The transcription factor c-Myb and coregulator p300 have a key role in maintaining production of controlled numbers of megakaryocytes and platelets. In mice, mutations in c-Myb or p300 cause thrombocytosis in otherwise wild-type animals and can ameliorate the thrombocytopenia in mice lacking the thrombopoietin receptor, c-Mpl, a model for human congenital amegakaryocytic thrombocytopenia. To examine whether inhibition of c-Myb/p300 is effective in other models of thrombocytopenia, the effect of the c-MybPlt4 mutation on thrombocytopenia associated with reduced platelet life span in Bcl-XPlt20/Plt20 mice was assessed, as were responses in c-MybPlt4 and/or p300Plt6 mutant mice to thrombocytopenia associated with antiplatelet antibodies, chemotherapy, or bone marrow transplantation. Homozygosity of the c-MybPlt4 allele ameliorated thrombocytopenia associated with reduced platelet life span, and c-MybPlt4/+ mice exhibited more rapid than normal recovery from thrombocytopenia caused by antiplatelet serum or bone marrow transplantation. Recovery to pretreatment platelet levels was unaltered in 5-fluorouracil–treated c-MybPlt4/+ mice relative to wild-type controls, but enhanced platelet production during subsequent thrombocytosis was evident. More modest enhancement of platelet recovery after 5-fluorouracil or bone marrow transplantation was also evident in p300Plt6/+ animals. The data suggest potential utility of c-Myb/p300 as a target for therapeutic intervention in thrombocytopenia of diverse origins.
Introduction
Thrombocytopenia, or low circulating platelet count, can be life-threatening when associated with bleeding episodes and requires careful clinical management. On rare occasions, thrombocytopenia occurs congenitally; several inherited mutations in genes encoding platelet surface molecules, transcription factors, cytoskeletal components, or cytokine receptors have been linked with reduced or dysmorphic megakaryocytopoiesis.1 More commonly, thrombocytopenia is associated with autoimmune or hematologic diseases, including idiopathic thrombocytopenia purpura and myelodysplastic syndrome. Dangerously low platelet counts may also occur in patients undergoing cytotoxic therapy for cancer, not only creating acute risk of hemorrhage but also often preventing ongoing treatment.2 To date, the only effective acute treatment for thrombocytopenia is platelet transfusion, which provides immediate relief but can be associated with immunologic sequelae and risk of infection.2
We have used large-scale N-ethyl-N-nitrosourea (ENU) mutagenesis screens to isolate mutations that elevate platelet counts in c-Mpl−/− mice, a model of human congenital amegakaryocytic thrombocytopenia.3,4 Using this strategy, we have isolated alleles of the transcription factor c-Myb, its coactivator p300, and the epigenetic regulator Suz12, which suppress c-Mpl−/− platelet deficiency,5,,–8 implying that pharmacologic modulation of these proteins may benefit thrombocytopenia. To explore further the potential benefit of modulating c-Myb/p300 in the context of low platelet count, we have examined the effect of c-Myb or p300 mutations on thrombocytopenia associated with reduced platelet life span, as well as platelet recovery after immune or cytotoxic drug-induced thrombocytopenia or after bone marrow transplantation. We show that, in addition to ameliorating the thrombocytopenia associated with c-Mpl deficiency, mutation in c-Myb elevates platelet counts in Bcl-x mutant mice. Moreover, on a wild-type background where mutation of one allele of Myb or p300 does not significantly elevate steady-state platelet number, there is amelioration of immune or bone marrow transplantation-induced thrombocytopenia. These data suggest that pharmaceutical inhibition of the c-Myb/p300 complex may be useful in elevating platelet production and that even modest reductions in c-Myb/p300 activity may have benefit in thrombocytopenia induced as a side effect of cancer therapy.
Methods
Mice
Hematology
Peripheral blood was collected from the retro-orbital sinus and analyzed using the Advia 2120 hematology system (Bayer, Tarrytown, NY). Megakaryocyte counts were performed by manual counting from histologic sections of sternum and spleen after staining with hematoxylin and eosin. Ten or more high-power fields were scored. Clonal cultures of hematopoietic cells were performed as described.3 Briefly, cultures of 2.5 × 104 adult bone marrow cells or 5 × 104 spleen cells in 1 mL of 0.3% agar in Dulbecco modified Eagle medium supplemented with newborn calf serum (20%) were stimulated with a cocktail of 100 ng/mL murine stem cell factor, 10 ng/mL murine interleukin-3, and 4 U/mL human erythropoietin and incubated for 7 days at 37°C in a fully humidified atmosphere of 10% (vol/vol) CO2 in air. Agar cultures were fixed, stained for acetylcholinesterase, and then with Luxol fast blue and hematoxylin, and the cellular composition of each colony was determined at ×100 to ×400 magnification.
Bone marrow transplantation
Recipient wild-type, c-MybPlt4/+, or p300Plt6/+ mice were irradiated with 11 Gy γ-irradiation split into 2 equal doses given several hours apart and then injected intravenously with 2 × 105 genotype-matched bone marrow cells. All recipient mice were maintained on antibiotic (neomycin sulfate at 1.1 g/L in the drinking water).
Induction of thrombocytopenia
Hematopoietic recovery was assessed in mice after a single injection of 5-fluorouracil (5-FU; 150 mg/kg) administered intraperitoneally. Immune thrombocytopenia was induced by administration of rabbit anti–mouse platelet serum (Inter-Cell Technologies, Hopewell, NJ). The serum was diluted 1:30 in phosphate-buffered saline and 100 μL was injected intravenously as a single dose.
Results
Mutation in c-Myb ameliorates Bcl-xL–deficient thrombocytopenia
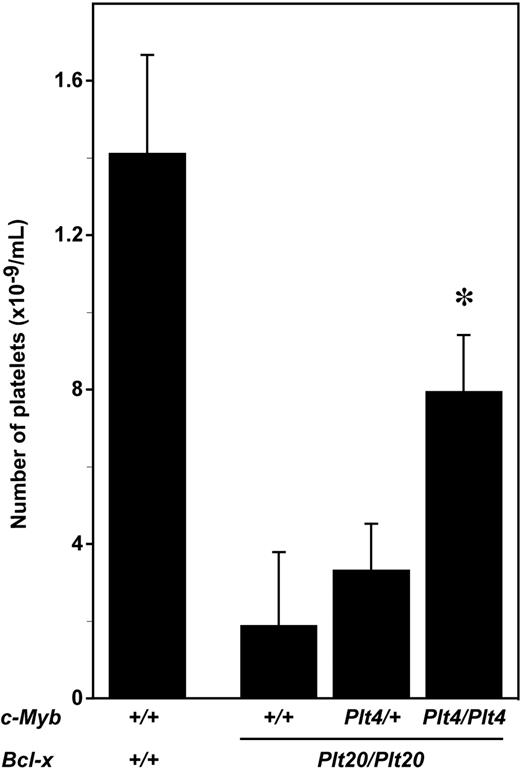
To determine whether disruption of c-Myb/p300 could ameliorate the thrombocytopenia associated with reduced platelet life span, mice with the c-MybPlt4 and Bcl-xPlt20 mutations were intercrossed. Consistent with previous data demonstrating the requirement for Bcl-xL in maintaining platelet survival,9 c-Myb+/+Bcl-xPlt20/Plt20 mutant mice exhibited thrombocytopenia, with platelet counts approximately 15% that observed in wild-type mice (Figure 1). This platelet deficiency was ameliorated in c-MybPlt4/Plt4Bcl-xPlt20/Plt20 mice, with 4-fold increase in platelet counts relative to c-Myb+/+Bcl-xPlt20/Plt20 controls (Figure 1).
Mutation in c-Myb ameliorates thrombocytopenia in Bcl-x mutant mice. Platelet counts in wild-type mice (c-Myb+/+Bcl-x+/+) or mice homozygous for the Plt20 allele of Bcl-x (Plt20/Plt20) and wild-type (+/+), heterozygous (Plt4/+), or homozygous (Plt4/Plt4) for the c-MybPlt4 allele. The mean ± SD of platelet counts from 4 to 14 mice per genotype are shown. *P < .05 in Student t test for comparison of data from c-MybPlt4/Plt4 Bcl-xPlt20/Plt20 mice with that of c-Myb+/+Bcl-x Plt20/Plt20 controls.
Mutation in c-Myb ameliorates thrombocytopenia in Bcl-x mutant mice. Platelet counts in wild-type mice (c-Myb+/+Bcl-x+/+) or mice homozygous for the Plt20 allele of Bcl-x (Plt20/Plt20) and wild-type (+/+), heterozygous (Plt4/+), or homozygous (Plt4/Plt4) for the c-MybPlt4 allele. The mean ± SD of platelet counts from 4 to 14 mice per genotype are shown. *P < .05 in Student t test for comparison of data from c-MybPlt4/Plt4 Bcl-xPlt20/Plt20 mice with that of c-Myb+/+Bcl-x Plt20/Plt20 controls.
Hematology in c-Mpl+/+c-MybPlt4/+ and c-Mpl+/+p300Plt6/+ mice
To explore further the utility of c-Myb/p300 inhibition for treatment of low platelet counts, methods of induced thrombocytopenia that model immune destruction or cytotoxic cancer treatments were established. Mice heterozygous for mutations in c-Myb or p300 on a wild-type genetic background were chosen for these studies. Whereas mice heterozygous for mutations in c-Myb or p300 on a c-Mpl−/− background exhibit higher platelet counts relative to unmutated c-Mpl−/− controls,5,6 normal circulating platelet numbers were observed in c-MybPlt4/+ and p300Plt6/+ mice on a C57BL/6 c-Mpl+/+ background (Table 1). Indeed, in all hematologic parameters examined, c-MybPlt4/+ and p300Plt6/+ mice were not significantly different from wild-type animals. Hematocrit and red blood cell count, white blood cell number, and relative proportions of lymphocytes, granulocytes, monocytes, and eosinophils were all within the normal range (Table 1). Consistent with the normal platelet counts, numbers of morphologically recognizable megakaryocytes in the bone marrow and spleens of c-MybPlt4/+ and p300Plt6/+ mice were no different from wild-type (Table 1), and the numbers of megakaryocyte progenitors were also normal (data not shown). Similarly, the total number of hematopoietic progenitor cells was unaltered, as was the distribution of clonogenic cells committed to production of myeloid cell lineages (data not shown). Thus, the responses of c-MybPlt4/+ and p300Plt6/+ mice to thrombocytopenia were examined to allow the impact of inhibition of c-Myb/p300 to be assessed in the absence of grossly abnormal hematopoiesis.
Hematologic profile of Plt4/+ and Plt6/+ mutant mice
| . | Genotype . | ||
|---|---|---|---|
| +/+ . | Plt4/+ . | Plt6/+ . | |
| Peripheral blood (Advia) | n = 13 | n = 9 | n = 16 |
| Platelet count, ×109/L | 1093 ± 133 | 1157 ± 167 | 1166 ± 185 |
| Red cell count, ×1012/L | 10.9 ± 0.4 | 10.9 ± 0.3 | 10.8 ± 0.4 |
| Hematocrit, % | 49.6 ± 1.4 | 49.3 ± 1.2 | 50.2 ± 1.4 |
| White cell count, ×109/L | 9.9 ± 1.8 | 6.9 ± 2.5 | 8.8 ± 2.1 |
| Neutrophils | 0.90 ± 0.20 | 0.64 ± 0.26 | 0.93 ± 0.18 |
| Lymphocytes | 8.4 ± 1.6 | 5.8 ± 2.1 | 7.2 ± 1.9 |
| Monocytes | 0.12 ± 0.05 | 0.08 ± 0.04 | 0.11 ± 0.03 |
| Eosinophils | 0.19 ± 0.05 | 0.18 ± 0.07 | 0.23 ± 0.11 |
| Bone marrow | n = 4 | n = 4 | n = 4 |
| Megakaryocytes (per 10 hpf)* | 77 ± 9 | 87 ± 18 | 73 ± 12 |
| Spleen | n = 4 | n = 4 | n = 4 |
| Megakaryocytes (per 10 hpf)* | 20 ± 7 | 24 ± 18 | 49 ± 18 |
| . | Genotype . | ||
|---|---|---|---|
| +/+ . | Plt4/+ . | Plt6/+ . | |
| Peripheral blood (Advia) | n = 13 | n = 9 | n = 16 |
| Platelet count, ×109/L | 1093 ± 133 | 1157 ± 167 | 1166 ± 185 |
| Red cell count, ×1012/L | 10.9 ± 0.4 | 10.9 ± 0.3 | 10.8 ± 0.4 |
| Hematocrit, % | 49.6 ± 1.4 | 49.3 ± 1.2 | 50.2 ± 1.4 |
| White cell count, ×109/L | 9.9 ± 1.8 | 6.9 ± 2.5 | 8.8 ± 2.1 |
| Neutrophils | 0.90 ± 0.20 | 0.64 ± 0.26 | 0.93 ± 0.18 |
| Lymphocytes | 8.4 ± 1.6 | 5.8 ± 2.1 | 7.2 ± 1.9 |
| Monocytes | 0.12 ± 0.05 | 0.08 ± 0.04 | 0.11 ± 0.03 |
| Eosinophils | 0.19 ± 0.05 | 0.18 ± 0.07 | 0.23 ± 0.11 |
| Bone marrow | n = 4 | n = 4 | n = 4 |
| Megakaryocytes (per 10 hpf)* | 77 ± 9 | 87 ± 18 | 73 ± 12 |
| Spleen | n = 4 | n = 4 | n = 4 |
| Megakaryocytes (per 10 hpf)* | 20 ± 7 | 24 ± 18 | 49 ± 18 |
Data are mean plus or minus SD.
hpf indicates high-power field (×600 for bone marrow, ×200 for spleen).
Determined from histologic sections.
Recovery from APS- or 5-FU–induced thrombocytopenia in c-MybPlt4/+ mice
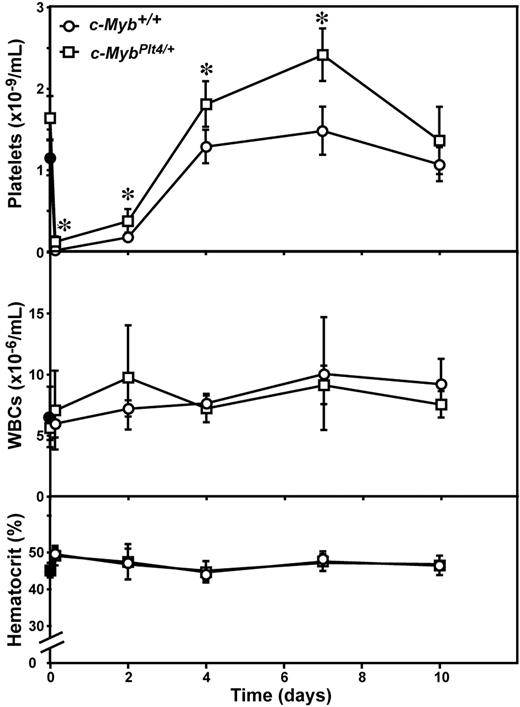
As previously reported,10,–12 single injection of antiplatelet serum (APS) in wild-type mice resulted in severe thrombocytopenia evident as early as 4 hours after administration (Figure 2). Recovery commenced within 2 days; and by 4 days after APS, the number of circulating platelets had recovered to the normal range. A mild thrombocytosis followed, peaking at 7 days after APS, after which platelet numbers returned to baseline levels by day 10 (Figure 2). As in control mice, in c-MybPlt4/+ mice treated with APS, a severe acute thrombocytopenia was evident; however, the platelet nadir in c-MybPlt4/+ mice was modestly but significantly less severe than in wild-type controls and recovery was more rapid and the peak platelet counts were higher (Figure 2). These data show that platelet production during recovery from immune-mediated thrombocytopenia is enhanced in c-MybPlt4/+ mice relative to wild-type animals. There were no significant changes in white blood cell count or hematocrit in either c-Myb+/+ or c-MybPlt4/+ mice over the course of the experiment (Figure 2).
Enhanced platelet recovery in c-MybPlt4/+ mice after APS-induced thrombocytopenia. Platelet count (top panel), white blood cell count (middle panel), and hematocrit (bottom panel) in c-MybPlt4/+ (squares) or wild-type (c-Myb+/+, circles) control mice were determined after administration of APS. Data points represent the mean ± SD from separate cohorts of mice analyzed at the times indicated. *P < .05 in Student t test for comparison of data from c-MybPlt4/+ mice with that of c-Myb+/+ controls. n = 2 (filled symbols) or 3 to 7 mice (open symbols) per point.
Enhanced platelet recovery in c-MybPlt4/+ mice after APS-induced thrombocytopenia. Platelet count (top panel), white blood cell count (middle panel), and hematocrit (bottom panel) in c-MybPlt4/+ (squares) or wild-type (c-Myb+/+, circles) control mice were determined after administration of APS. Data points represent the mean ± SD from separate cohorts of mice analyzed at the times indicated. *P < .05 in Student t test for comparison of data from c-MybPlt4/+ mice with that of c-Myb+/+ controls. n = 2 (filled symbols) or 3 to 7 mice (open symbols) per point.
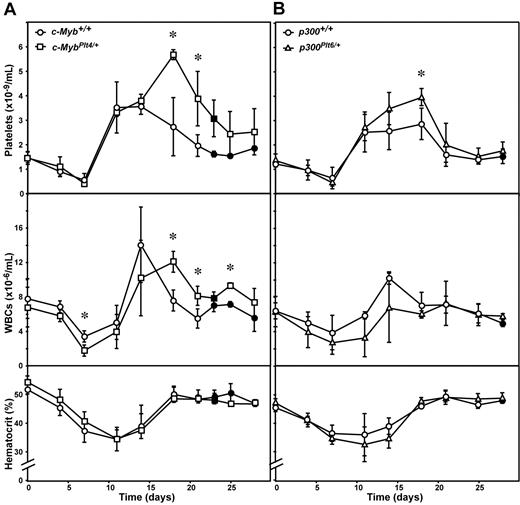
As previously described,12,13 administration of 5-FU in wild-type mice caused a 50% reduction in circulating platelet number with recovery to normal values 7 to 10 days after injection. As is characteristic with this agent, a significant thrombocytosis followed, with platelet numbers finally returning to baseline 3 to 4 weeks after treatment (Figure 3A). The thrombocytopenia resulting from exposure to 5-FU was indistinguishable in c-MybPlt4/+ mice, with the kinetics and extent of the platelet nadir, as well as the time for recovery, similar to that in c-Myb+/+ controls. However, the subsequent thrombocytosis was protracted in 5-FU–treated c-MybPlt4/+ mice, and the peak platelet counts were up to 2-fold higher than observed in wild-type mice (Figure 3A). The effects of 5-FU on circulating white and red blood cells were similar in c-MybPlt4/+ mice compared with responses in c-Myb+/+ mice, although a modest delay in recovery of white blood cell numbers was evident (Figure 3A).
Recovery in c-MybPlt4/+ and p300Plt6/+ mice after 5-FU–induced thrombocytopenia. Platelet count (top panel), white blood cell count (middle panel), and hematocrit (bottom panel) in (A) c-MybPlt4/+ (squares) or wild-type (c-Myb+/+, circles) mice, and (B) p300Plt6/+ (triangles) or wild-type (p300+/+, circles) mice were determined after administration of 5-FU. Data points represent the mean ± SD from separate cohorts of mice analyzed at the times indicated. *P < .05 in Student t test for comparison of data from c-MybPlt4/+ mice with that of c-Myb+/+ controls, or p300Plt6/+ mice with that of p300+/+ controls. n = 2 (closed symbols) or 3 to 12 mice (open symbols) per point.
Recovery in c-MybPlt4/+ and p300Plt6/+ mice after 5-FU–induced thrombocytopenia. Platelet count (top panel), white blood cell count (middle panel), and hematocrit (bottom panel) in (A) c-MybPlt4/+ (squares) or wild-type (c-Myb+/+, circles) mice, and (B) p300Plt6/+ (triangles) or wild-type (p300+/+, circles) mice were determined after administration of 5-FU. Data points represent the mean ± SD from separate cohorts of mice analyzed at the times indicated. *P < .05 in Student t test for comparison of data from c-MybPlt4/+ mice with that of c-Myb+/+ controls, or p300Plt6/+ mice with that of p300+/+ controls. n = 2 (closed symbols) or 3 to 12 mice (open symbols) per point.
Recovery from bone marrow transplantation in c-MybPlt4/+ mice
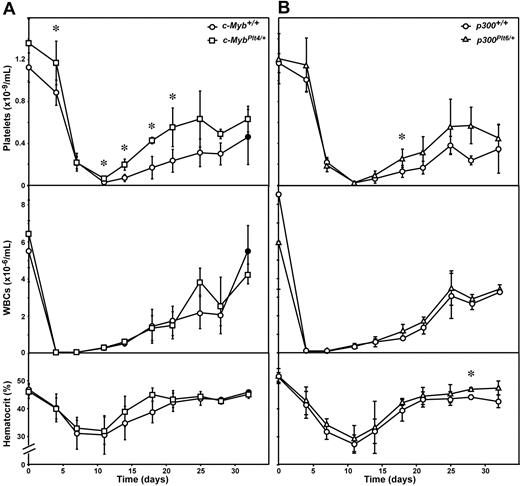
Recovery from thrombocytopenia associated with bone marrow transplantation was assessed by irradiation of c-MybPlt4/+ mutant mice followed by intravenous injection of 2 × 105 genotype-matched bone marrow cells. In wild-type controls, irradiation and transplantation resulted in more severe thrombocytopenia than observed after 5-FU injection, with platelet counts falling to less than 5% of pretransplantation levels within 2 weeks, followed by gradual recovery. c-MybPlt4/+ transplantation recipients also suffered severe thrombocytopenia, but the platelet count at nadir was modestly higher than c-Myb+/+ controls and the rate of platelet recovery was accelerated. The augmented recovery meant that c-MybPlt4/+ mice were severely thrombocytopenic (platelet count < 200 × 109/L) for a period 7 days shorter than their c-Myb+/+ counterparts (Figure 4A). Significant reductions in circulating white and red cells also followed transplantation, but the kinetics and magnitude of the changes were no different between c-MybPlt4/+ and wild-type mice (Figure 4A).
Recovery in c-MybPlt4/+ and p300Plt6/+ mice after bone marrow transplantation. Platelet count (top panel), white blood cell count (middle panel), and hematocrit (bottom panel) in (A) c-MybPlt4/+ (squares) or wild-type (c-Myb+/+, circles) mice, and (B) p300Plt6/+ (triangles) or wild-type (p300+/+, circles) mice were determined after whole body irradiation (11 Gy) and intravenous transfusion of 2 × 105 isogenic bone marrow cells. Data points represent the mean ± SD from separate cohorts of mice analyzed at the times indicated. *P < .05 in Student t test for comparison of data from c-MybPlt4/+ mice with that of c-Myb+/+ controls, or p300Plt6/+ mice with that of p300+/+ controls. n = 2 (closed symbols) or 3 to 8 mice (open symbols) per point.
Recovery in c-MybPlt4/+ and p300Plt6/+ mice after bone marrow transplantation. Platelet count (top panel), white blood cell count (middle panel), and hematocrit (bottom panel) in (A) c-MybPlt4/+ (squares) or wild-type (c-Myb+/+, circles) mice, and (B) p300Plt6/+ (triangles) or wild-type (p300+/+, circles) mice were determined after whole body irradiation (11 Gy) and intravenous transfusion of 2 × 105 isogenic bone marrow cells. Data points represent the mean ± SD from separate cohorts of mice analyzed at the times indicated. *P < .05 in Student t test for comparison of data from c-MybPlt4/+ mice with that of c-Myb+/+ controls, or p300Plt6/+ mice with that of p300+/+ controls. n = 2 (closed symbols) or 3 to 8 mice (open symbols) per point.
Recovery from thrombocytopenia in p300Plt6/+ mice
The transcriptional regulatory activity of c-Myb in megakaryocytopoiesis is dependent on interaction with the coactivator p300.14 Consistent with a profound role for the c-Myb/p300 complex in regulation of platelet production, as for mutations in c-Myb, mutation in p300 ameliorates c-Mpl−/− thrombocytopenia.6 To complement the analysis of recovery from induced thrombocytopenia in c-Myb mutant mice, responses in p300Plt6/+ mice were also examined. Like that observed in c-MybPlt4/+ mice, the kinetics and degree of thrombocytopenia resulting from exposure to 5-FU were indistinguishable in p300Plt6/+ and wild-type mice. A more profound thrombocytosis was evident in p300Plt6/+ mice after 5-FU treatment, although the duration and peak platelet counts in these mice were not as pronounced as observed in c-MybPlt4/+ mutants (Figure 3B). Similar observations were made in p300Plt6/+ mice recovering from bone marrow transplantation. Although the rate of platelet recovery in p300Plt6/+ mice was greater than wild-type controls, it was not as significant as in c-MybPlt4/+ mice (Figure 4B). No significant differences in recovery of white blood cells and red blood cells (hematocrit) were observed in p300Plt6/+ mice recovering from 5-FU treatment or bone marrow transplantation, relative to controls (Figures 3B, 4B).
Discussion
An understanding of the molecular and biologic regulation of megakaryocyte and platelet homeostasis underpins development of new therapeutics for thrombocytopenia. The identification of soluble regulators that stimulate megakaryocytopoiesis has led to clinical evaluation and use of cytokines and their mimetics or related agents, most notably thrombopoietin and interleukin-11.15,16 Studies in mouse models have provided profound insights into the molecular regulation of megakaryocyte and platelet homeostasis and identified potential new therapeutic strategies. For example, in vivo administration of stromal derived factor 1 or fibroblast growth factor 4, via adenoviral infection or injection of recombinant protein, can ameliorate chemotherapy-induced thrombocytopenia.17 Conversely, the absence of PF4 in knockout mice or administration of anti-PF4 antibodies reduced the time of platelet recovery after exposure to 5-FU,18 implying that manipulation of specific chemokine activities may be beneficial in thrombocytopenia. Administration of a peroxisome proliferation-activated receptor-γ ligand was also shown to accelerate platelet recovery after radiation exposure, suggesting that manipulation of megakaryocyte redox status is an alternative route for therapeutic consideration.19
Because most drugs act by inhibiting their targets and most ENU-induced mutations cause loss of gene function, we have exploited large-scale ENU mutagenesis screens to isolate mutations that ameliorate thrombocytopenia in c-Mpl−/− mice as a means of identifying new biologically validated drug targets. Using this strategy, we have isolated alleles of the transcription factor c-Myb and its coactivator p300 that suppress c-Mpl−/− platelet deficiency.5,–7 The capacity of mutations in c-Myb and p300 to ameliorate thrombocytopenia in c-Mpl−/− mice implies that pharmacologic modulation of these proteins may benefit thrombocytopenia.
Although the c-Mpl−/− mouse is an excellent model of congenital thrombocytopenia associated with failure of megakaryocyte production, the inherited thrombocytopenias are relatively rare in comparison with thrombocytopenia associated with autoimmunity or chemotherapy. To assess the potential of modulating c-Myb/p300 in other noncongenital thrombocytopenic contexts, we examined platelet recovery in mice carrying mutations in c-Myb or p300 in models of thrombocytopenia associated with reduced platelet life span, antiplatelet antibodies, or after chemotherapy or bone marrow transplantation. Homozygosity of the c-MybPlt4 allele ameliorated the thrombocytopenia associated with reduced platelet life span in mice also carrying mutations in Bcl-x. c-MybPlt4/+ mice displayed accelerated recovery from reduced platelet counts secondary to APS treatment or myeloablative bone marrow transplantation. In addition, although the time to recovery to normal platelet levels was unaltered, enhanced platelet production after thrombocytopenia induced by 5-FU chemotherapy was also observed in c-MybPlt4/+ mice. Although to a reduced extent relative to c-MybPlt4/+ mice, similar responses to 5-FU and bone marrow transplantation were also evident in p300Plt6/+ mice. Together, these data are consistent with potential utility of c-Myb/p300 as a target in thrombocytopenia of diverse origins. In the models of induced thrombocytopenia, mice heterozygous for mutation in c-Myb or p300 were used to allow the impact of inhibition of c-Myb/p300 to be assessed in the absence of the grossly abnormal hematopoiesis present in mice homozygous for these mutations. Because the heterozygous mice probably retain significant c-Myb/p300 activity, approaches that further reduce the function of this complex below these levels may yield still improved responses.
The c-Myb/p300 complex is not only required to restrain megakaryocyte and platelet production but is positively required for production of lymphocytes, red blood cells, and eosinophils, as evidenced by reduced numbers of these cells in mouse mutants.5,6,14,20,,,,–25 A potential side effect of therapeutic inhibition of c-Myb/p300 might therefore be cytopenia within these hematopoietic lineages. Interestingly, only a very moderate reduction in recovery of white blood cells was observed in c-MybPlt4/+ mice after 5-FU treatment. Recovery of white blood cells after bone marrow transplantation was normal in MybPlt4/+ mice, as was recovery of red blood cells after both treatments. No changes in recovery of red or white blood cells accompanied responses in p300Plt6/+ mice. Consistent with this observation, our previous studies suggested that regulation of platelet number may be more sensitive to changes in c-Myb/p300 activity than erythropoiesis and lymphopoiesis.6 Thus, these observations not only imply that c-Myb/p300 may be an effective therapeutic target but further suggest that a level of inhibition of c-Myb/p300 may be achievable that promotes platelet recovery from thrombocytopenia without significant deleterious effects on other blood cell lineages.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kelly Trueman, Jason Corbin, Janelle Lochland, Craig Hyland, and Katya Henley for expert technical assistance.
This work was supported by the Australian National Health and Medical Research Council (Canberra, Australia; Program Grant 461219, Project Grant 461247, Fellowships, D.J.H., W.S.A.; and Independent Research Institutes Infrastructure Support Scheme Grant 361646), an Australian Research Council (Canberra, Australia) Fellowship (B.T.K.), the National Heart, Lung, and Blood Institute (Bethesda, MD; RO1 grant HL080019), MuriGen Pty, Ltd (Melbourne, Australia; collaborative research grant), the Australian Cancer Research Fund, and a Victorian State Government (Melbourne, Australia) Operational Infrastructure Support grant.
National Institutes of Health
Authorship
Contribution: D.J.H., B.T.K., and W.S.A. designed and performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors hold shares in and/or are consultants to a company developing intellectual property derived from this study.
Correspondence: Warren S. Alexander, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, 3050, Australia; e-mail: alexandw@wehi.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal