Abstract
Originally cloned from hematopoietic stem cell (HSC) populations and its ligand being extensively used to promote ex vivo HSC expansion, the FMS-like tyrosine kinase 3 (FLT3; also called FLK2) receptor and its ligand (FL) were expected to emerge as an important physiologic regulator of HSC maintenance and expansion. However, the role of FLT3 receptor and ligand in HSC regulation remains unclear and disputed. Herein, using Fl-deficient mice, we establish for the first time that HSC expansion in fetal liver and after transplantation is FL independent. Because previous findings in Flk2−/− mice were compatible with an important role of FLT3 receptor in HSC regulation and because alternative ligands might potentially interact directly or indirectly with FLT3 receptor, we here also characterized HSCs in Flk2−/− mice. Advanced phenotypic as well as functional evaluation of Flk2−/− HSCs showed that the FLT3 receptor is dispensable for HSC steady-state maintenance and expansion after transplantation. Taken together, these studies show that the FLT3 receptor and ligand are not critical regulators of mouse HSCs, neither in steady state nor during fetal or posttransplantation expansion.
Introduction
Although hematopoietic stem cells (HSCs) are mostly quiescent and sustained at relatively constant numbers in steady-state adult hematopoiesis, mechanisms are in place to promote extensive HSC expansion during fetal development and after transplantation of limiting HSC numbers into myeloablated recipients.1-3 Extracellular cues are thought to be involved in HSC regulation, as shown for the hematopoietin receptor c-mpl and its ligand thrombopoietin and the cytokine tyrosine kinase receptor C-KIT and its ligand.4-9
Another cytokine tyrosine kinase receptor, the FMS-like tyrosine kinase 3 (FLT3; also termed FLK2), having originally been cloned from highly enriched HSC populations,10 was also expected to emerge as a key HSC regulator. However, despite extensive investigations, it remains unclear as to what degree FLT3 and its ligand (FL) are involved in regulation of HSCs, whereas its role in the earliest stages of lymphoid-primed multipotent progenitors (LMPPs) and lymphoid development is well established.11-19
Previous studies showed that FL-deficient (Fl−/−) mice have normal HSC numbers in adult steady-state bone marrow (BM),13 suggesting that FL has little or no role in regulating steady-state HSC numbers. However, those studies did not preclude or investigate a role of FL in regulating HSCs during development and in regenerating BM, when they expand extensively.2,3 In fact, previous studies suggested that the mouse FLT3 receptor is up-regulated on cycling HSCs in fetal liver and adult BM,20-22 and yet other studies provided strong evidence in support of FL promoting in vitro expansion of HSCs.15,22-26 Although those studies pointed to potential stimulatory effects of FL on cycling HSCs, they did not establish (or explore) a potential physiologic role of FL in regulation of HSC expansion, such as during development and after transplantation.
In studies of FLK2 receptor–deficient (Flk2−/−) mice, Mackarehtschian et al11 reported a multilineage reconstitution defect of Flk2−/− BM cells after transplantation, a finding compatible with a deficiency of Flk2−/− HSCs. However, whether the observed deficiency in generation of mature blood cells of multiple lineages reflected a role in HSC maintenance, in progenitor regeneration and differentiation, or in both could not be established. Further, because the FLK2 deficiency is cell intrinsic, it could affect not only steady- state HSC maintenance but potentially also their posttransplantation expansion, maintenance, or differentiation.
The seeming discrepancy between the apparent HSC phenotype in FLT3 receptor (Flk2−/−), but not FLT3 ligand–deficient mice,11,13 could potentially also be explained by other (yet to be identified) ligands that could directly bind to and activate the FLT3 receptor in Fl−/− mice. Alternatively, the FLT3 receptor in Fl−/− mice could potentially be transactivated by other signaling pathways or ligands that would not bind to the FLT3 receptor. In support of such a possibility, evidence has recently been provided for such transactivation as an explanation for the more severe lymphoid phenotype in IL-7 receptor– than IL-7 ligand–deficient mice.27
Resolving the conflicting results with regard to a potential role of FLT3 receptor and ligand in regulation of mouse HSCs has considerable implications for normal and leukemic hematopoiesis in man. Several studies have suggested that FLT3 is expressed on candidate normal human HSCs,28-30 and greater than 30% of acute myeloid leukemias (AMLs) have activating mutations in the FLT3 receptor.31,32 Thus, it is important to establish the normal cellular targets of FLT3 mutations, because, if these include HSCs, it might affect not only the biology but also the therapeutic targeting and resistance of FLT3-mutated leukemic clones.33
Herein, to establish whether FL plays an important role in regulation of the HSC expansion that takes place during normal development and after transplantation,2,3 we used distinguishable Fl−/− HSC donor and recipient mice. Through this approach we could establish that Fl deficiency does not significantly affect engraftment or fetal or posttransplantation expansion of mouse HSCs. Further, HSC numbers were also not affected in Flk2−/− mice, neither in steady state nor after transplantation, supporting that neither the FLT3 ligand nor receptor is critically involved in regulation of HSCs in mice.
Methods
Animals
Fl−/− mice on a pure C57BL/6 (CD45.2) background were previously described.12 Fl−/− CD45.1 mice were obtained by backcrossing Fl−/− CD45.2 with wild-type (WT) C57BL/6 CD45.1 mice. Flk2−/− mice were generated as previously reported11 and backcrossed with C57BL/6 (CD45.2) WT mice for 9 generations. All offspring from Fl−/− and Flk2−/− breeders used in these studies were genotyped to establish homozygosity for Fl−/− and Flk2−/−, respectively, as previously described.11,12 All animal protocols were approved by the local ethics committee at Lund University.
Antibodies
All antibodies were from Becton Dickinson (Franklin Lakes, NJ) unless otherwise indicated. Monoclonal antibodies (conjugated with different fluorochromes) used to stain cell-surface antigens were CD16/32 (clone 2.4G2), B220 (RA3-6B2), CD11b/Mac-1 (M1/70), CD4 (H129.9), CD8α (53-6.7), Ter-119 (LY-76), SCA-1 (E13-161.7), C-KIT (2B8), CD45.1 (A20), CD45.2, (104) FLT3 (AZF10.1), CD34 (RAM34), CD150 (TC15-12F12.2), Gr-1 (RB6-8C5), CD5 (53-7.3), CD3 (17A2), NK1.1 (PK136), and anti-IgM (R6-60.2). Biotinylated antibodies were visualized with streptavidin-PE or streptavidin-PECy7, and purified lineage (lin) antibodies were visualized with polyclonal goat anti–rat-Tricolor (both Invitrogen, Carlsbad, CA). In some experiments 7-amino-actinomycin D (7-AAD; Sigma-Aldrich, Poole, United Kingdom) was added to exclude dead cells from the analysis.
Transplantation assays
Lethally irradiated (900-975 cGy) CD45.2 WT or Fl−/− primary recipient mice were transplanted with 2 × 105 unfractionated BM (9- to 11-week-old) or day 14.5 unfractionated fetal liver cells from CD45.1 WT or Fl−/− mice, respectively. At 16 weeks after transplantation, 5 × 106 unfractionated BM cells from primary recipients were transplanted into secondary lethally irradiated WT (CD45.2) recipients along with 5 × 105 WT competitor BM cells (CD45.2). In other experiments, lethally irradiated (900-975 cGy) CD45.1 WT recipient mice were transplanted with 106 unfractionated BM cells from (10-14 weeks old) CD45.2 WT or Flk2−/− mice together with 106 unfractionated WT CD45.1 BM cells, respectively. At 16 weeks after transplantation, 20 × 106 unfractionated BM cells from primary recipients were transplanted into secondary lethally irradiated WT (CD45.1) recipients. To determine multilineage reconstitution34 nucleated peripheral blood (PB) cells or BM cells were stained with antibodies against CD45.1, CD45.2, Mac-1, Gr-1 (myeloid cells, also defined as negative for NK1.1), B220 and anti-IgM (B cells), and CD4 and CD8α (T cells). Positively reconstituted mice were as previously described,35 defined as having a minimum of 0.1% total and 0.02% of each of the B, T, and myeloid test cell contribution toward total PB cells. Donor-derived blood and BM lineage reconstitution in secondary recipients were analyzed at 12 to 28 weeks after the secondary transplantation. In addition, evaluation of the donor-derived HSC compartment was performed in recipients that received a transplant as described in “Transplantation assays.”
Analysis of HSC compartment in Fl−/− and Flk2−/− mice by flow cytometry
BM cells were enumerated and incubated with lineage (Lin) cocktail (purified rat antibodies against B220, CD5, CD8α, Gr-1, Ter-119, CD4, and Mac-1), stained with goat anti–rat-Tricolor, anti–mouse-FLT3-PE, or anti–mouse CD150-PE, and anti–CD34-FITC, anti–KIT-APC, anti–SCA1-biotinylated, or isotype-matched control antibodies and subsequently SA-PECy7 before analysis on FACSCalibur, FACSDiva, or FACS LSRII (Becton Dickinson) using FlowJo software (TreeStar, Ashland, OR). The same antibodies were used to evaluate HSCs in day 14.5 fetal liver except for exclusion of anti–Mac-1 and anti-CD4 from the Lin cocktail.1,36 In experiments evaluating donor-derived HSC reconstitution, anti–CD45.2-FITC antibody was included to detect donor cells.
Statistics
Statistical significances were determined with the use of the 2-tailed Mann Whitney test.
Results
Role of FLT3 ligand in HSC maintenance and expansion after transplantation
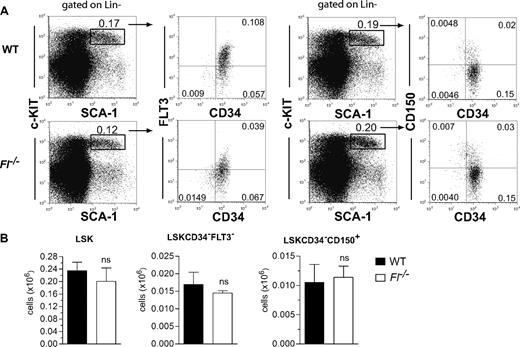
Although in vivo multilineage reconstitution assays remain the “gold standard,” recent improvements in phenotypic identification of HSCs have made direct evaluation of HSC numbers more feasible.34,37 We previously showed that the size of the LIN−SCA1+KIT+ (LSK) compartment is unaffected in adult Fl−/− mice,13 and we here analyzed more stringently defined HSCs and found LSKCD34−FLT3− as well as LSKCD34−CD150+ cells to be present at normal levels in adult Fl−/− mice (Figure 1A,B). Thus, maintenance of phenotypically defined HSCs in steady-state adult hematopoiesis is FL independent.
FLT3 ligand is dispensable for steady-state maintenance of adult HSCs. (A) Representative fluorescence-activated cell sorting (FACS) plots for analysis of LSKCD34−FLT3− and LSKCD34−D150+ cells in 9- to 10-week-old WT and Fl−/− mice. Numbers indicate frequencies of populations within indicated gates or quadrants of total BM cells. Cells have first been gated on Lin− cells as indicated. (B) Mean (SEM) numbers of LSK, LSKCD34−FLT3−, and LSKCD34−CD150+ cells in BM (per 2 tibiae and 2 femora) of 9- to 10-week-old WT and Fl−/− mice (n = 5 per genotype). ns indicates not significant.
FLT3 ligand is dispensable for steady-state maintenance of adult HSCs. (A) Representative fluorescence-activated cell sorting (FACS) plots for analysis of LSKCD34−FLT3− and LSKCD34−D150+ cells in 9- to 10-week-old WT and Fl−/− mice. Numbers indicate frequencies of populations within indicated gates or quadrants of total BM cells. Cells have first been gated on Lin− cells as indicated. (B) Mean (SEM) numbers of LSK, LSKCD34−FLT3−, and LSKCD34−CD150+ cells in BM (per 2 tibiae and 2 femora) of 9- to 10-week-old WT and Fl−/− mice (n = 5 per genotype). ns indicates not significant.
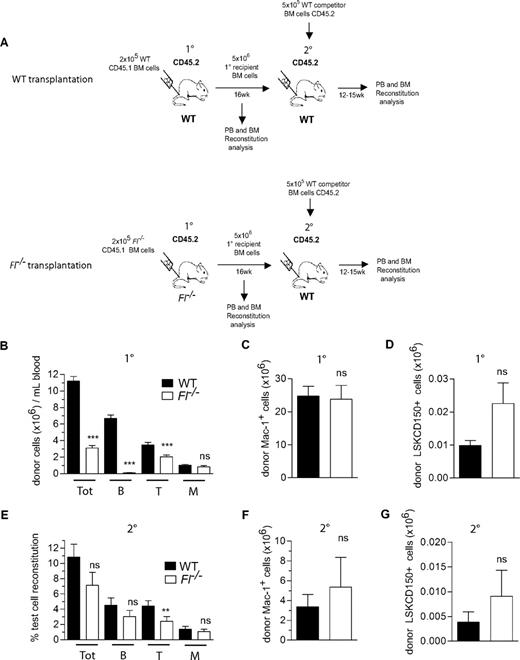
Next, to investigate the potential role of FL in promoting HSC engraftment and expansion after transplantation, lethally irradiated WT and Fl−/− (CD45.2) recipients were transplanted with a limited number (2 × 105) of WT and Fl−/− BM cells (CD45.1), respectively (Figure 2A). It was essential to also use Fl−/− recipients, because hematopoietic cells have been shown to be a major source of FL production.13,38,39 Notably, compared with WT mice a reduction was observed in total PB reconstitution in Fl−/− mice at 16 weeks after transplantation (3.6-fold; Figure 2B). This was almost entirely due to a failure to regenerate primarily B cells and partially T cells in the absence of FL, in agreement with recent studies,19,40 whereas myeloid reconstitution, representing the best measure of HSC activity because of the short half-life of the myeloid lineage, was not significantly reduced in peripheral blood (Figure 2B) or in BM (Figure 2C) of Fl−/− recipients. Furthermore, the regeneration of donor-derived LSKCD150+ HSC compartment was not different in Fl−/− and WT recipients (Figure 2D).
FLT3 ligand is dispensable for adult HSC reconstitution and expansion after BM transplantation. (A) Experimental design for HSC reconstitution and expansion experiments: 2 × 105 BM cells from 9- to 11-week-old WT and Fl−/− mice (CD45.1) were transplanted into lethally irradiated (900-975 cGy) adult WT and Fl−/− primary (1°) recipients (CD45.2), respectively. At 16 weeks after transplantation, 5 × 106 BM cells from 1° recipients were transplanted into secondary (2°) lethally irradiated WT (CD45.2) recipients along with 5 × 105 WT competitor BM cells (CD45.2). (B) Test cell–derived total (Tot), B, T, and myeloid (M) reconstitution in PB at 16 weeks after transplantation in 1° recipients, presented as total number of each cell type per milliliter blood. Data represent mean (SEM) values from 30 to 35 recipient mice of each genotype, from 5 independent experiments. (C) Mean (SEM) total number of test cell–derived myeloid (Mac-1+) cells in the BM (2 tibiae and 2 femora) of WT and Fl−/− 1° recipients at 16 weeks after transplantation (13 recipient mice per genotype from 2 independent experiments). (D) Mean (SEM) total number of test cell–derived LSKCD150+ cells in the BM (2 tibiae and 2 femora) of WT and Fl−/− 1° recipients at 16 weeks after transplantation (7-8 recipient mice per genotype). (E) Test cell–derived PB total, B-, T-, and M-cell reconstitution 12 to 15 weeks after transplantation of 2° recipients. Data are expressed as mean (SEM) percentage of test cell reconstitution (20-22 recipient mice per genotype, from 3 independent experiments). (F) Mean (SEM) total number of test cell–derived myeloid (Mac-1+) cells in the BM (2 tibiae and 2 femora) of WT and Fl−/− 2° recipients at 14 weeks after transplantation (7-8 recipient mice per genotype). (G) Mean (SEM) total number of test cell–derived LSKCD150+ cells in the BM (2 tibiae and 2 femora) of WT and Fl−/− 2° recipients at 14 weeks after transplantation (7-8 recipient mice per genotype). ns indicates not significant; **P < .01, ***P < .001.
FLT3 ligand is dispensable for adult HSC reconstitution and expansion after BM transplantation. (A) Experimental design for HSC reconstitution and expansion experiments: 2 × 105 BM cells from 9- to 11-week-old WT and Fl−/− mice (CD45.1) were transplanted into lethally irradiated (900-975 cGy) adult WT and Fl−/− primary (1°) recipients (CD45.2), respectively. At 16 weeks after transplantation, 5 × 106 BM cells from 1° recipients were transplanted into secondary (2°) lethally irradiated WT (CD45.2) recipients along with 5 × 105 WT competitor BM cells (CD45.2). (B) Test cell–derived total (Tot), B, T, and myeloid (M) reconstitution in PB at 16 weeks after transplantation in 1° recipients, presented as total number of each cell type per milliliter blood. Data represent mean (SEM) values from 30 to 35 recipient mice of each genotype, from 5 independent experiments. (C) Mean (SEM) total number of test cell–derived myeloid (Mac-1+) cells in the BM (2 tibiae and 2 femora) of WT and Fl−/− 1° recipients at 16 weeks after transplantation (13 recipient mice per genotype from 2 independent experiments). (D) Mean (SEM) total number of test cell–derived LSKCD150+ cells in the BM (2 tibiae and 2 femora) of WT and Fl−/− 1° recipients at 16 weeks after transplantation (7-8 recipient mice per genotype). (E) Test cell–derived PB total, B-, T-, and M-cell reconstitution 12 to 15 weeks after transplantation of 2° recipients. Data are expressed as mean (SEM) percentage of test cell reconstitution (20-22 recipient mice per genotype, from 3 independent experiments). (F) Mean (SEM) total number of test cell–derived myeloid (Mac-1+) cells in the BM (2 tibiae and 2 femora) of WT and Fl−/− 2° recipients at 14 weeks after transplantation (7-8 recipient mice per genotype). (G) Mean (SEM) total number of test cell–derived LSKCD150+ cells in the BM (2 tibiae and 2 femora) of WT and Fl−/− 2° recipients at 14 weeks after transplantation (7-8 recipient mice per genotype). ns indicates not significant; **P < .01, ***P < .001.
To more stringently assess the maintenance of HSCs in Fl−/− recipients, secondary competitive transplantation experiments were performed in which WT CD45.2 unfractionated BM cells were transplanted together with BM from primary recipients as a source of competitor cells (Figure 2A) into secondary WT CD45.2 recipients (Figure 2A). Importantly, also in this setting, Fl−/− BM cells from primary recipients were as efficient at reconstituting the myeloid, B- and T-cell lineages as those from WT primary recipients (Figure 2E). Further, there was no difference in the number of donor-derived myeloid cells (Figure 2F) or the LSKCD150+ HSC compartment (Figure 2G) in the BM of mice transplanted with WT and Fl−/− BM cells, further supporting that expansion of adult mouse HSCs after transplantation does not depend on FL.
Role of FLT3 ligand in HSC expansion during fetal development
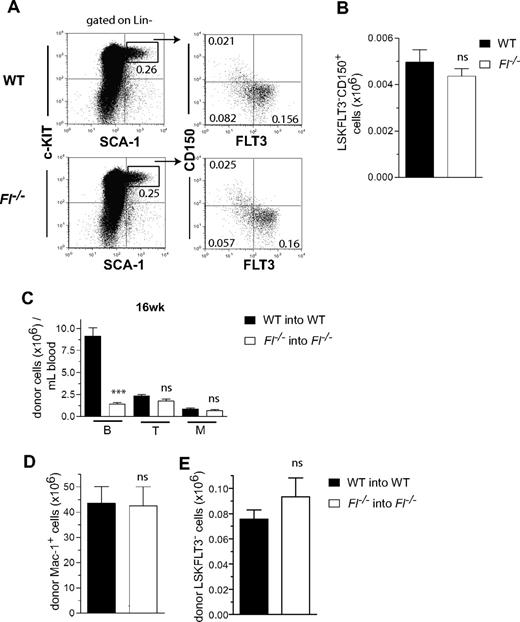
Because HSCs in fetal liver have been suggested to express FLT3,20 we next investigated whether FL might play a role in regulation of the extensive HSC expansion that takes place in the fetal liver2 and/or reconstitution of fetal HSCs after transplantation. Analysis of the HSC compartment in day 14.5 fetal liver41 showed no deficiency in Fl−/− mice (Figure 3A,B), supporting a redundant role of FL also in regulation of fetal HSC expansion.
FLT3 ligand is dispensable for generation, reconstitution, and posttransplantation expansion of fetal liver HSCs. (A) Representative FACS plots of LSKFLT3−CD150+ cells in day 14.5 fetal liver from WT and Fl−/− mice. Cells have first been gated as Lin− as indicated. Numbers indicate frequencies of populations within indicated gates or quadrants of total fetal liver cells. (B) Mean (SEM) numbers of LSKFLT3−CD150+ cells in day 14.5 fetal liver of WT and Fl−/− mice (n = 5 per genotype). (C-E) Liver cells (2 × 105) from WT and Fl−/− day 14.5 fetuses (CD45.1) were transplanted into lethally irradiated adult WT and Fl−/− recipients (CD45.2), respectively. Fetuses from at least 2 different litters were used. (C) Mean (SEM) donor-derived total B, T, and myeloid (M) reconstitution in PB at 16 weeks after transplantation from 9 recipient mice of each genotype. (D,E) Mean (SEM) total number (per 2 tibiae and 2 femora) of donor-derived Mac-1+ myeloid cells (D) and LSKFLT3− cells (E) in the BM of recipient mice (9 per genotype in 2 experiments) 16 weeks after transplantation. ns indicates not significant; ***P < .001.
