Abstract
Protease activated receptor 1 (PAR1) signaling can play opposing roles in sepsis, either promoting dendritic cell (DC)–dependent coagulation and inflammation or reducing sepsis lethality due to activated protein C (aPC) therapy. To further define this PAR1 paradox, we focused on the vascular effects of PAR1 signaling. Pharmacological perturbations of the intravascular coagulant balance were combined with genetic mouse models to dissect the roles of endogenously generated thrombin and aPC during escalating systemic inflammation. Acute blockade of the aPC pathway with a potent inhibitory antibody revealed that thrombin-PAR1 signaling increases inflammation-induced vascular hyperpermeability. Conversely, aPC-PAR1 signaling and the endothelial cell PC receptor (EPCR) prevented vascular leakage, and pharmacologic or genetic blockade of this pathway sensitized mice to LPS-induced lethality. Signaling-selective aPC variants rescued mice with defective PC activation from vascular leakage and lethality. Defects in the aPC pathway were fully compensated by sphingosine 1 phosphate receptor 3 (S1P3) deficiency or by selective agonists of the S1P receptor 1 (S1P1), indicating that PAR1 signaling contributes to setting the tone for the vascular S1P1/S1P3 balance. Thus, the activating proteases and selectivity in coupling to S1P receptor subtypes determine vascular PAR1 signaling specificity in systemic inflammatory response syndromes in vivo.
Introduction
Tissue factor (TF)–induced disseminated intravascular coagulation is a hallmark of diverse systemic inflammatory response syndromes associated with infectious diseases.1-3 The crucial role of the TF and protein C (PC) pathways in regulating sepsis outcome has been established in primate models,4-6 paving the way for the successful clinical testing of activated protein C (aPC) in sepsis therapy.7 Experiments in mice further underscored the importance of the coagulation pathways in sepsis. Genetic reductions of TF or its ligand coagulation factor VIIa reduce coagulation, inflammation, and lethality in murine LPS models,8,9 whereas deficiencies of PC, the endothelial cell PC receptor (EPCR), or disabling mutation of thrombomodulin (TM) sensitize animals to challenges of the innate immune system.10-14 In particular, vascular membrane EPCR is crucial for attenuation of LPS-induced local inflammation in the lungs.15,16 However, primate studies and clinical trials raised doubts whether anticoagulation is sufficient to prevent sepsis lethality,17-19 shifting the focus of recent interest to coagulation protease signaling pathways through protease activated receptors (PARs).
PAR1 contributes to signaling by proteases of both the procoagulant and anticoagulant pathways. PAR1 is a major thrombin receptor of vascular cells20 and is also cleaved and activated by the TF coagulation initiation complex.21 In addition, aPC uses EPCR as a cosignaling receptor to activate PAR1,22 and aPC-PAR1 signaling is neuroprotective in stroke and may regulate progression of multiple sclerosis.23-25 However, the cellular responses resulting from proteolytic activation of PAR1 by thrombin and aPC are distinct and often opposing, in particular in cytokine-perturbed endothelial cells.26 In vitro assays of endothelial barrier function have further provided strong support for the concept that aPC- and thrombin-mediated PAR1 signaling counterbalances crucial endothelial functions. aPC potently inhibits thrombin-induced vascular permeability,27-30 and endothelial barrier protective activities of aPC in vitro are dependent on raft localization of PAR1 and EPCR30,31 and on cross-activation of sphingosine 1 phosphate (S1P) receptor 1 (S1P1).27,32
However, in vivo studies are needed to clarify roles for endogenous EPCR/aPC-PAR1 signaling that may protect animals from adverse outcomes in systemic inflammatory response syndromes. aPC-treated mice show reduced vascular leakage in sepsis,33 but there may be alternative, aPC-independent pathways in which PAR1 cooperates with PAR2 to enhance barrier function.34 Despite compelling evidence for a role of PAR1 in the response to therapeutically administered aPC, it remains unclear how PAR1 responds to endogenously generated proteases in the escalation of sepsis syndrome.8,33-35
We have recently shown that PAR1−/− mice are protected from very severe, LD90 lipopolysaccharide (LPS) challenge and that intervention with a PAR1 antagonist can rescue wild-type animals from lethality in the commensal bacteria–induced cecal ligation and puncture (CLP) peritonitis model.36 In these models, thrombin-PAR1 signaling escalates systemic inflammation and disseminated intravascular coagulation by perturbing dendritic cells (DCs) in the lymphatic rather than the vascular compartment. In addition, proinflammatory PAR1 signaling sustains inflammatory and coagulant exacerbation specifically in the late stages of sepsis. The proinflammatory thrombin-PAR1 DC pathway is remarkably sensitive to the severity of the initial inflammatory stimulus, and systemic levels of DC-derived inflammatory cytokines, such as interleukin IL1β, are not regulated by PAR1 signaling under less severe, LD50 LPS challenge conditions.36 Consequently, PAR1−/− mice do not fare better than wild type and die with the same frequency.33,35,36 The absence of an appreciable phenotype of PAR1 deficiency in these partially compensated inflammatory models may be explained by the dual role of endothelial expressed PAR1 as a signaling receptor for thrombin as well as for EPCR/aPC.
In vitro studies have provided mechanistic insight into distinct downstream targets that may account for differential signaling outcomes when PAR1 is activated by these alternative protease pathways in endothelial cells (Figure 1). Endothelial barrier-enhancing aPC signaling involves S1P1 transactivation,27,32 whereas thrombin-PAR1 signaling is barrier disruptive and is dependent on another S1P receptor, S1P3, that is also involved in barrier disruption induced by LPS.37 Barrier-disruptive thrombin signaling thus resembles the proinflammatory thrombin-PAR1-S1P3 DC pathway, which also requires DC-expressed sphingosine kinase 1 (Sphk1).36
Coupling of thrombin-PAR1 and EPCR/PC-PAR1 signaling to barrier-regulating S1P receptor subtypes. In vitro studies indicated that thrombin-induced S1P3 activation promotes vascular leakage, whereas aPC-mediated S1P1 cross activation can diminish vascular leak and enhances barrier protection. S1P3 is also required for LPS-induced vascular leakage.
Coupling of thrombin-PAR1 and EPCR/PC-PAR1 signaling to barrier-regulating S1P receptor subtypes. In vitro studies indicated that thrombin-induced S1P3 activation promotes vascular leakage, whereas aPC-mediated S1P1 cross activation can diminish vascular leak and enhances barrier protection. S1P3 is also required for LPS-induced vascular leakage.
Here, we address the 2 opposing roles for vascular PAR1 signaling in systemic inflammatory response syndromes in vivo. We use a combination of genetic mouse models and pharmacological modulators to demonstrate that EPCR/aPC-PAR1 signaling has protective functions and promotes survival by preventing vascular leakage in escalating systemic inflammation. In contrast, thrombin-PAR1-S1P3 signaling is endothelial barrier disruptive. Our data further show that impairments of the endogenous EPCR/aPC-PAR1 pathway can be compensated for by therapy with either a signaling-selective aPC variant or a S1P receptor agonist that is selective for S1P1. Both therapies attenuate vascular leakage and improve survival following an inflammatory challenge.
Methods
Proteins
Recombinant wild-type murine aPC, signaling-selective variant aPC5A (carrying 5 Ala substitutions for basic residues involved in factor Va recognition),33 anticoagulant-selective aPC149A (with a single Glu149Ala mutation),38 and catalytically inactive aPC195A (carrying a catalytic triad Ser195Ala mutation) were purified under low endotoxin conditions.
Rat antimouse PC monoclonal antibody (TVM1) was generated using standard hybridoma technology and characterized for inhibition of catalytic and anticoagulant activities of aPC in vitro. Dose response curves were established for binding of TVM1 to immobilized mouse PC by enzyme-linked immunosorbent assay (ELISA), using detection of bound antibody with biotinylated anti–rat IgG (1 μg/mL) followed by streptavidin-conjugated horseradish peroxidase using octophenyl diamine substrate for color development read at 450 nm. Inhibition of the amidolytic activity of mouse aPC by TVM1 antibody was measured with 0.5 mM Pefachrome PCa substrate (Pentapharm, Basel, Switzerland), and inhibition of anticoagulant activity was characterized in a modified activated partial thromboplastin time (aPTT) clotting assay.
Sepsis models
The following mouse strains were extensively backcrossed into C57BL/6J and maintained under pathogen-free conditions: C57BL/6J (wild-type), PAR1−/−,39 S1P3−/−,40 Sphk1−/−,41 EPCRlow,42 TMPro,14 and the cross with PAR1−/− (TMPro/PAR1−/−). Under approved protocols of the Scripps Research Institute IACUC, mice were challenged by intraperitoneal injection of 3.5 mg/kg LPS (nonlethal in wild-type mice) or 5 mg/kg LPS (LD50). LPS was from E coli O111:B4 (Calbiochem, San Diego, CA).36 All interventions were performed at 10 hours after LPS challenge by single bolus tail vein injection of wild-type or mutant aPC (0.8 mg/kg), anti-PC antibody TVM1 (10 mg/kg), S1P1 agonist AUY954 (1 mg/kg), or the thrombin inhibitor hirudin (120 mg/kg lepirudin, 1 mg/kg PEG-hirudin).36
Vascular permeability in vivo assay
Vascular permeability was assessed after intravenous injection of the cell-impermeable Evans blue dye (Sigma-Aldrich, St Louis, MO) following the procedure described by Yano et al.43 Briefly, 0.1 mL of 1% Evans blue dye in normal saline was injected 45 minutes prior to blood drawing from the vena cava inferior. Animals were perfused from the heart with 40 mL normal saline and organs were harvested. Tissue dry weights were determined after dehydration and Evans blue was extracted with formamide for 3 days. Evans blue in the plasma and tissue was quantified by dual-wavelength spectrophotometric analysis at 620 and 740 nm,44 and 620-nm values were corrected for absorbance of contaminating heme pigments, using the following formula: (corrected absorbance at 620 nm = actual absorbance at 620 nm − [1.426 (absorbance 740 nm) + 0.03]), followed by normalization to organ dry weights. Pictures of organs were taken with a Sony Cybershot DSC-P8 camera (Sony, Tokyo, Japan) and reproduced at similar magnification based on the size of a calibrating scale in the pictures in Adobe Photoshop version CS3 10.0.1 (Adobe Systems, San Jose, CA).
Analytic procedures
IL1β (Quantikine; R&D Systems, Minneapolis, MN) and TAT (Enzygnost; Dade Behring, Marburg, Germany) were determined in plasma samples obtained from the inferior vena cava.
Statistical analysis
Data were normally distributed and evaluated by ANOVA followed by Fisher PLSD. Survival was analyzed by Kaplan-Meier curves and log-rank test with Bonferroni correction as needed.
Results
Thrombin-PAR1 and EPCR/aPC-PAR1 signaling have opposing effects on the lethality of LPS-challenged mice
PAR1 deficiency protects mice from thrombin-dependent very severe inflammation but not from less severe LD50 LPS challenge.35,36 Under these conditions, therapy with aPC rescues wild-type mice from lethality, but aPC has no protective activity in PAR1−/− or EPCRlow mice.33 We used pharmacological interventions at 10 hours in different genetic mouse strains to probe the role of the endogenous aPC pathway and alterations in the thrombin-aPC balance on survival in escalating inflammatory response syndromes. In very severe LPS challenge, inflammatory cytokine levels have reached a peak at 12 hours,36 and the interventions at 10 hours are therefore not expected to alter the direct effects of LPS, but rather modulate subsequent compensatory host response pathways.
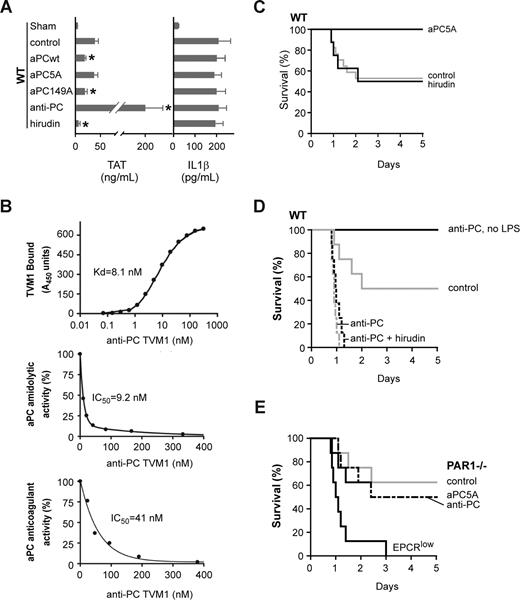
At 18 hours, we measured plasma IL1β levels to monitor systemic inflammation and TAT levels to assess coagulation activation and the efficacy of anticoagulants. Hirudin reduced plasma levels of TAT essentially to levels of sham-treated mice. A significant anticoagulant effect was also obtained by a single dose of wild-type murine aPC or anticoagulant-selective aPC149A, but not the signaling-selective aPC5A mutant (Figure 2A). We further acutely blocked endogenous aPC with the newly developed anti-PC antibody (TVM1) that inhibited aPC's catalytic and anticoagulant activities (Figure 2B). Intravenous injection of TVM1 at 10 hours after LPS produced a pronounced elevation of circulating TAT levels determined 8 hours later (Figure 2A). All pharmacological interventions had no effect on plasma levels of the DC-derived IL1β that is regulated by PAR1 signaling in more severe sepsis models.
The endogenous EPCR/aPC pathway contributes to protection from lethality in LD50 LPS-challenged mice. (A) Plasma TAT and IL1β levels 18 hours after LD50 LPS challenge in wild-type mice treated at 10 hours with wild-type aPC, aPC5A,44 aPC149A, anti-PC antibody (TVM1), or hirudin (mean ± SD; n = 4/group; * indicates different from untreated control; P < .02 by ANOVA). (B) Characterization of antimouse PC antibody (TVM1) dose responses for antibody binding to immobilized mouse PC (top panel) and for inhibition of mouse aPC amidolytic (middle panel) and anticoagulant (bottom panel) activities. (C-E) Survival after intervention with the indicated agents at 10 hours after LD50 intraperitoneal LPS (5 mg/kg) challenge of wild-type (WT) mice (C,D) or PAR1−/− or EPCRlow mice (E). n = 8–17/group; P < .01 for aPC5A, anti-PC TVM1, or EPCRlow vs WT control.
The endogenous EPCR/aPC pathway contributes to protection from lethality in LD50 LPS-challenged mice. (A) Plasma TAT and IL1β levels 18 hours after LD50 LPS challenge in wild-type mice treated at 10 hours with wild-type aPC, aPC5A,44 aPC149A, anti-PC antibody (TVM1), or hirudin (mean ± SD; n = 4/group; * indicates different from untreated control; P < .02 by ANOVA). (B) Characterization of antimouse PC antibody (TVM1) dose responses for antibody binding to immobilized mouse PC (top panel) and for inhibition of mouse aPC amidolytic (middle panel) and anticoagulant (bottom panel) activities. (C-E) Survival after intervention with the indicated agents at 10 hours after LD50 intraperitoneal LPS (5 mg/kg) challenge of wild-type (WT) mice (C,D) or PAR1−/− or EPCRlow mice (E). n = 8–17/group; P < .01 for aPC5A, anti-PC TVM1, or EPCRlow vs WT control.
A dose of hirudin that attenuated severe systemic inflammation and lethality in a LD90 LPS challenge36 did not improve survival of wild-type mice in a LD50 challenge (Figure 2C). Because high-dose hirudin inhibits both the anticoagulant and the procoagulant effects of thrombin, the balance of lethality promoting and protective PAR1 signaling may have remained unchanged under these partially compensated conditions. Alternatively, PAR1 signaling may not contribute to survival outcome under the conditions used. Consistent with previous data and sepsis-protective effects of EPCR/aPC-PAR1 signaling,33 wild-type, but not PAR1−/−, mice were rescued from LD50 LPS-induced death by a single dose of the signaling-selective mutant aPC5A (Figure 2C,E). Conversely, acute antibody blockade of aPC increased lethality in wild-type mice, but the same dose of anti-PC antibody did not cause excess death in the absence of a LPS challenge (Figure 2D). These data support a model in which protective as well as detrimental effects of PAR1 signaling are at play under these partially compensated conditions to regulate inflammation-induced lethality.
Although hirudin did not reverse the detrimental effects of acute antibody blockade of aPC in wild-type mice (Figure 2D), experiments in PAR1−/− mice demonstrated that this antibody's increased lethality required the presence of PAR1 (Figure 2E). Perhaps, hirudin did not completely block detrimental thrombin-PAR1 signaling in anti-PC–treated wild-type mice; however, activation of PAR1 by alternative proteases, such as the ternary TF-VIIa-Xa complex,21 cannot be ruled out. The outcome of the combined treatment with hirudin plus anti-PC antibody nevertheless emphasized the crucial role of the endogenous aPC pathway to counteract inflammation-induced lethality and possibly detrimental PAR1 signaling. EPCRlow mice also succumbed to the LPS challenge similar to anti-PC antibody–treated wild-type mice (Figure 2E), as expected from the selective loss of the anticoagulant aPC generation pathway in EPCRlow mice and/or of protective EPCR/aPC-PAR1 signaling.
Deregulated PAR1 signaling in TMPro mice
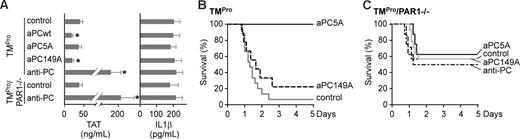
We analyzed TMPro mice to further test the concept that the balance of PAR1 signaling was crucial for survival in systemic inflammatory response syndromes. TMPro mice are genetically impaired in PC activation, but retain an intact EPCR-PAR1 receptor repertoire. TMPro mice were indistinguishable from wild-type in TAT and IL1β levels under all pharmacological interventions (Figure 3A vs Figure 2A), but TMPro mice showed more than 90% mortality in a LD50 LPS challenge (Figure 3B), as previously reported.14 Comparable TAT levels of TMPro and wild-type mice indicated either that the thrombomodulin mutation minimally impaired anticoagulant PC generation or that the DC-dependent coagulation activation at these late stages of the LPS challenge36 was less sensitive to genetic impairments of the endogenous PC activation pathway.
TMPro mice have a shifted thrombin-aPC signaling balance in systemic inflammation. (A) Plasma TAT and IL1β levels 18 hours after LD50 LPS challenge of TMPro and TMPro/PAR1−/− mice; the indicated treatments were given at 10 hours after LPS (mean ± SD; n = 4/group; * indicates different from respective control; P < .02 by ANOVA). (B,C) Survival of LD50 LPS-challenged TMPro (B) or TMPro/PAR1−/− (C) mice following treatment by a single bolus injection of the indicated agents at 10 hours (n = 8–15/group; P < .001 for aPC5A vs TMPro control).
TMPro mice have a shifted thrombin-aPC signaling balance in systemic inflammation. (A) Plasma TAT and IL1β levels 18 hours after LD50 LPS challenge of TMPro and TMPro/PAR1−/− mice; the indicated treatments were given at 10 hours after LPS (mean ± SD; n = 4/group; * indicates different from respective control; P < .02 by ANOVA). (B,C) Survival of LD50 LPS-challenged TMPro (B) or TMPro/PAR1−/− (C) mice following treatment by a single bolus injection of the indicated agents at 10 hours (n = 8–15/group; P < .001 for aPC5A vs TMPro control).
Notably, treatment with signaling selective aPC5A, but not with anticoagulant-selective aPC149A, rescued TMPro mice from lethality. TMPro mice were also partially rescued from increased lethality by PAR1 deficiency (Figure 3C), indicating that deregulated PAR1 signaling contributed to the increased susceptibility of TMPro mice to LPS challenge. As seen in PAR1−/− mice (Figure 2E), all pharmacological interventions did not alter mortality of LD50 LPS-challenged TMPro/PAR1−/− mice. Thus, impaired PC activation can be compensated for by therapeutic intervention with signaling-selective aPC, and EPCR/aPC-PAR1 signaling counterbalances detrimental effects of inflammation as well as of PAR1 signaling.
Modulation of selective downstream targets of PAR1 signaling reduces LPS-induced lethality
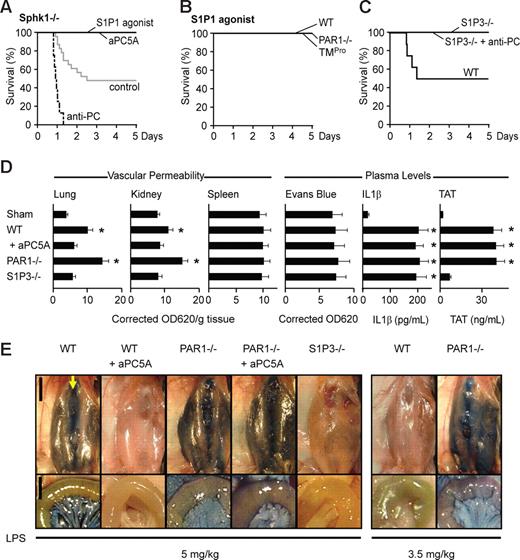
In DCs, proinflammatory PAR1 signaling couples to S1P3 and requires Sphk1.36 In endothelial cells, genetic loss of Sphk1 is partially compensated by Sphk2 during development.45 Because we did not observe differences in DC-derived inflammatory cytokines, we assumed that PAR1 signaling predominantly regulated endothelial cell function and predicted that Sphk1 was required neither for detrimental nor for protective PAR1 signaling. Indeed, LD50 LPS lethality of Sphk1−/− mice was indistinguishable from wild-type mice, and Sphk1−/− mice similarly succumbed to anti-PC antibody treatment and were rescued by therapy with signaling-selective aPC5A (Figure 4A). These data are in line with the concept that PAR1 signaling on vascular cells, rather than on DCs, was responsible for the modulation of lethality in this model.
Roles of S1P1 and S1P3 signaling in protection from LD50 LPS-induced lethality. (A) Sphk1−/− mice exhibit mortality reduction by aPC5A or S1P1 agonist AUY954 treatments and mortality enhancement following anti-PC TVM1 antibody treatment (n = 8–22 mice/group; P < .001, aPC5A, S1P1 agonist, and TVM1 treatment vs control). (B) Intervention at 10 hours with S1P1 agonist AUY954 rescued wild-type, PAR1−/−, and TMPro mice from death after LD50 challenge (n = 8/group; P < .02 vs respective control). (C) Protection of LD50-challenged S1P3−/− mice from anti-PC TVM1 antibody treatment (n = 8/group; P < .01 vs wild-type control). (D) Vascular leakage in lungs, kidney, and spleens, and plasma levels of Evans blue, IL1β, and TAT in LD50-challenged wild-type (WT), PAR1−/−, and S1P3−/− mice treated with aPC5A (mean ± SD; n = 3-7 mice/group; * indicates P < .01 different from sham receiving no LPS, ANOVA). (E) Macroscopic views at 18 hours after LPS of the anterior abdominal wall 45 minutes after Evans blue injection were prepared by blunt dissection of the superficial fascia. For orientation, the arrow points to the sternal xiphoid where the linea alba (running top to bottom) originates and the skin flap is turned to the left. The close-up views of the mesentery and small intestine (bottom panels) were taken after whole-body perfusion. Scale bars equal 1 cm for overviews and 5 mm for close-ups.
Roles of S1P1 and S1P3 signaling in protection from LD50 LPS-induced lethality. (A) Sphk1−/− mice exhibit mortality reduction by aPC5A or S1P1 agonist AUY954 treatments and mortality enhancement following anti-PC TVM1 antibody treatment (n = 8–22 mice/group; P < .001, aPC5A, S1P1 agonist, and TVM1 treatment vs control). (B) Intervention at 10 hours with S1P1 agonist AUY954 rescued wild-type, PAR1−/−, and TMPro mice from death after LD50 challenge (n = 8/group; P < .02 vs respective control). (C) Protection of LD50-challenged S1P3−/− mice from anti-PC TVM1 antibody treatment (n = 8/group; P < .01 vs wild-type control). (D) Vascular leakage in lungs, kidney, and spleens, and plasma levels of Evans blue, IL1β, and TAT in LD50-challenged wild-type (WT), PAR1−/−, and S1P3−/− mice treated with aPC5A (mean ± SD; n = 3-7 mice/group; * indicates P < .01 different from sham receiving no LPS, ANOVA). (E) Macroscopic views at 18 hours after LPS of the anterior abdominal wall 45 minutes after Evans blue injection were prepared by blunt dissection of the superficial fascia. For orientation, the arrow points to the sternal xiphoid where the linea alba (running top to bottom) originates and the skin flap is turned to the left. The close-up views of the mesentery and small intestine (bottom panels) were taken after whole-body perfusion. Scale bars equal 1 cm for overviews and 5 mm for close-ups.
EPCR/aPC-PAR1 in vitro signaling is coupled to S1P1,27,32 whereas thrombin-PAR1 signaling reduces barrier function by activating S1P3-dependent signaling in endothelial cells.37 Consistent with these counterbalancing roles for endothelial S1P1 and S1P3, a single dose of a receptor-selective agonist for S1P1 given at 10 hours rescued wild-type, Sphk1−/−, PAR1−/−, and TMPro mice from the lethal effects of LD50 LPS challenge (Figure 4A-B). Conversely, the loss of S1P3 downstream of thrombin-PAR1 signaling predicted the observed protection of S1P3−/− mice from LD50 LPS-induced lethality (Figure 4C). Importantly, in S1P3−/− mice, the pronounced elevation of thrombin levels caused by anti-PC antibody (Figure 5B) did not cause lethality (Figure 4C), consistent with in vitro results that S1P3 is required for barrier-disruptive activities downstream of both LPS and thrombin.37
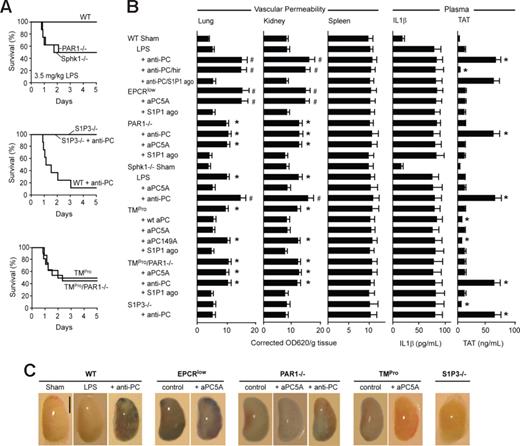
Loss of the endogenous aPC-PAR1 signaling pathway sensitizes mice to increased vascular leakage and lethality in sublethal LPS challenge. (A) Wild-type (WT), PAR1−/−, Sphk1−/−, S1P3−/−, TMPro, or TMPro/PAR1−/− mice were challenged with low dose, 3.5 mg/kg LPS and, where indicated, received anti-PC TVM1 antibody at 10 hours after LPS administration (n = 8-12; P < .01 survival difference between challenged WT and Sphk1−/−, PAR1−/−, TMPro, or TMPro/PAR1−/−). (B) Vascular permeability in lung, kidney, and spleen, and plasma levels of IL1β and TAT were determined at 18 hours after 3.5-mg/kg LPS challenge. Where indicated, treatments were given 10 hours after LPS challenge (mean ± SD; n = 3–5 mice/group; * indicates different from LPS-treated WT, P < .001; # indicates different from anti-PC TVM1-treated PAR1−/− or Sphk1−/− LPS-challenged groups, P < .005 by ANOVA). (C) Representative views of lower right lung lobes after Evans blue injections and perfusion. Scale bar equals 5 mm.
Loss of the endogenous aPC-PAR1 signaling pathway sensitizes mice to increased vascular leakage and lethality in sublethal LPS challenge. (A) Wild-type (WT), PAR1−/−, Sphk1−/−, S1P3−/−, TMPro, or TMPro/PAR1−/− mice were challenged with low dose, 3.5 mg/kg LPS and, where indicated, received anti-PC TVM1 antibody at 10 hours after LPS administration (n = 8-12; P < .01 survival difference between challenged WT and Sphk1−/−, PAR1−/−, TMPro, or TMPro/PAR1−/−). (B) Vascular permeability in lung, kidney, and spleen, and plasma levels of IL1β and TAT were determined at 18 hours after 3.5-mg/kg LPS challenge. Where indicated, treatments were given 10 hours after LPS challenge (mean ± SD; n = 3–5 mice/group; * indicates different from LPS-treated WT, P < .001; # indicates different from anti-PC TVM1-treated PAR1−/− or Sphk1−/− LPS-challenged groups, P < .005 by ANOVA). (C) Representative views of lower right lung lobes after Evans blue injections and perfusion. Scale bar equals 5 mm.
aPC prevents vascular leakage in LD50-challenged mice
The effects of pharmacological and genetic modulations of the S1P1/S1P3 balance suggested that vascular leakage was a crucial factor contributing to lethality. To address whether reduced lethality correlated with protection from inflammation-induced vascular leakage, we measured extravasation of the cell-impermeable Evans blue dye 18 hours after LD50 LPS challenge. LPS-treated wild-type and PAR1−/− mice displayed increased Evans blue dye extravasation compared with sham-treated wild-type mice or LPS-treated S1P3−/− mice (Figure 4D). Treatment of wild-type but not PAR1−/− mice with aPC5A prevented vascular leakage (Figure 4E).
Figure 4E illustrates the degree of vascular leakage in macroscopic views of the abdomen of mice injected intravenously with Evans blue dye 45 minutes prior to killing at 18 hours. The close-up views of mesentery and small intestine below were taken after vascular perfusion to visualize specifically extravasated dye. Wild-type and PAR1−/− mice showed increased vascular permeability, and aPC5A reduced vascular leakage in wild-type but not PAR1−/− mice (Figure 4E). Thus, PAR1 is required for aPC's ability to reduce vascular leakage and mortality in this LD50 LPS model.
Deficiencies in the endogenous EPCR/aPC-PAR1 pathway sensitize to inflammation-induced vascular leakage and lethality
Interestingly, challenging wild-type mice with a low, sublethal dose of 3.5 mg/kg LPS produced no signs of vascular leakage at 18 hours, whereas extensive vascular leakage was apparent in PAR1−/− mice (Figure 4E).
To further address this issue, we performed a series of additional experiments at this typically nonlethal LPS challenge dose of 3.5 mg/kg. No lethality was observed in wild-type or S1P3−/− mice, but 50% of PAR1−/− or Sphk1−/− mice died under these challenge conditions (Figure 5A). Lethality was also increased in TMPro mice, but there was no difference in lethality for TMPro mice versus TMPro/PAR1−/− mice. Anti-PC antibody treatment induced high lethality of wild-type mice but not of S1P3−/− mice. TAT levels were also markedly elevated in anti-PC antibody–treated mice (Figure 5B) similar to the LD50-challenged mice (Figures 2A, 3A). No differences in plasma levels of the DC-derived cytokine IL1β were observed between any of these mouse strains or under any of the experimental manipulations at the low LPS dose (Figure 5B).
Lung and kidney permeability measured by Evans blue extravasation showed no significant difference between sham and low-dose LPS–challenged wild-type mice, but antibody blockade of endogenous aPC induced significant vascular leakage in low-dose LPS-challenged wild-type mice (Figure 5B,C). Simultaneous administration of hirudin did not reverse increased vascular leakage, indicating that even under these typically nonlethal LPS challenge conditions, the aPC pathway is required to rebalance inflammation-induced vascular leakage. However, a direct S1P1 agonist reversed anti-PC antibody–induced vascular leakage without effects on systemic TAT levels, demonstrating that it is sufficient to increase the tone of S1P1 signaling, implicated as a downstream target of EPCR/aPC-PAR1 signaling, to prevent the detrimental effects of pharmacological blockade of the PC pathway.
Consistent with barrier-protective effects of endogenous EPCR/aPC-PAR1 signaling, EPCRlow mice displayed increased vascular leakage that was not rescued by signaling-selective aPC5A, but this leakage was reversed by the S1P1 agonist. Similarly, PAR1−/− mice displayed vascular leakage that was not suppressed by aPC5A intervention, but reversed by the S1P1 agonist. However, blockade of endogenous aPC with TVM1 antibody in PAR1−/− mice elevated thrombin levels, but did not exacerbate vascular leakage to levels seen in wild-type mice receiving anti-PC antibody or to levels observed in EPCRlow mice. This indicated that thrombin-PAR1 signaling contributed to the amplification of vascular leakage when the EPCR/aPC pathway was disabled.
Previous studies had shown that Sphk1−/− mice are prone to vascular leakage due to reduced S1P levels in endothelial cells which may decrease basal S1P1 tone.47,48 Sphk1−/− mice showed increased lethality and vascular leakage relative to wild-type at the low LPS dose (Figure 5A-C). In contrast to PAR1−/− mice, the administration of signaling-selective aPC5A prevented Evans blue extravasation in Sphk1−/− mice and vascular leakage increased upon anti-PC antibody administration in Sphk1−/− mice. Although these data cannot exclude that Sphk1 is involved in barrier-protective activities of the endogenous PC pathway, Sphk1 is clearly not required for pharmacological actions of aPC and, consistent with previous studies,48 of barrier-disruptive effects of thrombin.
Lower dose LPS also caused vascular leakage in TMPro mice and leakage was comparable with that seen in PAR1−/− mice (Figure 5B). Vascular leakage of TMPro mice was reversed similarly by treatment with wild-type aPC or with signaling-selective aPC5A, but not with the anticoagulant-selective aPC149A mutant that attenuated TAT levels as seen in mice treated with wild-type aPC. The double transgenic TMPro/PAR1−/− mice showed no change in vascular leakage relative to PAR1−/− or TMPro mice, providing additional evidence that the 2 genetic mutations were not additive and presumably function via the same pathway in this injury model. In addition, anti-PC antibody or intervention with aPC5A left the degree of vascular leakage unchanged in TMPro/PAR1−/− mice. Consistent with the proposed barrier-disruptive coupling of thrombin-PAR1 signaling to S1P3, S1P3−/− mice showed no vascular leakage and were resistant to anti-PC antibody treatment. Conversely, the S1P1 agonist reversed the vascular leakage seen in TMPro and TMPro/PAR1−/− mice.
Discussion
Initial studies did not uncover a role for PAR1 in LPS-induced inflammatory response syndromes,8,35 but our recent murine sepsis studies33,36 documented paradoxical roles for PAR1, acting either as a proinflammatory receptor for thrombin-PAR1 signaling on DCs or as a beneficial receptor when aPC is administered therapeutically. Here, we expand on these studies and demonstrate that the paradoxical effects of PAR1 in sepsis cannot solely be explained by cell type–specific effects of PAR1 signaling. By focusing on the effects of endogenously generated proteases during inflammatory exacerbation, we document that PAR1 activations mediated by thrombin versus EPCR/aPC have opposing roles in regulating vascular leakage. The combination of pharmacological interventions and genetic models presented here supports the overall concept that the protease selectivity of PAR1 signaling in the vascular system in vivo is a consequence of coupling to specific S1P receptor subtypes, namely to S1P1 versus S1P3.
PAR1−/− mice are shown to be more susceptible to die from an inflammatory challenge that is typically not lethal in wild-type mice. Following low-dose LPS challenge, PAR1−/− mice display significant vascular leakage, and this pathology was similarly found in mouse strains with genetic defects in the PC pathway (TMPro, EPCRlow) or when endogenous aPC was acutely blocked by a potent inhibitory antibody 10 hours after LPS challenge. In vitro data showed that aPC is barrier protective by transactivating S1P1.27,32 Unfortunately, S1P1−/− mice are not viable due to vascular failure.49 Therefore, one cannot directly address the role of S1P1 in the vascular-protective aPC pathway with simple genetic tools. However, a selective agonist for S1P1 prevented inflammation-induced vascular leakage and lethality in mice with a disabled PC pathway, even when systemic thrombin levels were highly elevated in mice treated with anti-PC antibody. Thus, restoring vascular tone by S1P1 agonism is sufficient to protect from the effects of detrimental thrombin signaling and to compensate for the loss of EPCR/aPC-PAR1 signaling. These data indirectly implicate S1P1 in the selectivity of aPC signaling in vivo.
Our data also provide mechanistic insights into the signaling specificity of thrombin by demonstrating an essential role of S1P3 for thrombin-induced vascular leakage. These data extend our previous findings for DCs36 to the vascular system and identify the thrombin-PAR1-S1P3 axis as a major detrimental pathway that worsens sepsis outcome via both vascular and innate immune cells. Accordingly, S1P3−/− mice are completely protected from lethality induced by acute blockade of the endogenous PC pathway with a potent anti-PC antibody. The remarkably complete resistance of S1P3−/− mice to anti-PC antibody indicates that the endogenous EPCR/aPC-PAR1 signaling pathway has a crucial role to counterbalance detrimental S1P3 signaling in inflammatory disorders affecting the vascular system.
Surprisingly, PAR1−/− mice, unlike wild-type mice, were protected from increased lethality when the endogenous anticoagulant PC pathway was blocked by anti-PC antibody. Thus, a major function of the PC pathway is to counteract detrimental, inflammation-related PAR1 signaling in the vascular compartment, rather than simply to inhibit intravascular thrombin generation. Although these findings in mice do not exclude that anticoagulant effects of aPC may contribute to the demonstrated clinical efficacy of aPC in human sepsis, they emphasize the crucial protective role of endogenous aPC-PAR1 signaling in inflammation. Consistently, the aPC5A variant that produced no measurable anticoagulant effect in vivo was as efficient as wild-type aPC to reverse vascular leakage and lethality of TMPro mice, whereas the anticoagulant-selective aPC149A variant was without effect on these parameters.
Sepsis impairs PC activation on the endothelium, and the experiments in TMPro mice show that therapy with the signaling-selective aPC5A variant can rebalance defective PC activation. Moreover, defects in EPCR/aPC-PAR1 signaling can be compensated for by increasing the tone of S1P1 signaling with a specific S1P1 agonist. These new data on the coupling of protease-specific PAR1 signaling in vivo have significant implications for therapy, because it may become feasible to modulate coagulation signaling without altering hemostatic pathways. This is particularly important in severely ill patients in which traditional anticoagulant strategies can raise safety issues about potential bleeding complications. Selective modulators of coagulation protease signaling pathways have the potential to find wider acceptance and allow for appropriate dosage in acute and severe inflammatory response syndromes in the clinic.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Cindi Biazak, Jennifer Royce, Nora Leaf, Xiao Xu, and Ngan Pham-Mitchell for expert technical assistance.
This study was supported by National Institutes of Health (NIH, Bethesda, MD) grants HL-78614, HL-16411 (W.R.), HL-31950, HL-52246 (J.H.G.), HL-73750 (F.J.C.), HL-60655 (H.W.), HL-87618 (L.O.M.), and AI-55509 (H.R.) and an American Heart Association Postdoctoral Fellowship (C.F.F.). S1P3−/−, Sphk1−/−, and PAR1−/− mice were kindly provided by Drs Jerold Chun, Richard Prioa, and Patricia Andrade-Gordon.
National Institutes of Health
Authorship
Contribution: F.N., C.F.-F., and J.A.F. designed experiments, performed research, analyzed data, and wrote the paper; L.O.M., F.J.C., H.W., and H.R. provided vital new reagents; J.H.G. analyzed data and wrote the paper; W.R. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: J.H.G., L.O.M., and H.W. have pending patent applications that are potentially related to this study. J.H.G. is a consultant for Socratech LLC.
Correspondence: Wolfram Ruf, Department of Immunology and Microbial Science, SP258, The Scripps Research Institute, La Jolla, CA 92037; e-mail: ruf@scripps.edu.
References
Author notes
*F.N. and C.F.-F. contributed equally to this work.