Abstract
Mutations of the CCAAT/enhancer binding protein alpha (CEBPA) gene have been associated with a favorable outcome in patients with acute myeloid leukemia (AML), but mainly in those with a normal karyotype. Here, we analyzed the impact of associated cytogenetic abnormalities or bad-prognosis fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) in 53 patients with CEBPA+ de novo AML treated in the Acute Leukemia French Association trials. We found that only those with a normal karyotype and no FLT3-ITD displayed the expected favorable outcome. In this context, relapse-free, disease-free, and overall survival were significantly longer than in corresponding patients without the CEBPA mutation (P = .035, .016, and .047, respectively). This was not observed in the context of an abnormal karyotype or associated FLT3-ITD. Furthermore, after adjustment on age, trial, and mutation type, these features were independently predictive of shorter overall survival in the subset of patients with CEBPA+ AML (multivariate hazard ratio = 2.7; 95% confidence interval, 1.08-6.7; and 2.9; 95% confidence interval, 1.01-8.2; with P = .034 and .05, for abnormal karyotype and FLT3-ITD, respectively).
Introduction
The prognosis of patients with acute myeloid leukemia (AML) has been deeply refined because of the impact of new molecular markers. If standard cytogenetics remains a strong factor for both complete remission (CR) achievement and relapse, several gene mutations have been described and demonstrated as significantly influencing the outcome of these patients.1 In this new context, most cooperative groups recommend to screen for some gene mutations at diagnosis, including the fms-like tyrosine kinase 3 (FLT3), the nucleophosmine (NPM1), and the CCAAT/enhancer binding protein alpha (CEBPA) genes.2
We have previously reported that CEBPA mutations are associated with a favorable outcome in younger patients with newly diagnosed AML.3 This was confirmed by other groups, but mainly in patients with cytogenetically normal (CN) AML.4-7 The issue of whether this favorable impact is still observed in patients with AML carrying cytogenetic abnormalities remains open. Another open issue is to determine whether cooperating bad-prognosis FLT3 internal tandem duplication (FLT3-ITD) may alter the prognosis of CEBPA mutated (CEBPA+) AML.3,5,7 Finally, it has been recently reported that only CEBPA double mutations, but not single, might be associated with a good outcome.8 In the present study, we thus analyzed the role of these factors in 53 patients with CEBPA+ AML treated in Acute Leukemia French Association (ALFA) trials.
Methods
A total of 1529 patients (15-70 years of age) with newly diagnosed AML entered the ALFA-9000, ALFA-9801, and ALFA-9802 trials between 1990 and 2006. Trial designs have been already reported.9-11 Briefly, the first ALFA-9000 trial demonstrated the superiority of an intensified timed-sequential over a standard (3 + 7) induction in terms of relapse incidence in the subgroup of patients 50 years of age or younger.9 From 1998, these younger patients were thus treated with timed-sequential induction in the ALFA-9802 trial, followed by a randomization between another timed-sequential consolidation course or the high-dose cytarabine postremission therapy from the Cancer and Leukemia Group B.10 Older patients until the age of 70 years entered the ALFA-9801 study, which tested different anthracyclines, as well as interleukin-2 maintenance.11 Studies were approved by an Institutional Review Board of Hôpital Claude Huriez and conducted with informed consent and in accordance to the Declaration of Helsinki.
Among these 1529 patients, 703 patients were screened for CEBPA mutation and FLT3-ITD (137, 249, and 317 patients from the ALFA-9000, ALFA-9801, and ALFA-9802 study, respectively). Most of themwere also tested for NPM1 mutation. Tested patients had lower median age (P = .01) and higher median white blood cell count (P < .001), but relapse-free survival (RFS), disease-free survival (DFS), and overall survival (OS) did not differ between tested and untested groups after adjustment on trial. Mutation screening was centrally performed according to previously described procedures.3,12,13 CEBPA mutations were classified as (1) single N-terminal stop mutation, (2) single C-terminal in-frame insertion/deletion, (3) double type 1 and 2 mutation, and (4) other single mutation.14 Among these 703 patients, 638 had an informative karyotype (84 favorable-risk, 471 intermediate-risk, and 83 unfavorable-risk according to the British MRC classification)15 and were included in the present study. A karyotype was considered as normal if at least 20 normal marrow metaphases were present without any abnormal marrow metaphases.
Standard statistical methods, including Fisher exact test and Mann-Whitney test, were used. Failure time data, including OS (death as event in all patients), DFS (relapse or death as events in CR patients), and RFS (relapse as event in CR patients) were estimated by the Kaplan-Meier method and then compared by the log-rank test. The multivariate analysis performed in CEBPA+ AML patients included the 3 covariates that were all associated with a P value of .10 or less in univariate analysis for OS, namely, abnormal karyotype, FLT3-ITD, and double versus single CEBPA mutation. Trial and age (as a continuous variable) were also systematically included as explanatory covariates. Analysis was adjusted with the Cox model and tested by the likelihood-ratio test. The proportional hazards assumption was checked for each variable individually. All calculations were performed using the STATA/SE software, version 10.0 (Stata, College Station, TX). The median follow-up was as long as 5 years.
Results and discussion
Among these 638 patients, 53 (8%) had CEBPA+ AML (20 single N-terminal, 7 single C-terminal, 24 double, and 2 other single). A comparison between CEBPA+ and CEBPA wild-type (CEBPA-wt) patients is shown in Table 1. The CR rate was similar in both subgroups. RFS and DFS were longer in CEBPA+ patients, but no significant difference emerged in OS, even after adjustment on trials and/or censoring patients receiving allogeneic stem cell transplantation (SCT) in first CR at SCT time.
Patient characteristics and outcomes
| . | CEBPA+ subset 1 (n = 26) . | CEBPA+ subset 2 (n = 10) . | CEBPA+ subset 3 (n = 17) . | All CEBPA+ (n = 53) . | All CEBPA wt (n = 585) . | P* . |
|---|---|---|---|---|---|---|
| Patient characteristic | ||||||
| Median age, y (range) | 44 (16-62) | 55 (24-64) | 51 (23-64) | 48 (16-64) | 47 (16-70) | .73 |
| Male/female, n | 17/9 | 6/4 | 12/5 | 35/18 | 305/280 | .06 |
| Median WBC, ×109/L (range) | 21 (0.5-250) | 56 (2-170) | 9 (2-96) | 20 (0.5-250) | 13 (0.6-400) | .17 |
| Secondary AML, n (%) | 0 | 0 | 0 | 0 (0%) | 12 (2%) | .61 |
| Cytogenetics, n (%) | ||||||
| Favorable | 0 | 0 | 2 | 2 (4%)† | 82 (14%) | .05 |
| Intermediate | 26 | 10 | 10 | 46 (87%)‡ | 425 (73%) | |
| Unfavorable | 0 | 0 | 5 | 5 (9%)§ | 78 (13%) | |
| CN-AML, n (%) | ||||||
| Yes | 26 | 10 | 0 | 36 (68%) | 285 (49%) | .01 |
| No | 0 | 0 | 17 | 17 (32%) | 300 (51%) | |
| FLT3-ITD, n (%) | ||||||
| Yes | 0 | 10 | 1 | 11 (21%) | 94 (16%) | .44 |
| No | 26 | 0 | 16 | 42 (79%) | 491 (84%) | |
| NPM1 mutation, n (%) | ||||||
| Yes | 3 | 1 | 0 | 4 (7%) | 139 (24%) | .008 |
| No | 19 | 4 | 16 | 39 (74%) | 376 (64%) | |
| Not available | 4 | 5 | 1 | 10 (19%) | 70 (12%) | |
| ALFA trial, n (%) | ||||||
| 9000 | 6 | 5 | 2 | 13 (24%) | 109 (19%) | .53 |
| 9801 | 6 | 4 | 9 | 19 (36%) | 212 (36%) | |
| 9802 | 14 | 1 | 6 | 21 (40%) | 264 (45%) | |
| Patient outcome | ||||||
| Patients, n‖ | 26 | 10 | 15 | 51 | 491 | |
| CR1 induction, n (%) | ||||||
| CR | 23 (88%)¶ | 7 (70%)# | 11 (73%)** | 41 (80%) | 404 (82%) | .54 |
| Refractory AML | 2 | 1 | 2 | 5 (10%) | 58 (12%) | |
| Early death | 1 | 2 | 2 | 5 (10%) | 29 (6%) | |
| Events in CR1, n (%) | ||||||
| Allo-SCT in CR1 | 3 | 0 | 1 | 4 (9%) | 71 (16%) | .27 |
| Relapse | 8 | 4 | 6 | 18 (44%) | 254 (63%) | .03 |
| Death in CR | 0 | 0 | 2 | 2 (5%) | 31 (8%) | .76 |
| 5-year RFS (95% CI) | 62% (38-79)¶ | 43% (10-73)# | 34% (8-63)** | 52% (35-67) | 27% (22-32) | .04 |
| 5-year DFS (95% CI) | 62% (38-79)¶ | 43% (10-73)# | 27% (7-54)** | 50% (33-64) | 24% (19-29) | .03 |
| 5-year OS (95% CI) | 64% (36-82)¶ | 30% (7-58)# | 32% (11-56)** | 47% (31-61) | 31% (27-36) | .11 |
| . | CEBPA+ subset 1 (n = 26) . | CEBPA+ subset 2 (n = 10) . | CEBPA+ subset 3 (n = 17) . | All CEBPA+ (n = 53) . | All CEBPA wt (n = 585) . | P* . |
|---|---|---|---|---|---|---|
| Patient characteristic | ||||||
| Median age, y (range) | 44 (16-62) | 55 (24-64) | 51 (23-64) | 48 (16-64) | 47 (16-70) | .73 |
| Male/female, n | 17/9 | 6/4 | 12/5 | 35/18 | 305/280 | .06 |
| Median WBC, ×109/L (range) | 21 (0.5-250) | 56 (2-170) | 9 (2-96) | 20 (0.5-250) | 13 (0.6-400) | .17 |
| Secondary AML, n (%) | 0 | 0 | 0 | 0 (0%) | 12 (2%) | .61 |
| Cytogenetics, n (%) | ||||||
| Favorable | 0 | 0 | 2 | 2 (4%)† | 82 (14%) | .05 |
| Intermediate | 26 | 10 | 10 | 46 (87%)‡ | 425 (73%) | |
| Unfavorable | 0 | 0 | 5 | 5 (9%)§ | 78 (13%) | |
| CN-AML, n (%) | ||||||
| Yes | 26 | 10 | 0 | 36 (68%) | 285 (49%) | .01 |
| No | 0 | 0 | 17 | 17 (32%) | 300 (51%) | |
| FLT3-ITD, n (%) | ||||||
| Yes | 0 | 10 | 1 | 11 (21%) | 94 (16%) | .44 |
| No | 26 | 0 | 16 | 42 (79%) | 491 (84%) | |
| NPM1 mutation, n (%) | ||||||
| Yes | 3 | 1 | 0 | 4 (7%) | 139 (24%) | .008 |
| No | 19 | 4 | 16 | 39 (74%) | 376 (64%) | |
| Not available | 4 | 5 | 1 | 10 (19%) | 70 (12%) | |
| ALFA trial, n (%) | ||||||
| 9000 | 6 | 5 | 2 | 13 (24%) | 109 (19%) | .53 |
| 9801 | 6 | 4 | 9 | 19 (36%) | 212 (36%) | |
| 9802 | 14 | 1 | 6 | 21 (40%) | 264 (45%) | |
| Patient outcome | ||||||
| Patients, n‖ | 26 | 10 | 15 | 51 | 491 | |
| CR1 induction, n (%) | ||||||
| CR | 23 (88%)¶ | 7 (70%)# | 11 (73%)** | 41 (80%) | 404 (82%) | .54 |
| Refractory AML | 2 | 1 | 2 | 5 (10%) | 58 (12%) | |
| Early death | 1 | 2 | 2 | 5 (10%) | 29 (6%) | |
| Events in CR1, n (%) | ||||||
| Allo-SCT in CR1 | 3 | 0 | 1 | 4 (9%) | 71 (16%) | .27 |
| Relapse | 8 | 4 | 6 | 18 (44%) | 254 (63%) | .03 |
| Death in CR | 0 | 0 | 2 | 2 (5%) | 31 (8%) | .76 |
| 5-year RFS (95% CI) | 62% (38-79)¶ | 43% (10-73)# | 34% (8-63)** | 52% (35-67) | 27% (22-32) | .04 |
| 5-year DFS (95% CI) | 62% (38-79)¶ | 43% (10-73)# | 27% (7-54)** | 50% (33-64) | 24% (19-29) | .03 |
| 5-year OS (95% CI) | 64% (36-82)¶ | 30% (7-58)# | 32% (11-56)** | 47% (31-61) | 31% (27-36) | .11 |
For the CEBPA+ versus CEBPA-wt comparison.
Both had inv(16) AML.
Including 36 normal karyotype, 3 del(9q), 1 trisomy 13, 1 trisomy 8, 1 trisomy 21, 1 abnormality 11q23, and 3 other various single abnormalities.
Including 2 complex karyotypes, 2 monosomy 7, and 1 abnormality 3q.
Patients with favorable cytogenetics as those with secondary AML were not included in outcome estimations and comparisons.
P values: .99, .030, .013, and .035 for CR rate, RFS, DFS, and OS, respectively, compared with the corresponding subset of 221 CEBPA-wt patients with de novo CN-AML and no FLT3-ITD, in whom the CR rate was 87%, and 5-year RFS, DFS, and OS, 29% (22%-37%), 26% (19%-34%), and 37% (30%-45%), respectively.
P = .69, .51, .33, and .85 for CR rate, RFS, DFS, and OS, respectively, compared with the corresponding subset of 59 CEBPA-wt patients with de novo CN-AML and FLT3-ITD, in whom the CR rate was 78%, and 5-year RFS, DFS, and OS, 27% (14%-43%), 23% (11%-37%), and 28% (17%-41%), respectively.
P = .75, .91, .55, and .80 for CR rate, RFS, DFS, and OS, respectively, compared with the corresponding subset of 211 CEBPA-wt patients with de novo AML and non-CBF abnormal karyotype, in whom the CR rate was 78%, and 5-year RFS, DFS, and OS, 23% (15%-31%), 20% (14%-28%), and 26% (20%-33%), respectively.
As expected, CEBPA mutations were more frequently observed in CN or intermediate-risk AML. Nevertheless, almost one-third of CEBPA+ patients had an abnormal karyotype, including 2 patients with favorable core binding factor (CBF) AML. As previously reported,2 CEBPA and NPM1 mutations were relatively mutually exclusive. Conversely, FLT3-ITD was observed in more than 20% of CEBPA+ cases. According to these 2 factors (CN and FLT3-ITD), only 26 of the 53 CEBPA+ patients had CN-AML without FLT3-ITD (subset 1, 49%), whereas 10 had CN-AML with FLT3-ITD (subset 2, 19%) and 17 had an abnormal karyotype without FLT3-ITD in 16 of them (subset 3, 32%). Of note, neither of the 2 CBF-AML had FLT3-ITD. Double CEBPA mutations were mostly observed in CN-AML (53% vs 29%; P = .14) and in subset 1 (58% vs 33%; P = .10), even if these associations were not statistically significant.
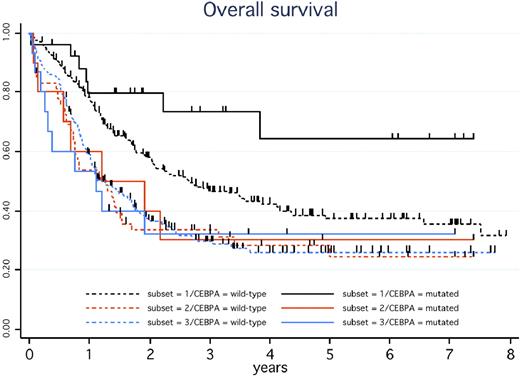
We then analyzed the outcome of these 3 subsets of CEBPA+ patients, but excluding the 2 with CBF-AML. CR rates did not differ significantly (88%, 70%, and 73% in subsets 1, 2, and 3, respectively; P = .33). As indicated in Figure 1, only those CEBPA+ patients with CN-AML and no FLT3-ITD (subset 1) displayed the expected favorable outcome. In the other CEBPA+ patients (subsets 2 and 3), the outcome was closer to that observed in intermediate-risk AML in general. In univariate analysis, single mutation did not significantly influence CR rate, RFS, or DFS, but a trend toward a shorter OS was observed compared with patients with double mutation (36% vs 60% at 5 years, P = .09). In multivariate analysis, only abnormal karyotype (hazard ratio [HR] = 2.7; 95% confidence interval [CI], 1.08-6.7; P = .034) and FLT3-ITD (HR = 2.9; 95% CI, 1.01-8.2; P = .05) were independent bad-prognosis factors for OS.
OS in CEBPA+ versus CEBPA-wt patients according to the presence of a normal karyotype (CN-AML) or FLT3-ITD. In the presence of the CEBPA mutation, estimated 5-year OS was 64% (95% CI, 36%-82%) in the 26 patients with CN-AML and no FLT3-ITD (solid black curve), 30% (95% CI, 7%-58%) in the 10 patients with CN-AML and FLT3-ITD (solid orange curve), and 32% (95% CI, 11%-56%) in the 15 patients with non-CBF abnormal karyotype (solid blue curve), with a hazard ratio of 0.30 (95% CI, 0.12-0.72) in the former compared with the 2 latter patient subsets (P = .004). In these 3 patient subsets, estimated 5-year DFS was 62% (95% CI, 38%-79%), 43% (95% CI, 10%-73%), and 27% (95% CI, 7%-54%), respectively (P = .028 for the former vs 2 latter subsets comparison), whereas estimated 5-year RFS was 62% (95% CI, 38%-79%), 43% (95% CI, 10%-73%), and 34% (95% CI, 8%-63%), respectively (P = .07 for the former vs 2 latter subsets). In the context of de novo CN-AML without FLT3-ITD, estimated 5-year OS in the 221 patients without CEBPA mutation (dashed black curve) was 37% (95% CI, 30%-45%), which significantly differed from the solid black curve (P = .035). In the context of de novo CN-AML with FLT3-ITD, estimated 5-year OS in the 59 patients without CEBPA mutation (dashed orange curve) was 28% (95% CI, 17%-41%), not significantly different from the solid orange curve (P = .85). In the context of de novo AML with non-CBF abnormal karyotype, estimated 5-year OS in the 211 patients without CEBPA mutation (dashed blue curve) was 26% (95% CI, 20%-33%), not significantly different from the solid blue curve (P = .80). Patients with favorable CBF-AML as well as those with secondary AML were not included in these estimations.
OS in CEBPA+ versus CEBPA-wt patients according to the presence of a normal karyotype (CN-AML) or FLT3-ITD. In the presence of the CEBPA mutation, estimated 5-year OS was 64% (95% CI, 36%-82%) in the 26 patients with CN-AML and no FLT3-ITD (solid black curve), 30% (95% CI, 7%-58%) in the 10 patients with CN-AML and FLT3-ITD (solid orange curve), and 32% (95% CI, 11%-56%) in the 15 patients with non-CBF abnormal karyotype (solid blue curve), with a hazard ratio of 0.30 (95% CI, 0.12-0.72) in the former compared with the 2 latter patient subsets (P = .004). In these 3 patient subsets, estimated 5-year DFS was 62% (95% CI, 38%-79%), 43% (95% CI, 10%-73%), and 27% (95% CI, 7%-54%), respectively (P = .028 for the former vs 2 latter subsets comparison), whereas estimated 5-year RFS was 62% (95% CI, 38%-79%), 43% (95% CI, 10%-73%), and 34% (95% CI, 8%-63%), respectively (P = .07 for the former vs 2 latter subsets). In the context of de novo CN-AML without FLT3-ITD, estimated 5-year OS in the 221 patients without CEBPA mutation (dashed black curve) was 37% (95% CI, 30%-45%), which significantly differed from the solid black curve (P = .035). In the context of de novo CN-AML with FLT3-ITD, estimated 5-year OS in the 59 patients without CEBPA mutation (dashed orange curve) was 28% (95% CI, 17%-41%), not significantly different from the solid orange curve (P = .85). In the context of de novo AML with non-CBF abnormal karyotype, estimated 5-year OS in the 211 patients without CEBPA mutation (dashed blue curve) was 26% (95% CI, 20%-33%), not significantly different from the solid blue curve (P = .80). Patients with favorable CBF-AML as well as those with secondary AML were not included in these estimations.
In the context of de novo CN-AML without FLT3-ITD, RFS, DFS, and OS were significantly longer in the 26 CEBPA+ patients than in the 221 corresponding CEBPA-wt patients (subset 1; P = .030, .013, and .035, respectively). This positive impact of CEBPA mutations was no longer observed in de novo CN-AML patients with FLT3-ITD (subset 2; P = .51, .33, and .85, respectively) or in those with de novo AML with non-CBF chromosomal abnormalities (subset 3; P = .91, .55, and .80, respectively; Figure 1). Again, all these results remained unchanged after adjustment on trials and/or censoring patients receiving allogeneic SCT in first CR at transplantation time.
We think that, despite the selection biases and the limited number of CEBPA+ patients analyzed in the present study, these results suggest that CEBPA mutations are predictive of a favorable outcome mostly in patients with a normal karyotype and no FLT3-ITD. Awaiting results from larger studies or overviews, it seems reasonable to consider patients with CEBPA+ AML but either an abnormal karyotype or FLT3-ITD as intermediate-risk patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Regional Clinical Research Offices of Paris Ile-de-France and Lyon, the Medical Biostatistics Departement of the Hôpital Saint-Louis, Paris, for their implication in ALFA trials sponsoring and monitoring, and Prof Sylvie Chevret for her statistical input and Karine Celli-Lebras for her indispensable help. All authors are members of the ALFA Scientific Board.
This work was supported by the Fondation de France (Leukemia Committee) and by the Canceropoles Ile-de-France and Nord-Ouest.
Authorship
Contribution: A.R., N.G., D.N., C.B., O.N., and C. Preudhomme performed mutation screening and validation; C. Pautas, X.T., and S.C. were principal investigators of the ALFA-9801, ALFA-9802, and ALFA-9000 trials, respectively; N.B., S.d.B., C. Pautas, O.R., S.C., X.T., C.G., and H.D. enrolled patients; C.T. centrally reviewed all cytogenetic results; and N.B., C.G., and H.D. analyzed the data and wrote the manuscript. All authors approved the study design and the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of ALFA investigators who participated in the 9000, 9801, and 9802 studies appears in the online Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Hervé Dombret, Department of Hematology, Hôpital Saint-Louis, 1 avenue Claude Vellefaux, 75010 Paris, France; e-mail: herve.dombret@sls.aphp.fr.
References
Author notes
*A.R. and N.B. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal