Advances in the understanding of the cells of the hematopoietic system have provided a rich basis for improving clinical hematopoietic cell transplants; finding and using proteins and molecules to amplify or suppress particular blood cell types; understanding the stepwise progression of preleukemic stages leading first to chronic myeloid disorders, then the emergence of acute blastic leukemias; and treating malignant and nonmalignant diseases with cell subsets. As a result of intense scientific investigation, hematopoietic stem cells (HSCs) have been isolated and their key functional characteristics revealed—self-renewal and multilineage differentiation. These characteristics are now found to be present in all tissue/organ stem cell studies, and even in the analysis of pluripotent embryonic, nuclear transfer, and induced pluripotent stem cells. Studies on HSC have identified hematopoiesis as one of the best systems for studying developmental cell lineages and as the best for understanding molecular changes in cell fate decision-making and for finding preclinical and clinical platforms for tissue and organ replacement, regeneration, and oncogenesis. Here we review the steps, from our viewpoint, that led to HSC isolation and its importance in self-nonself immune recognition.
First evidence of long-lived blood progenitor
Two years stand out as the starting point of the science that led to an understanding of hematopoietic stem cells: 1945 and 1961, which, coincidentally, are arguably the two finest vintages of Bordeaux in the 20th century. In 1945, Ray Owen observed that fraternal twin cattle that shared a placenta shared for life the blood cell types of both calves.1 He wrote, “Since many of the twins in this study were adults when they were tested, and since the interchange of formed erythrocytes alone between embryos could be expected to result in only a transient modification of the variety of circulating cells, it is further indicated that the critical interchange is of embryonal cells ancestral to the erythrocytes of the adult animal. These cells are apparently capable of becoming established in the hemapoietic tissues of their co-twin hosts and continuing to provide a source of blood cells distinct from those of the host, presumably throughout his life.” This finding, he considered, deserved more thought. “Several interesting problems in the fields of genetics, immunology and development are suggested by these observations.” By serendipity, experiments in cattle twins were again the source of major discovery as Medawar and Billingham carried out skin grafts between heterozygous (mixed-sex) and monozygotic (identical) twins, with the unexpected outcome (published in 1952) that the grafts were almost invariably accepted in both circumstances.2 It was in the process of trying to make sense of these findings that Medawar and Billingham were led to Owen's earlier work. As a result of their studies from 1953 on induction of tolerance by hematopoietic cell infusions in fetal and neonatal mice, Medawar was awarded the Nobel Prize in Medicine in 1960.3
In 1945, civilian populations in Hiroshima and Nagasaki were exposed to atomic bomb explosions and radiation, and in retrospect, those who died from the lowest lethal dose of irradiation almost certainly died of hematopoietic failure. When it was found that the radiation syndrome in mice could be prevented by shielding the spleen with lead,4 then by injecting spleen or marrow cells,5,6 the field of hematopoietic cell transplantation began.7 In 1955, Main and Prehn showed that transplantation of allogeneic marrow into lethally radiated adult mice using cells from donors that were different at the major histocompatibility complex (MHC) than the host could be successful, and that subsequently the transplant hosts accepted skin from the marrow donor without further immune suppression,8 implying that these mice, like Owen's cattle, were tolerant chimeras. Chimerism as the mechanism for marrow transplant radioprotection was verified in 1956 by chromosomal markers by Nowell et al9 and Ford et al.10 Trentin proved that the allogeneic chimeras were specifically tolerant of donor strain skin in a repeat of Main and Prehn's work, and he also reported a secondary wasting syndrome in these hematologically recovered mice.11 [See author profiles for the evidence that this was a manifestation of adult graft-versus-host disease.]
Prospective search for the hematopoietic stem cell
In 1961, Till and McCulloch published the first of their breakthrough series of experiments that indicated that (1) hematopoiesis could be studied as a quantitative science, (2) clonal hematopoietic cells in the marrow existed that could give rise to mixed myeloerythroid progeny (granulocytes, macrophages, red cells, megakaryocytes), (3) some of these cells made more of themselves, and (4) in the spleens of these mice, cells existed that could also make lymphocytes.12,,,–16 In perhaps the most brilliant experiment of the series, they induced clonal markers in donor marrow by sublethal irradiation, then transplanted cells in numbers that made visible day 10 spleen colonies; each colony that had a chromosomal marker had one that was distinctive for that colony and that existed in all dividing cells of the colony, proving definitively that the spleen colony forming unit (CFU-S) was a single clonogenic cell.13 Day 10 CFU-S cells made clonal colonies of myeloerythroid cells in the spleen 10 days after transfer to lethally irradiated hosts; some colonies produced more myeloerythroid colony-forming cells, and some spleens contained the potential to also repopulate lymphoid tissues. Till and McCulloch proposed that these latter cells were hematopoietic stem cells, that these cells could self-renew, and that they could make all blood cell types. Wolf and Trentin showed that colonies formed in the marrow also, and that while the majority of day 7 spleen colonies were predominantly erythropoietic, the marrow colonies were predominantly myelomonocytic.17 In a dramatic experiment, Wolf and Trentin placed an explant of irradiated bone marrow stroma into the spleen of irradiated and marrow-injected mice and showed predominantly myelomonocytic colonies in the ectopic marrow, while the surrounding spleen tissue contained mainly erythroid colonies; a few colonies at the spleen/marrow interface were myelomonocytic on the bone side and erythroid on the spleen side in the same colony. Because the spleen colony was taken then as an assay for hematopoietic stem cells (HSCs), this experiment was mainly interpreted as showing the effect of different microenvironments on the same multipotent cell population, HSCs. But in the 1970s, Iscove, a colleague of Till and McCulloch, showed that spleen colonies could be found from day 7 through day 14 and that the later colonies were not derived from the earlier colonies.18 This finding opened the question as to which cell type produced colonies, stem or progenitor; later studies with highly purified HSCs, multipotent progenitors, and oligopotent progenitors showed that the early colonies came mainly from oligopotent progenitors, while day 12 through day 14 colonies came from more multipotent cells, some of which were from HSCs.19
By the late 1970s and early 1980s, clinical bone marrow transplantation was in full swing, using both autologous and allogeneic hematopoietic cell transplants. While transplanters were aware that HSCs probably existed and played a role in successful hematopoietic reconstitution, the conventional wisdom acknowledged the contribution of many different cell types, including progenitor cells and stem cells, and it was not even clear whether self-renewal was a property of only one hematopoietic cell type. The only way to answer these questions was to transition from the retrospective genetic marker evidence that such cells existed13,20 to the prospective isolation of stem and progenitor cells of the blood-forming system. In that context, HSCs were defined as single cells with lifelong ability to self-renew as well as differentiate to produce all blood cell lineages (multipotency). Any discovery of HSCs or populations of cells enriched for HSCs should show full multipotency as well as self-renewal.
Prospective isolation of mouse HSCs
The search for HSCs began with groups using colony assays and various methods of mouse bone marrow cell separation, initially by size and density21,22 and then with reagents to surface markers.23 None of the early groups looked for populations of cells that could produce all blood lineages from cells of a single phenotype, much less a single cell; most used the spleen colony assay, or in vitro factor–dependent myeloerythroid colonies, and some rescue of lethally irradiated mice. Probably the best enrichment of d12 CFU-S was carried out by Mulder and Visser and colleagues, using wheat germ agglutinin binding and high-level H2 expression.23 However, finding candidate stem cells required the establishment of clonal assays for each of the blood cell outcomes—T cells, B cells, and myeloerythroid cells—as well as production of more HSCs. In the 1970s and early 1980s, clonal assays for thymic T-cell development from early marrow precursors were developed.24,,–27 However, there were no B-lineage colony assays in vivo until Owen Witte and his postdoctoral fellow, Cheryl Whitlock, established Whitlock-Witte bone marrow stromal cultures,28 an adaptation of the famous Dexter marrow mixed stromal cultures that support hematopoiesis.29 In 1986, Muller-Sieberg, Whitlock, and Weissman found that clonal lines established from Whitlock-Witte stromal cells would support long-term growth of hematopoietic cells in vitro.30 When limiting numbers of marrow cells were added, most of these allowed the formation of discrete colonies, initially cobblestones of stroma with a growing clonal colony above and below the stroma in culture; a subset of these began with myeloid output and finished with B lymphopoiesis, allowing the isolation of stem and progenitor cells that include B lymphoid output. Because the ultimate function of HSCs and progenitors was linked from the beginning, with marrow reconstitution of irradiated hosts with donor cells, there was a requirement to measure engraftment from donor cells in a setting wherein immune rejection of the graft was precluded by nearly complete donor host matches, and that allowed mixtures of cells from more than a single donor to be assessed in the same host. Boyse and colleagues had developed mouse strains on the C57BL background congenic for 2 alleles of the cell surface Ly5/CD45, the leukocyte common antigen,31 and monoclonal antibodies to both Ly5 alleles were raised so that one could transplant cells in vivo and distinguish donor from host, either in competitive reconstitution assays or in radiation rescue assays.32
Identification and isolation of a hierarchy of progenitors
To define mature progeny of all blood cell lineages, we produced monoclonal antibodies (mAbs) that detected B lineage and granulocyte lineage cells,33 and we obtained mAbs to T cells and their subsets; later we characterized an mAb to an early erythroid cell surface marker, Ter 119.34 With these tools in hand, we focused on subsetting cells by fluorescence-activated cell sorting and reading their developmental potential both in vitro in the clonal assays, and in vivo.35,,,,–40 The most informative early experiment defined the clonal precursors of Whitlock-Witte culture B lineage–engrafting cells. Amazingly, the cells that produced these myeloid/B clones lacked expression of the B-lineage marker B220, the B-cell form of the CD45 molecule.35,36 If B-cell markers are not present on the earliest B-lineage progenitors, then markers of other committed blood lineages (T, granulocyte, macrophage/monocyte, erythroid, etc) were unlikely to be there also; this finding was the origin of the 7-10 lineage (Lin) antibodies for negative selection of marrow cells. As expected, the Lin− cells also contained all defined clonal lineage progenitors, and also enriched for marrow reconstituting cells.35 Next it was shown that all reconstituting and clonal progenitor cells expressed mouse Thy1(CD90) as well.41 But the frequency of colony-forming cells was only about 500-fold enriched in 1986, so other antibodies were sought that could subset these. Because the enriched HSC population was Thy1+, a library of monoclonal antibodies were obtained from Jan Klein and Yuko Aihara that were produced against marrow Thy1+ cells made into hybridomas with T cells.42 One antibody, now called Sca1 (a CD59/Ly6 family member43 ), separated the cells into about 25% Sca1-positive and 75% Sca1-negative cells; only the Sca1-positive cells read out in all clonal and in vivo reconstitution assays.44 Later, Morrison et al showed that the Thy1.1lo, Lin−, Sca1+ population was a mixture of long-term (LT) self-renewing HSCs, short-term (ST) HSCs (they self-renewed for ∼10 weeks and produced all blood lineages at limit dilution),40 and their progeny, multipotent progenitors (MPP)39 ; LT-HSCs give rise to ST-HSCs, which give rise to MPP, with no recorded dedifferentiations from these multipotent subsets to LT-HSCs. By that time, c-kit (CD117) had also been identified as a marker on HSCs.40,45 Clonal competitive reconstitutions with Thy1lo (T) Lin−(L) Sca1+ (S; TLS) cells, some including the ckit (K) marker (KTLS cells), gave rise in some clones to massive HSC expansion as well as formation of all blood lineages for life.40,46,47 Further experiments showed that the KTLS cells were the only functional marrow cells to give long-term multilineage reconstitution (LTMR) in syngeneic and allogeneic transplants48 ; that the dose of HSCs was inversely related to the time of multilineage donor-derived reconstitution49 ; and that with the exception of memory T and B cells, HSCs were the only long-term self-renewing cells in marrow.
The demonstration that only LT-HSCs provide life-long LTMR in conditioned hosts became clear when the oligolineage progenitors were prospectively isolated: cells committed to lymphoid fates (T, B, NK, and all classes of dendritic cells) resided in the IL7 receptor–positive clonal common lymphoid progenitor (CLP) population, which produces only these lymphoid fates and not any myeloerythroid fate in vivo.38,50 Meanwhile, cells of the myeloerythroid hierarchy in mice begin with IL7 receptor–negative clonal common myeloid progenitors (CMP), cells that produce both granulocyte-macrophage progenitors (GMP) and megakaryocyte/erythroid progenitors (MEP)37 (Figure 1). Transplantation of CMP gave rise to all myeloerythroid but not any lymphoid progeny (except for rare B cells), while MEP gave only red cells and platelets, and GMP gave rise only to granulocytes, monocytes, and cells of the macrophage lineage, as well as all types of dendritic cells.37,50,,,,–55 In these studies it was shown that the day 7 to day 10 myeloerythroid spleen colonies derived from CMP and MEP, while HSCs and multipotent progenitors gave rise to day 12 to day 14 myeloerythroid spleen colonies.19 MEP give rise also to megakaryocyte progenitors (MkP)51 and erythroid progenitors (Ep).56 In healthy uninfected mouse colonies, CMP and MEP can radioprotect mice until residual HSCs take over.19 CMP and GMP can provide effective protection against Aspergillus fungal and Pseudomonas bacterial infections of irradiated, minimally HSC-reconstituted hosts,57 while CLP can similarly protect such hosts from otherwise lethal infections with cytomegalovirus.58 Transplantation of all of these multipotent or oligolineage progenitors gives time-limited but robust production of the daughter cells their names define, but only LT-HSCs produce sustained LTMR that can be serially transferred.19,37,38,51,59
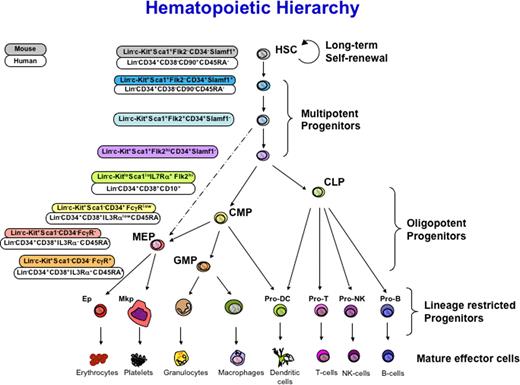
Schematic of hematopoietic development indicating intermediates in the hierarchy of hematopoietic differentiation. Surface markers used for isolation are indicated at left for human (top) and mouse (bottom) for each stem and progenitor cell. HSC indicates long-term reconstituting, self-renewing; MPP, multipotent progenitors with limited self-renewal leading to transient but multilineage reconstitution; CMP, common myeloid progenitor; CLP, common lymphoid progenitor; BLP, B lymphocyte protenitor; ProT, T-cell progenitor; GMP, granulocyte/macrophage progenitor; MEP, megakaryocyte/erythroid progenitor; MkP, megakaryocyte progenitor; EP, erythroid progenitor. Adapted from Figure 1 in Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: The paradigmatic tissue specific stem cell. Am J Pathol. 2006;169:338-346, with permission from the American Society for Investigative Pathology.
Schematic of hematopoietic development indicating intermediates in the hierarchy of hematopoietic differentiation. Surface markers used for isolation are indicated at left for human (top) and mouse (bottom) for each stem and progenitor cell. HSC indicates long-term reconstituting, self-renewing; MPP, multipotent progenitors with limited self-renewal leading to transient but multilineage reconstitution; CMP, common myeloid progenitor; CLP, common lymphoid progenitor; BLP, B lymphocyte protenitor; ProT, T-cell progenitor; GMP, granulocyte/macrophage progenitor; MEP, megakaryocyte/erythroid progenitor; MkP, megakaryocyte progenitor; EP, erythroid progenitor. Adapted from Figure 1 in Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: The paradigmatic tissue specific stem cell. Am J Pathol. 2006;169:338-346, with permission from the American Society for Investigative Pathology.
The search for human HSCs
In 1984, Civin and colleagues reported a marker shared by KG1A leukemia cells and about 1% to 4% of bone marrow, first called the My10 marker, later shown to be CD34.60 These CD34+ cells were enriched for in vitro CFC. In 1988, Bernstein and colleagues showed that CD34+ cells reconstituted hematopoiesis in lethally irradiated baboons.61 In 1990, the Thomas group carried out a CD34-enriched autologous graft in a patient with a brain neoplasm, and achieved engraftment.62 The extrapolation of these studies to the eventual isolation of highly enriched, or purified human-candidate HSCs, required in addition to the previously described kinds of clonal assays, a method to show in vivo LTMR. SCID-Hu mice were developed from SCID or NOD-SCID mice that received human fetal organs, including fetal thymus, fetal liver, fetal bone with marrow cavity, fetal spleen, and/ or fetal lymph nodes.63 In irradiation-reconstitution experiments, human CD34+Thy1 (CD90)+Lin− cells were the only cells in human adult and fetal marrow, cord blood, fetal liver, and mobilized peripheral blood (MPB) that achieved LTMR in SCID-Hu mice, as well as highly enriched B/myeloid cobblestone clonal colonies on the AC6 Whitlock-Witte clone of mouse marrow stromal layer, and myeloerythroid colonies in colony-forming cell and Dexter culture stroma.30,64,65 Similarly, in 1989, Sutherland, Eaves, and colleagues used irradiated human bone marrow stroma as a feeder layer to support long-term culture of hematopoietic cells sorted from human bone marrow,66 and in 1997, Bhatia et al showed in vivo repopulating activity of Lin−CD34+CD38− cells in NOD-SCID mice (SCID repopulating cells [SRC]) in cord blood and bone marrow.67 In humans and in mice, HSCs and progenitors are mobilized into the blood, and only HSCs are the operative cells in LTMR from MPB.68,–70 Human MPB CD34+CD90+ cells with or without Lin− selection are functional in human autologous transplants, with a dose-response to time of absolute neutrophil count (ANC) of more than 500 cells/μL and platelet count above 20 000 that approximated the mouse syngeneic HSC studies.49,71,–73
Human hematopoietic progenitors have also been characterized (Figure 1). Manz et al identified the human counterparts of the CMP, GMP, and MEP from both adult bone marrow and cord blood within the CD34+CD38+ fraction of bone marrow by the differential expression of CD45 RA and IL3Ra.74 As in the mouse, the CMP (IL3Ra+CD45RA−) was shown to give rise to both the GMP (IL3Rα+CD45RA+) and the MEP (IL3RαloCD45RA−). These cells contained no lymphoid developmental potential. A common lymphoid progenitor has also been characterized from humans. In 1995, Galy et al described a CD34+CD38+Lin−CD10+CD45RA+ cell that could give rise to T, B, natural killer (NK), and dendritic cells.75 In 2001, Hao et al also described a lymphoid progenitor from human cord blood with the expression profile of CD34+CD38−CD7+ that gave rise to B, NK, and dendritic cells,76 and more recently Hoebeke described T-cell development from these cells as well.77 Interestingly, this CD34+CD38−CD7+ population does not exist in adult bone marrow, and we have shown that the CD34+CD38+Lin−CD10+CD45RA+ CLP described by Galy are within the human IL7Rα marrow subset of CD34+38+Lin− cells, encompassing both CD10+ and CD10− subsets (M. Manz, K. Akashi, T. Miyamoto, and I.W., unpublished data, January 1, 2003).
Application of this method to the isolation other stem cells
The isolation of mouse and human HSCs and progenitor cells by the method described has served as the template for the prospective isolation of a number of other stem cells. Anderson et al isolated a peripheral nervous system stem cell,78 which Morrison and Anderson showed persisted into postnatal life.79 Uchida et al isolated a human central nervous system stem cell and showed that it was a neurosphere-initiating cell capable of self-renewal and production of at least some neurons, oligodendrocytes, and astrocytes from a single cell80 ; and, by transplantation to immunodeficient mice engrafted into the neurogenic zones, from which these human neural stem cells gave amazingly site-appropriate migration, differentiation, and integration into the mouse architecture.81 Sherwood et al isolated the mouse skeletal muscle stem cell, a subset of muscle satellite cells,82 and Cerletti, Wagers, and colleagues have recently shown that only this cell robustly regenerates mouse dystrophin mutant mice (a model of muscular dystrophy).83 Visvader, Eaves, and Clarke recently isolated mouse mammary gland stem cells and showed that a single cell regenerates the full mammary gland in a cleared mammary fat pad.84,–86 In addition to these normal tissue stem cells, these methods have also been applied to the identification of cancer stem cells. Bonnet and Dick showed using NOD-SCID mice that the CD34+CD38lo cells were enriched for human acute myeloid leukemia stem cells,87 and subsequently, Miyamoto et al purified CD34+CD38loCD90−Lin−88 human acute myeloid leukemia stem cells (capable of forming leukemic blast colonies), which have their preleukemic progression in the HSC stage, but which emerge as blastic leukemias at the MPP89 stage of development.88,90 In chronic myeloid leukemia stem cells, similarly, the chronic phase is at the HSC level, but the myeloid blast crisis leukemia stem cells are at the stage of GMP.91 Clarke et al were the first to prospectively isolate or enrich human breast cancer stem cells,92 and their studies have been followed by prospective enrichment of tumor-initiating candidate cancer stem cells from head and neck squamous cell cancers,93 glioblastomas,94,95 colorectal cancers,96,97 and pancreatic cancers.98 These findings from primary cancers at primary sites fit with the notion that they are multistep alterations of cells normally in the differentiation pathway of tissues, and still at this stage make daughter cells of the tissue; but some cancers may progress, especially at metastatic sites, to self-renewing immortal cells that do not differentiate at all, and in these a high proportion of cells in the tumor are tumorigenic.
Clinical uses of HSCs
What might be the value of prospective isolation of HSCs for human therapies? In studies of experimental hematopoietic cell transplantation (HCT) and clinical HCT, grafts contain heterogeneous populations of HSCs as well as downstream progenitors and mature blood cells. For patients with certain hematologic or advanced-stage malignancies who undergo autologous HCT, their grafts can additionally contain contaminating cancer cells. For this latter group of patients, prospectively isolated HSCs could serve as a tumor free graft that would rescue patients from supralethal doses of chemotherapy/radiation. In addition to the cytoreductive effects of the conditioning, allogeneic HCT is credited with a multitude of clinical effects, both positive and negative. The positive effects include replacement of a defective hematopoietic organ, the conference of antitumor effects (graft-versus-tumor [GVT]), and induction of immune tolerance. The negative features, which have certainly precluded the more widespread use of HCT, are the morbidity of the conditioning for transplantation, graft-verus-host disease (GVHD), and the likely related problem of impaired immune reconstitution. In sorting out the cellular elements responsible for effects of allogeneic HCT, postthymic T cells are identified as the major perpetuators of GVHD on the one hand and of GVT on the other. HSCs are the only cells required to function in a recipient in order to maintain continuous formation of the blood. However, until an HSC population was purified to homogeneity, it was not possible to know the capability of HSC grafts separated from all hematopoietic elements to regenerate blood formation in a robust and clinically meaningful way, or what the effects of hematopoiesis derived solely from HSCs would have on immune tolerance.
The process of identifying the Thy-1loLin−Sca1+ cells as an HSC-containing population utilized in vivo assays of rescue of lethally irradiated mice with limiting doses of candidate HSCs. Transplantation across nonhistocompatible CD45 congenic barriers in mice demonstrated that as few as 200 congenic KTLS cells per mouse (∼104 cells/kg) resulted in restoration of all blood cell lineages for the life of the animal, which was proof of concept that HSCs as a single population could be used in the clinical setting. However, clinical transplantation in patients who undergo myeloablative radiation and/or chemotherapy to rid them of their malignancy requires optimized engraftment times for restoration of protective immunity as conferred by neutrophils in the immediate posttransplantation phase, followed promptly thereafter by red cell and platelet engraftment to minimize transfusion dependency. To address these concerns, Uchida et al48,49 examined the engraftment kinetics of increasing doses of KTLS HSCs as compared with unmanipulated bone marrow that contained an equivalent amount of HSCs. Engraftment times of neutrophils, platelets, and red blood cells were nearly identical between the 2 groups, demonstrating that (1) the purified population had the capacity to rapidly regenerate the peripheral blood system; (2) the progenitor and mature cells contained in the whole bone marrow inoculum conferred a 1- to 2-day advantage in engraftment times over HSCs; and (3) a threshold in time to engraftment of 10 days was observed at a level of more than 5000 HSCs per mouse (∼2.5 × 105 cells/kg), likely reflecting saturation of the HSC niche. These studies gave credence to the clinical trials that ensued following the identification of the analogous CD34+90+ human population.64
Autologous HSC transplantation
High-dose radiation or chemotherapy and rescue of hematopoiesis with autologous cells is a treatment strategy routinely used for certain hematologic malignancies and solid tumors that are proven or deemed likely to recur after standard therapy. For a significant proportion of patients, their bone marrow and MPB grafts contain measurable quantities of malignant cells; however, the biologic significance of these contaminants is still debated. Three separate phase I/II clinical trials of transplanting 2 × 105 to 1 × 107/kg sorted autologous hematopoietic cell transplant into patients with advanced malignancies (stage IV breast cancer, non-Hodgkin lymphoma [NHL], multiple myeloma) were performed to test the safety of transplanting HSC grafts into myeloablated recipients. Manipulation of MPB by selection of CD34+CD90+ cells allowed the preparation of cancer-free transplants in the breast cancer patients71 and the NHL patients,72 and, although not completely devoid of detectable tumor cells, a 105-fold depletion of plasma cells in the multiple myeloma patients grafts was achieved,73 and comparable multilog reductions in tumor cells were obtained in the breast and NHL studies.71,72 Experiments specifically addressing reduction of tumor cells were performed by spiking in 1.2 × 108 cancer (breast, non-Hodgkin lymphoma, or myeloma) cells into MPB prior to sorting revealed more than 105-fold depletion of cancer cells when CD34 and Thy1.1 were used to enrich for HSC (see Hanania unpublished data discussed in Prohaska and Weissman99 ). The small phase I/II trials with breast cancer and NHL indicated showed promising results. Prompt engraftment occurred in the neutrophil, red blood cell, and platelet lineages, as predicted by the preclinical studies in mice. Slower T-cell recovery was noted as compared with MPB, but no significant differences were noted in the frequency or severity of opportunistic infections. Favorable results were particularly evident in the metastatic breast cancer study, in which 73% of patients (16 of 22) were alive and 41% (9 of 22) were disease free in the initial report.71 However, because of the small number of patients studied, it is not yet possible to determine whether or not purified HSCs result in improved outcome due to the reduction in reinfused metastatic malignant cells, although the results obtained to date are provocative and deserve follow-up with larger studies. It should be noted that despite the aggressive therapies used to eliminate the malignancies, patients may still harbor tumor cells. Thus, a measurable impact of a cancer-free hematopoietic cell graft on disease relapse may not be evident until the systemic therapy against the cancer is improved.
Allogeneic HSC transplantation
Transplantation of allogeneic HSCs is a unique endeavor. The risks, benefits, and spectrum of diseases that will be best treated by this approach differ substantially from allogeneic HCT as currently practiced. In the absence of T cells, problems of GVHD are gone, but so too is GVT. Thus, the application of allogeneic HSC transplantation is more suited for nonmalignant disease states including tolerance induction. The most important distinction of HSC transplantation as compared with standard HCT is that the modified cellular content of the graft changes the dynamics of host-versus-donor immunity, resulting in marked differences in the levels of donor cell chimerism and increasing the likelihood of graft failure. Whereas transplantation of unmanipulated bone marrow or MPB generally results in conversion of fully myeloablated recipients to complete donor type, transplantation of purified HSCs results in only partial donor/host T-cell chimerism.100,–102 Both conventional CD8+ T cells and CD8+ non–T cells have been identified to facilitate engraftment of HSCs101 and this facilitating effect is associated with elimination of residual host immune cells not purged by the radiation or other forms of host conditioning.101,102 In addition, in the absence of graft-facilitating cells, purified HSCs are more vigorously resisted by the host, and it is this increased resistance to engraftment that constitutes the major obstacle in using allogeneic HSCs in clinical transplantation.
Since the late 1950s and early 1960s, investigators have recognized that resistance to engraftment of allogeneic hema-topoietic cells is biologically different from rejection of other tissues.103,–105 Rejection of conventional solid organ grafts is mediated by T cells that utilize one clonal receptor type to recognize nominal antigen presented by MHC gene products or allogeneic MHC molecules. In contrast, classic genetic animal studies of bone marrow transplantations from parent into F1 offspring were inconsistent with resistance to transplanted hematopoietic cells solely by T cells.103,–105 This phenomenon, termed hybrid resistance, is proposed to be determined by a set of antigens expressed only on hematopoietic cells and inherited in a recessive manner.106 A large body of evidence suggests that Hh resistance is mediated by NK cells.106,–108 NK cells each utilize a constellation of receptors that interact in an integrated fashion, and their role in hematopoietic cell resistance is most relevant when MHC differences exist between donor and recipient. Consistent with the idea that HSCs and/or their progeny are targets of NK cells were our studies demonstrating that vigorous engraftment resistance encountered upon transplantation of purified HSCs across MHC barriers is significantly reduced by the use of the polyclonal anti–NK-cell antibody anti-asialoGM1.102 In more recent experiments, we observed that the immune barrier to transplanted KTLS HSCs is completely lacking in recipients who congenitally lack all 3 lymphoid lineages—T, B, and NK cells—as is the case for RAG2γc−/− mice.109 In contrast, RAG2−/− mice that lack T and B cells demonstrate only strong HSC resistance, reinforcing the primacy of NK cells in the immune barrier to engraftment.
In addition to the need to overcome immune-mediated resistance, successful engraftment of HSCs is contingent upon HSCs making their way to the correct microenvironment and establishing a foothold in that milieu. The notion that marrow “space” must be created by cytoreductive agents or radiation in order to accommodate transplanted HSCs was first proposed by Schofield in 1978.110 This hypothesis is based on the assumption that there are specific marrow areas or niches to which engrafting HSCs home, and that if these are occupied by host resident stem cells, donor engraftment will not take place. The requirement to create space to achieve engraftment has been challenged by Quesenberry et al111 and Brecher et al,112 who have suggested that either host HSCs can be displaced or excess niche space is available for incoming cells.111 Studies from our laboratories lead us to a modified view of these 2 stances.109,113 Specifically, cytoreduction is not an absolute requirement for obtaining engraftment because at any given point a limited number of niche sites (50-500 in the mouse) become available because of a steady-state trafficking of HSCs in and out of the bone marrow and blood stream. However, HSCs that remain in the niche are not easily unseated nor is there excessive niche space since high numbers of HSCs infused at one time do not lead to improved donor engraftment beyond the few percent threshold. HSC occupancy by host cells therefore presents a significant barrier to donor cell engraftment, and manipulations targeting the host HSCs should be included in any clinical preparative regimen aimed at achieving robust donor-derived multilineage hematopoiesis. Indeed, by treatment of RAG2γc−/− mice with a monoclonal antibody that depletes c-Kit–expressing cells, Czechowicz et al114 showed that targeting HSCs in this manner led to high levels of donor HSC engraftment. Interestingly, in diseases such as SCID, the engraftment of only a few donor HSCs is sufficient to rescue the lymphoid deficiency because of pronounced expansion of donor lymphoid progenitors, which do not develop in the SCID recipients.113
Tolerance induction
We began this review with the seminal studies of Ray Owen1 ; Billingham, Brent, and Medawar2,3 ; and Main and Prehn,8 whose combined discoveries in the 1940s and 1950s led to the principles of immunologic tolerance actively induced by inoculation of allogeneic hematopoietic cells. Those studies formed the cornerstone for understanding self-/non–self-reactivity and fostered the hope that one day modified tolerance induction protocols might be applied to clinical transplantation. An important extension of this research took place in the late 1960s and early 1970s, when it was demonstrated that transfer of hematopoietic cells could alter the course of autoimmune disease in rodents.115,–117 Specifically, Morton and Siegel showed that bone marrow transplantations could prevent disease if the hematopoietic cells were transplanted from unaffected rodents to susceptible ones.116,117 Several decades have passed since the potential of allogeneic HCT as a tolerance-inducing strategy was first revealed, and yet life-long pharmacologic immune suppression remains the standard of care for organ transplantation patients and for those severely affected by autoimmune disease. The translation of these discoveries to clinical practice has been hampered by the very real concerns of morbidity and even mortality that can occur as a result of hematopoietic cell allografting. The past decade has seen major strides in reducing HCT-related mortality. The approach of nonmyeloablative or reduced-intensity conditioning that permits engraftment of allogeneic hematopoietic cells, brought to fruition in the late 1990s by Storb and colleagues118,119 and others,120,121 has resulted in significant reduction of treatment-related risks. Given that human hematopoietic grafts can be manipulated to yield pure HSCs that have no possibility to induce GVHD, the central question we have asked regarding the use of HSCs for tolerance-induction protocols is whether HSCs themselves, devoid of all other donor blood elements, can give rise de novo to populations that will mediate immune tolerance.
To test whether allogeneic grafts of purified HSCs could tolerize recipients to donor-matched organs, we performed transplantations in mice conditioned with lethal122,123 and sublethal (nonmyeloablative) radiation (T.M. Cao, unpublished observations, December 29, 2004). Under both conditions, recipients retained significant levels of host T cells, which were therefore potentially alloreactive. The other hematopoietic lineages in the lethally irradiated mice converted to full donor type, whereas the nonmyeloablated mice developed multilineage chimerism. All stably allografted mice, regardless of whether they were or were not mixed-lineage chimeras, were tolerant to donor-matched but not third-party heart grafts, proving that the safer approach of nonmyeloablative conditioning and engraftment of HSCs is sufficient to induce organ transplantation tolerance. One mechanism responsible for this effect is that HSC-derived cells can mediate deletion of alloreactive T cells.100,122 To follow T-cell deletion, mice that express different endogenous superantigens were used as donor and recipient pairs. Because superantigen expression results in negative selection of specific Vβ T-cell subsets, it was possible to determine the fate of superantigen reactive cells by analyzing the peripheral blood. The HSC grafts were observed to mediate not only central deletion but deletion of postthymic host-derived T cells that had survived conditioning, showing that HSCs give rise to populations, presumably antigen-presenting cells that mediate T-cell deletion in the periphery as well as in the thymus.100
The principles of tolerance induction conferred by allogeneic HCT for the treatment of autoimmune disease differ from those conferred in organ transplantation tolerance (reviewed in Shizuru124 ). Autoimmune disease pathology is based on a person's genetic predisposition combined with the stochastic interaction of his/her immune response to environmental antigens. The major susceptibility genes linked to autoimmunity are certain alleles of the MHC, and because these molecules are responsible for T-cell selection and antigen presentation, it is not surprising that when MHC-mismatched hematopoietic grafts are transplanted into autoimmune prone animals, the repertoire of antigen-specific T cells changes with presumed deletion of autoreactive cells, and on that basis provides protection from disease. By following this reasoning, it is expected that the protective effects of MHC-matched grafts would be lesser than those of MHC-mismatched grafts. These expectations have proved only partially true as protection conferred by MHC-matched grafts is highly protective across some strain combinations in our preclinical models of transplantations in spontaneously arising, as well as antigen-induced, autoimmune disease (Beilhack et al,100 Beilhack et al,125 and L. Lee, unpublished data, May 3, 2007). Our experience includes prevention of the development of type 1 diabetes in NOD mice, as well as curative cotransplantation of HSCs and islets from mice genetically resistant to the disease in a few cases to already diabetic mice.100,125 Early-stage and advanced-stage mouse lupus in (NZBxNZW)F1 mice was halted by (DBA/2xC57BL)F1 MHC haploidentical transplants (one H2 allele shared, one not), even with sublethal conditioning that included anti-CD4 T-cell and anti-NK mAbs.126 The critical points derived from these preclinical studies when considering allogeneic HSC transplantation as therapy for autoimmune disease are as follows: (1) in many but not all cases the non-MHC background genes expressed on hematopoietic cells that differ between donor and recipient are sufficient to confer protection from autoimmunity; (2) a single copy of these background gene alleles such as the case of haploidentical transplantation will likely yield protection from autoimmunity; (3) nonmyeloablative conditioning and engraftment of HSCs that results in the establishment of multilineage partial chimerism is sufficient to confer protection from autoimmune disease; and (4) the preparative regimen influences the transplantation outcome. Regarding this last point, we have observed that mice affected with a form of multiple sclerosis can be cured of their paralysis by the combination of a nonmyeloablative regimen employing total lymphoid irradiation (TLI) and antithymocyte globulin (ATG) and engraftment of MHC-matched HSCs, whereas myeloablative radiation used as the regimen and rescue with HSCs in the same strain combination was ineffective (L. Lee, unpublished data, May 3, 2007). Of note, loss of the hematopoietic allograft results in relapse of the autoimmune disease. The TLI plus ATG regimen results in a lymphodepleted host whose residual lymphocyte profiles are dominated by regulatory cells.127 Thus, we conclude that the conventional approach of myeloablation for allogeneic HCT with the consequence of complete replacement of hematopoiesis by the donor may not be the optimal way to abrogate autoimmunity. Rather, since host regulatory cells are capable of suppressing autoreactivity, nonmyeloablative conditioning that favors the survival of regulatory cells coupled with the transplantation of allografts that permit mixed donor/host chimerism such as HSCs are the most promising way to approach autoimmune disease by cellular therapy.
Future directions
The technologies of selection and sorting will allow the cellular “engineering” of hematopoietic grafts of defined cell types. HSCs that are free of cancer can form the foundation for autologous and allogeneic grafts aimed at rescuing patients from the unwanted effect of high-dose radiation and chemotherapy. Immune populations with specific antitumor activity can be incorporated into the grafts to capitalize on the cellular therapeutic effects. The mystery of T-cell specificity has been solved, and tetramer reagents that specifically recognize targeted antigenic peptides embedded in known allelic HLA class I and II molecules reduce the separation of GVT from GVHD to 2 issues: (1) will modern genomic and epigenomic analyses of cancers, more specifically of cancer stem cells, reveal new targets for T cells? and (2) can reasonably cost-effective cell sorters isolate the tetramer- or plate-presented HLA peptide T cells in sufficient quantity to replace unselected T cells from the donor cells that cause GVHD? As in chemotherapy, we suggest that at least 3 independent tetramer-selected T-cell specificities (inflammatory and/or killer) be used to target the tumor cells so that single-epitope antigen loss variants will nevertheless be killed.
The treatment of a host of nonmalignant diseases by haplo-identical and HLA-mismatched HSCs awaits the advent of new conditioning regimens wherein the host is not in danger from the conditioning, yet pure HSCs, incapable of any GVHD, can engraft sufficiently to reverse the genetic or acquired blood cell disease. We have begun the process to find such regimens.102,114,128 With the establishment of appropriate conditioning regimens that permit engraftment of purified HSCs, the field will open for HSC-based therapy from HLA-matched or haplo-identical donors to patients suffering from a wide range of disorders. Beneficiaries of this approach will include patients with genetically defective hematopoiesis such as SCID,113 thalassemias and sickle cell disease, in addition to genetic and rarer pathologic blood formation. Our preclinical studies125,126 have shown that engraftment of allogeneic HSCs from haplo-identical or MHC-matched donors effectively induces organ transplantation and blocks the pathogenesis of autoimmune disease. Finally, we envision that infectious diseases of the blood system, such as AIDS, tuberculosis, malaria, and leprosy, might be treated with autologous gene-modified HSCs to produce T cells, red blood cells, and macrophages that resist colonization by the infectious agent.
These advances we now know cannot be brought to patients by any conventional or biotechnology company that requires small risk and large and rapid return. It will be the responsibility of national and state funding organizations to take the place of venture and angel investments so that such therapies, the simple transplantation of purified HSCs, can lead to advances in regenerative medicine.
A word on nomenclature
A very strange phenomenon concerning nomenclature has evolved in clinical hematopoietic cell transplantation. Papers in the early mid-1990s described the transplants by the cells given—bone marrow, mobilized peripheral blood, and so on—whereas sometime thereafter all transplants became “stem cell transplants,” regardless of the type of cells used. This weakening of the appropriate designation of the transplant has consequences, as clinicians not familiar with the difference between unmanipulated marrow or MPB grafts and purified or semipurified populations will be impaired in their ability to know what hematopoietic population was given, and whether cancer cells, T cells, progenitor cells, and so on comprised the transplant. We hope that a more rational and rigorous nomenclature is accepted, for example, unmanipulated MPB, or CD34-enriched MPB, or CD34+90+-enriched cells. We no longer talk about the helper to suppressor T-cell ratio, but now correctly cite the type of CD4 and the type of CD8 cells; transplanters of hematopoietic cells should be equally rigorous.
Acknowledgments
This work was supported by National Institutes of Health grants CA86017, CA086065, and HL058770 (to I.L.W.) and grants HL087240, HL075462, AI049331, and DK067559 (to J.A.S.).
National Institutes of Health
I read Paul De Kruif's great book on the lives of biomedical scientists, Microbe Hunters, at age 10 and was determined to find a laboratory biomedical research career. As a high school student in Great Falls, Montana, I began working in the laboratory of Ernst Eichwald, a transplantation biologist and pathologist who had just discovered the H-Y antigen. Within a few months I decided to understand why some mouse strain females rejected syngeneic male skin transplants, and why other females could not. I thought alternatively of strain-specific variations of the extent of intra-fetal cell traffic of male fetus cells in utero to induce tolerance (it was not), or genetic unreactivity that was strain-specific, which I helped show and published in 1958. I was inspired by the Billingham, Brent, and Medawar demonstration of actively acquired transplantation tolerance by injection of adult allogeneic marrow and spleen cells into fetal mice in utero, and I began reperforming these experiments for H2-mismatched and HY tolerance induction. I was sufficiently stimulated by John J. Trentin's findings on radiation chimeras to write him in 1957 to ask whether he thought the wasting observed in many such mice was due to an immune reaction of the donor against the host; he affirmed that notion, and kindly wrote in reply. That encouraged me that even as a high school student in a rural town without a university, I was still qualified to do experimental research. I showed that male donor marrow cells injected into sublethally irradiated syngeneic females induced transplant tolerance to syngeneic male skin, but not to allogeneic male or female skin. I also showed that transplant tolerance to syngeneic male skin could be induced in neonatal females with injections of spleen cells from either syngeneic or allogeneic male (but not female) spleen cells and showed that adoptive transfer of lymphocytes that eliminated the allogeneic hematolymphoid chimerism ended the tolerant state. I wondered what kinds of hematopoietic cells could persist to maintain the tolerance. So very early, I was drawn into the field of experimental hematopoietic cell transplantation as well as the mystery of adoptively acquired immunologic tolerance. Two other key figures played key roles in my career. Henry Kaplan, the great leukemia researcher and radiation oncologist gave me a lab at Stanford as a medical student in the 5-year plan and supported my continuing studies on tolerance and the thymus. During that time in 1962, at a meeting of the New York Academy of Sciences, I heard Jim Gowans reveal to the world the functions of highly purified small and large lymphocytes, and with Kaplan's support, worked in Gowans' lab and showed that the thymus functioned largely by producing and exporting cells to particular domains of peripheral tissues. Together, Eichwald (genetics as a tool in transplantation science), Kaplan (research discoveries can be translated to clinical therapies), and Gowans (you can purify cells like biochemists purify molecules) formed the environment that led to what I am still trying to do.
I was seduced into becoming a hematologist on the first day of my laboratory practical on this subject while a medical student at Stanford. I recall poring over a slide set of blood and bone marrow smears from patients with various forms of anemias and leukemias. Until that day I had planned to become an endocrinologist, focusing on the treatment of childhood diabetes (type 1 diabetes mellitus [T1DM]). However, while sitting in front of those slides, I realized how elegantly powerful the combination of a simple blood smear and scientific observation could be, and I was hooked. A few years previously, under the mentorship of C. Garrison Fathman, I had completed my doctoral thesis, which centered on immune tolerance induction strategies using monoclonal antibodies (mAbs) targeting T-cell subsets. We had shown that mAbs directed against CD4 cells could block autoimmune pathogenesis in spontaneously diabetic NOD mice, a model for T1DM, and further that transient treatment with these mAbs led to long-term survival of allogeneic pancreatic islets in wild-type mice. The rub was that antibody therapy alone was not sufficiently potent to both block autoimmunity and permit islet survival in overtly diabetic NOD mice, suggesting that similar difficulties would be encountered in treating human T1DM. The seminal observations of Ray Owen, Billingham, Brent and Medawar, and Main and Prehn from the 1940s and 1950s, which led to the concept that blood cell transplantation will result in immune tolerance, were well known to me. So, too, was the work of Morton and Siegel from the 1970s and later Ikehara and Good, showing that allogeneic bone marrow transplantation can cure autoimmune prone strains, including those found in prediabetic NOD mice. But the allure of becoming a hematologist, much less a hematopoietic cell transplanter, alluded me until I experienced the thrill of staring at those slides, and the revelation that the development of blood—unlike other tissues—could be envisaged on slides, and that obtaining such samples was astonishingly accessible. In the months that followed, it seemed that the universe conspired to draw me inexorably to a career in hematology. Chief among the tempters were Stanley Schrier and Karl Blume. Listening to Stan Schrier on clinical rounds or while reading bone marrow slides at the multiheaded microscope was like listening to a Mozart aria in his perceptiveness and clarity. Hearing Karl Blume describe the patients he treats and the program he built in Bone Marrow Transplantation conveyed all the passion, elegance, and expansive structure of a Beethoven sonata (cello and piano). My course was set in the summer of that year (1988) when my good friend and colleague, Irving Weissman, isolated the candidate hematopoietic stem cell from mouse bone marrow. Shortly thereafter, Irv (whom, by the way, I consider the Picasso of biomedical research) and I began our long collaboration dedicated to the idea that the safe transfer of the mighty blood organ will, among other things, restore and control an overzealous immune system. This goal we hope to realize in the near future, and once realized will lead me back to those patients with T1DM.
Authorship
Contribution: I.L.W. and J.A.S. both wrote the paper.
Conflict-of-interest disclosure: I.L.W. was a member of the scientific advisory board of Amgen and owns significant Amgen stock. I.L.W. cofounded and consulted for Systemix, is a cofounder and director of Stem Cells, Inc, and cofounded Cellerant Inc. J.A.S. declares no competing financial interests.
Correspondence: Dr Irving L. Weissman, Stanford Institute for Stem Cell Biology and Regenerative Medicine, 1050 Arastradero Road, A152, Mailcode 5542, Stanford, CA 94304-1334; e-mail: irv@stanford.edu.