Abstract
Activation of the pre-B-cell receptor (pre-BCR) in the bone marrow depends on both tonic and ligand-induced signaling and leads to pre-BII-cell proliferation and differentiation. Using normal mouse bone marrow pre-BII cells, we demonstrate that the ligand-induced pre-BCR activation depends on pre-BCR/galectin-1/integrin interactions leading to pre-BCR clustering at the pre-BII/stromal cell synapse. In contrast, heparan sulfates, shown to be pre-BCR ligands in mice, are not implicated in pre-BCR relocalization. Inhibition of pre-BCR/galectin-1/integrin interactions has functional consequences, since pre-BII-cell proliferation and differentiation are impaired in an in vitro B-cell differentiation assay, without affecting cellular apoptosis. Most strikingly, although galectin-1–deficient mice do not show an apparent B-cell phenotype, the kinetics of de novo B-cell reconstitution after hydroxyurea treatment indicates a specific delay in pre-BII-cell recovery due to a decrease in pre-BII-cell differentiation and proliferation. Thus, although it remains possible that the pre-BCR interacts with other ligands, these results highlight the role played by the stromal cell–derived galectin-1 for the efficient development of normal pre-BII cells and suggest the existence of pre-BII–specific stromal cell niches in normal bone marrow.
Introduction
Precursor B cells differentiate and proliferate in close contact with stromal cells that are key constituents of the bone marrow (BM) microenvironment. Stage-specific cellular niches and movement between the different niches have been identified for part of the B lymphopoiesis.1 The earliest pre-pro-B precursors were found in contact with CXCL12-expressing reticular cells, whereas pro-B/pre-BI cells, undergoing IgH gene rearrangements, locate near IL7-producing stromal cells. When functional Igμ chains are produced, they associate with the surrogate light chain (SLC), composed of λ5 and VpreB, and with the signaling molecules CD79a and CD79b, to form the pre-B-cell receptor (pre-BCR) expressed at the surface of pre-BII cells. At this stage, cells leave the IL7-expressing stromal cells2 but no pre-BII–specific cellular niche has been identified so far. However galectin-1 (GAL1) and heparan sulfates (HSs), produced by the BM microenvironment, have been described to bind to the pre-BCR.3-5
Pre-BCR expression represents a crucial step in B-cell development as it controls pre-BII-cell proliferation and differentiation.6 Pre-BCRs also mediate allelic exclusion at the IgH locus7,8 and selection of the Igμ chain repertoire,9 leading to the counterselection of autoreactive Igμ chains.10 Although the signaling pathways downstream of the pre-BCR are well characterized,11 the mechanisms implicated in pre-BCR activation are not completely understood. Both constitutive12,13 and ligand-induced3,14 signaling have been described. In the mouse, Bradl et al4 and Vettermann et al5 showed that pre-BCR binding to stromal cells required the N-terminal unique region of λ5 (λ5UR) and stroma cell–associated HS. In humans, we reported that GAL1 binds to the λ5UR by a protein/protein interaction but also to glycosylated integrins present on stromal and pre-B cells.3,15 We also determined that pre-B-cell integrin clustering in the presence of GAL1 is sufficient to promote pre-BCR relocalization at the contact zone between pre-B and stromal cells leading to pre-BCR activation.15 In both mice and humans, biochemical characterization of pre-BCR/ligand interactions has been performed using only pre-B-cell lines, which overexpressed the pre-BCR. Moreover, the physiological consequences of ligand-induced pre-BCR clustering and the respective roles of HS and GAL1 in this process remain to be elucidated.
In this study, using normal murine pre-BII cells, we demonstrated that pre-BCR clustering and activation depend on specific contacts between stromal cells and pre-BII cells, and most precisely on pre-BCR/GAL1/integrin interactions. Most importantly, we showed that these interactions are required for efficient in vivo development of normal pre-BII cells.
Methods
Mice and cell lines
GAL1-deficient mice16 and C57Bl/6 mice were housed under specific pathogen-free conditions and handled in accordance with European directives, with the approval of the Institutional Review Board of Inserm/CNRS. GAL1−/− mice were backcrossed for 6 generations onto a C57Bl/6 background. Normal pre-B cells and the B62c pre-B-cell line (kind gift from C. Paige, Ontario Cancer Institute, Toronto, ON) were cultivated in IMDM, 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μM β-mercaptoethanol, and IL7 supernatant (produced by J558-IL7 cells17 ; kind gift from A. Cumano, Pasteur Institute, Paris, France). The murine OP9 BM stromal cell line18 and the Phoenix-eco retroviral packaging cell line19 were grown in MEMα, 20% FCS and DMEM, 10% FCS, respectively.
Hydroxyurea treatment of mice
GAL1−/− and C57Bl/6 mice (10-16 weeks old) received 4 intraperitoneal injections of hydroxyurea (each of 1 mg/g body weight) at the rate of 2 injections separated by 7 hours per day for 2 days. BM B-cell differentiation was analyzed by flow cytometry 2, 7, and 35 days after treatment.
Production and purification of recombinant proteins
Production and purification of the human GAL1 (hGAL1) was previously described.15 The murine Vpre-B1 (mVpB) and λ5 (mλ5) coding sequences were amplified using cDNA from B62c cells with primers 1 + 2 and 4 + 5, respectively (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). To produce a murine single-chain recombinant surrogate light chain (mSLC), mVpB was linked to mλ5 with a linker peptide. The mλ5 and mVpB mutants deprived of their unique region (UR) were amplified from the mSLC vector using 4 + 6 and 1 + 3 primers, respectively. The mλ5 and mVpB mutants were cloned together to form a mutant mSLC deprived of both URs (mSLCΔΔ). Alternatively, the mλ5 mutant without its UR (primers 4 + 6) was cloned with the complete mVpB (primers 1 + 2) to form the mSLCΔ mutant. Normal and mSLC mutants were cloned in pET3a vector, expressed in Escherichia coli Rosetta strain, and purified as previously described.3
Mouse and human λ5 URs were amplified from the mλ5 and the hλ5 (kind gift from H. M. Jäck, Nikolaus-Fiebiger-Center for Molecular Medicine, Erlangen, Germany) encoding vectors and cloned into pGEX2T. The mλ5 UR and hλ5 URs coupled to GST (GST-mλ5UR, oligos 7 + 8 and GST-hλ5UR, oligos 9 + 10, respectively), and the GST control (GST, oligos 11 + 12) was expressed in the Rosetta strain and purified on Talon superflow Metal Affinity Resin (CLONTECH, Palo Alto, CA) followed by a Superdex S75 size exclusion column (AKTA system; Pharmacia biosensor; Uppsala, Sweden).
Flow cytometry and cell sorting
Single-cell suspensions were stained using standard protocols for flow cytometry using the Abs listed in Table S2. In the proliferation assay, bromodeoxyuridine (BrdU) incorporation was revealed using the BrdU Flow Kit (BD Biosciences, Pont de Claix, France). Apoptosis was evaluated using annexin V (BD Biosciences) and 7-amino-actinomycin D (7-AAD). Cell-cycle analysis was performed by staining double-stranded nucleic acids with TO-PRO-3 (Invitrogen, Cergy Pontoise, France) following fixation and permeabilization of the cells with the Cytofix/Cytoperm kit (BD Biosciences).
Sorting of pro-B/pre-BI and large pre-BII cells used for in vitro differentiation or confocal microscopy were performed on single BM cell suspensions, initially enriched for B cells using B220 microbeads, and separated on the autoMACS Separator (Miltenyi Biotec, Paris, France). Cells were stained and gated as shown in Figure S1. For Western blotting, pro-B/pre-BI and large and small pre-BII were sorted similarly and immature and recirculating B cells, as B220+/Igκ/λ+.
Fluorescence-activated cell sorting (FACS) analysis was performed on a FACSCantoII and cell sorting on a FACSAria (BD Biosciences) apparatus. Data were analyzed with the DiVa (BD Biosciences) or FlowJo (TreeStar, Ashland, OR) software and represented when required with the logical display.20
Biochemical and immunoblot analysis
The GST-fusion proteins (20 μg) were incubated with sepharose glutathione beads (Amersham, Arlington Heights, IL) at 4°C in lysis buffer (Tris 20 mM, NP40 1%, NaCl 150 mM, EDTA 1 mM, β-mercaptoethanol 1 mM, and protease inhibitors) and the hGAL1 (200 ng) was then added to these beads. Proteins were separated on SDS–polyacrylamide gel electrophoresis (PAGE), followed by Western blotting using the anti-GAL1 AS15 or anti-GST mAb (Sigma-Aldrich, Lyon, France) and detection by protein-A HRP (Sigma-Aldrich) or goat anti–mouse Ab conjugated to HRP (Sigma-Aldrich), respectively.
Proteins (50 μg) from OP9 cells or B-cell subpopulations were separated on SDS-PAGE and GAL1 detection was performed as above. The relative GAL1 expression level was normalized by comparison with β-actin, detected by the AC15 Ab (Sigma-Aldrich), revealed with a goat anti–mouse HRP Ab, and quantified using the Multi Gauge v2.3 software (Fujifilm; Bedford, United Kingdom).
Stable inhibition of GAL1 expression by RNA interference
The 19-nt-long siGAL1 sequence (5′-GGGAATGTCTCAAAGTTCG-3′) was selected using the SMARTselection algorithm (Dharmacon, Brebiéres, France) and did not present any matches with other transcripts in the mouse GenBank database.21 The sequence was cloned into thepSUPERretro.neo+gfp retroviral vector (pSR; OligoEngine, Seattle, WA) following the manufacturer's instructions. The pSR empty vector and the shGAL1 construct were transfected separately into the Phoenix retroviral producer cell line, using the FuGene 6 reagent (Roche, Meylan, France). Forty-eight hours after transfection, supernatants were used to infect OP9 cells in presence of 8 μg/mL sequabrene (Sigma-Aldrich). Retrovirally transduced OP9 cells were then sorted on the basis of GFP expression.
Immunofluorescence imaging
OP9 cells seeded on glass coverslips were allowed to grow for 24 hours. Sorted normal mouse pre-BII cells were cultured on OP9 cells for 2 hours in the presence or absence of the indicated proteins, carbohydrates, or blocking antibodies. For heparitinase treatment, stromal cells were incubated for 2 hours with 5 to 50 mU/mL heparitinase before being cocultured with the B62c cells in the continuous presence of heparitinase. After fixation, cells were stained in PBS/BSA 0.5% with the Abs listed in Table S2, mounted, and analyzed on a LSM510 Carl Zeiss confocal microscope (Heidelberg, Germany). Lactose, thiodigalactoside, and maltose were purchased from Sigma-Aldrich and heparitinase was purchased from Seikagaku (Tokyo, Japan).
In vitro pre-B-cell proliferation and differentiation on OP9 stromal cells
The in vitro pre-B-cell differentiation/proliferation protocol used was adapted from Rolink et al.22 Sorted (105) pro-B/pre-BI cells were cocultivated with irradiated (40 Gy) OP9-derived stromal cells in 24-well plates, for 2 days in supplemented IMDM. Cell cultures were performed in the presence of 50 μg/mL recombinant proteins. Experiments were also performed after an initial pro-B/pre-BI cell expansion on irradiated OP9 cells in the presence of IL7. Then, live cells were selected on the basis of 7-AAD exclusion and the few CD2+ cells, which passed the pre-BCR checkpoint, were excluded by cell sorting experiments. Pro-B/pre-BI cells (2 × 105) were then cultured for differentiation as described in “Results.”
For the proliferation assay, BrdU incorporation into pro-B/pre-BI cells was performed by incubating the cells with 10 μM BrdU for 1 hour at 37°C in the presence of IL7. CD2+ cells were excluded before starting the in vitro differentiation/proliferation assay.
Pre-BCR activation during large pre-BII/OP9 cocultures
Sorted CD2− large pre-BII cells (0.5 to 1 × 105) were incubated for 1 hour at 37°C without OP9 cells, with OP9-pSR cells in presence of 50 μg/mL mSLCΔΔ, or with OP9-shG1 cells in presence of 50 μg/mL mSLC. Cells were fixed in 2% PFA and permeabilized in ice-cold methanol. Phosphorylated BLNK was detected using an anti–phospho-BLNK (pY84) mAb (J117-1278; BD Biosciences) and compared with its isotype control. FACS acquisition was performed on a LSRII equipped with a laser 561 nm (BD Biosciences).
Results
Pre-BCRs, GAL1, and pre-B-cell integrins relocalize in the synapse formed between normal large pre-BII cells and stromal cells
To analyze the physiological consequence of GAL1-induced pre-BCR signaling, we first studied the formation of the pre-BII/stromal cell synapse. We used normal murine large pre-BII cells that were purified from the bone marrow by negative cell sorting (purity > 92%; Figure S1). These cells were cocultured with OP9 stromal cells for 2 hours. Figure 1A left shows one representative confocal section of such cultures. Red and white stars indicate pre-BII cells with and without a relocalized pre-BCR, respectively. Eighty to 90 percent of pre-BII cells expressed detectable amounts of surface pre-BCRs. By counting large numbers of cells (> 900), we observed that the frequency of cells with relocalized pre-BCRs reached 55%. Moreover, large pre-BII cells were labeled by the pre-BCR–specific SL156 monoclonal antibody (mAb)23 (Figure 1A right) and not by an anti-Igκ mAb (data not shown), confirming that these cells are pre-BCR+ pre-BII cells. By contrast, using the same culture conditions, the BCR expressed by normal Igκ+ immature B cells did not relocalize (data not shown). Finally, as previously observed with pre-B-cell lines, the pre-BCR and GAL1 were colocalized in the murine pre-BII/stromal cell synapse (Figure 1A right).
The pre-BCR, GAL1, and pre-B-cell integrins relocalize in the synapse formed between normal large pre-BII cells and stromal cells. (A) Normal murine BM pre-BII cells (B220+CD19+CD117−Igκ/λ− large forward scatter) were cocultured on OP9 stromal cells and fixed before staining. (Left) Representative view of cocultures stained with an anti-IgM Ab. Red and white stars indicate cells with relocalized and nonrelocalized pre-BCR, respectively. (Right) Cocultures were stained with the SL156 anti–pre-BCR Ab (green) and anti-GAL1 AS (red). (B) Costaining of cocultures using antibodies specific for GAL1, IgM, and CD24 and α4, β1, αL, and β2 integrins (63×/1.4 NA oil objective). (C) Western blot analysis of GAL1 expression by the BM B-cell subpopulations revealed by anti-GAL1 AS and anti–β-actin mAb.
The pre-BCR, GAL1, and pre-B-cell integrins relocalize in the synapse formed between normal large pre-BII cells and stromal cells. (A) Normal murine BM pre-BII cells (B220+CD19+CD117−Igκ/λ− large forward scatter) were cocultured on OP9 stromal cells and fixed before staining. (Left) Representative view of cocultures stained with an anti-IgM Ab. Red and white stars indicate cells with relocalized and nonrelocalized pre-BCR, respectively. (Right) Cocultures were stained with the SL156 anti–pre-BCR Ab (green) and anti-GAL1 AS (red). (B) Costaining of cocultures using antibodies specific for GAL1, IgM, and CD24 and α4, β1, αL, and β2 integrins (63×/1.4 NA oil objective). (C) Western blot analysis of GAL1 expression by the BM B-cell subpopulations revealed by anti-GAL1 AS and anti–β-actin mAb.
Since pre-B-cell integrins drive the pre-BCR relocalization process and initiate pre-BCR signaling in humans, we determined which integrin members are expressed by murine pre-BII cells. Confocal microscopy analysis of large pre-BII/OP9 cocultures showed that α4, αL, β1, as well as β2 integrins are expressed and colocalized with the pre-BCR and GAL1 at the contact zone between pre-BII and stromal cells (Figure 1B). In contrast, CD24, which does not bind to GAL1, was never recruited at the contact zone between the 2 cells (Figure 1B). Integrin expression was confirmed by flow cytometry and showed that the α4β1 (VLA4) and αLβ2 (LFA1) integrins present in the pre-B/stromal cell synapse originated from pre-BII cells since, among the tested integrins, VLA5 is the only one expressed by OP9 stromal cells (Figure S2).
Because the existence of a pre-BCR ligand produced by pre-B cells was proposed,12 we investigated whether BM B-cell subpopulations express GAL1 at the protein level. Interestingly, GAL1 was not detected in any sorted B-cell subpopulations, either by Western blot (Figure 1C) or by flow cytometry (data not shown). By contrast, GAL1 is strongly expressed by the OP9 (Figure 1C) and the MS5.1 stromal cell lines.3
Altogether, these data indicate that synapse formation between normal murine pre-BII and stromal cells involves pre-BCR, GAL1, and pre-B-cell integrins. In addition, as stromal cells, but not pre-B cells, express GAL1, these results show the importance of the BM microenvironment for pre-BCR clustering.
Relocalization of the pre-BCR at the pre-B/stromal cell synapse depends on pre-BCR/GAL1/integrin interactions
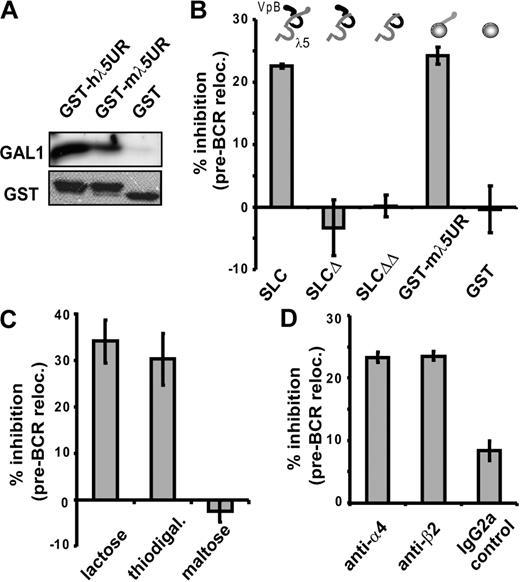
To determine whether pre-BCR relocalization depends on SLC/GAL1/integrin interactions as in humans,3 we first assessed whether the unique region of the mouse λ5 interacts with GAL1. Recombinant proteins corresponding to the mouse and the human unique region of λ5 fused to GST (GST-mλ5UR and GST-hλ5UR, respectively) used in pull-down experiments showed that both proteins bind to hGAL1, in contrast to the control GST (Figure 2A). These interactions involve direct peptide recognition, since these recombinant proteins are not glycosylated.
Relocalization of the pre-BCR at the pre-B/stromal cell synapse depends on pre-BCR/GAL1/integrin interactions. (A) GST-hλ5UR, GST-mλ5UR, and GST proteins were preincubated with sepharose glutathione beads before incubation with recombinant hGAL1. Coprecipitated proteins were revealed by Western blot using anti-GAL1 AS or anti-GST mAbs. (B-D) Large pre-BII/OP9 cocultures were performed without or with the following: 40 μg/mL mSLC, mSLCΔ, or mSLCΔΔ; 100 μg/mL GST-mλ5UR or control GST (B); 0.2 M of the indicated carbohydrate (C); 20 μg/mL anti-α4 and -β2 blocking Abs or an isotype control (D). The percentage of cells with a relocalized pre-BCR was determined by confocal microscopy after anti-IgM staining. For each condition, 121 to 487 cells were examined. The histograms represent the percentage of inhibition of pre-BCR relocalization compared with controls (at least 3 independent experiments). The recombinant proteins used in panel B are schematically represented above the histogram. Errors bars represent SD.
Relocalization of the pre-BCR at the pre-B/stromal cell synapse depends on pre-BCR/GAL1/integrin interactions. (A) GST-hλ5UR, GST-mλ5UR, and GST proteins were preincubated with sepharose glutathione beads before incubation with recombinant hGAL1. Coprecipitated proteins were revealed by Western blot using anti-GAL1 AS or anti-GST mAbs. (B-D) Large pre-BII/OP9 cocultures were performed without or with the following: 40 μg/mL mSLC, mSLCΔ, or mSLCΔΔ; 100 μg/mL GST-mλ5UR or control GST (B); 0.2 M of the indicated carbohydrate (C); 20 μg/mL anti-α4 and -β2 blocking Abs or an isotype control (D). The percentage of cells with a relocalized pre-BCR was determined by confocal microscopy after anti-IgM staining. For each condition, 121 to 487 cells were examined. The histograms represent the percentage of inhibition of pre-BCR relocalization compared with controls (at least 3 independent experiments). The recombinant proteins used in panel B are schematically represented above the histogram. Errors bars represent SD.
The role of λ5/GAL1 interactions on synapse formation was analyzed by adding different inhibitory proteins to pre-BII/stromal cell cocultures (Figure S3 for protein purity). Pre-BCR relocalization was inhibited by 22.5% in the presence of recombinant soluble mSLC (Figure 2B). This inhibition was highly significant since the P value reached .001 when calculated on the percentage of cells with a relocalized pre-BCR in the presence of recombinant mSLC versus without mSLC (Table S3). On the contrary, recombinant mSLC mutants from which either the unique region of λ5 (mSLCΔ) or of both the unique regions of λ5 and VpreB (mSLCΔΔ) were truncated had no inhibitory effect on pre-BCR relocalization (Figure 2B). Addition of GST-mλ5UR to the cocultures confirmed the role of this region in synapse formation, since the percentage of inhibition of the pre-BCR relocalization reached 24.2% (P = .007).
In addition to protein/protein interaction with the pre-BCR, GAL1 also interacts with glycosylated chains of integrins.3,15 Hence, GAL1 interaction with carbohydrate residues was assessed by addition of β-galactoside competitors, such as lactose and thiodigalactoside to pre-BII/stromal cell cocultures. These treatments resulted in significant inhibition of the pre-BCR relocalization, reaching 34% (P = .007) and 30.2% (P = .013), respectively, whereas addition of maltose as a control had no effect on the process (Figure 2C; Table S3).
The role of integrins in pre-BCR clustering was also characterized by the addition to the cocultures of neutralizing anti-α4 and anti-β2 integrin antibodies, which impair, respectively, VLA4 and LFA1 interactions with their stromal ligands. As shown in Figure 2D and Table S3, neutralizing anti-α4 and anti-β2 integrin mAbs led to a significant inhibition of pre-BCR relocalization, in comparison with an isotypic control mAb. Thus, interaction between VLA-4 and LFA-1 integrins with their ligands and the resulting “outside-in” integrin activation are required for driving pre-BCR relocalization into the pre-B/stromal cell synapse.
Altogether, these results indicate that relocalization of the pre-BCR expressed by normal mouse pre-BII cells depends on the interactions between GAL1, pre-BCR, and pre-B-cell integrins but also on the interactions between pre-B-cell integrins and their stromal ligands.
GAL1, but not HS, is required for the pre-BCR relocalization process
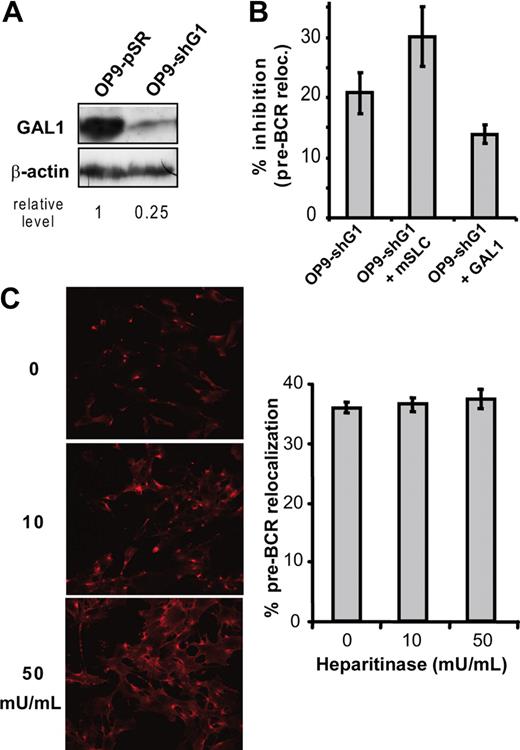
The direct demonstration of the implication of GAL1 in the pre-BCR relocalization was obtained by knocking down GAL1 expression in OP9 stromal cells. Compared with OP9 cells infected with the empty vector (OP9-pSR), GAL1 expression was decreased by 75% using a GAL1-specific shRNA (shGAL1; Figure 3A). When pre-BII/stromal cell cocultures were performed using shGAL1-infected OP9 cells (OP9-shG1), the pre-BCR relocalization was decreased by 20% with P = .001 (Figure 3B; Table S3). Furthermore, addition of mSLC to such cocultures enhanced the inhibition of the pre-BCR relocalization to 30% (P = .017). Finally, since addition of recombinant GAL1 to cocultures with OP9-shG1 significantly reversed the inhibition of pre-BCR relocalization (P = .02), we concluded that GAL1 was directly implicated in this process.
Galectin-1, but not heparan sulfates, is required for pre-BCR clustering. (A) The level of GAL1 expression in OP9-shG1 cells was evaluated by Western blotting in comparison with OP9-pSR control cells. Relative GAL1 expression level was normalized by comparison with β-actin. (B) Large pre-BII cells were cocultured with OP9-pSR or OP9-shG1 cells. The percentage of cells with a relocalized pre-BCR was determined by confocal microscopy after anti-IgM staining (at least 3 independent experiments). Data are expressed as percentage of inhibition of pre-BCR relocalization, compared with the OP9-pSR control. (C) OP9 stromal cells were preincubated with increasing amount of heparitinase before cocultures with B62c cells; (left) cellular staining with the anti–neo-hep mAb; (right) percentage of cells with a relocalized pre-BCR. Data are representative of 3 independent experiments. Cells (144 to 543) were examined for pre-BCR relocalization in each condition. Errors bars represent SD.
Galectin-1, but not heparan sulfates, is required for pre-BCR clustering. (A) The level of GAL1 expression in OP9-shG1 cells was evaluated by Western blotting in comparison with OP9-pSR control cells. Relative GAL1 expression level was normalized by comparison with β-actin. (B) Large pre-BII cells were cocultured with OP9-pSR or OP9-shG1 cells. The percentage of cells with a relocalized pre-BCR was determined by confocal microscopy after anti-IgM staining (at least 3 independent experiments). Data are expressed as percentage of inhibition of pre-BCR relocalization, compared with the OP9-pSR control. (C) OP9 stromal cells were preincubated with increasing amount of heparitinase before cocultures with B62c cells; (left) cellular staining with the anti–neo-hep mAb; (right) percentage of cells with a relocalized pre-BCR. Data are representative of 3 independent experiments. Cells (144 to 543) were examined for pre-BCR relocalization in each condition. Errors bars represent SD.
In addition to GAL1, HSs have been reported to bind mouse pre-BCRs.4 To determine whether HSs may also be involved in pre-BCR relocalization, we analyzed B62c pre-B cells cocultured with OP9 stromal cells treated with increasing amount of heparitinase, which cleaves surface HS. The efficiency of the heparitinase treatment was checked using the 3H10 antibody that detects specifically neo-epitopes (neo-Hep) created following HS cleavage, both by FACS (not shown) and by confocal microscopy (Figure 3C left). However, this treatment had no effect on the number of cells with a relocalized pre-BCR (Figure 3C right), even using 5 times more heparitinase than previously described.4
These results demonstrate that, of the 2 described mouse pre-BCR ligands, GAL1 but not HS is involved in the relocalization of pre-BCRs expressed by normal murine pre-BII cells.
Pre-BCR/GAL1/integrin interactions trigger pre-BII-cell proliferation and differentiation
To test the physiological relevance of the pre-BCR relocalization, we used the in vitro B-cell differentiation/proliferation system described by Rolink et al.22 In this assay, purified pro-B/pre-BI cells (Figure S1B) cocultured with OP9 stromal cells proliferate in the presence of IL7, and differentiate toward immature B cells upon IL7 removal. However, a small amount of pro-B/pre-BI cells may differentiate into pre-BII cells even in the presence of IL7.24 These pre-BII cells, which expressed CD2, shown to be up-regulated upon pre-BCR activation,25 were sorted out before the differentiation step in the absence of IL7 (Figure S1C).
In cocultures without IL7, cell proliferation was determined by measuring the loss of BrdU incorporated into pro-B/pre-BI cells. When pre-BCR/GAL1/integrin interactions were inhibited by adding recombinant mSLC in the medium, cell proliferation was highly impaired compared with cultures with recombinant mSLCΔΔ or mSLCΔ that do not inhibit pre-BCR relocalization (Figure 4A,B and data not shown). The proliferation was also significantly decreased in cocultures performed on OP9-shG1 knockdown stromal cells. Moreover, when recombinant mSLC was added in pro-B/pre-BI cocultures performed on OP9-shG1 stromal cells, inhibition of proliferation was further increased (Figure 4A,B).
Inhibition of pre-BCR relocalization impairs pre-BII-cell proliferation. (A) After BrdU incorporation, pro-B/pre-BI cells (B220+CD117+Igκ/λ−) were allowed to differentiate for 2 days in the absence of IL7 by coculture with stromal cells. Control experiments were performed using OP9-pSR cells in the presence of mSLCΔΔ. Pre-BCR relocalization was inhibited by adding mSLC in the medium, by performing cocultures with OP9-shG1 cells in the presence of mSLCΔΔ, or by adding mSLC and using OP9-shG1 cells. Data are representative of at least 4 independent experiments. The progressive loss of BrdU expression is representative of the proliferative activity of the cells. The mean fluorescence intensity (MFI) is indicated in each histogram. (B) Representation of BrdU expression (normalized MFI) for each condition described in panel A. Each point represents an individual experiment and bars represent the average values. P values were determined using the Mann-Whitney unpaired test with a risk of 5% (*P = .013, **P = .005).
Inhibition of pre-BCR relocalization impairs pre-BII-cell proliferation. (A) After BrdU incorporation, pro-B/pre-BI cells (B220+CD117+Igκ/λ−) were allowed to differentiate for 2 days in the absence of IL7 by coculture with stromal cells. Control experiments were performed using OP9-pSR cells in the presence of mSLCΔΔ. Pre-BCR relocalization was inhibited by adding mSLC in the medium, by performing cocultures with OP9-shG1 cells in the presence of mSLCΔΔ, or by adding mSLC and using OP9-shG1 cells. Data are representative of at least 4 independent experiments. The progressive loss of BrdU expression is representative of the proliferative activity of the cells. The mean fluorescence intensity (MFI) is indicated in each histogram. (B) Representation of BrdU expression (normalized MFI) for each condition described in panel A. Each point represents an individual experiment and bars represent the average values. P values were determined using the Mann-Whitney unpaired test with a risk of 5% (*P = .013, **P = .005).
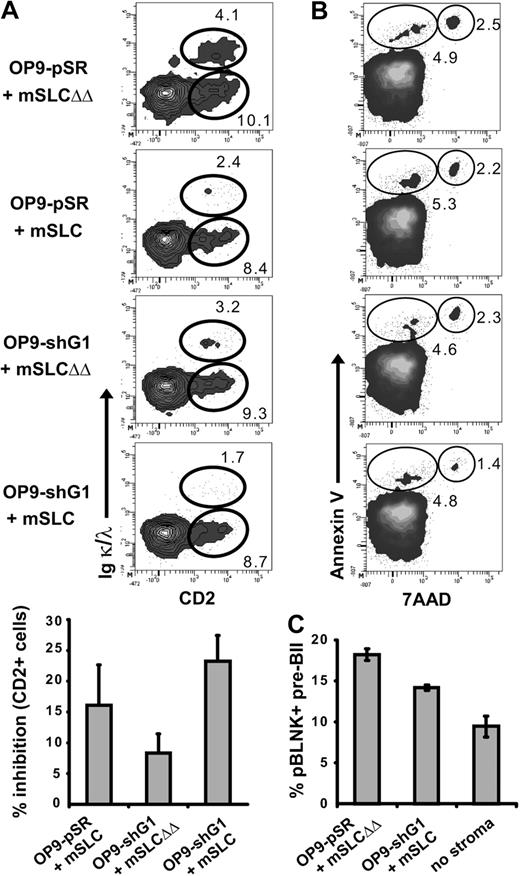
B-cell differentiation was simultaneously assessed in this in vitro assay by analyzing the appearance of CD2 and of Igκ/λ expression. CD2+ Igκ/λ− cells correspond to pre-BII cells, whereas CD2+ Igκ/λ+ correspond to immature B cells. By adding recombinant mSLC in the medium or by using OP9-shG1 stromal cells, pro-B/pre-BI cell differentiation was reproducibly impaired, as the number of pre-B and immature B cells decreased (Figure 5A top and bottom; P ≤ .032). Most importantly, the differentiation blockage increased in the case of pro-B/pre-BI/OP9-shG1 cocultures performed in the presence of recombinant mSLC. To confirm that the differentiation blockage was not due to the pro-B/pre-BI cell amplification step in the presence of IL7, freshly sorted BM pro-B/pre-BI cells were directly cocultured with OP9 stromal cells. As expected, using the same inhibitors, B-cell differentiation was similarly impaired (Figure S4A). Furthermore, we observed that inhibition of pre-BCR relocalization did not increase cellular apoptosis. Indeed, the proportion of annexin-V+ 7-AAD− apoptotic cells was not increased in presence of recombinant mSLC and/or OP9-shG1 stromal cells (Figure 5B). Finally, since pre-B-cell differentiation is dependent on BLNK activation,26 we determined the status of pre-BCR activation by following the phosphorylation of BLNK during pre-BII/stromal cell cocultures. When the pre-BCR relocalization is not inhibited, as in the case of large pre-BII cells cultured on OP9-pSR in the presence of mSLCΔΔ, BLNK is phosphorylated in about 19% of the cells (Figures 5C, S4B). By contrast, the percentage of pBLNK+ cells is consistently decreased in the case of cocultures performed with OP9-shG1 cells in the presence of mSLC. The decrease was even stronger when pre-BII cells were cultured in the absence of stromal cells.
Inhibition of pre-BCR relocalization impairs pre-BCR activation and pre-BII-cell differentiation. (A) Pro-B/pre-BI cells were allowed to differentiate as described in Figure 4. Differentiation was evaluated by following CD2 and IgL (Igκ + Igλ) expression, by flow cytometry (top). The histogram shows the percentage of inhibition (± SD) of total CD2+ cells compared with the mSLCΔΔ control (bottom). For each inhibitory condition, the percentage of CD2+ and CD2+IgL+ subpopulations was compared with the control situation (OP9-pSR+mSLCΔΔ) and P values were determined using the Mann-Whitney unpaired test with a risk of 5% (P ≤ .032). (B) Cellular apoptosis was evaluated by annexin V/7-AAD staining after 1 day of culture. The percentage of apoptotic cells (annexin V+ 7-AAD−) and dead cells (annexin V+ 7-AAD+) is shown. For panels A and B, data are representative of at least 4 independent experiments. (C) Sorted CD2− large pre-BII cells were incubated for 1 hour with OP9-pSR cells in the presence of mSLCΔΔ, with OP9-shG1 cells in the presence of mSLC, or without stromal cells. The percentage of phospho-BLNK+ pre-BII cells plus or minus SD was determined by flow cytometry (3 independent experiments).
Inhibition of pre-BCR relocalization impairs pre-BCR activation and pre-BII-cell differentiation. (A) Pro-B/pre-BI cells were allowed to differentiate as described in Figure 4. Differentiation was evaluated by following CD2 and IgL (Igκ + Igλ) expression, by flow cytometry (top). The histogram shows the percentage of inhibition (± SD) of total CD2+ cells compared with the mSLCΔΔ control (bottom). For each inhibitory condition, the percentage of CD2+ and CD2+IgL+ subpopulations was compared with the control situation (OP9-pSR+mSLCΔΔ) and P values were determined using the Mann-Whitney unpaired test with a risk of 5% (P ≤ .032). (B) Cellular apoptosis was evaluated by annexin V/7-AAD staining after 1 day of culture. The percentage of apoptotic cells (annexin V+ 7-AAD−) and dead cells (annexin V+ 7-AAD+) is shown. For panels A and B, data are representative of at least 4 independent experiments. (C) Sorted CD2− large pre-BII cells were incubated for 1 hour with OP9-pSR cells in the presence of mSLCΔΔ, with OP9-shG1 cells in the presence of mSLC, or without stromal cells. The percentage of phospho-BLNK+ pre-BII cells plus or minus SD was determined by flow cytometry (3 independent experiments).
Altogether our results show that GAL1-dependent pre-BCR relocalization improves pre-BCR activation, leading to a stronger pro-B/pre-BI-cell proliferation and differentiation toward the immature B-cell stage.
Altered development of pre-BII-cell differentiation in galectin-1 knockout mice
The physiological relevance of the pre-BCR clustering was also tested in vivo, using GAL1−/− mice, backcrossed onto a C57Bl/6 background. As shown in Figure 6A and quantified in Figure 6B, no alteration in bone marrow B-cell subpopulations was observed in GAL1−/− compared with WT mice. In particular, there is no significant difference for CD19+B220+IgM−CD25+CD2+ pre-BII-cell numbers. To destabilize the cellular equilibrium, GAL1−/− and WT mice were treated with hydroxyurea (HU), a cytostatic agent that kills cycling cells. The kinetics of de novo B-cell differentiation was monitored at days 2, 7, and 35 after treatment and for all B-cell subpopulations (Figure 6C-E). The most striking feature was obtained at day 7 after HU injection, as the percentage and the total number of B cells were significantly decreased in the GAL1-deficient mice (1.67% ± 0.7% and 0.42 ± 0.3 × 106 B cells) compared with WT controls (3.85% ± 2.1% and 1.1 ± 0.6 × 106 B cells). Moreover, as expected and shown in Figure 6C, the decrease affected specifically B220+CD19+ B cells (circle) and not the noncycling recirculating B220hiCD19+ B cells (dashed circle). Although the kinetics of pro-B/pre-BI recovery after HU treatment were comparable between GAL1−/− and WT mice, a 5-fold decrease in pre-BII-cell numbers was observed in the GAL1-deficient mice (Figure 6C,D, P = .022), indicating that the cellular defect was due to a specific alteration of the pre-BII-cell compartment. Finally, the pre-BII-cell subpopulation was restored 5 weeks after treatment in GAL1−/− mice and to a level similar to that of control mice (Figure 6E). Similarly, pre-BII-cell recovery after sublethal irradiation was impaired in GAL1−/− compared with WT mice (Figure S5). To determine whether pre-BII-cell proliferation was affected after HU treatment, we analyzed the cell-cycle status of pre-BII cells. As revealed by the TO-PRO-3 nucleic acid stain, we found that proliferation of the pre-BII cells was strongly impaired in GAL1−/− mice (Figure 6F).
De novo pre-BII-cell differentiation is impaired in GAL1−/− mice. (A) BM B-cell differentiation from WT (n = 6) and GAL1-deficient mice (n = 4) was analyzed by FACS using antibodies specific for CD19, B220, IgM, CD23, CD25, and CD2. The gating strategy is shown in the figure and the B-cell subsets were defined as follows: pro-B/pre-BI, CD19+IgM−CD23−CD2−CD25−; pre-BII, CD19+IgM−CD23−CD2+CD25+; immature B, CD19+IgM+CD23−; recirculating B, CD19+IgM+CD23+. (B) Absolute cell numbers of the BM B-cell subsets. (C) BM B-cell differentiation from HU-treated WT and GAL1-deficient mice was analyzed 7 days after injection as described in panel A. (D) Absolute cell numbers of the BM B-cell subsets, 7 days after HU treatment. (E) Kinetics of recovery of the BM B-cell subsets at different time points following HU treatment (n = 4 for each condition). (F) Cell-cycle analysis was performed for HU-treated mice 7 days after injection, using TO-PRO-3. The DNA content of pre-BII cells and the percentage of cells in S/G2/M are shown in the histograms. In the different panels, WT mice are shown in gray and GAL1−/−, in black. Error bars represent SD. P values were determined using the Mann-Whitney unpaired test with a risk of 5% (*P = .022).
De novo pre-BII-cell differentiation is impaired in GAL1−/− mice. (A) BM B-cell differentiation from WT (n = 6) and GAL1-deficient mice (n = 4) was analyzed by FACS using antibodies specific for CD19, B220, IgM, CD23, CD25, and CD2. The gating strategy is shown in the figure and the B-cell subsets were defined as follows: pro-B/pre-BI, CD19+IgM−CD23−CD2−CD25−; pre-BII, CD19+IgM−CD23−CD2+CD25+; immature B, CD19+IgM+CD23−; recirculating B, CD19+IgM+CD23+. (B) Absolute cell numbers of the BM B-cell subsets. (C) BM B-cell differentiation from HU-treated WT and GAL1-deficient mice was analyzed 7 days after injection as described in panel A. (D) Absolute cell numbers of the BM B-cell subsets, 7 days after HU treatment. (E) Kinetics of recovery of the BM B-cell subsets at different time points following HU treatment (n = 4 for each condition). (F) Cell-cycle analysis was performed for HU-treated mice 7 days after injection, using TO-PRO-3. The DNA content of pre-BII cells and the percentage of cells in S/G2/M are shown in the histograms. In the different panels, WT mice are shown in gray and GAL1−/−, in black. Error bars represent SD. P values were determined using the Mann-Whitney unpaired test with a risk of 5% (*P = .022).
Altogether, the results obtained after de novo B-cell differentiation demonstrate that GAL1 plays a determinant and specific role in pre-BII-cell development, underlying again the importance of the stromal cell microenvironment in supporting early B-cell development.
Discussion
We previously characterized synapse formation between human pre-B and BM stromal cells, leading to pre-BCR activation.3,15 To study the functional consequence of this phenomenon, we used normal pre-BII cells isolated from mouse BM and demonstrated that formation of the pre-BII/stromal cell synapse depends on the direct interactions between the λ5UR of the pre-BCR and GAL1, and between GAL1 and glycosylated integrins (Figure 2). Moreover, pre-B-cell integrin “outside-in” activation was shown to be crucial for this phenomenon. The implication of GAL1 was directly demonstrated, since GAL1 knockdown in BM stromal cells significantly decreased pre-BCR relocalization. Moreover, adding back recombinant GAL1 to the culture medium reversed the inhibition (Figure 3B). These results confirmed that the pre-BCR relocalization is not due to aberrant pre-BCR surface expression, as it could be the case for pre-B-cell lines, and allowed us to address the physiological significance of pre-B/stromal cell synapse formation.
The percentage of normal pre-BII cells with a relocalized pre-BCR was reproducibly around 55%. It is likely that the remaining percentage of cells has already formed and resolved a synapse. Indeed, our analyses were performed at a unique time point (after 2 hours of coculture with stromal cells) and synapse formation is a dynamic process. Moreover, we observed that large pre-BII cells isolated from normal BM form a heterogeneous population regarding CD2 expression, indicating that part of the cells may have already undergone pre-BCR activation in the BM prior to their purification (Figure S1A).
The inhibition of pre-BCR relocalization observed by altering the interactions implicated in synapse formation was statistically significant whatever the inhibitors used but did not exceed 35%. One possible explanation is that the formation of the lattice containing the pre-BCR, GAL1, and the integrins is thermodynamically favored, as it was already demonstrated for other galectin-containing lattices.27 In line with this assumption is the observation that lactose or SLC treatments are able to efficiently inhibit the pre-BCR relocalization only if the inhibitors are added to the culture medium prior to pre-B-cell deposition on stromal cells. Addition or combination of various inhibitors was not possible, as these treatments resulted in the loss of pre-B-cell adhesion to stromal cells, especially when anti-integrin blocking Abs were used. Although HSs have been shown to be pre-BCR ligands in mice, we demonstrated that they do not participate in synapse formation and thus in pre-BCR activation (Figure 3C). However, as pre-BCRs have been shown to interact with self-antigens,28 we cannot exclude that synapse formation depends also on the presence of other molecules.
When inhibitors of pre-BCR/GAL1/integrin interactions were used in the in vitro B-cell differentiation assay, cell proliferation and differentiation, but not apoptosis, were significantly impaired (Figures 4, 5). Moreover, the combination of mSLC and GAL1 knockdown stromal cells further increased the inhibition of both pre-BCR relocalization and pre-B-cell proliferation and differentiation. As mentioned in the previous paragraph, mSLCs cannot compete completely with an established lattice, but when GAL1 expression level is strongly decreased (as is the case with GAL1 knockdown stromal cells), this competition becomes more efficient. Although the in vitro experiments indicate that GAL1 potentiates pre-BII-cell proliferation and differentiation, no alteration in BM B-cell subpopulations was observed in GAL1-deficient mice, suggesting the existence of redundancies between galectin members, as already reported.3 However, by measuring the kinetics of de novo B-cell differentiation after HU treatment or sublethal irradiation of the mice, we observed a specific delay in pre-BII-cell recovery and a defect in pre-BII-cell proliferation in the GAL1-deficient mice (Figure 6 and Figure S5). Moreover, CD2 is expressed at the transition from large cycling to small resting pre-BII cells,29 and its decreased expression after HU treatment (Figure 6C) indicates that pre-BII-cell differentiation is also impaired. In line with this result, the phosphorylation of BLNK, which is crucial for pre-BII-cell differentiation,26 is reduced in absence of GAL1 (Figures 5C, S4B). Thus, both in vitro and in vivo experiments demonstrate a clear role of GAL1 in pre-BII-cell proliferation and differentiation.
Our results are in accordance with previous reports indicating that SLC is necessary for efficient pre-BII-cell proliferation and differentiation. Indeed, λ5-deficient pre-BI cells cultured in the absence of stromal cells are unable to proliferate in vitro.17 Moreover, the analysis of SLC-deficient mice revealed that B-cell proliferation is blocked in the absence of λ5 and/or VpreB proteins and that pre-B-cell differentiation is impaired.30 Finally, the λ5UR was recently shown in vivo to control the clonal expansion of pre-B cells expressing functional Igμ chains.5
Finally, a constitutive activation of the pre-BCR, the so-called tonic signaling, was shown to be sufficient to induce pre-BII-cell differentiation.17,31 However, although BLNK phosphorylation was observed in pre-BII cells cultivated in the absence of stromal cells, this phosphorylation increased upon pre-BCR relocalization following pre-BII/stromal cell interaction (Figures 5C, S4B). We propose that both pre-BCR activation modes are required to generate a large pool of pre-BII cells expressing a diverse repertoire of Igμ chains. Indeed, in vitro experiments by Kawano et al showed that increasing the level of pre-BCR surface expression enhanced pre-BCR activation and pre-B-cell differentiation and proliferation.32 Moreover, the level of pre-BCR surface expression depends on the potential of Igμ chains to interact with SLC. Thus, in the case of strong Igμ/SLC association, the tonic signal would be sufficient to promote pre-B-cell differentiation and proliferation. In line with this assumption, expression of the pre-BCR is very strong on pre-B cells from experimental models, showing that the pre-BCR tonic signal is sufficient to induce the complete set of pre-BCR functions. However, in case of a weaker Igμ/SLC expression and thus low pre-BCR surface expression, as is mainly the case for normal pre-B cells in vivo, ligand-induced signaling would be required to amplify pre-BCR functions. Interestingly, normal pro-B/pre-BI cells cultivated without stromal cells or in transwell poorly differentiate and massively die26 (and data not shown), pinpointing the importance of pre-B-cell interactions with the BM microenvironment for pre-B-cell development. Finally, Kawano et al32 showed that cooperative signaling between the pre-BCR and IL7R is necessary to promote pre-B-cell expansion in case of low level of pre-BCR expression. Thus, by inducing formation of a pre-B/stromal cell synapse, GAL1 may facilitate pre-B-cell proliferation by maintaining pre-BII cells in contact with IL7-expressing stromal cells.
In conclusion, this study dissects for the first time the formation of the pre-B/stromal cell synapse in a situation where pre-BII cells express physiological levels of pre-BCRs. Moreover, our results unequivocally demonstrate that pre-BCR/GAL1/integrin interactions are required for efficient pre-BII-cell development in vivo and highlight the importance of the interactions between pre-BII cells and the BM microenvironment in physiological conditions.
Understanding of the molecular organization of the pre-B/stromal cell synapse may have crucial consequences in pathology. Indeed, our results demonstrate that GAL1-induced pre-BCR relocalization leads to BLNK phosphorylation, and alterations in BLNK signaling have been implicated in the development of early B acute lymphoblastic leukemias (B-ALLs).33,34 Moreover, as bone marrow stromal cells have been shown to control B-ALL survival and growth,35,36 it would be of particular interest to investigate whether GAL1-dependent activation of the pre-BCR influences these processes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the flow cytometry facility and the PICsL imaging core for expert technical assistance. We gratefully acknowledge C. Paige, A. Cumano, F. Batista, and H. M. Jäck for providing us cells and reagents and L. Gauthier, E. Meffre, and L. Leserman for critical reading of the paper.
This work was supported by Inserm, CNRS, ANR (Agence Nationale de la Recherche; (05-BLANC-0035-01), and ARC (Association pour la Recherche contre le Cancer) contract nos. 3656 and 3854. M.E. was funded by ARC.
Authorship
Contribution: M.E., S.J.C.M., and C.B. performed research; M.E., S.J.C.M., and C.S. designed research, analyzed data, and wrote the paper; and F.P. provided essential material.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stéphane J. C. Mancini and Claudine Schiff, CIML, Case 906, 13288 Marseille Cedex 09, France; e-mail: mancini@ciml.univ-mrs.fr and schiff@ciml.univ-mrs.fr.
References
Author notes
*M.E. and S.J.C.M. contributed equally to this work.