FLT3 ligand is dispensable for generation, reconstitution, and posttransplantation expansion of fetal liver HSCs. (A) Representative FACS plots of LSKFLT3−CD150+ cells in day 14.5 fetal liver from WT and Fl−/− mice. Cells have first been gated as Lin− as indicated. Numbers indicate frequencies of populations within indicated gates or quadrants of total fetal liver cells. (B) Mean (SEM) numbers of LSKFLT3−CD150+ cells in day 14.5 fetal liver of WT and Fl−/− mice (n = 5 per genotype). (C-E) Liver cells (2 × 105) from WT and Fl−/− day 14.5 fetuses (CD45.1) were transplanted into lethally irradiated adult WT and Fl−/− recipients (CD45.2), respectively. Fetuses from at least 2 different litters were used. (C) Mean (SEM) donor-derived total B, T, and myeloid (M) reconstitution in PB at 16 weeks after transplantation from 9 recipient mice of each genotype. (D,E) Mean (SEM) total number (per 2 tibiae and 2 femora) of donor-derived Mac-1+ myeloid cells (D) and LSKFLT3− cells (E) in the BM of recipient mice (9 per genotype in 2 experiments) 16 weeks after transplantation. ns indicates not significant; ***P < .001.
With exception of reduced B cells, PB reconstitution (T cell and myeloid) was unaffected in Fl−/− recipients of fetal liver cells at 16 weeks (Figure 3C) after transplantation, and long-term reconstitution in the BM of myeloid (Figure 3D) and LSKFLT3− (Figure 3E) cells were also unaffected. These results further supported that FL is dispensable for HSC expansion during fetal development and after transplantation.
Role of FLT3 receptor in HSC maintenance and posttransplantation expansion
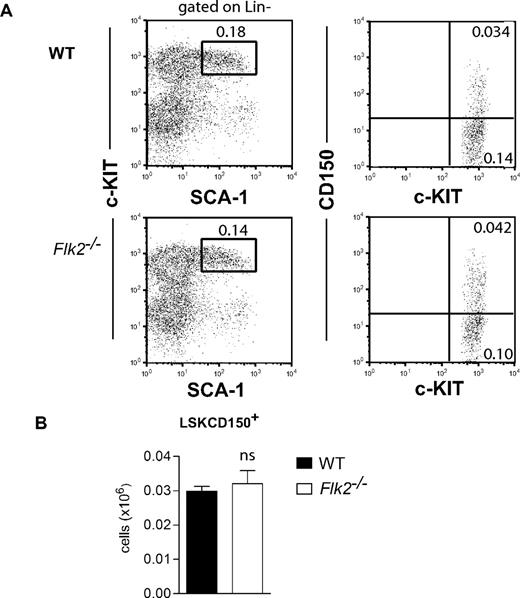
Previous studies of adult Flk2−/− mice showed a multilineage reconstitution defect,11 compatible with an important role of FLT3 receptor in regulation of HSCs. Herein, we first analyzed the HSC compartment of Flk2-deficient mice backcrossed to CD45.2 C57BL/6 mice for 9 generations. In agreement with previous studies,11 BM cellularity was not significantly affected in Flk2−/− mice (data not shown), and phenotypic analysis showed that also the LSKCD150+ HSC compartment was unaltered in Flk2−/− mice (Figure 4), compatible with also the FLT3 receptor being dispensable for HSC maintenance in steady-state adult hematopoiesis.
FLT3 receptor is dispensable for steady- state maintenance of adult HSCs. (A) Representative FACS plots of LSK and LSKCD150+ cells in BM of 9- to 18-week-old WT and Flk2−/− mice. Numbers indicate frequencies of populations within indicated gates or quadrants of total BM cells. Cells have first been gated as Lin− as indicated. In the right panels, only gated c-KIT+ cells are displayed. (B) Mean (SEM) numbers of LSKCD150+ cells in BM (per 2 tibiae and 2 femora) of 9- to 18-week-old WT and Flk2−/− mice (n = 8-10 per genotype), from at least 2 different litters. ns indicates not significant.
FLT3 receptor is dispensable for steady- state maintenance of adult HSCs. (A) Representative FACS plots of LSK and LSKCD150+ cells in BM of 9- to 18-week-old WT and Flk2−/− mice. Numbers indicate frequencies of populations within indicated gates or quadrants of total BM cells. Cells have first been gated as Lin− as indicated. In the right panels, only gated c-KIT+ cells are displayed. (B) Mean (SEM) numbers of LSKCD150+ cells in BM (per 2 tibiae and 2 femora) of 9- to 18-week-old WT and Flk2−/− mice (n = 8-10 per genotype), from at least 2 different litters. ns indicates not significant.
