Abstract
The tyrosine kinase JAK3 plays a well-established role during normal lymphocyte development and is constitutively phosphorylated in several lymphoid malignancies. However, its contribution to lymphomagenesis remains elusive. In this study, we used the newly identified activating JAK3A572V mutation to elucidate the effect of constitutive JAK3 signaling on murine lymphopoiesis. In a bone marrow transplantation model, JAK3A572V induces an aggressive, fatal, and transplantable lymphoproliferative disorder characterized by the expansion of CD8+TCRαβ+CD44+CD122+Ly-6C+ T cellsthat closely resemble an effector/memory T-cell subtype. Compared with wild-type counterparts, these cells show increased proliferative capacities in response to polyclonal stimulation, enhanced survival rates with elevated expression of Bcl-2, and increased production of interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα), correlating with enhanced cytotoxic abilities against allogeneic target cells. Of interest, the JAK3A572V disease is epidermotropic and produces intraepidermal microabscesses. Taken together, these clinical features are reminiscent of those observed in an uncommon but aggressive subset of CD8+ human cutaneous T-cell lymphomas (CTCLs). However, we also observed a CD4+ CTCL-like phenotype when cells are transplanted in an MHC-I–deficient background. These data demonstrate that constitutive JAK3 activation disrupts T-cell homeostasis and induces lymphoproliferative diseases in mice.
Introduction
Lymphoid malignancies have been associated with mutations that result in altered function or overexpression in transcription factors, such as NOTCH1, LMO2, or TAL-1/SCL.1-3 Myeloid malignancies are frequently associated with activating alleles of tyrosine kinases, including FLT3ITD or KITD816V in acute myeloid leukemia,4 BCR-ABL1 in chronic myelogenous leukemia,5 FIP1L1-PDGFRA in chronic eosinophilic leukemia,6 TEL-PDGFRB in chronic myelomonocytic leukemia,7 KITD816V in systemic mastocytosis,8 and JAK2V617F in the most cases of primary myelofibrosis, polycythemia vera, and essential thrombocythemia.9 In sharp contrast, only a few mutations in tyrosine kinases have been identified to date in lymphoid malignancies. Examples include fusions involving the anaplastic lymphoma kinase (ALK) gene and different partners, including the nucleophosmin (NPM) gene, which are found in a subset of anaplastic large cell lymphomas (ALCLs),10 and the fusion between the nuclear pore protein NUP214 and the ABL1 kinase, which is present in approximately 5% of patients with T-cell acute lymphoblastic leukemia (T-ALL).11 These examples and the frequent activation of downstream effectors of kinases in lymphoma12-14 suggest that other mutations that activate kinases are likely to be found in lymphoid malignancies.
The JAK3 tyrosine kinase plays a crucial and unique role in normal lymphocyte development and function.15,16 JAK3 differs from the other members of the JAK family that include JAK1, JAK2, and TYK2, in several ways. Its expression is developmentally regulated and restricted to the hematopoietic compartment,17 and it is the only member of the JAK family that interacts with the common gamma chain (γc) of several interleukin receptors, including interleukin (IL)–2, IL-4, IL-7, IL-9, IL-15, and IL-21. Since these cytokine receptors are necessary for normal lymphopoiesis, loss of JAK3 or γc function results in an early and severe block in T-cell and natural killer (NK) cell development and impaired B-cell function in mice and humans.18 Collectively, diverse mutations in JAK3 account for approximately 7% to 14% of human severe combined immunodeficiency cases.19
Conversely, JAK3 activation has been reported in several lymphoproliferative disorders, including mantle-cell lymphoma (MCL),12 Burkitt lymphoma,20 HTLV-1–induced adult T-cell lymphoma/leukemia (ATLL),14 cutaneous T-cell lymphoma (CTCL),21,22 and anaplastic large-cell lymphoma (ALCL).23 Interestingly, JAK3 physically interacts with the NPM-ALK fusion protein in ALCL24 and inhibition of JAK3 results in apoptosis of NPM-ALK+ lymphoma cells,25 suggesting that JAK3 is an essential mediator of the transforming properties of NPM-ALK. However, the mechanistic basis for constitutive JAK3 activation, as well as its precise contribution to other lymphoid disorders, is not well understood. We recently used a mass spectrometry approach to identify a JAK3A572V mutation (JAK3-AV) that resides in the JH2 pseudokinase domain of JAK3.26 JAK3-AV transforms the pre-B lymphoid cell line Ba/F3 to factor-independent growth and activates a spectrum of downstream effectors that include STAT5, the PI3K/AKT pathway, and the RAS/MAPK pathway. Although the JAK3-AV allele was initially found in a megakaryoblastic leukemia cell line, the most prominent phenotype of this mutation in a murine bone marrow transplantation (BMT) model is a fatal lymphoproliferative disorder. Inasmuch as prior studies of other constitutively active tyrosine kinases in BMT models have produced myeloid malignancies almost exclusively, we further investigated the nature of this lymphoid disorder and the role of JAK3-AV in its genesis.
Methods
Viral infection and BMT
The MSCV-JAK3WT-GFP and MSCV-JAK3A572V-GFP constructs and viral supernatants were obtained as described previously.26 Equivalent viral titers were used for each retroviral construct, and transduction efficiency in primary cells was confirmed by flow cytometric analysis of green fluorescent protein (GFP) content. For BMT, 8- to 10-week-old wild-type C57BL/6 donor mice (Taconic, Germantown, NY) were injected with 5-fluorouracil (5-FU; Sigma-Aldrich, St Louis, MO) 6 days prior to bone marrow collection from femurs and tibiae. After an overnight incubation in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 10 ng/mL murine (m) IL-3, 20 ng/mL mIL-6, and 10 ng/mL mSCF, cells were spin-infected with viral supernatants 2 times over 2 days, and 106 cells were injected into the tail-vein of lethally irradiated wild-type C57BL/6 or C57BL/6-H-2Kbtm1 H-2Dbtm1 (National Institute of Allergy and Infectious Disease [NIAID] Exchange Program, NIH:4215; Taconic) recipient mice. Animals were analyzed 4 to 10 weeks after transplantation. For secondary transplantations, 106 splenocytes from sick animals were injected into 6- to 8-week-old, sublethally irradiated syngeneic recipients. Approval for the use of animals in this study was granted by the Children's Hospital Boston Animal Care and Use Committee.
Histopathology, immunohistochemistry, and microscopy
Paraffin-embedded tissue sections from all organs were prepared at the Dana-Farber/Harvard Cancer Center Specialized Histopathology Services Core or at the Rodent Histopathology Core at Harvard Medical School and stained with hematoxylin and eosin (H&E) or with rabbit polyclonal anti-TdT, anti-CD3, anti-B220 (Dako, Carpinteria, CA), or CCR10 (Abcam, Cambridge, MA) antibodies. Images were obtained using a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan) and a SPOT RT color digital camera (model 2.1.1; Diagnostic Instruments, Sterling Heights, MI). Low-power images, magnification ×40 and ×100, were acquired with 4×/0.13 NA and 10×/0.25 NA objective lenses, respectively. Intermediate-power images, magnification ×400 and ×600, were acquired with 40×/1.3 NA and 60×/1.4 NA objective lenses with oil. High-power images (×1000) were acquired with a 100×/1.4 NA objective lens with oil (Trak 300; Richard Allan Scientific, Kalamazoo, MI). All images were analyzed with Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
Single-cell suspensions and cell-subset enrichment
Single-cell suspensions were obtained from spleen, lymph nodes, thymus, bone marrow, or blood and treated with red blood cell (RBC) lysis buffer (Puregene; QIAGEN, Valencia, CA). Splenocytes were enriched for specific cell subsets by incubation with a cocktail of specific lineage antibodies for 30 minutes on ice: for CD8+ T cells, anti-CD4, anti-Ter119, anti-B220, anti-CD19, anti–Mac-1, anti-CD11c, and anti-CD16/32 antibodies were used; for antigen-presenting cells (APCs), anti-CD3 and anti-Thy1.2 antibodies were used. After washing, the cells were incubated with magnetic bead–coupled sheep anti-rat antibody (Dynal-Invitrogen, Carlsbad, CA) for 30 minutes at 4°C on a rocker. By placing the tubes on a magnet for 2 to 3 minutes, antibody-bound cells were separated from antibody-free cells.
Southern blot and PCR for clonality analysis
Genomic DNA from splenocytes was extracted following the Puregene DNA Isolation procedure (QIAGEN). A total of 10 μg of DNA were digested with BamH1 restriction endonuclease at 37°C overnight, and subjected to Southern blot analysis using a GFP-specific probe. For polymerase chain reaction (PCR) analysis, RNA was extracted from frozen cell pellets with TRIzol (Invitrogen) and reverse transcription (RT)–PCR was performed using the SuperScriptII system (Invitrogen) according to the manufacturer's protocol. Each PCR reaction consisted of 2.5 μL of resulting cDNA and 20 pmol of different combinations of T-cell receptor β (TCRβ)–specific primers.27
Flow cytometry
All antibodies were obtained from BD Pharmingen (San Diego, CA), and staining procedures were performed in phosphate-buffered saline (PBS)/2% FBS. For apoptosis assays, freshly harvested thymocytes were prestained with APC-conjugated anti-CD8 antibody for 30 minutes at 4°C. After 2 washes with PBS/1% FBS, cells were resuspended in 100 μL 1× annexin-V buffer and 5 μL each of PE-conjugated annexin-V and 7-AAD were added. Cells were incubated at room temperature in the dark for 30 minutes and analyzed by flow cytometry.
For intracellular staining, 106 splenocytes or CD8+ T cells were either left untreated or stimulated with 10 nM PMA and 1 μM ionomycin for 24 hours. A fraction of the cells was incubated with JAK inhibitor I (Calbiochem, La Jolla, CA). Intracellular staining was performed using APC-conjugated anti-CD8 antibody and PE-conjugated anti-TNFα, anti-IFNγ, anti–Bcl-2 (BD Pharmingen), p-STAT5 (p-Tyr 694), p-STAT3 (p-Tyr 705), p-AKT (p-Ser 473), or p-S6rp (p-Ser 235/236; Cell Signaling, Beverly, MA). All data were acquired on a BD FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) and analyzed using FlowJo software (TreeStar, Ashland, OR).
Proliferation assay and sandwich ELISA
Unfractionated splenocytes (0.2 × 106) or purified CD8+ T cells (0.1 × 106) were plated into round-bottom tissue culture plates in a final volume of 200 μL of RPMI-1640/10% FBS, supplemented with 2 mM l-glutamine, 100 μg/mL penicillin, and 100 μg/mL streptomycin (RPMI + media). The cells were either mock-treated or stimulated with PMA (10 nM) and ionomycin (1 μM) for 60 hours at 37°C/5% CO2. Culture supernatants were removed from each well, and cell pellets were pulsed with 3H-thymidine (0.037 MBq/well [1 μCi/well]) for the last 12 to 16 hours of incubation. Cells were then harvested onto glass fiber filters, and 3H-thymidine incorporation was measured with a 1450 Microbeta Trilux scintillation counter (Wallac-PerkinElmer, Waltham, MA). Proliferation is displayed as mean counts per minute of triplicate cultures.
For enzyme-linked immunosorbent assay (ELISA), plates were coated with 100 μL coating buffer (0.1 M Na2CO3/NaHCO3, pH 9.5) containing 5 μg/mL monoclonal anti-IFNγ (BD Pharmingen) at 4°C overnight. The assays were performed following manufacturer's recommendations (BD OptEIA; BD Pharmingen) using the supernatants from the proliferation assay.
Immunoprecipitation and Western blot analysis
A total of 107 primary bone marrow cells from JAK3-WT or JAK-AV animals were lysed directly, or treated with JAK inhibitor I for 4 hours, and either immunoprecipitated using anti-HA tag antibody (Cell Signaling) or directly electrophoresed and immunoblotted as described elsewhere.26 Membranes were probed with anti-phosphoJak3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-HA antibody or STAT5 antibody (Santa Cruz Biotechnology), or anti-STAT3, anti-AKT, and anti-S6rp antibodies (Cell Signaling).
Redirected cytotoxicity assay
Purified CD8+ “effector” T cells (2 × 106 cells/mL of RPMI + media) were mixed with irradiated APCs (4 × 106/mL) at a 1:1 ratio and plated into 24-well plates with or without 1 μg/mL anti-CD3 in a total volume of 2 mL/well for 5 days. Target cells were labeled with 1.85 MBq (50 μCi) of Na2(51Cr)O4 for 1 hour at 37°C, washed 3 times with PBS, and plated (104/well) with effector cells in U-bottomed 96-well plates at different effector-target ratios in triplicate. After 4 hours of incubation, supernatants were harvested and mixed with 6 volumes of Optiphase Supermix, and counts were collected using a 1450 Microbeta Trilux scinitllation counter.
Sample collection and DNA sequence analysis
Skin biopsies from 30 patients with CTCL were collected from the Bordeaux University Hospital and Brigham and Women's Hospital. PCR amplification of genomic DNA and sequencing of exon 12 of human JAK3 was performed using M13-tailed primers as previously described.26 Sequence analysis of bidirectional sequence traces was performed using DNA Star (Madison, WI) and Mutation Surveyor (State College, PA) softwares. PCR products were cloned using the TOPO cloning kit (Invitrogen) following the manufacturer's recommendation. This study was approved by the Bordeaux University Hospital and Brigham and Women's Hospital Ethics Committee, and informed consent was obtained in accordance with the Declaration of Helsinki.
Statistical analysis
Statistical significance of differences between the different conditions was assessed using a 2-tailed unpaired t test.
Results
JAK3-AV induces a fatal lymphoproliferative phenotype in vivo
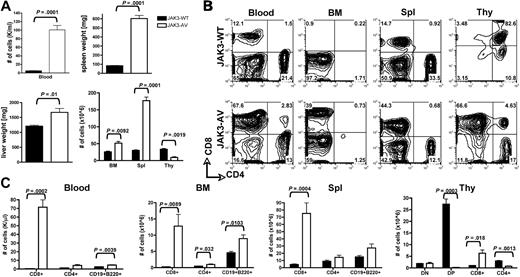
To address the role of constitutive JAK3 activation in normal and malignant lymphoid development and function, we performed a BMT assay in which cells from 5-FU–treated donor mice were transduced with either JAK3-WT or JAK3-AV retroviral constructs on 2 consecutive days before injection into lethally irradiated, syngeneic recipient mice. Primary bone marrow cells showed increased phosphorylation of JAK3 in JAK3-AV recipients in vivo, although JAK3-WT and JAK3-AV recipients presented similar expression of the JAK3 protein (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Expression of the JAK3-AV allele resulted in a rapid and severe lymphoproliferative disease, with 100% penetrance in both the C57BL/6 and Balb/c backgrounds. At 6 weeks after transplantation, JAK3-AV animals presented increased leukocyte counts and marked hepatosplenomegaly compared with JAK3-WT animals (Figure 1A). Histopathologic analyses showed a massive lymphocytic infiltration of most lymphoid and nonlymphoid organs. Interestingly, thymic size and total thymocyte numbers were significantly reduced in JAK3-AV animals compared with JAK3-WT animals. Flow cytometric analysis revealed that the expanded lymphoid populations in the blood, bone marrow, and spleen were composed predominantly of CD8+ T cells (Figure 1B), although the absolute numbers of CD4+ T cells and B220+CD19+ B cells were also significantly increased in JAK3-AV recipients (Figure 1C). In the thymus, in contrast, the number of CD8+ single-positive (SP) T cells was increased, whereas the numbers of CD4+SP and CD4+CD8+ double-positive (DP) cells were decreased. Of note, no lymphoproliferative phenotype was observed when JAK3-AV–transduced bone marrow cells were transplanted into syngeneic nude, athymic recipients (data not shown). These results show that constitutive activation of JAK3 in a murine BMT model induces a thymus-dependent fatal lymphoproliferative disease.
Expansion of lymphoid cells in JAK3-AV animals. (A) Comparison of total number of white blood cells (top left panel), spleen weights (top right panel), liver weights (bottom left panel), and total cells after RBC lysis in different organs (bottom right panel) in recipients of JAK3-WT–transduced bone marrow (■) versus JAK3-AV–transduced bone marrow (□). (B) Flow cytometric analysis of the different lymphoid compartments. All analyses are gated on GFP+ cells; plots are representative of at least 5 independent experiments. Percentages are indicated. (C) Subpopulation analysis of lymphocytes migrating to the different lymphoid organs in JAK3-AV or JAK3-WT recipients. BM indicates bone marrow; Spl, spleen; and Thy, thymus. Bar graphs represent the average and standard deviation obtained from at least 5 animals.
Expansion of lymphoid cells in JAK3-AV animals. (A) Comparison of total number of white blood cells (top left panel), spleen weights (top right panel), liver weights (bottom left panel), and total cells after RBC lysis in different organs (bottom right panel) in recipients of JAK3-WT–transduced bone marrow (■) versus JAK3-AV–transduced bone marrow (□). (B) Flow cytometric analysis of the different lymphoid compartments. All analyses are gated on GFP+ cells; plots are representative of at least 5 independent experiments. Percentages are indicated. (C) Subpopulation analysis of lymphocytes migrating to the different lymphoid organs in JAK3-AV or JAK3-WT recipients. BM indicates bone marrow; Spl, spleen; and Thy, thymus. Bar graphs represent the average and standard deviation obtained from at least 5 animals.
The expanded T-cell population closely resembles a mature effector-memory T cell
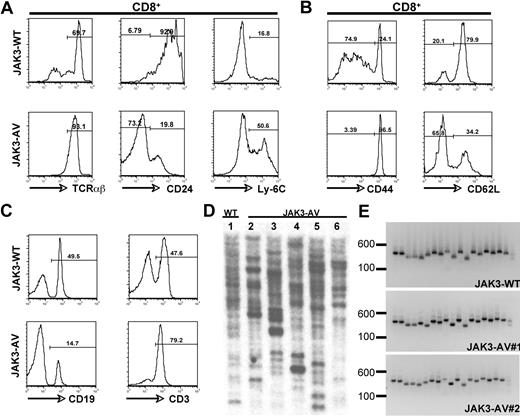
To further characterize the expanded CD8+ T-cell population, we performed immunophenotyping by flow cytometry of single-cell suspensions derived from thymus, spleen, blood, and bone marrow. The majority of the CD8+ SP T cells in the thymus of JAK3-AV mice showed increased expression levels of TCRαβ and Ly-6C, and low levels of heat-stable antigen (ie, CD24) compared with JAK3-WT counterparts (Figure 2A), while the numbers and percentages of γδ T cells were comparable in both groups (data not shown). In the spleen, the CD8+ T cells of mutant mice also showed increased expression of CD44 and CD122 and decreased expression of CD62L, respectively (Figure 2B; data not shown). The increase in splenic T cells in JAK3-AV mice was accompanied by a relative decrease in the percentage of B cells in this organ (Figure 2C), but no block in B-cell development was observed, as assessed by flow cytometric analysis of bone marrow cells for different markers of B-cell maturation, including B220, CD43, CD24, CD25, CD19, IgM, and IgD (data not shown). Of note, when CD8+ T-cell maturation was blocked by transplantation of JAK3-AV–transduced wild-type bone marrow cells into Kb−/−Db−/− (ie, MHC class I–deficient) syngeneic animals, recipients developed a lymphoproliferative disease, characterized by the accumulation of mature CD4+ T cells, with a latency of 2 to 3 months (Figure S2A,B).
Immunophenotypic characterization of the CD8+ T-cell population. Flow cytometric analysis of JAK3-WT versus JAK3-AV thymic (A) or splenic (B) CD8+CD4− T cells. (C) Flow cytometric staining for splenic T cells (CD3+) and B cells (CD19+) in JAK3-AV versus JAK3-WT mice. All analyses are gated on GFP+ cells and are representative of at least 5 independent experiments. Numbers indicate the percentage of cells. (D) Southern blot analysis from splenic genomic DNA of 1 JAK3-WT (lane 1) and 5 different JAK3-AV mice (lanes 2-6) using a GFP-specific probe. (E) PCR for the different variable TCRβ regions from splenocyte-derived cDNA of 1 JAK3-WT and 2 JAK3-AV animals (right panel). Each lane represents 1 PCR reaction with a different forward primer for the TCRβ variable regions (1-19 from left to right) and the same reverse primer for the constant region.
Immunophenotypic characterization of the CD8+ T-cell population. Flow cytometric analysis of JAK3-WT versus JAK3-AV thymic (A) or splenic (B) CD8+CD4− T cells. (C) Flow cytometric staining for splenic T cells (CD3+) and B cells (CD19+) in JAK3-AV versus JAK3-WT mice. All analyses are gated on GFP+ cells and are representative of at least 5 independent experiments. Numbers indicate the percentage of cells. (D) Southern blot analysis from splenic genomic DNA of 1 JAK3-WT (lane 1) and 5 different JAK3-AV mice (lanes 2-6) using a GFP-specific probe. (E) PCR for the different variable TCRβ regions from splenocyte-derived cDNA of 1 JAK3-WT and 2 JAK3-AV animals (right panel). Each lane represents 1 PCR reaction with a different forward primer for the TCRβ variable regions (1-19 from left to right) and the same reverse primer for the constant region.
To determine the clonality of the CD8+ lymphoproliferative disorder obtained in the wild-type background, we first performed Southern blot analysis on splenocyte-derived genomic DNA using a GFP-specific probe. DNA obtained from JAK3-WT and JAK3-AV splenocytes presented more than 15 retroviral integration sites in all animals analyzed (Figure 2D). We then performed a PCR-based assay on cDNA obtained from GFP+ splenocytes using primer pairs specific for each of the 19 possible variable/constant region junctions at the murine TCRβ locus. Similar to JAK3-WT animals, all 19 TCRβ junctions could be amplified from JAK3-AV splenocytes (Figure 2E). Together, these results indicate that constitutive JAK3 activation is sufficient to induce a T-cell lymphoproliferative disorder in this model. To assess transplantability of the disease, 106 splenocytes isolated from JAK3-AV animals were injected into sublethally irradiated secondary recipients. Secondary animals developed a similar disease to primary animals, characterized by the expansion of a polyclonal mature CD8+ T-cell population (Figure S1B,C) and a median latency of 5 months.
Altogether, JAK3-AV induces the abnormal expansion of a polyclonal CD8+TCRαβ+Ly-6C+CD44+CD122+CD24lo population that most closely resembles mature effector or memory T cells. In addition, JAK3-AV induces a CD4+ lymphoproliferative disease in the absence of CD8+ T-cell maturation, suggesting that constitutive activation of JAK3 can also transform the CD4+ T-cell lineage.
JAK3-AV T cells show increased proliferative and survival capacities
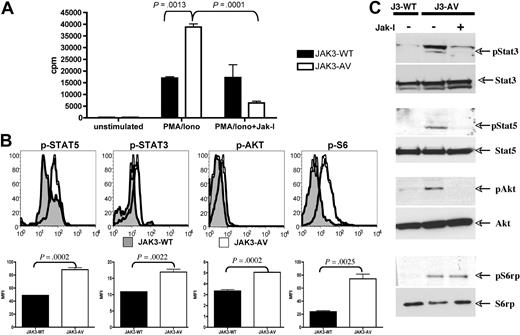
We next investigated whether the expansion of CD8+ T cells induced by JAK3-AV results from enhanced proliferative capacity or increased survival rate. To determine the proliferative properties of JAK3-AV cells, we stimulated freshly purified CD8+ T cells with PMA and ionomycin for 60 hours and measured their rate of 3H-thymidine incorporation during the last 12 to 16 hours of culture. The results indicate that the CD8+ T cells from JAK3-AV animals had increased proliferative capacities compared with JAK3-WT cells (Figure 3A). This effect could be abrogated by addition of a pan-JAK small molecule inhibitor (ie, JAK inhibitor I) at the beginning of the polyclonal stimulation, supporting a causal role for the JAK3-AV mutation in increased proliferation. We then examined in vivo activation of JAK3 and known downstream effectors in bone marrow cells and splenocytes using Western blot or flow cytometry with phosphospecific antibodies, respectively, and observed increased phosphorylation of JAK3, STAT5, STAT3, AKT, and rpS6 in JAK3-AV versus JAK3-WT CD8+ T cells (Figure 3B,C; Figure S1A). Incubation of cells with JAK inhibitor I for 4 hours before cell lysis significantly decreased the phosphorylation of these downstream targets in JAK3-AV cells as assessed by Western blot.
JAK3-AV confers enhanced proliferative capacities. (A) 3H-thymidine incorporation after 60 hours of stimulation with PMA/ionomycin of purified CD8+ T cells from JAK3-WT (■) and JAK3-AV (□) mice. Rightmost bars show effect of pharmacologic inhibition of JAK3 with JAK inhibitor I (JAK-I). Bar graphs represent the average and standard deviation of 3 independent experiments performed in triplicate. (B) Assessment of phosphorylation status of downstream effectors of JAK3 by intracellular staining of JAK3-WT (shaded histogram) and JAK3-AV (open histogram) splenocytes with phosphospecific antibodies. Histograms display a representative experiment gated on CD8+ T cells (n = 6). Bar graphs represent the average and standard deviation of 3 independent experiments for each phosphoprotein. (C) Western blot from bone marrow lysates from JAK3-WT or JAK3-AV animals shows constitutive phosphorylation of JAK3 downstream targets in the latter. The last lane shows inhibition of phosphorylation by JAK-I.
JAK3-AV confers enhanced proliferative capacities. (A) 3H-thymidine incorporation after 60 hours of stimulation with PMA/ionomycin of purified CD8+ T cells from JAK3-WT (■) and JAK3-AV (□) mice. Rightmost bars show effect of pharmacologic inhibition of JAK3 with JAK inhibitor I (JAK-I). Bar graphs represent the average and standard deviation of 3 independent experiments performed in triplicate. (B) Assessment of phosphorylation status of downstream effectors of JAK3 by intracellular staining of JAK3-WT (shaded histogram) and JAK3-AV (open histogram) splenocytes with phosphospecific antibodies. Histograms display a representative experiment gated on CD8+ T cells (n = 6). Bar graphs represent the average and standard deviation of 3 independent experiments for each phosphoprotein. (C) Western blot from bone marrow lysates from JAK3-WT or JAK3-AV animals shows constitutive phosphorylation of JAK3 downstream targets in the latter. The last lane shows inhibition of phosphorylation by JAK-I.
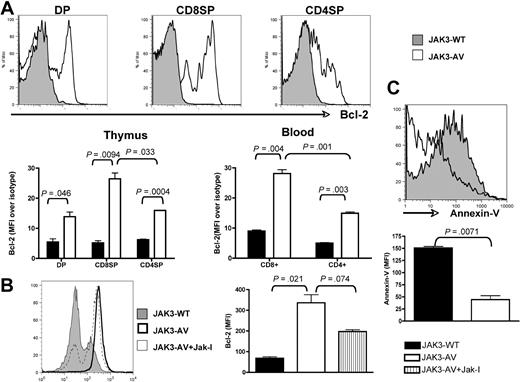
To assess the survival properties of JAK3-AV cells, we then performed ex vivo analysis of Bcl-2 expression, a critical antiapoptotic molecule, via flow cytometry of freshly isolated thymocytes and leukocytes. We observed a significant increase in Bcl-2 expression in JAK3-AV–bearing CD4+CD8+ DP, CD4+ SP, and CD8+ SP thymocytes compared with wild-type counterparts, which was also observed in both CD4+ and CD8+ peripheral T cells (Figure 4A). Of note, both thymic and circulating CD8+ T cells obtained from JAK3-AV recipients showed higher Bcl-2 expression compared with CD4+ T cells. Inhibition of JAK3-AV CD8+ cells with JAK inhibitor I in vitro significantly decreased the level of Bcl-2 expression in these cells (Figure 4B). In addition, JAK3-AV CD8+ thymocytes showed decreased apoptosis compared with JAK3-WT CD8+ thymocytes, as assessed by flow cytometry using annexin-V staining (Figure 4C). Taken together, these findings indicate that constitutive JAK3 activity leads to an increase in both proliferative and survival abilities of CD8+ T cells and is associated with activation or transcriptional up-regulation of several downstream targets in vivo, including STAT3, STAT5, PI3K/AKT, and Bcl-2.
JAK3-AV–expressing T cells show decreased apoptosis and increased Bcl-2 expression. (A) Flow cytometric analysis of intracellular Bcl-2 levels in 3 different thymocyte populations (DP and SP) of JAK3-WT (shaded histogram) versus JAK3-AV (open histogram) animals. Bottom panel shows mean fluorescence intensity of the Bcl-2 signal normalized against an isotype control antibody in thymus and blood of JAK3-WT (■) and JAK3-AV (□) mice. All analyses are gated on GFP; data represent the average and standard deviation of 4 independent experiments. (B) Effect of JAK inhibitor I on Bcl-2 levels in CD8 SP thymocytes shown as a histogram and as bar graphs representing the mean and standard deviation of 2 independent animals for each group performed in duplicate. (C) Annexin-V staining for assessment of apoptosis levels in JAK3-WT versus JAK-3 AV CD8+ thymocytes. Analyses are gated on GFP+CD8+7-AAD− thymocytes. Bar graphs represent the mean and standard deviation of at least 2 independent experiments each performed in duplicate.
JAK3-AV–expressing T cells show decreased apoptosis and increased Bcl-2 expression. (A) Flow cytometric analysis of intracellular Bcl-2 levels in 3 different thymocyte populations (DP and SP) of JAK3-WT (shaded histogram) versus JAK3-AV (open histogram) animals. Bottom panel shows mean fluorescence intensity of the Bcl-2 signal normalized against an isotype control antibody in thymus and blood of JAK3-WT (■) and JAK3-AV (□) mice. All analyses are gated on GFP; data represent the average and standard deviation of 4 independent experiments. (B) Effect of JAK inhibitor I on Bcl-2 levels in CD8 SP thymocytes shown as a histogram and as bar graphs representing the mean and standard deviation of 2 independent animals for each group performed in duplicate. (C) Annexin-V staining for assessment of apoptosis levels in JAK3-WT versus JAK-3 AV CD8+ thymocytes. Analyses are gated on GFP+CD8+7-AAD− thymocytes. Bar graphs represent the mean and standard deviation of at least 2 independent experiments each performed in duplicate.
The expanded CD8+ T-cell population shows increased production of inflammatory/cytotoxic cytokines and enhanced target cell cytotoxicity
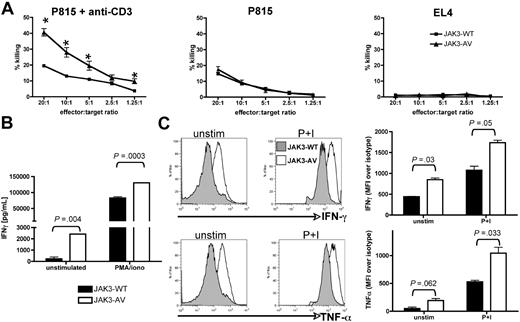
The best understood function of effector CD8+ T cells is cytotoxicity, mediated by binding and killing foreign antigen-bearing target cells via secretion of specific cytokine and effector molecules. To assess whether CD8+ T cells expanded in JAK3-AV animals were bona fide functional effector CD8+ T cells, we first tested activity of these cells in a “redirected killing assay.”28 This assay circumvents the inability to stimulate a polyclonal T-cell population, such as the JAK3-AV CD8+ T cells, with a specific peptide or peptide-loaded APC. Purified JAK3-WT or JAK3-AV CD8+ T cells were sensitized for 5 days with allogeneic APCs, and then cocultured with 51Cr-loaded allogeneic P815 mastocytoma target cells that express high levels of Fcγ-R. JAK3-AV CD8+ T cells killed P815 target cells at higher efficiencies than JAK3-WT CD8+ T cells when the coculture was supplemented with an anti-CD3 antibody, which both further activates the T cells and provides a close contact between effector and target cells via Fcγ-R binding (Figure 5A). In control experiments, JAK3-WT and JAK3-AV cells showed comparable killing activities in the absence of anti-CD3, whereas neither of them was able to efficiently kill syngeneic EL4 target cells.
JAK3-AV CD8+ T cells produce more inflammatory/cytotoxic cytokines and display enhanced cytotoxic activity. (A) Redirected 51Cr release assay of CD8+ “effector” T cells incubated with allogeneic (P815) target cells at different ratios in the presence (left panel) or absence (middle panel) of anti-CD3. Syngeneic EL4 target cells (right panel) were used as a negative control. Graphs display a representative of 4 experiments performed in triplicate. Percentage of killing was calculated as decribed in “Methods.” *P < .05. (B) ELISA assay for production of IFNγ in unstimulated (unstim) or PMA/ionomycin-treated (P+I) purified CD8+ T cells of JAK3-WT (■) and JAK3-AV (□) mice. Data (mean ± SD) represent the average of 5 experiments performed in duplicate. (C) Intracellular staining of splenic GFP+CD8+ T cells for IFNγ and TNFα confirm the increased production of these 2 cytokines in JAK3-AV (open histogram) compared with JAK3-WT cells (shaded histogram) at a single-cell level. Bar graphs indicate the mean and SD of 3 independent experiments.
JAK3-AV CD8+ T cells produce more inflammatory/cytotoxic cytokines and display enhanced cytotoxic activity. (A) Redirected 51Cr release assay of CD8+ “effector” T cells incubated with allogeneic (P815) target cells at different ratios in the presence (left panel) or absence (middle panel) of anti-CD3. Syngeneic EL4 target cells (right panel) were used as a negative control. Graphs display a representative of 4 experiments performed in triplicate. Percentage of killing was calculated as decribed in “Methods.” *P < .05. (B) ELISA assay for production of IFNγ in unstimulated (unstim) or PMA/ionomycin-treated (P+I) purified CD8+ T cells of JAK3-WT (■) and JAK3-AV (□) mice. Data (mean ± SD) represent the average of 5 experiments performed in duplicate. (C) Intracellular staining of splenic GFP+CD8+ T cells for IFNγ and TNFα confirm the increased production of these 2 cytokines in JAK3-AV (open histogram) compared with JAK3-WT cells (shaded histogram) at a single-cell level. Bar graphs indicate the mean and SD of 3 independent experiments.
To understand the molecular basis for the increased killing activity of JAK3-AV CD8+ T lymphocytes, we evaluated their ability to produce and secrete IFNγ, an inflammatory cytokine produced by mature CD8+ T lymphocytes. ELISA analysis of the supernatants obtained from proliferation assays of purified CD8+ T cells revealed significantly increased IFNγ production in JAK3-AV– versus JAK3-WT–bearing cells (Figure 5B). Intracellular cytokine staining (ICS) using flow cytometric analysis demonstrated that both unstimulated and PMA/ionomycin-treated JAK3-AV CD8+ T cells produce more IFNγ than JAK3-WT CD8+ T cells at the single-cell level (Figure 5C). Furthermore, production of TNFα, a cytotoxic molecule commonly secreted by cytotoxic T lymphocytes (CTLs), was also increased in JAK3-AV CD8+ T cells. The production of both IFNγ and TNFα was inhibited in JAK3-AV CD8+ T cells by treatment with the JAK inhibitor I (Figure S3). Of note, no significant difference in IFNγ and IL-4 production was observed in CD4+ T cells. These findings indicate that the JAK3-AV CD8+ T cells bear properties of fully functional CTLs and show enhanced killing activities associated with increased production of cytotoxic and inflammatory cytokines compared with control CD8+ T cells.
The JAK3-AV–induced T-cell lymphoproliferative disorder shows prominent cutaneous involvement
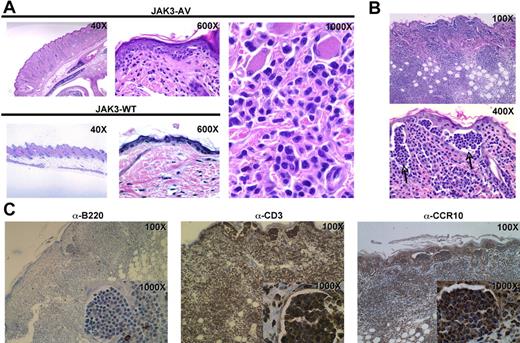
A notable and recurrent phenotype observed in recipients of JAK3-AV cells was the development of skin lesions as well as tail necrosis during the course of the disease. Examination of H&E-stained sections of skin lesions of the snout and tail demonstrated frequent tagging along the dermal-epidermal junction (Figure 6A) by highly atypical lymphoid cells with markedly irregular to convoluted and cerebriform nuclei. This prominent skin infiltration was even more marked in secondary recipient animals, with the appearance of frequent intraepidermal collections of these highly atypical lymphocytes reminiscent of Pautrier microabcesses (Figure 6B), a morphologic feature characteristic of several forms of human CTCL, such as mycosis fungoides (MF). Immunohistochemistry on skin sections showed that the infiltrates consisted almost exclusively of CD3+ cells, confirming the T-cell nature of these atypical lymphocytes (Figure 6C). These infiltrating T cells also stained positive for the chemokine receptor CCR10, which is associated with the homing of T cells to epidermal layers and is highly expressed on neoplastic T cells of cutaneous T-cell lymphomas.29 Of note, TUNEL assay on skin sections revealed only rare apoptotic T cells or keratinocytes (data not shown). Together, these observations indicate that cutaneous involvement due to infiltration of epidermotropic T lymphocytes is a major consequence of constitutive JAK3 activation in this mouse model.
Constitutive JAK3 activation induces a T-cell lymphoproliferation with prominent cutaneous involvement. (A) H&E-stained skin tissue sections from JAK3-AV (top row and rightmost panel) or JAK3-WT (bottom row) animals showing a dense atypical dermal infiltrate comprised of pleomorphic lymphoid cells tagging along the dermal-epidermal junction in the former. (B) H&E-stained sections of skin lesions from secondary recipients display even more pronounced cutaneous disease, with significant involvement of the dermis and extension into subcutaneous adipose tissue. Sections of the epidermis highlight frequent collections of atypical intraepidermal lymphocytes resembling Pautrier microabcesses (indicated by). (C) Immunohistochemistry of skin sections from secondary recipients with anti-CD3 (left panel), anti-B220 (middle panel), or anti-CCR10 (right panel) antibodies. Insets show staining of intraepidermal lymphocyte collections highlighted in panel B.
Constitutive JAK3 activation induces a T-cell lymphoproliferation with prominent cutaneous involvement. (A) H&E-stained skin tissue sections from JAK3-AV (top row and rightmost panel) or JAK3-WT (bottom row) animals showing a dense atypical dermal infiltrate comprised of pleomorphic lymphoid cells tagging along the dermal-epidermal junction in the former. (B) H&E-stained sections of skin lesions from secondary recipients display even more pronounced cutaneous disease, with significant involvement of the dermis and extension into subcutaneous adipose tissue. Sections of the epidermis highlight frequent collections of atypical intraepidermal lymphocytes resembling Pautrier microabcesses (indicated by). (C) Immunohistochemistry of skin sections from secondary recipients with anti-CD3 (left panel), anti-B220 (middle panel), or anti-CCR10 (right panel) antibodies. Insets show staining of intraepidermal lymphocyte collections highlighted in panel B.
The JAK3A572V mutation is found in human CTCL
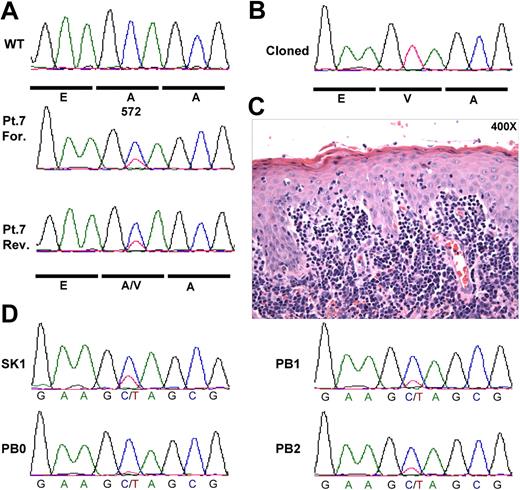
The close resemblance between the cutaneous phenotype of our JAK3-AV murine model and the clinical presentation of human patients with CD8+ CTCL led us to hypothesize that activating JAK3 mutations may be present in these patients. We were not able to obtain DNA derived from patients with CD8+ CTCL. However, based in part on the observation that JAK3A572V can cause a CD4+ CTCL-like phenotype in certain host contexts (ie, MHC-1 deficiency), we analyzed DNA samples obtained from the skin biopsy of 30 patients with CTCL, including 10 with MF, 10 Sézary syndrome, and 10 with ALK−CD30− ALCL for the presence of mutations at codon A572 of JAK3 by direct resequencing. One patient with MF showed the JAK3A572V mutation in a population-based DNA sequence trace generated from a skin biopsy obtained at time of large-cell transformation (Figure 7A).30 Cloning and sequencing of individual PCR products revealed that 11 of 45 PCR products contain the mutant JAK3A572V allele (Figure 7B). Therefore, the ratio of mutant to wild-type allele (0.24) is significantly lower than a ratio of 1:1 that would be expected if this was a germline allele, and is compatible with the percentage of lymphoma cells in the skin lesion (Figure 7C). The mutation was also present in an earlier skin biopsy prior to large-cell transformation (SK1), but nearly undetectable in peripheral blood (PB0) collected at diagnosis (Figure 7D), consistent with the epidermotropic nature of these cells. The mutation was readily observed in a later blood biopsy, collected at time of large-cell transformation (PB2). Of note, sequencing of the entire coding sequence of JAK3 (23 exons) in 12 additional CTCL samples did not identify additional activating mutations (data not shown). Together, these findings indicate that constitutive activation of JAK3 due to acquisition of the somatic JAK3A572V mutation is present at a low frequency in human CTCL.
JAK3A572V mutation is present in human CTCL. (A) DNA samples from 30 patients with CTCL were PCR amplified and resequenced using M13-tailed primers flanking exon 12 of the human JAK3 gene. Forward (For.) and reverse (Rev.) sequence traces from Patient 7 (Pt.7) sample as well as wild-type trace are shown. (B) PCR products obtained in (A) were cloned and individually sequenced. Eleven of 44 (25%) products showed the mutant JAK3A572V allele. (C) Hematoxylin&Eosin staining of the skin biopsy shown in (A). (D) Sequence traces from Patient 7 (Pt.7) biopsies collected during disease progression. SK1 indicates skin biopsy at diagnosis prior to large-cell transformation shown in panel A; PB0, peripheral blood at diagnosis; PB1, before diagnosis of large cell transformation; and PB2, after diagnosis of large cell transformation.
JAK3A572V mutation is present in human CTCL. (A) DNA samples from 30 patients with CTCL were PCR amplified and resequenced using M13-tailed primers flanking exon 12 of the human JAK3 gene. Forward (For.) and reverse (Rev.) sequence traces from Patient 7 (Pt.7) sample as well as wild-type trace are shown. (B) PCR products obtained in (A) were cloned and individually sequenced. Eleven of 44 (25%) products showed the mutant JAK3A572V allele. (C) Hematoxylin&Eosin staining of the skin biopsy shown in (A). (D) Sequence traces from Patient 7 (Pt.7) biopsies collected during disease progression. SK1 indicates skin biopsy at diagnosis prior to large-cell transformation shown in panel A; PB0, peripheral blood at diagnosis; PB1, before diagnosis of large cell transformation; and PB2, after diagnosis of large cell transformation.
Discussion
The crucial role of JAK3 in lymphoid development and function has been demonstrated by JAK3 loss of function alleles in mice and humans that result in immunodeficiency syndromes.16 Immunodeficiency in this context can be attributed to exclusive interaction of JAK3, but not other JAK family members, with the common γ-chain that is shared by receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. These cytokines are known to be important for efficient lymphopoiesis and for the proper functioning of the different arms of the immune system.31 Conversely, constitutive activation of JAK3 has been observed in a spectrum of lymphoid malignancies.12,22 Here, we report that constitutively activated JAK3 in a murine retroviral transduction/BMT model, but not wild-type JAK3, induces a fully penetrant and fatal polyclonal T-cell lymphoproliferative disorder that is transplantable into secondary recipients. Immunophenotypical characterization of the expanded T-cell population indicates that constitutive JAK3 activation disrupts T-cell homeostasis and is sufficient to induce an abnormal expansion of effector-memory CD8+ T cells.
Although JAK3 is essential for normal hematopoiesis, the fully penetrant lymphoproliferative syndrome obtained in this murine model is rather unexpected. Indeed, the conditions used for this BMT model, including pretreatment of donors with 5-FU and a 2-day culture of donor bone marrow cells with SCF, IL-3, and IL-6, strongly favor the development of myeloid malignancies by activated tyrosine kinases. Examples include BCR-ABL, TEL-PDGFRB, TEL-ABL, FIP1L1-PDGFRA, ZNF198-FGFR1, and FLT3-ITD.32-35 This generalization extends even to activating alleles of other JAK family members, such as JAK2,36,37 and the NPM-ALK fusion despite its association with lymphoid malignancies in humans.38 These observations together suggest that there are specific characteristics of JAK3A572V that delineate its biological activity from other constitutively activated tyrosine kinases associated with hematologic malignancies.
JAK3 is required for normal proliferation and survival of T cells at different stages of differentiation, including mature lymphocytes.16,39 Our results indicate that CD8+ T cells represent the main expanded population in animals with constitutive JAK3 activation in a wild-type background, suggesting that JAK3 plays a more specific role in the development of mature CD8+ T cells. However, JAK3-AV recipients also show an increase in the absolute numbers of CD4+ T cells in bone marrow and spleen. Accordingly, MHC class I–deficient recipients of JAK3-AV–transduced wild-type bone marrow fail to develop mature CD8+ T cells as expected, but in turn develop a CD4+ T-cell lymphoproliferative disorder. Together, these data indicate that constitutive JAK3 activation induces aberrant proliferation and survival of CD8+ and CD4+ T cells, leading to a lymphoproliferative disease.
In support of these observations, the expanded CD8+ T cells present abnormal activation of downstream effectors implicated in control of proliferation, including STATs and the PI3K/AKT pathway. Of note, JAK3-AV CD8+ T cells also show high Ly6C expression and IFNγ secretion, both of which have previously been associated with higher proliferative capacities of T cells.40 Interestingly, IFNγ itself induces Ly6C up-regulation and selective CD8+ T cells proliferation in vivo,41,42 suggesting that the T-cell lymphoma induced by constitutive JAK3 activation may in part result from a positive feedback loop involving IFNγ and Ly-6C, leading to aberrant proliferation of CD8+ T cells. In addition, constitutive JAK3 activation also increases resistance to apoptosis in thymocytes and induces up-regulation of the antiapoptotic effector Bcl-2 in DP, CD4+ SP, and CD8+ SP T cells, suggesting that the observed phenotype also results from enhanced survival of these cells. Interestingly, Bcl-2 up-regulation is significantly higher in CD8+ T cells than in CD4+ T cells, both in the thymus and the blood of JAK3-AV animals. In keeping, JAK3 deficiency results in reduction of Bcl-2 levels preferentially in CD8+ SP thymocytes.43 The observation that thymic size in JAK3-AV animals is reduced, despite the increased survival capacity of thymocytes, suggests that JAK3-AV–bearing T cells may be able to escape the thymus prematurely. Furthermore, our data are consistent with an increased CD4+/CD8+ T-cell ratio in the peripheral blood of JAK3−/− animals16,39 that can be rescued to wild-type levels by overexpression of Bcl-2.43 Finally, Bcl-2−/− animals present marked defects in CD8+ T cells, whereas CD4+ T cells are spared.44 Taken together, these finding indicate that constitutive JAK3 activation results in an increase in both proliferation and survival of CD8+ T lymphocytes and is associated with increased expression of Bcl-2 and IFNγ that may mediate in part the specific expansion of this CD8+ effector-memory T-cell subset.
A striking pathologic finding is the prominent epidermotropism of the CD8+ T-cell lymphoproliferation, which resembles that observed in some human T-cell malignancies.45,46 Infiltrated skin shows abnormal inflammation and presence of lymphoid clusters reminiscent of Pautrier microabcesses, intraepidermal collections of lymphocytes that are characteristic of epidermotropic human CTCLs.46 Of further interest, the expanded JAK3-AV CD8+ T cells demonstrate abnormal production of inflammatory or cytotoxic cytokines, including IFNγ and TNFα, that are likely to contribute to the development of skin lesions, mediated by massive infiltration of epidermotropic lymphocytes. Together, these results indicate that constitutive JAK3 activation in this model mimics some important phenotypic features of a rare subset of human CD8+ primary cutaneous peripheral T-cell lymphomas.45-47
Finally, the results obtained in this murine model informed a screen that identified JAK3A572V as an infrequent allele in human CD4+ CTCL. A larger cohort of patients will be required to determine its precise incidence in the different CTCL subsets, and whether other activating alleles in the JAK-STAT pathway contribute to human CTCL. There are aggressive forms of human CTCL associated with a CD8+ lymphoproliferation similar to that observed in this BMT model of JAK3A572V-induced disease. However, most cases of human CTCL are CD4+, and the patient with the JAK3A572V mutation described here presented with a CD4+ CTCL. Our experiments demonstrate that expression of the JAK3A572V mutation in cells with a compromised CD8+ T-cell differentiation potential (ie, in a MHC-I–deficient background) results in the development of a CD4+ lymphoproliferative disease, supporting a role for this allele in human CD4+ CTCL as well. However, further studies will be required to clarify the basis for the difference between the CD8+ phenotype observed in our murine model and the CD4+ phenotype in the patient presenting the JAK3A572V mutation.
Of note, the importance of these findings in the context of T-cell malignancies is also highlighted by the association of NPM1-ALK with ALCL and the recent reports of an ITK-SYK fusion in PTCL48 and JAK1 mutations in T-ALL.49 In addition, NPM1-ALK interacts with JAK3,24 and JAK3 has been reported to activate both SYK and JAK1 in response to cytokine stimulation,21,50 suggesting that constitutive activation of this pathway might be a common mechanism associated with malignant transformation of the T-cell lineage. Of note, concomitant MPL and JAK2 mutations are found in some patients with myeloproliferative disease. Therefore, further investigation of the presence of JAK3 mutations with other known mutations in signaling molecules associated with T-cell malignancies (ie, ALK+ or SYK+ lymphomas and T-ALL) may be warranted.
Current therapies and prognosis for PTCL/CTCLs are generally poor, but a number of JAK family inhibitors are under development for other indications, such as myeloproliferative or autoimmune diseases. Thus, this model could not only be useful to understand the molecular basis of human peripheral lymphoid malignancies, but also to explore the possibility of JAK3 or downstream target inhibition as a therapeutic approach to this challenging clinical entity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank E. Parganas and Dr J. N. Ihle for providing the wild-type Jak3 MSCV construct; Dr Roderick Bronson and Dr Béatrice Vergier for histopathologic analyses and data; and Dr Judy Liebermann, Dr Shannon Turley, Dr Scott Armstrong, Dr Raif S. Geha, and Dr Marc-André Wurbel for helpful discussions. D.G.G. is a Doris Duke Foundation Distinguished Clinical Scientist and a Howard Hughes Medical Institute investigator.
This work was supported in part by National Institutes of Health grants DK50654 and CA66996 and by the Leukemia & Lymphoma Society to D.G.G. T.M. is a recipient of a Leukemia & Lymphoma Society Award.
National Institutes of Health
Authorship
Contribution: M.C.G. designed and performed research and wrote the manuscript; M.G.K., M.B.W., and S.L.B. contributed vital reagents and performed research; S.A.M. and B.B. performed research; M.B.-B., S.J.R., J.C.A., and J.-P.M. provided patient samples; B.H.L. analyzed histopathology data; H.C. provided vital help; D.G.G. designed and funded research and revised the manuscript; and T.M. designed and performed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Mercher, Inserm EMI0210, Hôpital Necker–Tour Pasteur, 149 Rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: thomas.mercher@inserm.fr; or D. Gary Gilliland, Brigham and Women's Hospital, Karp Research Building, 1 Blackfan Circle, Boston, MA 02115; e-mail: ggilliland@rics.bwh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal