Abstract
Cytomegalovirus (CMV) continues to cause major complications after hematopoietic cell transplantation (HCT). Over the past decade, most centers have adopted preemptive antiviral treatment or prophylaxis strategies to prevent CMV disease. Both strategies are effective but also have shortcomings with presently available drugs. Here, we review aspects of CMV treatment and prevention in HCT recipients, including currently used drugs and diagnostics, ways to optimize preemptive therapy strategies with quantitative polymerase chain reaction assays, the use of prophylaxis, management of CMV disease caused by wild-type or drug-resistant strains, and future strategies.
Introduction
Cytomegalovirus (CMV) remains one of the most important complications after allogeneic hematopoietic stem cell transplantation (HCT). It can cause multiorgan disease in recipients of stem cell transplants, including pneumonia, hepatitis, gastroenteritis, retinitis, and encephalitis, and the disease can develop both early and late after the transplantation procedure.1-3 Seropositivity for CMV remains a risk factor for transplantation-related mortality in patients who receive a transplant from an unrelated donor despite major advances in early diagnosis and management.4-6 The pathogenesis of CMV infection and disease is complex with several interactions between CMV and the immune system. The interaction is mediated through several mechanisms, including the virus having effects on HLA expression, cytokine production, and expression of adherence molecules. These interactions can explain the increased risk of secondary bacterial and fungal infections in patients with CMV infection.7 Another possible effect of the interaction with the immune system is the described association between CMV and acute and chronic graft-versus-host disease (GVHD). It has been documented that patients with acute GVHD are at an increased risk of CMV disease.8-10 However, CMV infection has been reported as a risk factor for acute GVHD in patients receiving T cell–depleted grafts, and for chronic GVHD.4,11-13 CMV reactivation is controlled by CMV-specific T cells.14,15 However, recent studies also suggest that natural killer (NK) cells play a role in protecting against CMV, because donor-activating killer immunoglobulin–like receptor (KIR) genes have been associated with protection from CMV reactivation in the recipient.16,17
Prevention of primary CMV infection
Pretransplantation strategies
Determining the CMV serologic status.
CMV serologic status should be assessed as early as possible when a patient is being considered for allogeneic HCT. There is an advantage for patients who are CMV seronegative when coming to transplantation, and, in some situations, it might be logical to test the patient's status at the time of diagnosis of a disease that may require HCT in the future. If a patient is found CMV seronegative, a strategy to provide “CMV-safe” blood products should be used.
Donor selection.
Patients who are CMV seronegative before transplantation should, if possible, be retransplanted from a CMV-negative donor. In an HLA-identical sibling situation, a CMV-seronegative donor to a CMV-seronegative patient is clearly preferable. In an unrelated donor situation, an important question is how to weigh the factor of CMV serological status compared with other relevant donor factors, especially if more than one possible donor is available. The most important of these factors is the HLA match. Although no study has assessed the relative importance of HLA match versus CMV serology, an antigen-matched donor for HLA-A, -B, or -DR would most likely be preferred to a CMV-negative donor. However, for a lesser degree of mismatch, such as allele -mismatches or mismatches on HLA-C, -DQ, or -DP, the situation is different, and a CMV-negative donor could be considered even if the match was poorer. Compared with other donor factors such as age or blood group, a CMV match has preference. A special situation exists with cord blood donors because they can be seen as CMV negative.18,19
Posttransplantation strategies
Blood products.
CMV-seronegative patients receiving grafts from CMV-seronegative donors have a low risk of contracting CMV infection with proper management. The risk for CMV transmission in D−/R− patients is mainly through blood products.20 Today, 2 effective options exist for reducing the risk of CMV transmission: the use of blood products from CMV-seronegative donors or the use of leukocyte-reduced, filtered blood products.21-23 It is not settled which strategy is preferable.24,25 Leukocyte filtration should be performed at the blood bank, and the established quality standards followed.24,25 No controlled study has investigated whether there is an extra benefit from the use of both seronegative and filtered blood products. This is important because in many centers, and indeed in entire countries, leukocyte depletion of blood products is mandatory, and there is a significant cost and utilization of resources for the blood bank to provide CMV-negative blood products.
One important practical question is whether it is necessary to monitor CMV-seronegative patients receiving grafts from CMV-seronegative donors as well as CMV “safe” blood products. Monitoring is costly, and it could be argued that the cost-benefit ratio would not be favorable. However, treatment of CMV disease is also expensive, and neither testing for CMV antibodies nor the use of safe blood products is 100% effective. Furthermore, there is always the risk that mix-ups may occur, resulting in certain patients who should have been monitored, but were not. In our practices, we therefore use the same monitoring strategy for all patients. Although CMV infection is rare in D−/R− patients, such a monitoring strategy is highly effective in identifying CMV infection and preventing disease.21
Prevention of infection in a CMV-seronegative patient receiving a CMV-seropositive graft
If only a CMV-seropositive donor is available for a CMV-seronegative patient, the risk of transmission of CMV by the stem cell product to the recipient is approximately 20% to 30%.7,26
Two studies have been performed with intravenous immune globulin (IVIG) as prophylaxis against primary infection. Bowden et al27 showed a reduction in the rate of CMV infection but no reduction in CMV disease. In a similarly designed study performed by the Nordic BMT group, there was no reduction in CMV infection by the use of CMV hyperimmunoglobulin.28 A CMV-specific monoclonal antibody also failed to prevent CMV infection.29 We therefore do not use immunoglobulin to prevent CMV infection. It is possible that antiviral chemoprophylaxis can reduce the risk for primary CMV infection. In a randomized study, the risk of primary infection was 16% in patients receiving high-dose valacyclovir and 26% in patients receiving high-dose acyclovir.30 However, at the current time we do not use anti-CMV chemoprophylaxis in this situation.
None of the existing strategies eliminate the risk of primary CMV infection. If a CMV-seropositive donor is used, we consider these patients at risk for CMV disease and use preventive strategies similar to those in CMV-seropositive patients.
Prevention of CMV infection in CMV-seropositive patients
Choice of donor
In a seropositive patient, the choice of a proper donor is controversial. It was reported that seropositive patients undergoing unrelated, non–T cell–depleted HCT had an improved survival rate if they received a graft from a seropositive donor.31,32 Others studies have failed to confirm this finding.33,34 However, recent studies have shown that CMV-seropositive patients with CMV-seronegative donors have an increased risk of both repeated CMV reactivations and for CMV disease.10,35,36 Presently, at Huddinge, CMV donor serostatus is part of the selection algorithm for seropositive unrelated donor recipients, whereas in Seattle, CMV serostatus is presently not considered as a donor selection criterion for seropositive recipients. A large Center for International Blood and Marrow Transplant Research (CIBMTR) study is presently underway to reconcile the controversial findings with regard to donor CMV serostatus.
Immunoglobulin and anti-CMV monoclonal antibody to prevent recurrent infection.
The indications for IVIG in allogeneic HCT recipients have varied over the last decades. The effects on reducing CMV infection and disease both for standard and hyperimmunoglobulin are modest, at best.37-39 Some studies showed a reduction of bacteremia, non-CMV interstitial pneumonia, or acute GVHD, whereas other studies did not report such a difference. An improvement of survival has not been reported in any of the studies or in meta-analyses.39-42 Recent data suggest that the beneficial effects, with regard to the prevention of non-CMV interstitial pneumonia or GVHD, is counteracted by an increase in veno-occlusive disease, and that the net result does not favor the use of IVIG prophylaxis.39,43 In addition, a study using a CMV-specific monoclonal antibody failed to reduce the risk of CMV infection and disease.29 Therefore, the use of immune globulin to prevent CMV cannot be recommended.
Antiviral chemoprophylaxis
Several antiviral drugs with anti-CMV activity exist. All have their drawbacks, and, although studies have shown positive results, many centers do not use this strategy to prevent recurrent CMV infection and disease. However, it would be logical to use antiviral chemoprophylaxis in subgroups of patients (ie, patients at high risk for CMV disease). New developments might also change the situation in the future.
Acyclovir/valacyclovir prophylaxis.
High-dose acyclovir (500 mg/m2 intravenously 3 times daily, followed by 800 mg 4 times daily [adult dosing]) can reduce the risk of CMV infection and possibly CMV disease.44,45 The study by Prentice et al45 reported improved survival rates, although the mechanism for this improvement is not clear. Valacyclovir is the valin ester prodrug of acyclovir and is better absorbed and thereby gives better serum concentrations than acyclovir. A large, randomized study comparing high-dose valacyclovir (2 g 4 times daily) with high-dose acyclovir showed a reduction of CMV infection in valacyclovir recipients from 40% to 28% (HR, 0.59; 95% CI, 0.46, 0.76; P < .001).30 Furthermore, the use of preemptive therapy was reduced by almost half. There was no difference in CMV disease or survival. Another study by Winston et al46 compared high-dose valacyclovir with intravenous ganciclovir and found similar rates of CMV disease.
Ganciclovir prophylaxis.
Prophylaxis with intravenous ganciclovir has been tested in several randomized trials,46-50 all of which have shown a reduction of the risk of CMV disease compared with placebo but did not show improved survival. Ganciclovir given at engraftment causes prolonged neutropenia, leading to more invasive bacterial and fungal infections.47,49,51 In addition, a substantial number of patients not at risk for disease (ie, 60%-65%) will unnecessarily receive a potentially marrow-toxic drug. The neutropenia might at least, in part, be prevented or treated with G-CSF, but this adds to the cost. Nonrandomized studies have been reported and used a pretransplantation induction course of ganciclovir from day −8 to −1 followed by lower maintenance doses of ganciclovir (ie, 5 mg/kg, 3 times a week) starting at engraftment.52-55 The results have been varying, with some of the studies showing high rates of CMV disease.52,54 Thus, this strategy may be unsafe in high-risk patients.
Valganciclovir.
Valganciclovir is the valin ester prodrug of ganciclovir. No data exist for valganciclovir used as prophylaxis, and it cannot be recommended.
Foscarnet.
The role of foscarnet for prophylaxis of CMV disease remains undefined because no controlled studies have been published. Three uncontrolled studies have been reported,56-58 with some breakthroughs of CMV infection and disease. Foscarnet is associated with dose-dependent renal toxicity and electrolyte abnormalities.
Maribavir.
Maribavir is a new and, as yet, unlicensed antiviral agent that is presently being investigated as a prophylactic drug. A randomized, placebo-controlled dose-ranging phase 2 study has been published with promising results, showing significantly lower risk of CMV infection and borderline reduction of CMV disease, compared with placebo.59 Toxicity was also limited, mainly consisting of gastrointestinal side effects, but no marrow toxicity was found. Enrollment in a large phase 3 trial has been completed, and results are expected to be available in 2009.
The bottom line about antiviral chemoprophylaxis.
All prophylaxis strategies will result in the unnecessary treatment of patients who will not develop CMV infection or disease. None of the currently available antiviral drugs are ideal; ganciclovir and foscarnet due to toxicity, and acyclovir or valacyclovir due to low efficacy. Presently, intravenous ganciclovir prophylaxis appears to be the most effective way of preventing CMV disease. Because of the drawbacks of current available antiviral drugs, neither of our institutions use prophylaxis routinely. There might, however, be special situations when prophylaxis is indicated, especially in patients who are at high risk of CMV infection and disease (eg, after cord blood transplantation). However, myelotoxicity of ganciclovir products may also be increased early after cord blood transplantation.
CMV viral load and preemptive antiviral therapy
The preemptive treatment strategy is highly effective at managing CMV infection; however, its success depends largely on the presence of CMV in the blood before the onset of disease and the early detection of CMV. The latter depends on the adherence to the testing schedule. Occasionally, there will be missed cases of disease that are not preceded by CMV DNAemia or pp65 antigenemia. Disease may also occur because of missed surveillance tests or surveillance tests that are spaced too far apart.
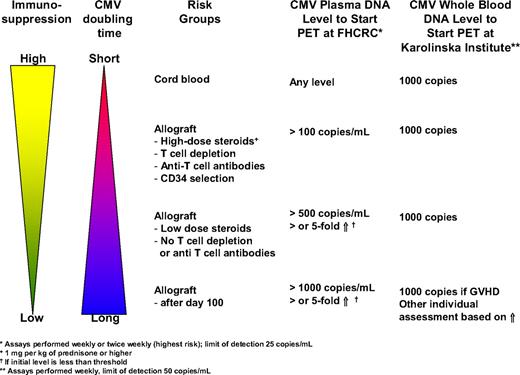
Preemptive therapy is typically initiated at first detection of CMV reactivation by a rapid diagnostic technique such as the pp65 antigenemia assay, the pp67 mRNA assay, or DNA detection methods. Quantitative real-time polymerase chain reaction (PCR) assays for CMV DNA are increasingly used because they offer 2 advantages. First, they are highly sensitive, thereby providing the opportunity to become positive in cases of CMV disease that have been missed with less-sensitive assays, such as the pp65 antigenemia assay. With the antigenemia assay, CMV disease rates during CMV surveillance may be as high as 7.7% during the first 100 days after HCT.49 Most of these breakthrough cases are due to gastrointestinal disease; PCR can be positive, even if antigenemia is negative.49 Second, the quantitative nature of the assay may increase its specificity by using a certain viral load threshold or increases over time, thereby avoiding unnecessary treatment of patients who are at low risk of progression to disease. There are no validated viral load thresholds. Furthermore, universally acceptable thresholds would be difficult to establish because of differences in assay performance and testing material (whole blood versus plasma). However, there are several biologic and assay-specific principles that can be used to design such thresholds. Studies by Emery and Griffiths60 have shown that the doubling time of CMV is only 1 to 2 days on average and that the degree of immunosuppression determines the in vivo replication dynamics. Thus, patients with a high degree of immunosuppression have a shorter doubling time, leading to a more rapid increase in viral load. It has also been shown that the initial viral load (and to some degree the peak viral load) is predictive for the risk of CMV disease and non–relapse mortality.60,61 The initial viral load is probably the best indicator of viral dynamics because it indicates the slope of CMV replication without intervention, provided that the surveillance testing is spaced evenly. Finally, if one uses viral load increases as a parameter to start preemptive therapy, one has to consider the assay variability to determine “true” increases. The coefficient of variation of most PCR assays for viral loads close to the limit of detection may be as high as 30%.62,63 Thus, increases of less than 0.5 log10 (or 3 times the baseline level) may not represent true increases. On the basis of these principles, we have instituted viral load thresholds and relative increases at the Fred Hutchinson Cancer Research Center (FHCRC) and are presently undergoing evaluation. At Huddinge, a preset viral threshold has been in use for some years without breakthroughs with disease (Figure 1).
CMV viral load to start preemptive therapy (PET) used at the FHCRC in Seattle, WA, and the Karolinska Institute, Stockholm, Sweden.
CMV viral load to start preemptive therapy (PET) used at the FHCRC in Seattle, WA, and the Karolinska Institute, Stockholm, Sweden.
Another way to further increase specificity of preemptive therapy is to combine it with monitoring for CMV-specific T-cell immunity. This is an interesting strategy because it may allow one to withhold preemptive therapy in patients with low-to-moderate levels of CMV DNA, if CMV-specific T-cell responses are detectable. The technology is available64 ; however, few studies have reported thresholds of T-cell immunity that can be considered protective.64 A small pilot study using T-cell responses as the guide for withholding therapy in patients more than 100 days after transplantation has been performed at Huddinge.65 Clearly, such strategy requires validation in a randomized trial before it can be recommended.
Special situations
Pretransplantation CMV infection and disease
Patients with CMV disease developing close in time to a planned allogeneic HCT have a high risk of death after transplantation.66 Because of the increasing use of alemtuzumab and other highly immunosuppressive therapies,67,68 the number of patients who will develop pretransplantation CMV disease will probably increase. The prevention of CMV disease is therefore important for HCT candidates.68
Secondary prophylaxis to prevent late CMV disease
Late CMV viremia and disease occur in a subset of high-risk patients and are associated with poor outcome.1,3 Late CMV disease occurs in approximately 4% to 15% of seropositive allograft recipients, and most cases occur between months 4 and 12 after HCT.1,49 Risk factors include CMV infection or disease during the first 3 months after HCT, low CD4 T-cell count, undetectable CMV-specific T-cell immunity, and GVHD or T-cell depletion in the graft or use of anti–T-cell agents.1 Recipients of umbilical cord blood transplant are also at risk for CMV complications throughout the first year after HCT.
Table 1 shows criteria used to identify patients at risk for late CMV disease and to discontinue preventative strategies. We continue CMV surveillance in patients at risk for late CMV disease and use preemptive therapy with valganciclovir if CMV is detected. The thresholds for intervention used at FHCRC is 1000 copies/mL or a more than 5-fold increase over baseline when lower DNA levels are detected. Initial data from a randomized trial of daily valganciclovir prophylaxis for 6 months showed that such a strategy was not superior to preemptive valganciclovir, but it is also not more toxic.69 Thus, valganciclovir prophylaxis (900 mg/day adjusted for renal dysfunction) could serve as an alternative if virologic testing is not feasible. However, monitoring for renal function and hematologic toxicity is still required. If valganciclovir cannot be used for long-term prevention because of toxicity, we sometimes recommend high-dose valacyclovir at 2 g 3 times per day. This strategy has not been evaluated specifically for prevention of late CMV disease but is generally well tolerated and has some effect on CMV infection.30 Virologic surveillance is still required with this approach.
Strategy to prevent late CMV disease (FHCRC approach)
| Patients at risk for disease, start and discontinuation of virologic surveillance, and preemptive therapy . |
|---|
| Risk factors for late CMV disease (CMV-seropositive allograft recipients or -seronegative recipients with a positive donor) |
| Virologic criteria (one required) |
| CMV infection or disease before day 100 or |
| Prophylaxis with ganciclovir/valganciclovir/foscarnet plus |
| Immunologic criteria (one required) |
| Undetectable CMV-specific T-cell responses or |
| GVHD requiring systemic treatment or |
| High-dose steroids for reasons other than GVHD or |
| T-cell depletion or |
| Cord blood transplantation or |
| Donor lymphocyte infusion or |
| CD4 T-cell count less than 50/mm3 |
| CMV surveillance* |
| Continue PCR weekly surveillance after day 100 if risk factors for late CMV disease are present |
| Discontinue CMV surveillance if |
| No or minimal immunosuppression (< 0.5 mg prednisone/kg/day) and |
| No anti–T-cell agents and |
| At least 3 negative weekly tests |
| Preemptive therapy |
| Start preemptive therapy with valganciclovir (900 mg twice daily) if CMV DNA is more than 1000 copies/mL |
| Continue induction dosing until viral load declines, at least 1 week |
| Treat with maintenance dose (900 mg/day) until viral load is undetectable |
| Patients at risk for disease, start and discontinuation of virologic surveillance, and preemptive therapy . |
|---|
| Risk factors for late CMV disease (CMV-seropositive allograft recipients or -seronegative recipients with a positive donor) |
| Virologic criteria (one required) |
| CMV infection or disease before day 100 or |
| Prophylaxis with ganciclovir/valganciclovir/foscarnet plus |
| Immunologic criteria (one required) |
| Undetectable CMV-specific T-cell responses or |
| GVHD requiring systemic treatment or |
| High-dose steroids for reasons other than GVHD or |
| T-cell depletion or |
| Cord blood transplantation or |
| Donor lymphocyte infusion or |
| CD4 T-cell count less than 50/mm3 |
| CMV surveillance* |
| Continue PCR weekly surveillance after day 100 if risk factors for late CMV disease are present |
| Discontinue CMV surveillance if |
| No or minimal immunosuppression (< 0.5 mg prednisone/kg/day) and |
| No anti–T-cell agents and |
| At least 3 negative weekly tests |
| Preemptive therapy |
| Start preemptive therapy with valganciclovir (900 mg twice daily) if CMV DNA is more than 1000 copies/mL |
| Continue induction dosing until viral load declines, at least 1 week |
| Treat with maintenance dose (900 mg/day) until viral load is undetectable |
Consider valganciclovir prophylaxis if virologic surveillance is not feasible (see “Secondary prophylaxis to prevent late CMV disease”).
Adoptive immunoprophylaxis
It is well recognized that patients lacking a specific immune response to CMV are at an increased risk of developing CMV disease.1,2,14,15,70 Monitoring of CD8 and/or CD4 CMV-specific T cells can be applied with the use of different techniques, including detection by tetramers or measurement of peptide-specific lymphocyte responses. However, none are standardized for routine use. Several groups have studied the usefulness of adoptive transfer of T cells71-73 or vaccination with CMV-primed dendritic cells.74 These technologies seem not to be associated with significant toxicity, but their effectiveness needs to be further assessed in controlled trials.
Diagnosis of CMV infection
Several techniques exist, allowing rapid diagnosis of CMV with a high sensitivity. Currently, the most used tests for diagnosis of CMV infection are detection of antigen (pp65; antigenemia assay), DNA, or mRNA. In regard to the detection of CMV DNA by PCR, the specimens vary, but today either whole blood or plasma is most commonly used. Moreover, the quantification of viral load by quantitative PCR can give important prognostic information, and this technique is now widely available.10,60,62,75,76 Detection of mRNA by nucleic acid sequence–based amplification (NASBA) is also a sensitive and rapid technique and has, in randomized trials, been shown to be as effective as pp65 antigenemia or detection of DNA by PCR.77,78 However, it is rarely used at transplantation centers.
Antiviral drug resistance
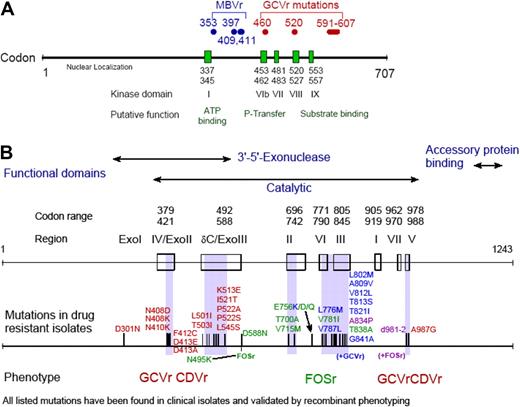
Drug resistance is relatively uncommon after HCT, but there are certain situations in which it should be suspected (Figure 2). Drug resistance can occur with all drugs used for the treatment and prophylaxis of CMV (ie, ganciclovir, valganciclovir, foscarnet, cidofovir); however, most reports are for ganciclovir resistance, because this drug (and its prodrug valganciclovir) is used in approximately 90% of patients as first-line agent. Many mutations have been mapped (Figure 3), and genotypic assays are available for diagnostic analysis in reference laboratories.79
CMV drug resistance. Development of drug resistance requires prolonged pre-exposure (usually weeks to months) to the antiviral drug and persistent reactivation in the presence of drug, which will ultimately lead to the selection of resistant strains.
CMV drug resistance. Development of drug resistance requires prolonged pre-exposure (usually weeks to months) to the antiviral drug and persistent reactivation in the presence of drug, which will ultimately lead to the selection of resistant strains.
CMV drug resistance mutation maps. CMV drug resistance mutation maps for the UL97 (A) and DNA polymerase (B) genes. Figure was obtained with permission from S. Chou.79
CMV drug resistance mutation maps. CMV drug resistance mutation maps for the UL97 (A) and DNA polymerase (B) genes. Figure was obtained with permission from S. Chou.79
In general, drug resistance should be suspected in patients who are on antiviral drugs and who have had load increases for more than 2 weeks. After start of preemptive therapy, viral load increases occur in approximately one-third of patients and are due to the underlying immunosuppression.80 Thus, in a drug-naive person (which is the case in most patients during the first 3 months after transplantation), it is unlikely that these increases are due to true drug resistance in adult patients; however, cases of early-onset resistance in pediatric patients have been reported. The situation is different if patients have received ganciclovir before transplantation or if viral load increases occur in the late setting when most patients are not antiviral drug naive anymore. Today, ganciclovir or foscarnet is used rarely before transplantation except in situations of pretransplantation CMV disease and in children with congenital immunodeficiency. Overall, one should be vigilant and test patients for genotypic resistance if viral load increases for more than 2 weeks, especially, if there is significant exposure to antiviral drugs.
Clinically, drug resistance can manifest as rising viral load or CMV disease. If drug resistance is suspected, we recommend sending samples for genotypic testing and switching to an alternative drug. In most cases, this means switching from a ganciclovir drug to foscarnet. Viral load can be used to monitor the response to treatment. In patients who do not respond or those who are critically ill, few options exist, and none are supported by good data. One option is to continue ganciclovir, in addition to foscarnet, in patients with ganciclovir resistance. The theoretical basis for this is an additive effect of ganciclovir and foscarnet in vitro81 and a mathematically predicted efficacy in viral load reduction of intravenous ganciclovir against strains with single UL97 mutations of approximately 60%.82 We also sometimes use ganciclovir monotherapy at increased doses (eg, 15 mg/kg per day divided in 2 doses; renally adjusted) with G-CSF support. Cidofovir may also be used, provided that a CMV DNA polymerase mutation that causes cross-resistance is not present79 (Figure 3B). However, there is limited experience with cidofovir for treatment of ganciclovir-resistant CMV. Drugs presently under evaluation, such as maribavir, may also provide therapeutic options in the future. Maribavir inhibits the CMV UL97 kinase and is active against wild-type and ganciclovir-resistant CMV strains83 (Figure 3A). Because maribavir inhibits UL97, and presumably impairs phosphorylation of ganciclovir, it has been shown to be antagonistic to ganciclovir in vitro, and both agents therefore should not be used in combination.84 However, maribavir (once it is available) could be used alone or in combination with foscarnet in patients with ganciclovir-resistant CMV infection or disease.79 It should be pointed out that it is presently unknown how maribavir performs in the treatment setting because no studies have been conducted for the treatment of CMV disease.
There are also licensed drugs with possible anti-CMV activity, including the arthritis drug leflunomide and the antimalaria compound artesunate.85,86 Leflunomide is available in most parts of the world but is not approved by European or American regulatory authorities for the treatment of CMV. Artesunate has not been approved by the US Food and Drug Administration but is available in other parts of the world with an indication for malaria treatment. In vitro studies have been performed, and the use of these drugs has been described in a small number of case reports.85-87 Another potentially useful approach is to use the immunosuppressive drug sirolimus as adjunct therapy because it may impair CMV replication inside host cells.88 Sirolimus has shown to reduce the risk of CMV reactivation after HCT and also in renal transplant recipients.89 We would like to emphasize that none of these options have been systematically studied with regard to efficacy and toxicity, but they may be options that can be considered in desperate clinical situations.
Diagnosis and treatment of CMV disease
CMV gastrointestinal disease and pneumonia are by far the most common manifestations of CMV disease in the current era. CMV retinitis is uncommon, but it should be suspected in patients with visual changes.90,91 Overall, the incidence of CMV disease during the first year after HCT has decreased from approximately 30% to 35% in the era before ganciclovir to 8% to 10% in seropositive recipients.92 The diagnosis of CMV pneumonia is established by detection of CMV in bronchoalveolar lavage (BAL) or lung biopsy in the presence of clinical signs and symptoms. International definitions of CMV disease have been published.93 Briefly, diagnostic methods to test for CMV in these samples include rapid cultures, direct fluorescent antibody tests, and cytology; there is presently no data on what level of CMV DNA correlates with CMV pneumonia; thus, presently, we do not recommend PCR to make the diagnosis. This is an important point because pulmonary shedding of CMV is common, even in asymptomatic patients with normal clinical and radiologic examinations.94 Because of its high sensitivity, however, PCR has a high negative predictive value and can be used to rule out the diagnosis of CMV pneumonia.95 The diagnosis of gastrointestinal disease relies on detection of CMV in biopsy specimens by culture (rapid or conventional), immunohistochemistry, or detection of inclusion bodies. The latter is highly specific but insensitive. Although each of these methods is sufficient to diagnose CMV gastrointestinal disease, the diagnostic yield is significantly increased if more than one method is used.96 We therefore believe that at least 2 different methods should be used on biopsy specimens to diagnose CMV disease, especially if PCR is used. Similar concerns as outlined for CMV pneumonia apply to the use of PCR as a single method in gastrointestinal biopsy specimens. Notably, CMV disease, especially gastrointestinal disease, can occur in the absence of CMV detection in the blood.96
We treat gastrointestinal disease with ganciclovir or foscarnet alone. Depending on the extent of the disease, prolonged courses of antiviral treatment at high doses may be required because ulcers may be deep, and the time to reepithelialization may be weeks or months. We therefore, treat for 2 to 3 weeks with induction dosing, followed by several weeks of maintenance. If severe immunosuppression continues, recurrence of gastrointestinal disease may occur in approximately 30% of patients. This may justify secondary prophylaxis, such as prolonged maintenance dosing, until immunosuppression has been reduced. Intravenous ganciclovir is used at the time of the initial treatment because clinical symptoms may be severe; oral medication has not been studied in this phase of treatment. Foscarnet is used as an alternative if neutropenia is present.97 Whether valganciclovir can be used during the maintenance treatment phase has not been studied. Two studies have shown similar bioavailability of valganciclovir and intravenous ganciclovir in the setting of mild-to-moderate gastrointestinal GVHD.98,99 We therefore believe that valganciclovir use can be justified if symptoms are improved, there is good oral intake, no concurrent severe GI GVHD is present, and systemic CMV viral load has been suppressed. Treatment of concurrent conditions, especially GVHD, and optimized supportive care with proton pump inhibitors are critical.
CMV pneumonia remains the most feared complication of CMV in HCT recipients because its attributable mortality continues to be high. Over the past 2 decades little progress has been made in the treatment of CMV pneumonia. In the late 1980s, 3 non–randomized studies established the current standard of care, ie, treatment with ganciclovir (or foscarnet as an alternative agent) in combination with intravenous immunoglobulin.100-102 These studies showed improved survival rates compared with historical outcome results. There does not appear to be a specific advantage of CMV-specific immune globulin (CMV-Ig) compared with pooled immunoglobulin.103 However, in specific clinical situations, CMV-Ig may be preferred. If volume overload is an issue, CMV-Ig can be used because the infused volume of CMV-Ig is lower (150 mg/kg vs 500 mg/kg of pooled IVIG). Another situation may be when IVIG is not available, because there is a chronic shortage in some parts of the world.
Since this standard of ganciclovir and immunoglobulin was established, several studies have raised doubt in the magnitude of the effect of concomitant immunoglobulin. A study from the European Bone Marrow Transplant (EBMT) group found that survival at 1 month after diagnosis was only 31%.104 Another study from Brazil concluded that there was no major effect of immunoglobulin.105 These studies were too small in size to account for other factors that may affect outcome of pneumonia, such as overall morbidity at the time of diagnosis, status of the immune reconstitution, and the presence of copathogens. Recently presented results from a large analysis of cases of CMV pneumonia add to the controversy because there was no statistically significant benefit of immunoglobulin.106 This and other large studies will have to determine whether there are specific subgroups that might benefit from immunoglobulin therapy. Until then, we continue to use immunoglobulin for the treatment of CMV pneumonia after HCT.
Conclusions and future needs
CMV treatment has been optimized in HCT recipients over the past decade, especially when used preemptively, but several questions remain. Issues that should be studied are the concepts of how viral load thresholds and dynamic changes can be used to take full advantage of quantitative PCR assays and how to combine virologic and immune surveillance. Another is the treatment of CMV pneumonia. Several studies now suggest that the overall benefit of concomitant treatment with immunoglobulin may be less impressive than originally thought, based on the original 3 nonrandomized studies. Other treatment strategies such as antiviral combination therapy with novel drugs may be more promising approaches, but studies are needed to examine this hypothesis.
New treatment options for CMV are urgently needed because the currently available drugs have major limitations. Novel drugs such as maribavir,59 lipid cidofovir,107 and novel a non–nucleoside inhibitor,108 as well as leflunomide86 and artesunate,87 deserve a systematic evaluation. These compounds are needed for current management indications because the downsides of presently available drugs. They are also critical for the management of drug-resistant CMV disease, which will probably increase with the increased use of antiviral drugs in some HCT candidates, such patients with chronic lymphocytic leukemia,68 and perhaps in immunocompetent patients in the future.109
Acknowledgment
This work was supported by the National Institutes of Health (NIH; Bethesda, MD; CA 18029; M.B.).
National Institutes of Health
Authorship
Contribution: M.B. and P.L. retrieved data and wrote the paper.
Conflict-of-interest disclosure: M.B. has served as consultant to Roche Laboratories, Viropharma, Alphavax, AiCuris, and Chimerix; has received research funding from Roche Laboratories, Viropharma, and Vical; and has received lecture fees from Viropharma and Roche Laboratories. P.L. has served as a consultant to Viropharma, Roche Laboratories, and AiCuris and has received research funding and lecture fees from Viropharma.
Correspondence: Michael Boeckh, Vaccine and Infectious Disease Institute and Program in Infectious Diseases, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: mboeckh@fhcrc.org.