The small GTPase Cdc42 is a key regulator of cell polarity. In this issue of Blood, Lämmermann and colleagues show that DCs without Cdc42 are still able to migrate fairly efficiently on 2-dimensional surfaces but become irreversibly entangled in 3-dimensional environments, both in vitro and in vivo.
Dendritic cells (DCs) are critical for the initiation of adaptive immune responses by taking up antigen in the periphery, such as skin, to present it to lymphocytes passing through draining peripheral lymph nodes (PLNs). To perform this task efficiently, activated DCs switch their sessile sampling behavior to a highly migratory one, characterized by the acquisition of a polarized phenotype and increased expression of the chemokine receptor CCR7, which responds to its ligands CCL19 and CCL21.1 These changes are prerequisites for efficient DC migration into afferent lymphatic vessels, which secrete CCR7 ligands and serve as a communication highway to draining PLNs.1
Small GTPases of the Rho and Ras families are key components of the induction and maintenance of a polarized phenotype and migration. Rac and Rho are involved in lamellipodia formation and uropod retraction, respectively. The Rho family member Cdc42 plays a role in induction and maintenance of polarity in various cell types, such as neutrophils and macrophages, in part through stabilization of the leading edge lamellipodia.2 It remains unclear, however, how Cdc42 affected DC motility.
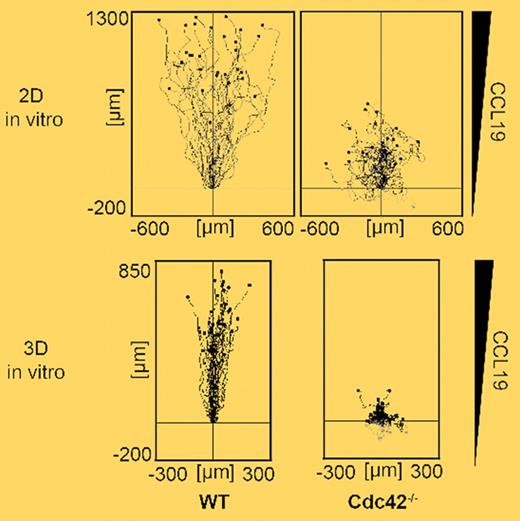
In this issue of Blood, Lämmermann et al report their findings on the role of Cdc42 during physiologic DC migration obtained in a series of elegant in vitro and in vivo assays.3 Using primary mouse DCs derived from Cdc42-deficient bone marrow cultures, the authors investigate the migratory properties of these cells on 2-dimensional surfaces. Despite defects in maintaining polarity, Cdc42-deficient DCs still managed to migrate toward an increasing concentration of CCL19, with only slightly reduced migration velocities as compared with wild-type DCs (see top panel of figure). The residual migratory capacity was likely due to largely intact Rac-induced spreading and lamellipodia formation. Thus, on 2-dimensional surfaces, Cdc42 was not absolutely required for directed cell motility.
In a second set of experiments, Lämmermann et al examine the importance of Cdc42 during DC migration in geometrically more complex environments, that is, the 3-dimensional fibrillar networks of collagen matrices in vitro and dermis in vivo. Somewhat unexpectedly, DCs lacking Cdc42 were strongly impaired in their directed motility in 3-dimensional environments in vitro (see bottom panel of figure). Similarly, Cdc42-deficient DCs were entirely blocked in their migration from skin to draining PLNs, due to impaired entry into afferent lymphatic vessels. A more detailed morphologic analysis of Cdc42-deficient DCs uncovered that these cells became rapidly entangled within the 3-dimensional meshwork, with multiple protrusions pulling in different directions. Therefore, whereas migration efficiency in absence of Cdc42 was partially rescued due to the “lack of alternative routes” on 2-dimensional surfaces, cell motility in 3-dimensional environments absolutely required Cdc42.
Although the function of Cdc42 in other leukocytes was not addressed in this study, recent studies provide solid evidence for a common integrin-independent, actin protrusion-dependent “amoeboid” migratory phenotype inside 3-dimensional environments in all hematopoietic cells.4-6 The data presented by Lämmermann et al support the notion that directional “decisiveness” conferred by Cdc42 is a critical element of this migration mode in complex 3-dimensional settings. The observations may also explain the reduced migration of DCs deficient in the Cdc42-effector Wiskott-Aldrich syndrome protein,7 although the more severe phenotype of Cdc42-deficient DCs reported by Lämmermann et al suggests the involvement of additional downstream effectors. Similarly, lymphocytes expressing a mutated form of the actin regulator Coronin1A, which results in excessive lamellipodia formation, show strongly impaired parenchymal motility.8 Together with this latest report from Lämmermann et al, these findings highlight the importance of tightly controlling actin cytoskeletal dynamics for efficient “decision-making” and maneuvering through complex 3-dimensional pore systems.
Single cell trajectories of wild-type (WT) and Cdc42-deficient DCs migrating along a CCL19 gradient in 2-dimensional (top panel) and 3-dimensional (bottom panel) settings. Despite reduced directionality, Cdc42-deficient DCs were still able to migrate with residual efficiency on 2-dimensional surfaces. In contrast, lack of Cdc42 dramatically reduced cell displacement in 3-dimensional environments due to irreversible cell entangling. See the complete figure in the article beginning on page 5703.
Single cell trajectories of wild-type (WT) and Cdc42-deficient DCs migrating along a CCL19 gradient in 2-dimensional (top panel) and 3-dimensional (bottom panel) settings. Despite reduced directionality, Cdc42-deficient DCs were still able to migrate with residual efficiency on 2-dimensional surfaces. In contrast, lack of Cdc42 dramatically reduced cell displacement in 3-dimensional environments due to irreversible cell entangling. See the complete figure in the article beginning on page 5703.
In conclusion, the authors demonstrate a requirement for Cdc42 in 3-dimensional environments to avoid “cellular trapping.” Their findings also highlight the importance of choosing the appropriate experimental system—in particular, 2-dimensional versus 3-dimensional settings—to dissect the physiologic role of signaling molecules orchestrating cellular motility.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal