two degrees of freedom.
Predictors of survival in patients with early-stage disease receiving IM before HCT
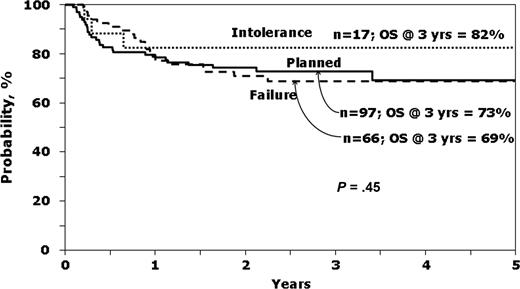
It was of interest to see which variables predicted survival in patients with early-stage disease receiving IM before HCT. The characteristics of this group according to the reason to proceed to HCT are presented in the online appendix (Table S3), whereas Figure 2 shows unadjusted survival curves. Ninety-seven (53.8%) underwent a planned transplantation, whereas IM intolerance (n = 17, 9.4%) and IM failure or no response (n = 66, 36.7%) were the listed reasons for HCT for the rest. Note that P values presented in the Table S3 compared planned transplantation to IM Failure/No response, as the small number (n = 17) transplanted for IM intolerance precludes comments on the characteristics and outcomes of this group.
Probability of survival according to reason to proceed to transplantation, for patients in first chronic phase receiving IM before HCT.
Probability of survival according to reason to proceed to transplantation, for patients in first chronic phase receiving IM before HCT.
Patients undergoing planned transplantation tended to be younger, have unrelated donors, receive myeloablative conditioning and bone marrow, have a shorter interval between diagnosis and transplantation and lower EBMT scores than patients transplanted for IM failure or no response. However, hematologic control at time of HCT was not demonstrably different as measured by hemoglobin, platelet count, white blood count, or peripheral blasts. Relapse was highest and LFS lowest in the group transplanted because of IM failure or no response (Table S3).
Multivariate models for survival and TRM were created for patients receiving IM and transplanted in first chronic phase (n = 180). Potential predictors included reason for proceeding to HCT, best response to IM before HCT, duration of IM treatment, and interval between IM discontinuation and HCT, in addition to the other potential clinical predictors. HLA matching status was the only variable associated with survival and TRM (data not shown). Specifically, reason to proceed to HCT, the variables making up the EBMT score, and the IM variables were not associated with these outcomes. Multivariate models including all IM+ patients, both early and advanced phase disease, were created for survival and TRM (n = 391). HLA match and CML disease phase were the only significant predictors of both survival and TRM; none of the IM-associated variables was associated with survival or TRM in advanced-phase patients (data not shown).
Discussion
Results from this observational study suggest that patients in first chronic phase who received IM before HCT have better survival than patients who did not receive IM before HCT. However, LFS was similar perhaps explained by nonstatistically significant trends toward less TRM but more relapses in patients receiving IM before HCT. Among patients transplanted with more advanced disease beyond first chronic phase, there were no detectable differences in survival, LFS, TRM, relapse, or acute GVHD.
Our results confirm prior observations that, for patients transplanted in first chronic phase, use of bone marrow rather than peripheral blood,24 transplantation within the first year after diagnosis,21 and better HLA matching between donor and recipient are also associated with improved survival. Notably, year of transplantation, intensity of conditioning regimen, patient age, and donor-recipient sex match were not identified as independent predictors for survival at P less than .01 in our analysis. Our results also appear consistent with prior studies about the lack of adverse effects observed with prior exposure to IM on the outcomes of HCT.15-20 However, in contrast to previous studies, we observed better survival for the group that received IM.
The population receiving IM before HCT had more unfavorable traditional prognostic characteristics than the IM− group. Specifically, IM+ patients had more unrelated donors, peripheral blood grafts, worse HLA matching, and a longer interval between diagnosis and transplantation. They were also less probably to have myeloablative conditioning, although conditioning regimen intensity was not associated with outcome. Thus, after statistical adjustment for these adverse prognostic factors, the improved survival difference with IM exposure before HCT was accentuated compared with the unadjusted survival estimates. Because of our concern that statistical adjustment may not adequately adjust for population differences, we also conducted a separate analysis among a subset matched for time from diagnosis to transplantation (± 3 months), degree of HLA matching, graft type, and sex matching. Results were consistent with the multivariate conclusions.
As treatment and monitoring for disease status in CML becomes more sophisticated with newer tyrosine kinase inhibitors, more widespread availability of mutational analysis, and standardization of disease burden quantification using molecular techniques, we will be better able to identify patients who are not expected to do well with available nontransplantation therapies. When these patients undergo HCT, they may have been exposed to IM or other tyrosine kinase inhibitors for much longer than the median duration of exposure in this study (8-9 months). Although we did not see any association between duration of IM exposure and outcomes in the current study, fewer than 10% of patients had been exposed to more than 30 months of IM before HCT. Additional studies evaluating longer-term use of IM before HCT, use of other tyrosine kinase inhibitors before HCT, and use of these drugs after HCT for prophylactic or preemptive treatment are necessary to address the evolving questions in CML management.
The trends toward lower rates of TRM and higher rates of relapse in patients receiving IM before transplantation are of interest. Because rates of grades II to IV acute GVHD were similar between IM+ and IM− patients, acute GVHD does not explain differential TRM rates. In contrast to 2 other previous reports,18,20 we did not see lower rates of chronic GVHD in patients receiving IM before HCT. The IM+ patients were more probably to have a nonmyeloablative conditioning regimen and be transplanted more recently. Although neither intensity of conditioning regimen nor year of transplantation was an independent predictor of any outcome, they may have contributed somewhat to the TRM differences.
Although absence of improved outcomes in patients with advanced-phase disease treated with IM before HCT may be disappointing, it is possible that availability of IM has allowed more patients with advanced disease to attain disease control to undergo HCT. Within the limited time frame of this study, there was no meaningful evidence to support this contention as the number of transplantations for advanced phase disease and the spectrum of disease phases was relatively constant. It is also important to emphasize that because IM was not associated with improved outcomes in patients with advanced disease, it is especially important that patients undergo HCT before they experience progression for HCT to be the most successful.
Because most patients now receive IM or another tyrosine kinase inhibitor starting when CML is diagnosed, most will be exposed to IM before HCT. Our results suggest that treatment with IM, as long as patients remain in first chronic phase at the time of HCT, is not detrimental and may even be associated with benefits.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Novartis (research grant). The CIBMTR is supported by Public Health Service Grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung, and Blood Institute; Office of Naval Research; Health Resources and Services Administration; and grants from the following: AABB; Aetna; American International Group; American Society for Blood and Marrow Transplantation; Amgen; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Baxter International; Bayer HealthCare Pharmaceuticals; BioOne Corporation; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Bristol-Myers Squibb Company; Cangene Corporation; Celgene Corporation; CellGenix; Cerus Corporation; Cubist Pharmaceuticals; Cylex; CytoTherm; DOR BioPharma; Dynal Biotech, an Invitrogen company; EKR Therapeutics; Enzon Pharmaceuticals; Gambro BCT; Gamida Cell; Genzyme Corporation; Gift of Life Bone Marrow Foundation; GlaxoSmithKline; Histogenetics; HKS Medical Information Systems; Hospira; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery; Merck & Company; Medical College of Wisconsin; MGI Pharma; Millennium Pharmaceuticals; Miller Pharmacal Group; Milliman USA; Miltenyi Biotec; MultiPlan; National Marrow Donor Program; Nature Publishing Group; Oncology Nursing Society; Osiris Therapeutics; Pall Life Sciences; PDL BioPharma; Pfizer; Pharmion Corporation; Roche Laboratories; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte; StemSoft Software; SuperGen; Sysmex; Teva Pharmaceutical Industries; Marrow Foundation; THERAKOS; University of Colorado Cord Blood Bank; ViaCell; Vidacare Corporation; ViraCor Laboratories; ViroPharma; and Wellpoint.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Authorship
Contribution: S.J.L., S.A.G., J.S., A.E.W., M.M.H., and R.T.M. designed research; M.K. and M.M.H. collected data; M.K. and T.W. performed statistical analysis; S.J.L., M.K., T.W., S.A.G., J.S., M. Arora, A.E.W., F.C., R.E.C., R.P.G., J.H., A.K., D.I.M., P.L.M., E.O., E.A.S., M. Abecasis, V.G., H.J.K., B.G., G.A.H., J.L.L., D.A.R., J.H.A., B.J.B., M.H.C., E.C., O.I., M.R.L., H.C.S., A.R.Z., M.M.H., and R.T.M. interpreted data; S.J.L. drafted the manuscript; S.J.L., M.K., T.W., S.A.G., J.S., M. Arora, A.E.W., F.C., R.E.C., R.P.G., J.H., A.K., D.I.M., P.L.M., E.O., E.A.S., M. Abecasis, V.G., H.J.K., B.G., G.A.H., J.L.L., D.A.R., J.H.A., B.J.B., M.H.C., E.C., O.I., M.R.L., H.C.S., A.R.Z., M.M.H., and R.T.M. critically revised the manuscript.
Conflict-of-interest disclosure: J.S. has been a member of the Novartis Australia imatinib advisory board and received travel support in the past. D.A.R. is a member of a Novartis-sponsored advisory board. The remaining authors declare no competing financial interests.
Correspondence: Stephanie J. Lee, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N, D5-290, Seattle, WA 98109; e-mail: sjlee@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal