Treatment of Epstein-Barr virus (EBV)–positive nasopharyngeal carcinoma (NPC) with EBV-specific cytotoxic T cells (EBV-specific CTL) has been promising, producing clinical responses. However, infused EBV-specific CTL did not expand in vivo, likely limiting their antitumor activity. Lymphodepleting patients with chemotherapy before T-cell transfer enhances in vivo T-cell expansion, but results in nonspecific destruction of the resident immune system and can have significant toxicity. To evaluate if monoclonal antibodies (mAbs) can produce a more selective lymphodepletion, we conducted a clinical study in which NPC patients received a pair of lymphodepleting mAbs targeted to the CD45 antigen (CD45 mAbs) before EBV-specific CTL infusion. Eight patients with recurrent NPC received CD45 mAbs followed by escalating doses of auto-logous EBV-specific CTL. Infusion of CD45 mAbs resulted in transient lymphopenia in all patients and an increase in interleukin-15 (IL-15) levels in 6 out 8 patients. All patients had an increase in their peripheral blood frequency of EBV-specific T cells after CTL infusion. Three patients with a persistent increase had clinical benefits including 1 complete response (> 24 months) and 2 with stable disease (for 12 and 15 months). Lymphodepleting mAbs prior CTL transfer may represent an alternative to chemotherapy to enhance expansion of infused CTL. This study is registered at http://www.clinialtrials.gov as NCT00608257.
Introduction
Nasopharyngeal carcinoma (NPC) arises from the epithelial cells of the nasopharynx, and almost all nonkeratinizing and undifferentiated forms of this tumor are associated with Epstein-Barr virus (EBV).1,2 NPC patients with limited local disease have a good prognosis when treated with chemotherapy and intensity-modulated radiation therapy, but outcomes in patients with loco-regional bulky or metastatic disease remain poor.1,3,4 In addition, patients who do survive frequently face severe short- and long-term treatment-related complications.5,6 Hence, there is a need for novel therapies to improve disease-free survival and reduce treatment-related complications. Targeted T cell–based immunotherapy clearly has the potential to meet these needs.7,8
Treatment of EBV-positive NPC with polyclonal EBV-specific cytotoxic T cells (EBV-specific CTL) has been promising, producing disease stabilization and complete remissions in patients with relapsed disease with low disease burden.9,–11 One of the primary obstacles in the treatment of NPC with EBV-specific CTL is the lack of expansion of the cells in the peripheral blood after infusion, so that the numbers of effector T cells available may be sufficient only for patients without bulky disease. This failure of CTL expansion in the periphery contrasts with the greater than 1000-fold expansion seen when EBV-specific CTL are given to patients during the period of lymphopenia after hematopoietic stem cell transplantation (HSCT)12 or to patients with lymphoid malignancies, who have a relative lymphopenia.13,14 Lymphoid depletion as a strategy to create space for the expansion of adoptively transferred cells has already shown evidence of success; melanoma patients receiving cyclophosphamide and fludarabine before the adoptive transfer of ex vivo expanded, melanoma-specific tumor-infiltrating lymphocytes (TILs), showed enhanced repopulation and proliferation of the transferred cells as well as regression of metastatic melanoma.15,16 However, some of these patients remained profoundly immunocompromised and failed to regenerate an effective immune system. This poor immune reconstitution resulted in part from the extensive and nonspecific destruction of the resident immune system by the lymphodepleting cytotoxic drugs.
Monoclonal antibodies (mAbs) that are cytolytic for lymphocytes may be an alternative means of producing lymphodepletion. The ideal antibody for T-cell depletion before CTL infusion should be effective but short lived in vivo, to permit rapid infusion and repopulation with infused CTL. We have used rat mAbs directed to the common leukocyte antigen CD45, which can deplete all leukocyte lineages.17 This depletion was prolonged only in lymphoid lineages, as neutrophils began to recover 48 hours after injection. For our clinical studies, we used a pair of rat immunoglobulin G2 (IgG2) mAbs, which have a short half-life in humans, permitting rapid subsequent infusion of CTL.18,19 To investigate if CD45 mAbs lymphodeplete NPC patients and allow for in vivo expansion of adoptively transferred EBV-specific CTL, we gave CD45 mAbs immediately before EBV-specific CTL infusion. Our results indicate that the approach is safe, results in transient lymphodepletion, and allows adoptively transferred CTL to expand even in NPC patients.
Methods
Study eligibility
This study was approved by the Institutional Review Board of Baylor College of Medicine and by the Food and Drug Administration. In accordance with the Declaration of Helsinki, informed consent was obtained from all patients before the study began. Patients were eligible if they had stage III or IV NPC at diagnosis (according to the American Joint Committee on Cancer) and had refractory or relapsed disease. Tumor EBV-positivity was verified by in situ hybridization for the EBV-encoded RNAs (EBERs). All patients were required to be off therapeutic/experimental treatments at least 4 weeks before study entry. Before CD45 mAbs and EBV-specific CTL administration, patients had functional imaging with fluorodeoxyglucose positron emission tomography (PET) and/or magnetic resonance imaging (MRI) to assess pretreatment disease burden.
Generation of EBV-transformed lymphoblastoid cell lines and EBV-specific CTL
Lymphoblastoid cell lines (LCL) and EBV-specific CTL were generated according to current Good Manufacturing Practice (cGMP) guidelines as previously described.9,12,13 After expansion, EBV-specific CTL were tested for sterility, human leukocyte antigen (HLA)–identity, immunophenotype, and EBV-specificity at the time of cryopreservation. Specificity was tested in a 4-hour 51chromium release cytotoxicity assay.
Study description
In this study, we used 2 unconjugated rat anti–human CD45 mAbs, YTH54.12 and YTH25.4, which are of the IgG2b subclass, bind to noncompeting but proximate CD45 epitopes present in all CD45 isoforms, and in combination, have a synergistic ability to lyse human hematopoietic and lymphoid cells by complement-mediated and cell-dependent mechanisms.18,19 Patients received CD45 mAbs (400 μg/kg per dose) as a daily 6- to 8-hour infusion for 4 days. Forty-eight to 72 hours after the end of the final infusion, CD45 mAb levels were determined, and EBV-specific CTL were given if the CD45 mAb level was less than 100 ng/mL, a CD45 mAb concentration that is 1000-fold below the concentration needed to induce complement-mediated lysis in vitro.19 Patients were treated on 3 escalating CTL dose levels and received one dose of either 2 × 107 cells/m2 (dose level 1), 5 × 107 cells/m2 (dose level 2), or 108 cells/m2 (dose level 3). Peripheral blood was obtained before infusion and at scheduled time points after infusion to evaluate for evaluation of toxicity and EBV immunity. To assess clinical response to CTL therapy, patients were reimaged 8 weeks after CTL infusion (or earlier if clinically indicated).
Enzyme-linked immunosorbent spot assay
Patients' peripheral blood mononuclear cells (PBMCs) were isolated using Lymphoprep and cyropreserved. Enzyme-linked immunosorbent spot (ELISPOT) assays were performed as previously published using a single plate for each individual patient.13,20 Briefly, a Multiscreen 96-well plate (Millipore, Bedford, MA) was coated with “capture” antibody against interferon (IFN)–γ (Mabtech AB, Nacka, Sweden) overnight at 4°C. The wells were washed 3 times with phosphate-buffered saline (PBS) and then blocked with ELISPOT media (RPMI 1640, 2 mM GlutaMAX-I, 5% human serum [C-6; Diagnostic Inc, Germantown, WI]) for at least 1 hour. The blocking media was removed from the Multiscreen plate, the wells were washed twice with PBS, and 100 μL cells in ELISPOT media were plated in triplicates; 2 × 105 cells per well for PBMC and 105 cells per well for CTL. Next, 100 μL media were added to each well containing LCL (105 cells), 5 μg/mL peptide (see list below) or 500 ng/mL overlapping peptide mixes (see list below). Unstimulated PBMCs served as negative control. After 16 to 20 hours, the plates were developed and analyzed (ZellNet Consulting, New York, NY). Spot-forming cells (SFCs) per CTL or PBMCs were calculated and expressed as SFC per 105 CTL or SFC per milliliter whole blood; the CD3+ lymphocyte count on the day of the blood draw was used for calculation of SFC per milliliter whole blood.
The following peptides (Genemed Synthesis, San Francisco, CA), listed by EBV antigen and HLA restriction, were used in ELISPOT assays to determine the frequency of epitope-specific T cells in CTL lines or patients' PBMCs21,–23 : BMLF1: HLA-A2: GLCTLVAML; BRLF1: HLA-A2: YVLDHLIVV; HLA-A3: RVRAYTYSK; HLA-24: DYCNVLNKEF; BZLF1: HLA-B8: RAKFKQLL; EBNA3A: HLA-A3: RLRAEQVK; HLA-24: RYSIFFDY; HLA-B8: FLRGRAYGL; EBNA3B: HLA-A11: AVFDRKSDAK, IVTDFSVIK, NPTQAPVIQLVHAVY, LPGPQVTAVLLHEES, DEPASTEPVHDQLL; HLA-A24: TYSAGIVQI; HLA-B44: VEITPYKPTW; EBNA3C: HLA-B27: RRIYDLIEL; HLA-B44: KEHVIQNAF, EGGVGWRHW, EENLLDFVRF; LMP1: HLA-A2: YLLEILWRL, YLQGNWWTL, LLLIALWNL, LLVDLLWLL; LMP2: HLA-A2: CLGGLLTMV, FLYALALLL, GLGTLGAAL, LTAGFLIFL, LLWTLVVLL: HLA-A11: SSCSSCPLSKI; HLA-A24: TYGPVFMCL, PYLFWLAAI; HLA-A25: VMSNTLLSAW; HLA-A68: FTASVSTVV; HLA-B27: RRRWRRLTV, RRLYDLIEL.
Overlapping peptide mixes for LMP1, LMP2, and cytomegalovirus protein pp65 (CMVpp65) were synthesized by JPT Peptide Technologies (Berlin, Germany). Peptide mixes contained 15 amino acid peptides scanning the entire length of the corresponding protein with an 11 amino acid overlap.
Flow cytometry
For all flow cytometric analyses, a FACScalibur instrument (Becton Dickinson, San Jose, CA) and CellQuest software (Becton Dickinson) were used. Cells were stained as previously described. In all cases, negative controls included isotype antibodies. All monoclonal antibodies were obtained from Becton Dickinson and included anti-CD3, -CD4, -CD8, -CD16, -CD19, -CD25, -CD27, -CD28, -CD45RA, -CD45RO, -CD56, -CD62L, -CD69, -CCR7, –T-cell antigen receptor (TCR)α/β, and -TCRγ/δ. The EBNA3B, HLA-A11–restricted AVF pentamer was manufactured by ProImmune (Springfield, VA). Pentamers were used at a 1:100 final dilution together with antibodies against CD3 and CD8. Intracellular FoxP3 staining was performed using the human FoxP3 staining kit from eBioscience (San Diego, CA) according to the manufacturer's instructions.
IL-7 and IL-15 ELISAs
Patients' plasma samples were collected and stored at −80°C. The plasma concentrations of interleukin-7 (IL-7) and IL-15 were measured using Quantikine Human IL-15 and Quantikine High Sensitivity Human IL-7 Immunoassay kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instruction. Briefly, a 96-well plate was coated with 100 μL of assay diluent; then 50 μL standard or control (both provided by the manufacturer) or sample were added to the appropriate wells. The plates were sealed and incubated for 2 hours at room temperature (RT). The wells were washed (×4) with wash buffer, and anti–IL-7 or –IL-15 conjugate was added to all wells, followed by a further incubation at RT. Again, the plate was washed (×4), and 200 μL substrate solution were added to each well. The plate was then incubated for 30 minutes at RT in the dark; 50 μL stop solution was added to each well, and absorption at 450 nm was determined using a 96-well plate reader (Elx808 Ultra Microplate Reader; Bio-Tek Instruments, Winooski, VT).
Determination of EBV-DNA and CMV-DNA levels in PBMC and plasma
For PBMCs, DNA was isolated from 3 to 5 × 106 PBMCs using an anion exchange column (QIAGEN, Valencia, CA). After isolation, 500 ng DNA were used for real-time polymerase chain reaction (PCR) to quantify the EBV genome copy number and was reported as copies/μg DNA.24 For plasma, DNA was isolated from 200 μL ethylenediaminetetraacetic acid (EDTA) plasma using an anion exchange column (QIAGEN). After isolation, EBV genome copy number was determined by real-time PCR and reported as copies/mL plasma. The CMV-DNA levels were measured by ViraCor Laboratories (Lee's Summit, MO) using real-time PCR.
Statistical analysis
The phase 1 trial used a standard 3 + 3, dose escalation design. Lymphocyte cell subset measurements, EBV-specific CTL frequency via IFN-γ ELISPOT assays, and IL-15, IL-7 ELISA assays were summarized over time using descriptive statistics. Data were initially assessed for normality. A log-transformation was applied to achieve normal distribution of data if appropriate. Paired analyses using paired Student t test or the Wilcoxon signed ranks test were performed to compare changes from pre-CD45 mAb or pre-CTL infusion.
Results
Patient characteristics
Eight patients were enrolled on this study and all had histologically confirmed EBV-positive poorly differentiated or undifferentiated NPC (World Health Organization [WHO] type II/III). Four were male and 4 female, and the median age was 38.5 years (range, 14-64 years). Two patients were Asian, 5 were Caucasian, and 1 was African American. All patients had failed at least one treatment regimen, and 6 of 8 patients had metastatic disease at the time of CTL infusion; 2 had loco-regional bulky, recurrent/refractory disease (Table 1).
Patient characteristics and clinical outcomes
| Patient ID . | Age . | Race . | Sex . | Stage at diagnosis . | Previous therapy . | Site of active disease at time of study enrollment . | Dose level . | EBV-CTL response . |
|---|---|---|---|---|---|---|---|---|
| P1507 | 39 | A | M | T4N1M0 (IVA) | CT ×2, XRT | Primary, bone, LN | 1 | SD × 12 mo |
| P1560 | 53 | C | F | T4N1M0 (IVA) | CT ×4, XRT | Primary, sinuses, temporal lobe | 1 | SD × 15 mo |
| P1641 | 14 | C | F | T4N1M1 (IVC) | CT ×1, XRT | Primary, bone, LN | 1 | PD |
| P1668 | 17 | B | M | T4N2M0 (IVA) | CT ×3, XRT | Bone, LN | 2 | PD |
| P1708 | 38 | C | F | T4N1M0 (IVA) | CT ×2* | Primary, bone, LN, lung, parotid gland | 2 | PD |
| P1740 | 15 | C | F | T2bN2M0 (III) | CT ×4, XRT | Primary | 2 | CR > 24 mo |
| P1790 | 64 | C | M | T4N2M0 (IVA) | CT ×2, XRT | Bone, LN, lungs | 3 | PD |
| P1798 | 40 | A | M | T4N1M0 (IVA) | CT ×4, XRT | Bone, LN, liver | 3 | PD |
| Patient ID . | Age . | Race . | Sex . | Stage at diagnosis . | Previous therapy . | Site of active disease at time of study enrollment . | Dose level . | EBV-CTL response . |
|---|---|---|---|---|---|---|---|---|
| P1507 | 39 | A | M | T4N1M0 (IVA) | CT ×2, XRT | Primary, bone, LN | 1 | SD × 12 mo |
| P1560 | 53 | C | F | T4N1M0 (IVA) | CT ×4, XRT | Primary, sinuses, temporal lobe | 1 | SD × 15 mo |
| P1641 | 14 | C | F | T4N1M1 (IVC) | CT ×1, XRT | Primary, bone, LN | 1 | PD |
| P1668 | 17 | B | M | T4N2M0 (IVA) | CT ×3, XRT | Bone, LN | 2 | PD |
| P1708 | 38 | C | F | T4N1M0 (IVA) | CT ×2* | Primary, bone, LN, lung, parotid gland | 2 | PD |
| P1740 | 15 | C | F | T2bN2M0 (III) | CT ×4, XRT | Primary | 2 | CR > 24 mo |
| P1790 | 64 | C | M | T4N2M0 (IVA) | CT ×2, XRT | Bone, LN, lungs | 3 | PD |
| P1798 | 40 | A | M | T4N1M0 (IVA) | CT ×4, XRT | Bone, LN, liver | 3 | PD |
A indicates Asian; B, black; C, Caucasian; M, male; F, female; mo, months; CT × n, where n is the number of previous chemotherapy regimens; XRT, radiotherapy; *, refused XRT; CR, complete response; PR, progressive disease; and SD, stable disease.
Analysis of EBV-specific CTL lines
EBV-specific CTL were successfully generated for all 8 patients. As previously reported, growth characteristics of EBV-specific CTL from NPC patients were comparable with normal donors.9 In 4-hour 51chromium release cytotoxicity assays, CTL lines of all patients showed a significantly higher killing of autologous LCL compared with mismatched LCL (P = .016), human T-cell leukemia (HSB-2) targets (P = .03), or autologous phytohemagglutinin (PHA) blasts (P = .008) at an effector to target ratio of 20:1 (data not shown). The CTL lines contained a high percentage of CD3+ T cells (median interquartile range [IQR] = 97.6% [94.3%-98.3%]); predominantly CD8 (median IQR = 88.6% [86.6%-91.8%]) with a small CD4 component (median IQR = 3.1% [2.0-6.2%]). NK cells (CD3−, CD16+, or CD56+) were present in low numbers (median IQR = 2.6% [1.4%-6.7%]; data not shown). To determine which EBV antigens were recognized by these EBV-specific CTL, we used IFN-γ ELISPOT assays to measure responses to published HLA-restricted peptides derived from EBV antigens. For patients in whom no HLA-restricted LMP1 and LMP2 peptide was described, we used peptide mixes that contained 15 amino acid peptides scanning the entire length of LMP1 or LMP2. CTL lines contained T cells specific for immunodominant EBV proteins (lytic and EBNAs) and LMP2 antigens, whereas no LMP1-specificity was detected (Table 2).
Specificity of EBV-specific CTL lines
| Patient ID . | HLA type . | Specificity . | ||
|---|---|---|---|---|
| EBNA 3A-C . | LYTIC . | LMP2 . | ||
| P1507 | A11, 24; B18, 40 | ++++ (A11, 24) | ||
| P1560 | A01, 24; B08, 47 | ++ (A24) | ||
| P1641 | A02, 68; B44, 49 | * | +(B68) | |
| P1668 | A24; B40, 45 | +++ (A24) | ++ (A24) | |
| P1708 | A3; B27, 45 | +++ (B27) | ||
| P1740 | A01, 02; B27, 37 | +++ (A2) | ++ (A2) | |
| P1790 | A01, 25; B08, 38 | + (B8) | +++ (B8) | ++ † |
| P1798 | A11, 24; B13, 51 | |||
| Patient ID . | HLA type . | Specificity . | ||
|---|---|---|---|---|
| EBNA 3A-C . | LYTIC . | LMP2 . | ||
| P1507 | A11, 24; B18, 40 | ++++ (A11, 24) | ||
| P1560 | A01, 24; B08, 47 | ++ (A24) | ||
| P1641 | A02, 68; B44, 49 | * | +(B68) | |
| P1668 | A24; B40, 45 | +++ (A24) | ++ (A24) | |
| P1708 | A3; B27, 45 | +++ (B27) | ||
| P1740 | A01, 02; B27, 37 | +++ (A2) | ++ (A2) | |
| P1790 | A01, 25; B08, 38 | + (B8) | +++ (B8) | ++ † |
| P1798 | A11, 24; B13, 51 | |||
Frequency: ++++, 1-2%; +++, 0.5-1%; ++, 0.25-0.5%; +, < 0.25%.
No epitopes.
HLA restriction unknown.
Infusion of CD45 mAbs and EBV-specific CTL is safe
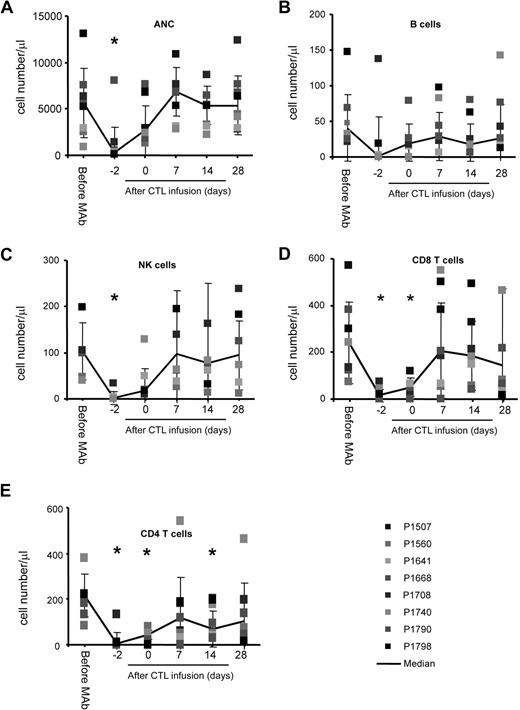
Infusion of CD45 mAbs was safe, and the only nonhematologic toxicities we observed were grade 1 and 2 toxicities according to the National Cancer Institute (NCI) Common Toxicity Criteria Scale version 2.0, including fever, abdominal pain, hypotension, and nausea. In addition, transient neutropenia (absolute neutrophil count [ANC] < 0.5 × 109/L), which resolved within 48 hours, was observed (Figure 1A). Subsequent adoptive transfer of EBV-specific CTL was safe, and no dose-limiting toxicity was observed. Within 48 hours after CTL infusion, one patient (P1708) on dose level 2 developed swelling at a metastatic cervical lymph node site that resolved within 2 weeks. The transient swelling was attributed to active tumor cell destruction by CTL, because it coincided with a near 2-log increase in plasma EBV-DNA levels from 6.3 × 104 copies/mL to 4.6 × 106/mL before returning to baseline, and an MRI showed central necrosis of the lymph node.
CD45 mAb infusion induces transient neutropenia and lymphodepletion. Counts are shown of (A) neutrophils, (B) B cells, (C) NK cells, (D) CD8pos T cells, and (E) CD4pos T cells (*P < .05).
CD45 mAb infusion induces transient neutropenia and lymphodepletion. Counts are shown of (A) neutrophils, (B) B cells, (C) NK cells, (D) CD8pos T cells, and (E) CD4pos T cells (*P < .05).
Infusion of CD45 mAbs results in transient lymphodepletion
CD45 mAb infusions profoundly decreased all lymphocyte subsets (B cells, natural killer [NK] cells, CD4+ and CD8+ T cells; Figure 1B-E). At the time of CTL infusion, 48 hours after the last CD45 mAb dose, patients remained lymphopenic with a significantly reduced CD4+ and CD8+ T-cell count (CD4, P = .008; CD8, P = .005). All lymphocyte subsets returned to baseline within 9 days of CD45 mAb infusion.
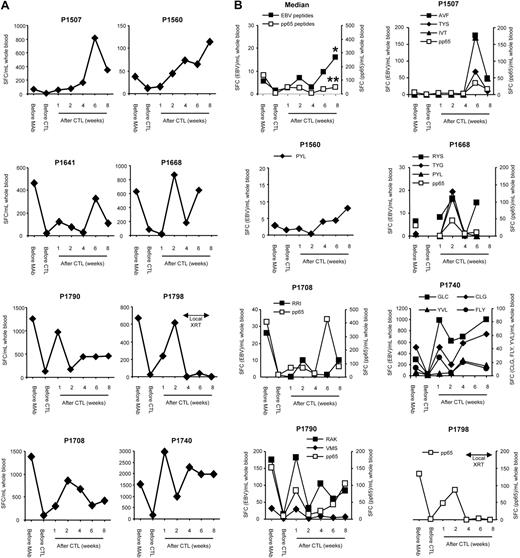
CD45 mAb-mediated lymphodepletion results in expansion of adoptively transferred EBV-specific CTL
To asses the global immune response to EBV, IFN-γ ELISPOT assays were used with autologous LCLs as stimulators. CD45 mAbs initially decreased the EBV-specific T-cell precursor frequency in all patients (P = .008), but at one week after infusion of EBV-specific CTL, the median EBV-specific T-cell precursor frequency increased significantly, indicating expansion of adoptively transferred T cells (P = .039; Figure 2A). At later time points, however, there was more variability in the total EBV-specific T-cell precursor frequency using LCL as stimulators, and we therefore focused on determining the frequency of EBV peptide-specific T cells in patients who had T cells recognizing known EBV-specific peptide epitopes in their CTL product (6 of 8 patients). T-cell responses were specific for lytic (3 antigens), EBNAs 3A, B, and C (5 antigens), and LMP2 (6 antigens) EBV antigens. The frequency of CMVpp65-specific T cells served as an internal control in patients who were CMV-seropositive (5 of 8 patients; Figure 2B). The median frequency of EBV peptide-specific T cells increased 2.8-fold from pre-CD45 values to 8 weeks after CTL infusion (P = .001), whereas no significant increase was observed in the frequency of CMVpp65-specific T cells (P = .25; Figure 2B).
Increase of EBV-specific T cells after lymphodepletion and EBV-specific CTL infusion. (A) The EBV-specific T-cell precursor frequency in patients' PBMCs was determined with IFN-γ ELISPOT assays using autologous LCL. (B) EBV peptide-specific T-cell responses were compared with CMVpp65-specific T-cell responses with IFN-γ ELISPOT assays using peptides (*EBV peptide-specific T cells before mAb vs 8 weeks, P = .001; **CMVpp65-specific T cells before mAb vs 8 weeks, P not significant).
Increase of EBV-specific T cells after lymphodepletion and EBV-specific CTL infusion. (A) The EBV-specific T-cell precursor frequency in patients' PBMCs was determined with IFN-γ ELISPOT assays using autologous LCL. (B) EBV peptide-specific T-cell responses were compared with CMVpp65-specific T-cell responses with IFN-γ ELISPOT assays using peptides (*EBV peptide-specific T cells before mAb vs 8 weeks, P = .001; **CMVpp65-specific T cells before mAb vs 8 weeks, P not significant).
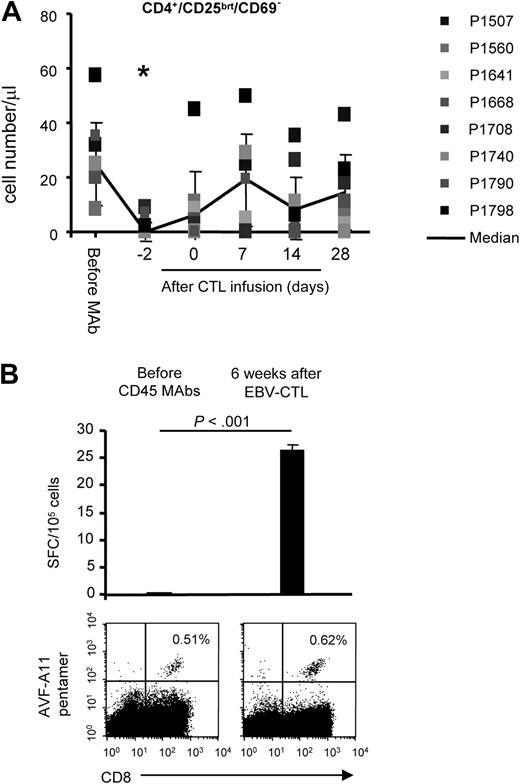
Mechanisms of CTL expansion
CD45 mAb-mediated lymphodepletion decreased the numbers of immunosuppressive T cells (defined as CD4+, CD25bright, CD69−).25,26 These cells recovered to baseline within 9 days of the last CD45 mAb infusion (Figure 3A). To insure this cell population correlated with the number of FoxP3+ Treg cells, we also determined the frequency of CD4+, CD25+, and FoxP3+ T cells in 3 patients on the day of and at 2 weeks after CTL infusion (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We observed no increase in the percentage of T cells with regulatory phenotype during the recovery phase, in contrast to the rebound of Tregs after chemotherapy-mediated lymphodepletion.27 The functional significance of these immunomodulatory effects were corroborated in one patient (P1507) for whom we had sufficient material to compare the frequency of pentamer-positive and IFN-γ–secreting T cells specific for the HLA-A11–restricted EBNA3B peptide AVF (Figure 3B) before lymphodepletion and 6 weeks after EBV-specific CTL infusion. Whereas the frequency of AVF-specific T cells was similar by pentamer analysis at both times, AVF-specific T cells only secreted IFN-γ upon AVF peptide stimulation 6 weeks after CTL infusion (P < .001), suggesting that anergic cells were replaced with functional cells after adoptive transfer.
Reversal of T-cell anergy after lymphodepletion and EBV-specific CTL infusion. (A) CD45 mAb-mediated lymphodepletion results in transient decrease of CD4+, CD25bright, and CD69− T cells (*P < .05). (B) The frequency of pentamer-positive and IFN-γ–secreting T cells specific for the HLA-A11–restricted EBNA3B peptide AVF were determined before lymphodepletion and 6 weeks after EBV-specific CTL infusion. Whereas the frequency of AVF-specific T cells was similar by pentamer analysis at both time points, AVF-specific T cells only secreted IFN-γ upon AVF peptide stimulation 6 weeks after CTL infusion.
Reversal of T-cell anergy after lymphodepletion and EBV-specific CTL infusion. (A) CD45 mAb-mediated lymphodepletion results in transient decrease of CD4+, CD25bright, and CD69− T cells (*P < .05). (B) The frequency of pentamer-positive and IFN-γ–secreting T cells specific for the HLA-A11–restricted EBNA3B peptide AVF were determined before lymphodepletion and 6 weeks after EBV-specific CTL infusion. Whereas the frequency of AVF-specific T cells was similar by pentamer analysis at both time points, AVF-specific T cells only secreted IFN-γ upon AVF peptide stimulation 6 weeks after CTL infusion.
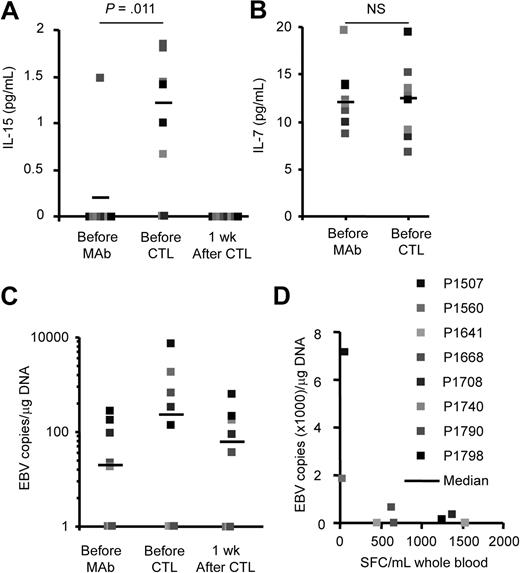
We next investigated whether CD45 mAb-mediated lymphodepletion also had a direct positive effect on lymphoid expansion, by enhancing production of cytokines that promote homeostatic lymphocyte expansion in the lymphopenic host. We measured the levels of IL-7 and IL-15 and found a significant increase (P = .031) in the plasma levels of IL-15 in 6 of 8 patients at the time of CTL infusion that returned to baseline with in 7 days (Figure 4A); in contrast, plasma IL-7 levels remained unchanged (Figure 4B).
CD45 mAb infusion induces a transient increase of IL-15 and EBV-DNA. Plasma (A) IL-15 and (B) IL-7 levels were determined with ELISAs. IL-15 levels increased significantly, whereas IL-7 levels remained unchanged. (C) EBV-DNA load increased transiently in 4 patients. (D) Patients with the lowest EBV-specific T-cell precursor frequency had the highest increase in EBV-DNA load.
CD45 mAb infusion induces a transient increase of IL-15 and EBV-DNA. Plasma (A) IL-15 and (B) IL-7 levels were determined with ELISAs. IL-15 levels increased significantly, whereas IL-7 levels remained unchanged. (C) EBV-DNA load increased transiently in 4 patients. (D) Patients with the lowest EBV-specific T-cell precursor frequency had the highest increase in EBV-DNA load.
Finally, we determined whether lymphodepletion produced EBV reactivation due to loss of immune surveillance by endogenous EBV-specific T cells, with relative sparing of the more CD45-resistant B-cell population infected with the virus.19 Such reactivation would expose the adoptively transferred T cells to high levels of EBV antigen in the residual B cells. We measured EBV-DNA in PBMCs before and after CD45 mAb infusion, and found an increase in 4 of 8 patients, which returned to baseline with in 7 days (Figure 4C). Of note, both patients (P1507, P1560) with the lowest pretreatment EBV-specific T-cell precursor frequency had the highest EBV-DNA increase after CD45 mAb treatment, supporting the effects of lymphodepletion on existing EBV regulation by endogenous T cells (Figure 4D).
Clinical benefit of EBV-specific CTL infusion
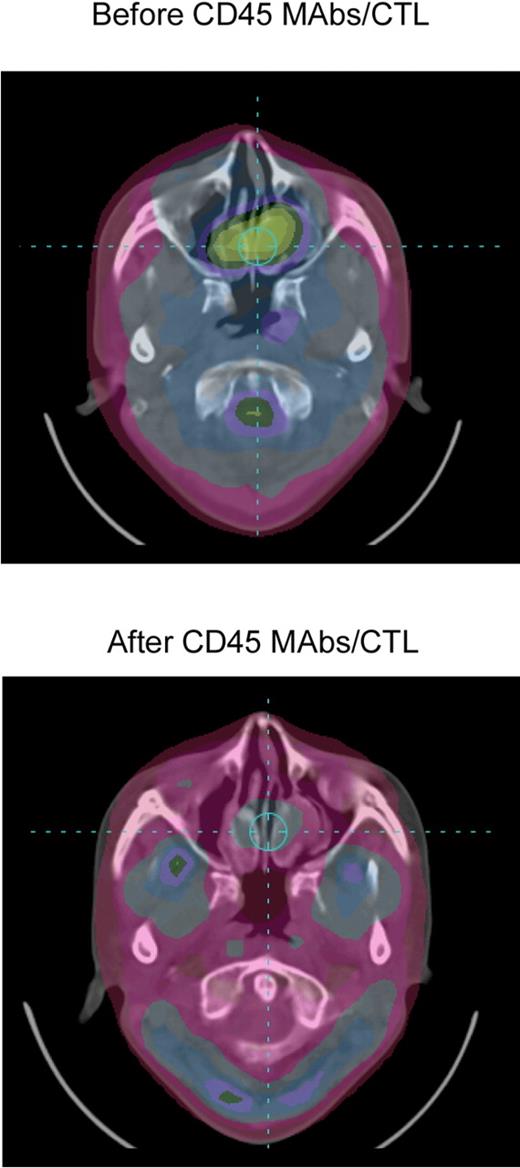
Patients were evaluated for disease 8 weeks after CTL infusion, using PET and/or MRI (Table 1). One patient (P1740) had a complete response by PET imaging (Figure 5). The patient received an additional dose of EBV-specific CTL (without CD45 mAb infusion) and remains in complete response more than 24 months later. A residual nasopharyngeal mass demonstrated by MRI (but not PET avid) was biopsied 10 months after CTL and showed no evidence of active disease. Two patients (P1507, P1560) had stable disease 8 weeks after CTL infusion. Both patients received additional infusions of CTL without CD45 mAb infusion (P1507, 2 doses; P1560, 4 doses), and stable disease was maintained for 12 and 15 months, respectively. Five patients had progressive disease; 6 patients died (at 3, 4, 8, 23, 25, and 26 months after CTL infusion), and one patient is alive with active disease at 27 months. This patient received palliative chemotherapy (capecitabine) after coming off study and had a long-lasting partial response (PR).
Complete response after lymphodepletion and EBV-specific CTL infusion. PET images of patient (P1740) before and 8 weeks after CD45 mAbs/EBV-CTL infusion.
Complete response after lymphodepletion and EBV-specific CTL infusion. PET images of patient (P1740) before and 8 weeks after CD45 mAbs/EBV-CTL infusion.
Discussion
In this study, we evaluated the safety and efficacy of CD45 mAbs for lymphodepleting patients with EBV-positive NPC before infusion of EBV-specific CTL. We found that that infusion of CD45 mAbs is safe and results in lymphodepletion, elevated IL-15 plasma levels, and expansion of adoptively transferred EBV-specific CTL. This treatment was associated with clinical benefits in 3 of 8 patients with relapsed refractory disease, including one complete response.
Adoptive transfer of EBV-specific T cells to patients with NPC can induce durable antitumor responses.9,10 As for other T-cell therapies, however, the antitumor activity of EBV-specific CTL is currently limited by several factors, including lack of in vivo expansion of infused EBV-specific CTL.28 Lymphodepletion to enhance expansion of adoptively transferred T cells has shown promise in animal models as well as in phase 1 clinical studies.15,16,29 In all studies so far, chemotherapy and/or radiation were used to lymphodeplete patients. Both approaches carry a significant risk of short- and long-term treatment-related complications, particularly in heavily treated relapsed or resistant patients. Lymphodepleting antibodies are an attractive, less toxic option because they are highly cell-specific and cause little bystander cell damage.
CD45 mAbs produced lymphodepletion in these patients, consistent with our previous study in which we gave the mAbs to 12 patients who were to receive HSC transplants.19 We were unable to determine the duration of lymphodepletion in these HSC transplant recipients, because CD45 mAbs were given before an ablative conditioning regimen. In the current series of NPC patients, however, lymphocyte counts fully recovered within 9 days of mAb administration. This transient lymphodepletion contrasts with our murine data, in which the animals remained lymphopenic for 4 weeks after CD45 mAbs administration.17 The differences between murine and human studies may reflect increased uptake in the liver resulting in incomplete depletion of lymphocytes in lymphoid organs and marrow, allowing rapid rebound of these cells to normal levels.30,31
In this study, the median frequency of EBV peptide-specific T cells increased by 2.8-fold after CD45 mAb infusion over an 8-week period. Such a persistent increase was not seen in our previous study in which we infused EBV-specific CTL without prior lymphodepletion to NPC patients.9 The observed increase in EBV-specific T-cell frequency likely underestimates the true level of expansion, because it assumes that all T cells remain in the circulation and that none enter lymphoid organs or tumor sites. Physiologically, however, lymphocytes traffic readily, and the blood only contains 2% of the entire lymphocyte pool.32,33 If we assume that the total number of lymphocytes is approximately 5 × 1011, the actual fold expansion of infused CTL would be greater than 100-fold.32,33
Could this increase in EBV peptide-specific immunity been solely due to endogenous EBV-specific immune recovery? An ideal control group to answer this question would have been patients receiving CD45 mAbs without the infusion of EBV-specific T cells. However, we cannot use CD45 mAbs alone in NPC patients, because the maximum tolerated dose in humans is already established, and NPC cells are themselves CD45−.19,34 To minimize this limitation, we measured the recovery of CMVpp65-specific T cells as a control for the endogenous rate of immune recovery to viruses. We observed an increase in the frequency of EBV peptide-specific T cells, whereas the frequency of CMVpp65-specific T cells was unchanged. This result argues that the observed expansion of EBV-specific T cells was due to the infused CTLs and not due to the general recovery of viral-specific immunity after CD45 mAb administration.
Three mechanisms likely contribute to the expansion of EBV-specific CTL observed after CD45 mAb infusion. Homeostatic restoration of lymphoid cell numbers after lymphodepletion is mediated in part by increased levels of cytokines, such as IL-7 and IL-15. These cytokines would also be expected to promote the proliferative expansion of adoptively transferred T cells in the lymphopenic host.29,35 Although prolonged lymphodepletion results in increased plasma levels of both IL-7 and IL-15,36 in our more transiently depleted subjects, we observed an increase only of IL-15. This cytokine is, however, critical for the expansion of adoptively transferred EBV-specific CTLs, since these are almost exclusively effector memory T cells. In contrast, IL-7 controls the proliferation and maintenance of naive T cells.37,38 Hence, EBV-specific CTLs should only expand in the presence of IL-15 and not IL-7,39,40 and an increase in the levels of IL-15 supports the observed expansion of adoptively transferred EBV-specific CTLs.
Loss of Tregs may be a second mechanism underlying EBV-specific CTL expansion. Removal of Tregs before vaccination or T-cell transfer has resulted in enhanced antitumor effects in preclinical animal models, and one clinical study has reported encouraging results in humans.41,42 We observed a decrease of Tregs after CD45 mAb infusion without rebound as seen after the administration of cytotoxic agents,27 so it is possible that perturbation of Treg also contributes to the observed expansion of infused EBV-specific CTL.
Finally, CD45 mAb infusion also increases the level of antigenic stimulation to the infused EBV-specific CTLs. Following mAb-mediated depletion of endogenous EBV-specific CTL, we observed an increase in the EBV-DNA in 4 of 8 patients, indicating EBV reactivation. That this increased viral replication may contribute to subsequent expansion of EBV-specific CTL is strongly suggested by patients P1507 and P1560, who had the lowest pretreatment EBV-specific T-cell precursor frequency, the highest EBV-DNA increase after mAb, and the greatest rise in EBV-CTL frequency after infusion. Hence T cell–depleting mAbs may have the advantage over cytotoxic chemotherapy (which profoundly depletes B cells as well), because these mAbs not only create space for T-cell expansion, but also induce viral reactivation.
Investigators have shown that the expansion of adoptively transferred tumor TILs correlates with antitumor activity in melanoma patients.16 Does CTL expansion similarly correlate with clinical benefits for patients with NPC? The 3 NPC patients (P1507, P1560, P1740) with the greatest and longest lasting rise in their EBV-specific immunity had clinical benefits, whereas no antitumor activity was observed in the other 5 patients, encouraging the pursuit of lymphodepletion before CTL transfer. While these results are encouraging, the study was not designed to answer the question whether CD45 Ab infusion before adoptive transfer of EBV-specific CTL results in enhanced antitumor activity in comparison to CTL alone; this can only be addressed in a future phase 2 clinical study comparing the antitumor efficacy of EBV-specific CTL infusion with or without lymphodepletion in a randomized fashion.
The foremost limitation of our study is the short duration of CD45 mAb-induced lymphodepletion. Increasing the number of infused T cells or the use of cytokines such as IL-2 might be strategies to overcome limited lymphodepletion. Clinical studies have shown that large number of infused T cells rapidly disappear and never enter the memory pool.43 In addition, preparing increased numbers of T cells would require prolonged ex vivo culture, which is associated with decreased T-cell performance in vivo.44 Adoptively transferred T cells expand in humans after profound lymphodepletion with and without IL-2.12,16 Because the administration of IL-2 may be associated with significant side effects and mediates the expansion of inhibitory Tregs in vivo,27 its routine use to enhance the expansion of adoptive transferred T cells is controversial. Other cytokines are actively being explored including the use of IL-15; in addition, the genetic modification of T cells with cytokine genes might enhance their ability to expand in vivo.39,45
Prolonged lymphodepletion induced by radiation and chemotherapy enhances the antitumor activity of adoptively transferred T cells in animal models and initial studies in humans corroborate this finding.16 Because prolonged lymphodepletion in humans can be achieved without the unwanted side effects of cytotoxic agents,36 we favor combining CD45 mAb infusion with a second short-lived mAb,46 or with chemotherapeutic agents such as fludarabine, which are currently being used in reduced-intensity transplantation regimens.47
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank E. Yvon and A. Durett for expert technical assistance and A. Leen, T. Lopez, and the staff of the GMP facility for assisting in CTL line production.
The authors were supported by National Institutes of Health (NIH; Bethesda, MD) grants P01 CA94237, R21CA108143 and the General Clinical Research Center (GCRC) at Baylor College of Medicine (RR00188; Houston, TX). C.U.L. is supported by NIH grant NIDDK T32 DK64717; C.M.B. is supported by awards from the Kimmel Foundation (Baltimore, MD) and the Gillson Longenbaugh Foundation (Houston, TX); S.G. is the recipient of a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (New York, NY); and H.E.H. is the recipient of a Distinguished Clinical Scientist Award from the Doris Duke Charitable Foundation.
National Institutes of Health
Authorship
Contribution: C.U.L. cared for the enrolled patients, analyzed generated CTL lines as well as patients' samples, and contributed to the writing of the manuscript. K.S. and C.M.B. participated in the development of the clinical study; C.G. analyzed generated CTL lines as well as patients' samples. H.M.H. supervised CTL preparation and quality assurance. M.V.G. reviewed tumor samples for EBV positivity. M.-F.W. and H.L.W. provided statistical support. A.P.G. performed quality assurance on all CTL lines before clinical use. M.K.B. participated in the development of the clinical study and contributed to the writing of the manuscript. C.M.R. participated in the development of the clinical study, was co–principal investigator on the clinical trial, and contributed to the writing of the manuscript. H.E.H. participated in the development of the clinical study, was co–principal investigator on the clinical trial, cared for some of the patients, and contributed to the writing of the manuscript. S.G. participated in the development of the clinical study, was co–principal investigator on the clinical trial, cared for the enrolled patients, analyzed generated CTL lines as well as patient samples, and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen Gottschalk, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin Street MC 3-3320, Houston, TX 77030; e-mail: smg@bcm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal