Abstract
This phase 3, multicenter, randomized (1:1), double-blind, placebo-controlled study evaluated the safety and efficacy of plerixafor with granulocyte colony-stimulating factor (G-CSF) in mobilizing hematopoietic stem cells in patients with multiple myeloma. Patients received G-CSF (10 μg/kg) subcutaneously daily for up to 8 days. Beginning on day 4 and continuing daily for up to 4 days, patients received either plerixafor (240 μg/kg) or placebo subcutaneously. Starting on day 5, patients began daily apheresis for up to 4 days or until more than or equal to 6 × 106 CD34+ cells/kg were collected. The primary endpoint was the percentage of patients who collected more than or equal to 6 × 106 CD34+ cells/kg in less than or equal to 2 aphereses. A total of 106 of 148 (71.6%) patients in the plerixafor group and 53 of 154 (34.4%) patients in the placebo group met the primary endpoint (P < .001). A total of 54% of plerixafor-treated patients reached target after one apheresis, whereas 56% of the placebo-treated patients required 4 aphereses to reach target. The most common adverse events related to plerixafor were gastrointestinal disorders and injection site reactions. Plerixafor and G-CSF were well tolerated, and significantly more patients collected the optimal CD34+ cell/kg target for transplantation earlier compared with G-CSF alone. This study is registered at www.clinicaltrials.gov as #NCT00103662.
Introduction
High-dose chemotherapy with autologous hematopoietic stem cell transplantation has been shown in clinical trials to increase complete remissions and overall survival in patients with multiple myeloma compared with conventional chemotherapy.1-3 Recent clinical studies have also reported that tandem transplantation may provide a longer disease-free survival than single transplantation in patients with multiple myeloma.4-6 Obtaining sufficient CD34+ cells for either single or tandem transplantation remains one of the challenges in using this treatment strategy. Approximately 20% of patients with multiple myeloma do not mobilize sufficient hematopoietic stem cell for transplantation with either cytokines alone or cytokines and chemotherapy.7-9 Patients unable to mobilize adequate hematopoietic stem cells will probably require multiple mobilization attempts, thus negatively affecting patients' quality of life and increasing healthcare costs.10
Plerixafor is the first in a new class of small molecules that reversibly inhibits chemokine stromal cell–derived factor-1α binding to its cognate receptor CXC chemokine receptor 4.11-13 In a phase 2 study conducted in patients with multiple myeloma and non-Hodgkin lymphoma, the addition of plerixafor to granulocyte colony-stimulating factor (G-CSF) was well tolerated and found to increase the likelihood of obtaining more than or equal to 5 × 106 CD34+ cells/kg in fewer aphereses compared with G-CSF alone.14 Because G-CSF (10 μg/kg per day) is the only approved agent for hematopoietic stem cells mobilization, this phase 3 study was designed to compare the safety and efficacy of plerixafor and G-CSF with placebo and G-CSF in mobilizing CD34+ cells in patients with multiple myeloma and to support plerixafor drug approval.
Methods
Study design and patients
We conducted a phase 3, multicenter, randomized, double-blind, placebo-controlled study. The study consisted of 5 main periods: period 1 (mobilization and treatment/apheresis period), from randomization to the day before myeloblative chemotherapy; period 2 (chemotherapy and transplantation period), from first day of chemotherapy to the first day of platelet and neutrophil engraftment (whichever was later) after first transplantation; period 3, from the first day after engraftment to the day before starting chemotherapy (for patients undergoing a tandem transplantation) or through 12 months after transplantation (for patients undergoing only 1 transplantation); period 4, from first day of the second chemotherapy to the first day of successful engraftment after the second transplantation; period 5, from first day after engraftment (after second transplantation) through 12 months after transplantation.
The study was conducted in accordance with Declaration of Helsinki and Good Clinical Practice guidelines and approved by the institutional review board or ethics committee of each participating institution. All patients provided written informed consent and could withdraw from the study at any time.
Patients between the ages of 18 and 78 years with biopsy-confirmed diagnosis of multiple myeloma before the first mobilization, in first or second complete or partial remission, and eligible for autologous hematopoietic stem cell transplantation were eligible to participate. Additional inclusion criteria were: at least 4 weeks since last cycle of chemotherapy, Eastern Cooperative Oncology Group performance status of 0 or 1, white blood cell count more than 2.5 × 109/L, absolute neutrophil count more than 1.5 × 109/L, platelet count more than 100 × 109/L, serum creatinine less than or equal to 2.2 mg/dL, normal liver function, cardiac and pulmonary status sufficient to undergo apheresis and transplantation, negative for HIV, and in agreement to use an approved form of contraception if of childbearing potential.
Patients were excluded if they had a comorbid condition which, in the view of the investigators, rendered the patient at high risk from treatment complications; had prior autologous or allogeneic transplantation; received bone-seeking radionuclides; received more than 2 cycles of alkylating agent combinations; were less than 6 weeks off 1,3-bis(2-chloroethyl)-1-nitrosourea before first dose of G-CSF; received granulocyte-macrophage colony-stimulating factor or pegfilgrastim within 3 weeks before the first dose of G-CSF for mobilization; received G-CSF within 14 days before the first dose of G-CSF for mobilization; had active central nervous system involvement; failed previous hematopoietic stem cell collections or collection attempts; received radiation therapy to more than or equal to 50% of the pelvis; anticipated posttransplantation chemotherapy and/or radiation therapy below the diaphragm; received thalidomide, lenalidomide, dexamethasone, and/or bortezomib within 7 days before the first dose of G-CSF; had previously received experimental therapy within 4 weeks of enrolling or currently enrolled in another experimental protocol; or patients whose apheresis product were to be further selected and purified.
Treatment plan
Patients were randomized 1:1 to receive either plerixafor or placebo. Randomization was stratified by study center, baseline platelet count (< 200 000/dL vs ≥ 200 000/dL), and type of transplantation planned (single vs tandem). Randomized patients underwent mobilization with G-CSF 10 μg/kg per day subcutaneously daily in the morning for up to 8 days. Beginning on the evening of day 4, patients received either plerixafor 0.24 mg/kg or placebo subcutaneously daily for up to 4 days or until more than or equal to 6 × 106 CD34+ cells/kg were collected. Apheresis (3.0 blood volume ± 10%) began on day 5 and continued daily for up to 4 days or until more than or equal to 6 × 106 CD34+ cells/kg were collected (Figure 1). Apheresis product was processed and stored according to local practice guidelines at each study center.
Rescue procedure
Patients who failed to collect either more than or equal to 0.8 × 106 CD34+ cells/kg after 2 days of apheresis or more than or equal to 2 × 106 CD34+ cells/kg in 4 apheresis days, or patients who were planned for tandem transplantation and did not collect more than or equal to 4 × 106 CD34+ cells/kg in 4 or fewer apheresis days were given the option to participate in an open-label rescue procedure. After a minimum 7-day rest period, the patients received a course of G-CSF and plerixafor identical to the treatment plan described (in Figure 1) and underwent aphereses. A separate informed consent was obtained. Study staff and patients remained blinded to the initial study treatment received before entering the rescue protocol.
Transplantation
Patients underwent ablative chemotherapy with or without total body irradiation in preparation for hematopoietic stem cell transplantation according to the institution's standard of care. Transplantation was performed within 5 weeks after the last apheresis using the collected CD34+ cells. A minimum of 2 × 106 CD34+ cells/kg was required for a single transplantation. If cells were to be used for tandem transplantation, then more than or equal to 50% of the cells, or at least 5 × 106 CD34+ cells/kg if available, were to be used for the second transplantation.
Efficacy
The primary efficacy endpoint was the proportion of patients collecting more than or equal to 6 × 106 CD34+ cells/kg in 2 or fewer apheresis days. The secondary efficacy endpoints were the proportion of patients collecting more than or equal to 6 × 106 CD34+ cells/kg in 4 or fewer apheresis days; the proportion of patients collecting more than or equal to 2 × 106 CD34+ cells/kg in 4 or fewer apheresis days; the number of apheresis days required to reach more than or equal to 6 × 106 CD34+ cells/kg; the number of days to neutrophil and platelet engraftment; the proportion of patients maintaining a durable graft at 100 days, 6 months, and 12 months after transplantation. The fold-increase in the number of peripheral blood CD34+ cells on each apheresis day was also analyzed as an exploratory endpoint.
Local laboratories and a central laboratory were used to determine CD34+ cell yield. Efficacy endpoints were calculated using the percentage of CD34+ cells determined by the central laboratory applied to the white blood cell count from the local laboratories. The local laboratory values were used for all clinical decisions, including the number of apheresis days and the decision to proceed to transplantation.
Neutrophil engraftment was defined as neutrophil count more than or equal to 0.5 × 109/L for 3 days or more than or equal to 1.0 × 109/L for 1 day. Platelet engraftment was defined as platelet count more than or equal to 20 × 109/L without a transfusion for the preceding 7 days. Graft durability was defined as maintenance of normal blood counts according to at least 2 of the following 3 criteria: platelet count more than 50 000 (50 × 109/L) without transfusion for at least 2 weeks before the follow-up visit, hemoglobin level more than or equal to 10 g/dL with no erythropoietin support or transfusions for at least 1 month before the follow-up visit, absolute neutrophil count more than 1000 (109/L) with no G-CSF for at least 1 week before the follow-up visit. To account for missing data at 100-day study visit, the definition of graft durability was adjusted to include all available follow-up information beyond the first 100 days. An observation of a later durable graft confirmed that the graft was durable at all previous time points since recovery from true graft failure is not possible.
If a tandem transplantation was performed within 6 months of the original transplantation, data from the second transplantation were used to compare outcomes between the 2 groups. If the tandem transplantation was performed more than 6 months after the first transplantation, then data from the first transplantation were used in the efficacy evaluation.
Safety
Safety was monitored by the incidence of adverse events, serious adverse events, and changes from baseline in medical history, clinical laboratory measurements, vital signs, and physical examination findings. Safety issues were evaluated throughout the study by an independent Data Safety Monitoring Board.
Statistical analysis
The data were analyzed by the study-sponsored biostatistician. All authors have access to the primary clinical trial data.
Power and sample size calculation.
Based on an estimate that 30% of patients in the placebo group would achieve the primary endpoint, a sample size of 93 patients per group was needed to provide a power of 80% to detect a 20% difference between the 2 groups for a per-protocol population. Assuming that 20% of the accrued patients would be excluded equally from both groups for a per-protocol analysis and that none of these patients would achieve the primary endpoint and the target treatment effect was 0.16, the final sample size estimate was 150 patients per group for the intent-to-treat population.
Efficacy and safety analyses.
The intent-to-treat analyses, which included all randomized patients, were used to establish efficacy. The Pearson χ2 test (unstratified), uncorrected for continuity, was used to compare the proportion of patients meeting the primary endpoint between the groups. A Cochran-Mantel-Haenszel statistic, stratified by study center, was used as a supportive analysis. The secondary endpoints were analyzed similarly. Time to achieving CD34+ cell collection target and time to neutrophil and platelet engraftment were estimated using Kaplan-Meier methods and tested for treatment effect by an unstratified log-rank statistic.
Safety analyses were performed on all patients who received at least 1 dose of G-CSF in the mobilization phase (period 1). The incidence of all adverse events and serious adverse events are reported as frequencies.
Results
Patients
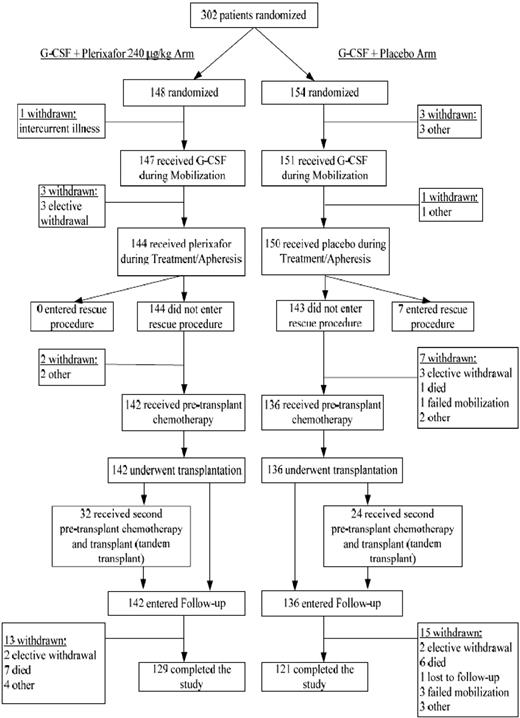
From February 4, 2005 to July 7, 2006, a total of 302 patients (148 plerixafor, 154 placebo) from 40 sites in 3 countries (38 in the United States, 1 in Canada, and 1 in Germany) were enrolled. We report herein the data for all patients through 12 months of follow-up. Patient disposition is shown in Figure 2. Overall, patient demographics and baseline characteristics were similar between the 2 groups (Table 1).
Patient demographics and baseline characteristics
| . | Plerixafor (n = 148) . | Placebo (n = 154) . | P . |
|---|---|---|---|
| Median (± SD) age, y | 58.2 ± 8.4 | 58.4 ± 8.6 | .83 |
| Male sex, n (%) | 100 (67.6) | 107 (69.5) | .72 |
| Ethnic origin, n (%) | .10 | ||
| White | 117 (79.1) | 128 (83.1) | |
| Black | 18 (12.2) | 14 (9.1) | |
| Asian | 1 (0.7) | 3 (1.9) | |
| Hispanic/Latino | 11 (7.4) | 4 (2.6) | |
| Other | 1 (0.7) | 5 (3.2) | |
| Time from initial diagnosis to randomization, mo* | 10.7 (16.5) | 11.5 (16.9) | .66 |
| Stage of disease at initial diagnosis, n (%) | .09 | ||
| I | 28 (18.9) | 19 (12.3) | |
| II | 29 (19.6) | 44 (28.6) | |
| III | 91 (61.5) | 90 (58.4) | |
| Missing | 0 (0.0) | 1 (0.6) | |
| Remission status at enrollment, n (%) | .40 | ||
| First complete remission | 11 (7.4) | 18 (11.7) | |
| First partial remission | 129 (87.2) | 126 (81.8) | |
| Second complete remission | 0 (0.0) | 0 (0.0) | |
| Second partial remission | 8 (5.4) | 10 (6.5) | |
| Prior chemotherapy, n (%) | N/A | ||
| Yes | 144 (97.3) | 148 (96.1) | |
| No | 0 (0.0) | 0 (0.0) | |
| Missing | 4 (2.7) | 6 (3.9) | |
| Prior cytotoxic chemotherapy,† n (%) | 51 (34.5) | 48 (31.2) | |
| Prior radiotherapy, n (%) | .53 | ||
| Yes | 40 (27.0) | 47 (30.5) | |
| No | 106 (71.6) | 106 (68.8) | |
| Missing | 2 (1.4) | 1 (0.6) |
| . | Plerixafor (n = 148) . | Placebo (n = 154) . | P . |
|---|---|---|---|
| Median (± SD) age, y | 58.2 ± 8.4 | 58.4 ± 8.6 | .83 |
| Male sex, n (%) | 100 (67.6) | 107 (69.5) | .72 |
| Ethnic origin, n (%) | .10 | ||
| White | 117 (79.1) | 128 (83.1) | |
| Black | 18 (12.2) | 14 (9.1) | |
| Asian | 1 (0.7) | 3 (1.9) | |
| Hispanic/Latino | 11 (7.4) | 4 (2.6) | |
| Other | 1 (0.7) | 5 (3.2) | |
| Time from initial diagnosis to randomization, mo* | 10.7 (16.5) | 11.5 (16.9) | .66 |
| Stage of disease at initial diagnosis, n (%) | .09 | ||
| I | 28 (18.9) | 19 (12.3) | |
| II | 29 (19.6) | 44 (28.6) | |
| III | 91 (61.5) | 90 (58.4) | |
| Missing | 0 (0.0) | 1 (0.6) | |
| Remission status at enrollment, n (%) | .40 | ||
| First complete remission | 11 (7.4) | 18 (11.7) | |
| First partial remission | 129 (87.2) | 126 (81.8) | |
| Second complete remission | 0 (0.0) | 0 (0.0) | |
| Second partial remission | 8 (5.4) | 10 (6.5) | |
| Prior chemotherapy, n (%) | N/A | ||
| Yes | 144 (97.3) | 148 (96.1) | |
| No | 0 (0.0) | 0 (0.0) | |
| Missing | 4 (2.7) | 6 (3.9) | |
| Prior cytotoxic chemotherapy,† n (%) | 51 (34.5) | 48 (31.2) | |
| Prior radiotherapy, n (%) | .53 | ||
| Yes | 40 (27.0) | 47 (30.5) | |
| No | 106 (71.6) | 106 (68.8) | |
| Missing | 2 (1.4) | 1 (0.6) |
N/A indicates not applicable.
Data available from 147 patients in the plerixafor group and 153 patients in the placebo group.
Cytotoxic chemotherapy included prior treatment with any of the following agents: anthracycline, bortezomide, cyclophosamide, melphalan, and vincristine.
Efficacy
Mobilization.
A significantly greater proportion of patients in the plerixafor group reached the primary endpoint of collecting more than or equal to 6 × 106 CD34+ cells/kg in 2 or fewer days of apheresis than patients in the placebo group, 106 of 148 (71.6%) versus 53 of 154 (34.4%), respectively (P < .001). The estimated treatment effect was 37.2% (95% confidence interval, 26.8%-47.6%). The median number of apheresis days required to collect more than or equal to 6 × 106 CD34+ cells/kg was 1.0 day in the plerixafor group compared with 4.0 days in the placebo group (P < .001; Figure 3A). A significantly greater proportion of patients in the plerixafor group than patients in the placebo group also met the secondary efficacy endpoints of collecting more than or equal to 6 × 106 CD34+ cells/kg in 4 or fewer apheresis days, 75.7% versus 51.3%, respectively (P < .001) and of collecting more than or equal to 2 × 106 CD34+ cells/kg in 4 or fewer apheresis days, 95.3% versus 88.3%, respectively (P = .031).
Kinetics of CD34/kg collection. (A) Kaplan-Meier estimate of proportion of patients reaching 6 × 106 or more CD34+ cells/kg. (B) Median CD34+ cells collected on each apheresis day.
Kinetics of CD34/kg collection. (A) Kaplan-Meier estimate of proportion of patients reaching 6 × 106 or more CD34+ cells/kg. (B) Median CD34+ cells collected on each apheresis day.
Plerixafor-treated patients collected a total of 12.97 (± 10.67) × 106 (median, 10.96; range, 0.66-104.57) CD34+ cells/kg, whereas placebo-treated patients collected a total of 7.31 (± 5.49) × 106 (median, 6.18; range, 0.11-42.66) CD34+ cells/kg (P < .001). Figure 3B shows the median number of CD34+ cells collected for each apheresis by treatment group.
Peripheral blood CD34+ cell counts.
Day 4 and day 5 peripheral blood CD34+ cell counts were available for analyses in 122 plerixafor-treated patients and 123 placebo-treated patients. The peripheral blood CD34+ cell count before study treatments (day 4) was similar between groups. On day 5, 10 to11 hours after study treatment, plerixafor-treated patients had significantly higher peripheral blood CD34+ cell count (median, 109.0 cells/μL) than placebo-treated patients (median, 33.0 cells/μL; P < .001). The fold increase in peripheral blood CD34+ cell count from day 4 to day 5 was also significantly higher in the plerixafor-treated patients than in the placebo-treated patients, 4.8-fold versus 1.7-fold, respectively (P < .001). On day 5, only 2 patients (1.6%) in the plerixafor group had peripheral blood CD34+ cell count less than 20 cells/μL compared with 30 patients (24.6%) in the placebo group.
Transplantation and engraftment.
A total of 142 of 148 patients (95.9%) in the plerixafor group and 136 of 154 patients (88.3%) in the placebo group underwent transplantation. For the 6 patients in the plerixafor group who did not proceed to transplantation, 4 patients withdrew from study during G-CSF mobilization (before any plerixafor) and 2 patients withdrew from the study during the treatment/apheresis. For the 18 patients in the placebo group who did not proceed to transplantation, 4 patients withdrew from study during G-CSF mobilization (before any placebo), 7 patients withdrew from the study during the treatment/apheresis, and 7 patients entered the rescue procedures because they failed to collect sufficient cells for transplantation. Tandem transplantation was planned for 71 of 148 (48.0%) of patients in the plerixafor group and for 67 of 154 (43.5%) of the patients in the placebo group. However, only 32 patients in the plerixafor group and 24 patients in the placebo group underwent tandem transplantation.
The mean number of CD34+ cells transplanted was 5.84 (± 2.64) × 106 cells/kg (median, 5.37; range, 1.90-16.74) for the plerixafor-treated patients compared with 4.41 (± 2.22) × 106 cells/kg (median, 3.98; range, 1.76-16.88) for the placebo-treated patients. For patients who underwent tandem transplantation, the mean number of CD34+ cells transplanted for the first transplantation was 4.61 (± 2.34) × 106 cells/kg for the plerixafor-treated patients (n = 32) and 3.54 (± 1.57) × 106 cells/kg for the placebo-treated patients (n = 24). The mean number of CD34+ cells for the second transplanted was 4.48 (± 2.34) × 106 cells/kg for the plerixafor-treated patients and 2.97 (± 1.79) × 106 cells/kg for the placebo-treated patients.
The transplantation outcomes were similar between groups. A total of 99.3% of patients in the plerixafor group, and 100% of patients in the placebo group had successful neutrophil engraftment. The median time to neutrophil engraftment was 11 days in each group. The one patient in the plerixafor group who did not engraft died 10 days after transplantation. A total of 99.3% of patients in each treatment group had successful platelet engraftment. The median time to platelet engraftment was 18 days in each group. One patient in each group died at 10 and 13 days after transplantation, before engraftment was fully assessable. Using both laboratory and clinical data, no patients were identified as having experienced graft failure in either group.
Efficacy outcomes for rescue patients.
Seven patients in the placebo group failed to collect sufficient number of CD34+ cells for transplantation and entered the rescue protocol; no patients from the plerixafor group entered the rescue protocol. All rescue patients collected more than or equal to 2 × 106 cells/kg in 4 days of apheresis. All rescue patients (n = 7) underwent transplantation and 4 of 7 underwent tandem transplantation. All rescue patients had successful neutrophil and platelet engraftment. Through 12 months of follow-up, all of the rescue patients were alive and all had durable grafts.
Patient survival.
Through 12 months' follow-up, 141 of 148 (95.3%) of the plerixafor-treated patients were alive compared with148 of 154 (96.1%) of the placebo-treated patients. A total of 13 patients died during the study: 7 in the plerixafor group and 6 in the placebo group. All except one death occurred after transplantation. The most common cause of death was disease progression (3 of 7 deaths in the plerixafor group and 5 of 6 deaths in the placebo group).
Safety
Adverse events.
A total of 147 patients in the plerixafor group and 151 patients in the placebo group were included in the primary safety population. One patient in the plerixafor group and 3 patients in the placebo group were enrolled and randomized but did not receive any G-CSF or plerixafor, and thus were excluded from the safety analyses. The overall incidence of adverse events was similar between the 2 groups in each of the study periods. During period 1, 140 of 147 (95.2%) of patients in plerixafor group and 140 of 151 (97.2%) of patients in the placebo group experienced at least one adverse event. Most of these adverse events were mild to moderate. The most common adverse events considered related to plerixafor in period 1 were gastrointestinal disorders and injection site reactions (Table 2). The majority of the adverse events that occurred in periods 2 to 5 are expected and common after ablative chemotherapy and hematopoietic stem cell transplantation.
Most common adverse events related to study treatment that occurred in 5% or more of patients in either treatment group during period 1
| . | Plerixafor (n = 147) . | Placebo (n = 151) . |
|---|---|---|
| Any related adverse events, n (%) | 95 (64.6) | 67 (44.4) |
| Gastrointestinal disorders | ||
| Diarrhea | 27 (18.4) | 8 (5.3) |
| Nausea | 24 (16.3) | 11 (7.3) |
| Vomiting | 8 (5.4) | 4 (2.7) |
| General disorders and administration site conditions | ||
| Fatigue | 12 (8.2) | 5 (3.3) |
| Injection site erythema | 30 (20.4) | 5 (3.3) |
| Musculoskeletal and connective tissue disorders | ||
| Bone pain | 14 (9.5) | 12 (7.9) |
| Nervous system disorders | ||
| Headache | 8 (5.4) | 13 (8.6) |
| Paresthesia | 11 (7.5) | 11 (7.3) |
| . | Plerixafor (n = 147) . | Placebo (n = 151) . |
|---|---|---|
| Any related adverse events, n (%) | 95 (64.6) | 67 (44.4) |
| Gastrointestinal disorders | ||
| Diarrhea | 27 (18.4) | 8 (5.3) |
| Nausea | 24 (16.3) | 11 (7.3) |
| Vomiting | 8 (5.4) | 4 (2.7) |
| General disorders and administration site conditions | ||
| Fatigue | 12 (8.2) | 5 (3.3) |
| Injection site erythema | 30 (20.4) | 5 (3.3) |
| Musculoskeletal and connective tissue disorders | ||
| Bone pain | 14 (9.5) | 12 (7.9) |
| Nervous system disorders | ||
| Headache | 8 (5.4) | 13 (8.6) |
| Paresthesia | 11 (7.5) | 11 (7.3) |
Serious adverse events.
During the study, 57 of 147 (38.8%) of the patients in the plerixafor group and 48 of 151 (31.8%) of the patients in the placebo group had at least one serious adverse event. The majority of these serious adverse events occurred in periods 2 to 5, and all were considered not to be related to study treatment.
Adverse events leading to discontinuation of study treatment.
One patient in the plerixafor group experienced diarrhea and fatigue during treatment, and study drug was discontinued after receiving 3 doses. Two patients in the placebo group discontinued study treatment, one resulting from enlarged spleen and the other resulting from nausea, abdominal pain, and vomiting. All 3 patients remained in the study.
Adverse events leading to study withdrawal.
Four patients in the plerixafor group withdrew from the study because of adverse events. Two patients withdrew because of disease progression during treatment/apheresis phase, and one patient withdrew because of cardiac disease before beginning of G-CSF and plerixafor mobilization.
Discussion
In this international, phase 3, multicenter, randomized, double-blind, placebo-controlled study, a significantly higher proportion of patients treated with plerixafor and G-CSF achieved the primary endpoint of collecting more than or equal to 6 × 106 CD34+ cells/kg in 2 or less apheresis days than patients treated with placebo and G-CSF, 71.6% vs 34.4%, respectively (P < .001). Plerixafor was well tolerated, and transplantation of hematopoietic stem cells mobilized by plerixafor and G-CSF resulted in prompt and durable engraftment. One of the most important findings of this study is that the addition of plerixafor allowed for collection of the optimal CD34+ cell dose in a median of 1 apheresis day compared with a median of 4 apheresis days with G-CSF alone. Eliminating the need for additional apheresis sessions may improve patients' quality of life, reduce cost, and allow for more efficient use of resources.15
The success of high-dose chemotherapy and hematopoietic stem cell transplantation is contingent on collecting sufficient CD34+ cells to support a single or tandem autologous transplantations in patients with multiple myeloma. Although most patients in this study collected sufficient cells for transplantation, 65.6% of patients mobilized with G-CSF alone failed to collect more than or equal to 6 × 106 CD34+ cells/kg within the first 2 days of apheresis and 48.7% failed to collect this target within 4 days of apheresis. The more than or equal to 6 × 106 CD34+ cells/kg target was selected to ensure that all the patients had the option to receive more than or equal to 3 × 106 CD34+ cells/kg per transplantation because tandem transplantation may provide a longer disease-free survival than single transplantation.4-6
These failure rates are higher than those reported in single-center studies (5%-23%).7-9,16,17 Direct comparison of the findings of this study with these single-center studies is difficult because we used a standard dose of G-CSF (10 μg/kg per day), a 3.0× blood volume apheresis, and a primary endpoint that included the number of apheresis days. Although the use of higher doses of G-CSF could result in a higher CD34+ cell yield,18 other studies have reported that the failure rates remained high despite the higher doses of G-CSF used.7-9,16,17 In addition, variable CD34+ cell collection targets were used in these studies, and the duration of apheresis required to achieve these collection targets often were not included in the definition of mobilization failure.
The addition of chemotherapy to G-CSF can result in higher CD34+ cell yield than G-CSF alone. This higher yield is obtained at the expense of significant toxicities and additional costs associated with management and treatment of chemotherapy-induced toxicities.8,19-21 We elected to use G-CSF alone as the comparative arm rather than G-CSF and chemotherapy because the clinical benefits of adding chemotherapy remain controversial in patients with multiple myeloma and there are no standard or approved chemotherapeutic agents for mobilization.7,22 Blinding of the study would not be feasible because of the known toxicities of chemotherapy and the need to continuously monitor either white blood cell count and/or peripheral blood CD34+ cell count to determine the optimal day to initiate apheresis.23-25
In contrast, the higher CD34+ cell yield achieved with plerixafor is not associated with significant toxicity or adverse events. Consistent with the phase 2 and the compassionate use program experience, plerixafor was generally well tolerated and the adverse events were generally mild.14,26 The most common adverse events that were related to plerixafor in this study were gastrointestinal and injection site reactions.
The impact of tumor cell contamination in peripheral blood stem cell products and patient survival remains controversial.27,28 Various methods have been used to reduce tumor cell contamination in the apheresis product for autologous transplantation, but these methods failed to improve disease-free or overall survival.29,30 To date, there is no evidence of tumor cell mobilization in plerixafor-treated patients in phase 2 studies. Plerixafor and G-CSF may cause mobilization of leukemic cells and subsequent contamination of the apheresis product.31 Therefore, plerixafor is not intended for mobilization and harvest in patients with leukemia. As such, there may be a risk that tumor cells may be released from the marrow and subsequently collected in the leukapheresis product when plerixafor is used with G-CSF. Additional studies are warranted.
Various risk factors, including previous chemotherapy, have been reported to be associated with suboptimal mobilization.9,16,32 Treatment with thalidomide-dexamethasone has, in contrast, not been shown to have any adverse effects on mobilization.33,34 Recent studies have reported that the use of lenalidomide may increase the risk of mobilization failures with either G-CSF alone or G-CSF and chemotherapy.35-37 In this study, 7 patients in the plerixafor group and 11 patients in the placebo group received lenalidomide before mobilization. Although all of these patients mobilized more than or equal to 2 × 106 CD34+ cells/kg, 5 of 7 (71.4%) of plerixafor-treated patients and 3 of 11 (27.3%) of placebo-treated patients mobilized more than or equal to 6 × 106 CD34+ cells/kg in 2 or fewer apheresis days, which are consistent with the entire study population. Future studies are warranted to evaluate the efficacy of plerixafor in patients previously exposed to lenalidomide.
Consistent with previous studies, the addition of plerixafor resulted in a median of 4.8-fold increase in peripheral blood CD34+ cell count compared with only a 1.7-fold increase with G-CSF alone.38-40 Only 1.6% of patients in the plerixafor group had peripheral blood CD34+ cell count of less than 20 CD34+ cells/μL compared with 24.6% of patients in the placebo group on the first day of apheresis or 10 to 11 hours after study treatment. This observation confirms that the current dosing regimen of plerixafor reliably and predictably mobilizes CD34+ cells in most patients.
In conclusion, results from this phase 3 study demonstrated that a mobilization regimen of plerixafor and G-CSF resulted in a statistically significantly higher probability of achieving the optimal CD34+ cell target for tandem transplantation in fewer apheresis procedures compared with placebo and G-CSF. This enhanced mobilization by plerixafor was well tolerated and resulted in successful and durable engraftment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the Data Safety Monitoring Board, including James C. Wade (chair), Donna S. Neuberg (statistician and member), Robert Nelson (secretary and member), Koen Van Besien (member), and Ginna G. Laport (member) for their time and commitment to this study; Jennifer Angell (Genzyme Corporation) for statistical support; Agnes Costello (Genzyme Corporation) for help with the content and coordinating the revisions of this manuscript; and the many research coordinators and nurses for their time and dedication to the execution of this study.
This work was wholly supported by research funding from Genzyme Corporation to all authors and to investigators listed in the Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Authorship
Contribution: J.F.D., E.A.S., A.N., I.N.M.M., J.L.K., P.J.S., R.T.M., C.H., S.F., M.H., and D.C. participated in the study design, performed the research, and interpreted the data; G.B. and G.C. participated in the study design and interpreted the data; J.F.D. and G.C. wrote the manuscript; and E.A.S., A.N., I.N.M.M., J.L.K., R.T.M., C.H., S.F., M.H., and D.C. edited and reviewed the manuscript.
Conflict-of-interest disclosure: J.F.D., E.A.S., A.N., I.N.M.M., J.L.K., R.T.M., C.H., and S.F. received research funding and honoraria from Genzyme Corporation. G.B. and G.C. were employees of the former AnorMed Inc; now both are consultants for Genzyme Corporation. All other authors declare no competing financial interests.
Correspondence: John F. DiPersio, Division of Oncology, Siteman Cancer Center, Washington University School of Medicine, 660 S Euclid, St Louis, MO 63110; e-mail: jdipersi@im.wustl.edu.