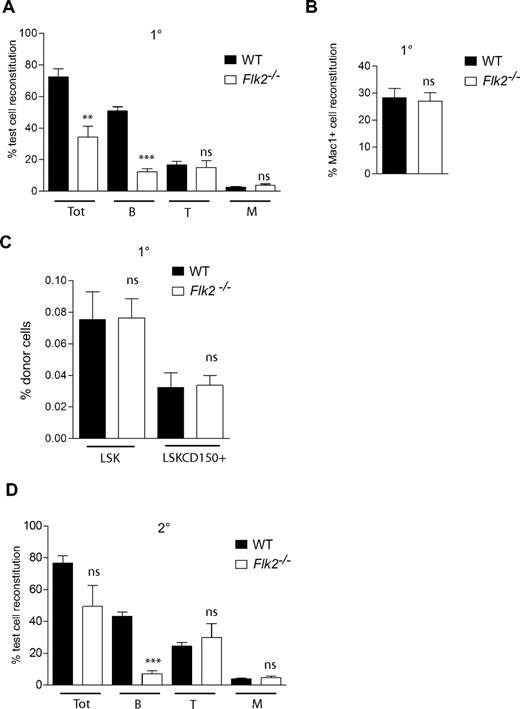
Next, a competitive transplantation experiment was performed to investigate whether the FLT3 receptor plays an important role in posttransplantation HSC expansion and maintenance. At 16 weeks after transplantation, total donor peripheral blood reconstitution by Flk2−/− BM cells was 2.1-fold lower compared with that of WT cells (Figure 5A). However, in agreement with the studies of Fl−/− mice, this reduction was entirely due to impaired B-cell regeneration, which was reduced as much as 4.1-fold in mice transplanted with Flk2−/− BM cells (Figure 5A). In contrast, peripheral blood reconstitution of both T cells and myeloid cells were not significantly affected in recipients of Flk2−/− BM cells (Figure 5A), and also myeloid reconstitution in the BM was normal (Figure 5B). Flk2−/− BM cells were as efficient as WT cells in regenerating the LSK and LSKCD150+ HSC compartments (Figure 5C). Finally, after transplantation into secondary recipients, Flk2−/− BM cells remained as efficient in sustaining long-term myeloid (and T cell) reconstitution at 24 to 28 weeks after transplantation as did WT cells (Figure 5D). Taken together, these findings show that steady state maintenance and posttransplantation expansion and self-renewal of HSCs occur independently of FLT3 ligand and receptor.
FLT3 receptor is dispensable for reconstitution and expansion of adult HSCs after BM transplantation. Lethally irradiated adult WT recipient (CD45.1) mice were transplanted with 106 unfractionated Flk2−/− (CD45.2) or WT (CD45.2) BM cells together with 106 unfractionated WT CD45.1 competitor BM cells. (A) Donor-derived PB total (Tot), B-cell, T-cell, and myeloid (M) reconstitution at 16 weeks after transplantation. Mean (SEM) values from 12 to 14 primary recipient mice of each genotype, from 2 independent experiments. At 16 weeks after transplantation recipient mice were killed, and BM cells were counted and analyzed for donor-derived myeloid (Mac-1+) reconstitution (B) as well as donor-derived regeneration of LSK and LSKCD150+ compartments (C). Mean (SEM) values for proportion of donor cells of 12 to 14 mice per genotype, from 2 independent experiments. (D) Test cell–derived PB total, B-, T-, and M-cell reconstitution 24 to 28 weeks after transplantation in secondary WT recipients. Data are expressed as mean (SEM) percentage of test cell reconstitution (9-10 recipient mice per genotype, from 2 independent experiments). ns indicates not significant; **P < .01, ***P < .001.
FLT3 receptor is dispensable for reconstitution and expansion of adult HSCs after BM transplantation. Lethally irradiated adult WT recipient (CD45.1) mice were transplanted with 106 unfractionated Flk2−/− (CD45.2) or WT (CD45.2) BM cells together with 106 unfractionated WT CD45.1 competitor BM cells. (A) Donor-derived PB total (Tot), B-cell, T-cell, and myeloid (M) reconstitution at 16 weeks after transplantation. Mean (SEM) values from 12 to 14 primary recipient mice of each genotype, from 2 independent experiments. At 16 weeks after transplantation recipient mice were killed, and BM cells were counted and analyzed for donor-derived myeloid (Mac-1+) reconstitution (B) as well as donor-derived regeneration of LSK and LSKCD150+ compartments (C). Mean (SEM) values for proportion of donor cells of 12 to 14 mice per genotype, from 2 independent experiments. (D) Test cell–derived PB total, B-, T-, and M-cell reconstitution 24 to 28 weeks after transplantation in secondary WT recipients. Data are expressed as mean (SEM) percentage of test cell reconstitution (9-10 recipient mice per genotype, from 2 independent experiments). ns indicates not significant; **P < .01, ***P < .001.
Discussion
The FLT3 ligand and receptor have received considerable attention in hematology, in part because FLT3-activating mutations are very common in hematologic malignancies, in particular in AML32 but also because FLK2 was identified and cloned as a cytokine tyrosine kinase receptor highly expressed on enriched mouse HSCs.10 When FL was cloned, it also became evident that it through activation of FLT3 was a potent synergistic factor for in vitro growth of enriched HSCs,15,42-44 and FL was also reported to promote ex vivo expansion of mouse HSCs.15,22-26 Because through these findings FL emerged as a candidate key HSC regulator,15 it was unexpected when HSCs in steady state adult BM were found to be unaltered in Fl−/− mice.13 However, several findings, including reports of FLT3 being expressed on cycling HSCs in fetal liver and regenerating BM,20-22 were compatible with FLT3 ligand and receptor perhaps having a distinct role in regulating actively cycling HSCs. Such a scenario could also explain why Flk2−/− BM cells would show reduced reconstituting ability after transplantation into ablated recipients.11
Herein, we demonstrate through studies of Fl−/− and Flk2−/− mice that both FL and FLT3 receptor are dispensable for regulation of HSCs in steady-state adult hematopoiesis in which HSCs are predominantly quiescent or slowly cycling,45,46 as well as during fetal development and after transplantation when HSCs expand extensively.2,3 These findings were corroborated by direct phenotypic analysis of the HSC compartment as well as through functional evaluation of HSC activity. Although our findings of normal HSC homeostasis in Flk2−/− mice might appear to be in conflict with the impaired multilineage reconstitution ability of Flk2−/− BM cells reported by Mackarehtschian et al,11 there were aspects of those studies which in fact would be most compatible with FLT3 being redundant for HSC self-renewal and maintenance. Specifically, in the studies of Mackarehtschian et al11 the hematopoietic activity of Flk2−/− HSCs being transplanted appeared stable over time, and most notably serial transplantation, the hallmark assay for HSC self-renewal, failed to show any further reduction in the ability of Flk2−/− HSCs to multilineage reconstitute secondary recipients.11 Thus, the reduced multilineage reconstitution ability observed on transplantation of Flk2−/− BM cells might rather have reflected a critical role of FLT3 in generation and maintenance of early progenitors, in particular of the lymphoid lineages,11,12,14,19,40 as confirmed here. Notably, the lack of a role of FLT3 receptor and ligand in HSC regulation is distinctly different from that of c-KIT, which has been shown to be critically required for maintenance of HSCs, in particular in HSC expansion after transplantation.6-8
Whereas our current studies show that FLT3 ligand and receptor have little or no role in regulation of mouse HSCs, previous studies have shown that FLT3 is critically involved in regulation of the earliest B- and T-cell progenitors,11-13 and in fact more recent studies also show a role of FLT3 in regulation of LMPPs with combined granulocyte-monocyte, B-cell and T-cell potential but no megakaryocyte-erythroid potential.17,19 Thus, in mice, FLT3 receptor and ligand might act at the very earliest stages in the lineage restriction pathway(s) from HSCs to lymphoid-restricted progenitors. However, it remains possible that FLT3 might be expressed and important already in the HSC compartment in man. There is in fact strong evidence in support of human candidate HSCs expressing FLT3,28-30 in contrast to mouse HSCs that are predominantly FLT3−.16,20
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Jacques Peschon for kindly providing the Fl−/− mice and Drs Ihor Lemischka and Christoph Schaniel for Flk2−/− mice. We also thank Lilian Wittmann, Christina T. Jensen, Kees-Jan Pronk, Stuart Walsh, Hong Qian, and Yanjuan Tang for their expert technical assistance and advice.
This work was supported by grants from The Swedish Paediatric Cancer Society, The Göran Gustafsson Foundation, The Swedish Research Council, and the Juvenile Diabetes Research Foundation (JDRF). E.S. has an assistant professor position from the Swedish Cancer Society. The Lund Stem Cell Center is supported by a Center of Excellence grant from the Swedish Foundation for Strategic Research. S.E.W.J. is supported by an international recruitment award from the Medical Research Council, United Kingdom.
Authorship
Contribution: N.B.-V. and E.S. designed and performed the research, analyzed data, and wrote the paper; M.C., S.D., and H.N.C. performed research and analyzed data; and S.E.W.J. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sten Eirik W. Jacobsen, Haematopoietic Stem Cell Lab, Weatherall Institute of Molecular Medicine, University of Oxford, Headington, Oxford OX3 9DS, United Kingdom; e-mail: sten.jacobsen@imm.ox.ac.uk.
References
Author notes
*S.E.W.J. and E.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal