Abstract
Patients not in complete cytogenetic response (CCyR) continuously face the competing possibilities of eventually achieving a cytogenetic response versus progressing. We analyzed the probability of achieving a CCyR, major molecular response, and progression in 258 patients with chronic myeloid leukemia in early chronic phase at 3, 6, and 12 months from imatinib start. The initial imatinib dose was 800 mg/day in 208 (81%) and 400 mg/day in 50 (19%) patients. For patients not in CCyR, the probability of achieving CCyR (P = .002) or major molecular response (P = .004) significantly decreased, whereas the risk of progression increased (P = .16) at each time point. Patients with a BCR-ABL1/ABL1 ratio greater than 1% to 10% after 3 months of imatinib had a 92% probability of achieving CCyR with continued therapy, similar to the 98% for those with 1% or less, but their risk of progression (11%) was almost 3-fold that of patients with a BCR-ABL1/ABL1 transcript ratio of 1% or less (4%) and similar to that of patients with transcript levels more than 10% (13%). These results suggest that patients not in CCyR after 12 months on imatinib have a higher risk of progression. This risk is discernible as early as 3 months into imatinib therapy by molecular analysis and may provide the rationale to institute therapies that render higher rates of early response.
Introduction
Chronic myelogenous leukemia (CML) is a clonal hematopoietic disorder driven by the constitutively activated BCR-ABL1 tyrosine kinase oncoprotein.1 Frontline therapy with the tyrosine kinase inhibitor (TKI) imatinib mesylate resulted in overall cumulative complete hematologic (CHR) and complete cytogenetic (CCyR) response rates of 98% and 82%, respectively, in patients with CML in chronic phase (CP).2 The use of high-dose imatinib (ie, 800 mg daily) has been associated with a CCyR rate of 89% in patients in CP after failure of interferon-α therapy3 and in more than 90% in those with newly diagnosed CML CP.4 The achievement of cytogenetic and molecular responses during imatinib therapy translates into improved event-free survival (EFS) and protection against progression to advanced phases of the disease.2 These excellent results have consolidated imatinib as standard therapy for patients with CML.
After 12 months of imatinib therapy, 69% of patients with CML in early CP are projected to achieve a CCyR.5 This response rate improves to 82% after 5 years of continuous therapy.2 However, it remains controversial whether achieving a CCyR early during the course of imatinib therapy confers a prognostic advantage over a CCyR attained at later time points. Initial reports showed that early responses predicted for an improved long-term outcome.5,6 However, recent analyses suggest that the risk of disease progression among patients who achieve a CCyR during the first 12 months of imatinib therapy is similar to that of patients who achieve this response at later time points.2,7 Although some patients may indeed improve their response with continuation of imatinib therapy, a patient not in CCyR at any time faces the dual competing possibilities of either achieving the intended CCyR or eventually progressing. Moreover, the depth of molecular responses obtained during imatinib therapy has important prognostic implications. Among patients in CCyR after 12 months of imatinib therapy, those with a BCR-ABL1/ABL1 ratio reduction of at least 3-log (ie, major molecular response [MMR]) have been reported to have significantly improved progression-free survival (PFS) compared with those with less than a 3-log reduction in BCR-ABL1 mRNA transcripts.8 By contrast, patients who fail to achieve an MMR during imatinib therapy have an increased risk of losing their cytogenetic response6 and developing resistance to imatinib and other TKIs.9 Because the attainment of molecular responses with imatinib is associated with improved long-term outcomes in CML, it is conceivable that measuring the magnitude of reduction in BCR-ABL1 mRNA transcripts may predict, early in the course of therapy, the probability of eventually achieving a CCyR or progressing.
In the present study, we investigated the probabilities of improving the cytogenetic response and of eventually progressing for patients not in CCyR at different time points. Our data indicate that failure to attain a CCyR within the first 12 months of imatinib therapy is linked to a higher risk of disease progression. This subset of patients at risk for progression may be identified early in the course of imatinib therapy based on their molecular response.
Methods
Patients and eligibility criteria
Patients 15 years of age or older with Philadelphia chromosome (Ph)–positive or BCR-ABL1–positive CML who received frontline therapy with imatinib in clinical trials at M. D. Anderson Cancer Center were eligible for this analysis. Patients were required to have CML in early CP (ie, time from diagnosis to treatment < 12 months). Patients with accelerated phase (AP) or blastic phase (BP) CML were excluded. Patients with cytogenetic clonal evolution were eligible if there were no other criteria of CML AP. Other eligibility criteria included (1) performance status 0 to 2 by the Eastern Cooperative Oncology Group scale, (2) serum creatinine and total bilirubin level lower than 1.5 times the upper limit of normal, and (3) signed informed consent. Pregnant and nursing women were excluded, and those of childbearing potential were required to practice effective methods of contraception. Studies were approved by the Internal Review Board of M. D. Anderson Cancer Center and conducted in accordance with the Declaration of Helsinki.
Treatment and monitoring
Imatinib was administered orally at a starting daily dose of 400 mg (n = 50) or 800 mg (n = 208). Except for hydroxyurea, patients should have received no or minimal prior therapy, defined as less than 1 month of prior interferon-α (IFN-α; with or without ara-C) and/or imatinib. Hydroxyurea within the previous 7 days and investigational agents 28 days before starting imatinib were not allowed. Before the start of therapy, all patients had a complete history taken and physical examination, a complete blood count, a comprehensive biochemistry panel, and a bone marrow (BM) aspirate with cytogenetics and fluorescence in situ hybridization (FISH). Patients were followed for progression and survival at least every 3 months. BM and peripheral blood samples for cytogenetics (or FISH when cytogenetic studies were inevaluable) and quantitative real-time polymerase chain reaction (RT-PCR) analyses were collected every 3 months for the first 12 months of therapy and at least every 6 months while on therapy. Patients were monitored with quantitative RT-PCR every 3 months for the first 12 months and every 6 months thereafter.
Patient evaluation
Response criteria were as previously described.10 Briefly, CHR was defined as a white blood cell count of less than 10 × 109/L, platelet count of less than 450 × 109/L, no immature cells (blasts, promyelocytes, myelocytes) in the peripheral blood, and disappearance of all signs and symptoms related to leukemia (including palpable splenomegaly) lasting for at least 4 weeks. A CHR was further categorized by the percentage of Ph− metaphases as CCyR (0% Ph+ cells in metaphase), partial ([PCyR]; ≤ 35% Ph+ cells in metaphase), and minor ([mCyR]; 36%-90% Ph+ cells in metaphase) cytogenetic response. A major cytogenetic response (MCyR) included CCyR plus PCyR (ie, ≤ 35% Ph+ cells in metaphase).
Cytogenetic and quantitative RT-PCR analyses
Cytogenetic analysis was performed by standard G-banding techniques, and at least 20 metaphases were analyzed. Marrow specimens were examined on direct 24-hour cultures. FISH was used only when conventional cytogenetics rendered insufficient metaphases; but for it to be considered for CCyR assessment, it had to be confirmed in a subsequent routine karyotype. BCR-ABL1 transcripts were detected by quantitative RT-PCR analysis on peripheral blood and BM aspirate. BM and/or peripheral blood samples with undetectable levels of BCR-ABL1 transcripts were confirmed by nested PCR as previously reported.6 For all samples, we extracted total RNA from 10 mL peripheral blood (after RBC lysis) containing approximately 50 × 106 cells for CML samples with at least 5000/μL leukocytes. We then converted 7.5 μg RNA into cDNA and used 1 μg cDNA equivalent in the quantitative RT-PCR reaction. Given the lower limit of the TaqMan assay, we established a sensitivity of at least a 1 BCR-ABL1+ cell in 105 cells (as established by periodic dilution studies) for posttreatment CML, although this is obviously influenced by the levels of BCR-ABL1 transcription in any given tumor. We set a limit of at least 10 000 copies of ABL1 detected by the normalizing probes as the lower limit of sensitivity needed for any given sample. For the purpose of this analysis, a CMR was defined as the presence of undetectable BCR-ABL1 transcript levels. An MMR was defined as a BCR-ABL1/ABL1 ratio of less than 0.05% in line with previous studies conducted at our institution.
Statistical considerations
Differences among variables were compared by the χ2 test and Mann-Whitney U test. EFS was calculated from the time of start of therapy until loss of CHR, loss of mCyR or an increasing white cell count (defined as a doubling of the count to more than 20 × 109/L on 2 occasions at least 1 month apart), transformation to AP (defined by the presence of ≥ 15% blasts in the blood or bone marrow, ≥ 30% blasts plus promyelocytes in the blood or BM, ≥ 20% peripheral basophils, or platelet count < 100 × 109/L unrelated to treatment), BP (defined by the presence of ≥ 30% blasts in the blood or BM or extramedullary involvement [eg, chloromas], but not hepatosplenomegaly), or death from any cause during imatinib treatment. Transformation-free survival (TFS) was calculated from the time of start of therapy to the time of transformation to AP or BP, or death from any cause. Survival was calculated from the time imatinib therapy began until death from any cause or last follow-up. Survival and time-to-event curves were estimated by the Kaplan-Meier method and compared by the log-rank test. Univariate and multivariable analyses were performed to identify potential prognostic factors associated with the achievement of CCyR. Multivariable analysis used the logistic regression method.
Results
Study group
A total of 258 patients with CML in early CP received imatinib as first-line therapy for their disease. The baseline characteristics of the 258 patients are presented in Table 1. The median age was 48 years (range, 15-84 years). All patients were Ph+, and all patients except one (e1a2) expressed b2a2, b3a2, or both transcripts. Seven patients with clonal evolution, but otherwise in CP, were included. Risk stratification on the basis of the Sokal prognostic score was representative of recent series in which most patients presented with low or intermediate risk scores.
Characteristics of 258 patients treated with imatinib in early chronic phase
| Parameter . | Value . |
|---|---|
| Median age, y (range) | 48 (15-84) |
| Median time from diagnosis to imatinib, mo (range) | 1 (0-12) |
| Median WBC, ×109/L (range) | 26.8 (2.2-283) |
| Median hemoglobin, g/dL (range) | 12.4 (6.2-16.7) |
| Median platelets, ×109/L (range) | 365 (100-1476) |
| Median percentage of PB blasts (range) | 0 (0-12) |
| Median percentage of PB basophils (range) | 3 (0-19) |
| Median percentage of BM blasts (range) | 2 (0-14) |
| Median percentage of BM basophils (range) | 3 (0-15) |
| Splenomegaly, n (%) | 70 (27) |
| Percentage of Ph+ > 90, n (%) | 241 (93) |
| Clonal evolution, n (%) | 7 (3) |
| Dose, mg, n (%) | |
| 400 | 50 (19) |
| 800 | 208 (81) |
| Sokal risk score, n (%) | |
| Low | 165 (64) |
| Intermediate | 71 (28) |
| High | 22 (8) |
| Parameter . | Value . |
|---|---|
| Median age, y (range) | 48 (15-84) |
| Median time from diagnosis to imatinib, mo (range) | 1 (0-12) |
| Median WBC, ×109/L (range) | 26.8 (2.2-283) |
| Median hemoglobin, g/dL (range) | 12.4 (6.2-16.7) |
| Median platelets, ×109/L (range) | 365 (100-1476) |
| Median percentage of PB blasts (range) | 0 (0-12) |
| Median percentage of PB basophils (range) | 3 (0-19) |
| Median percentage of BM blasts (range) | 2 (0-14) |
| Median percentage of BM basophils (range) | 3 (0-15) |
| Splenomegaly, n (%) | 70 (27) |
| Percentage of Ph+ > 90, n (%) | 241 (93) |
| Clonal evolution, n (%) | 7 (3) |
| Dose, mg, n (%) | |
| 400 | 50 (19) |
| 800 | 208 (81) |
| Sokal risk score, n (%) | |
| Low | 165 (64) |
| Intermediate | 71 (28) |
| High | 22 (8) |
WBC indicates white blood cell; PB, peripheral blood; BM, bone marrow; and Ph, Philadelphia chromosome.
Overall outcomes
The median follow-up from the time imatinib was started was 53 months (range, 2-81 months). A CCyR was achieved by 89% and MMR by 72% of patients, whereas 42% of them had undetectable BCR-ABL1 transcript levels. Similar rates of CCyR were observed between patients with (86%) or without (88%) clonal evolution (P = .60), and the median time to obtain such response was also similar in both groups (3.1 vs 3.2 months, P = .49). Eighty-eight patients (34%) were taken off imatinib because of hematologic resistance (n = 7), cytogenetic resistance (n = 14), AP (n = 5), BP (n = 5), toxicity (n = 17), allogeneic stem cell transplantation (n = 1), death of CML-unrelated cause while on treatment (n = 5), or other (n = 34; noncompliance, lost to follow-up, financial reasons, or intercurrent disease). Progression rates were higher among patients receiving 400 mg/day (11 of 50) than among those receiving 800 mg/day (20 of 208, excluding 5 patients who died of non–CML-related causes; P = .03).
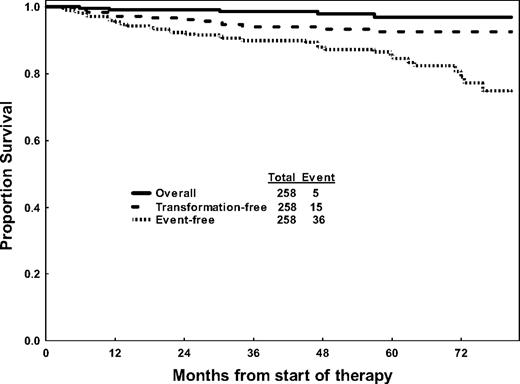
The Kaplan-Meier curves for overall survival, EFS, and TFS are shown in Figure 1. A total of 36 events, involving 14% of patients, were observed for a 5-year EFS rate of 85%. Ten patients transformed to AP or BP and 5 patients died of CML-unrelated reasons for a 5-year TFS rate on imatinib of 92%.
Overall, transformation-free, and event-free survival of 258 patients receiving imatinib therapy.
Overall, transformation-free, and event-free survival of 258 patients receiving imatinib therapy.
Probability of achieving a CCyR versus progression for patients not in CCyR
To evaluate whether the achievement of early responses confers a prognostic advantage, we analyzed the outcome of patients not in CCyR at 3, 6, and 12 months from the start of imatinib therapy. The probabilities of eventually achieving a CCyR and MMR as well as the probability of eventually developing an event were determined for all assessable patients at these time points. As shown in Table 2, the probability of eventually achieving a CCyR decreases steadily if CCyR has not been yet achieved after 3, 6, and 12 months on imatinib (P = .002), with a similar reduction in the probability of achieving an MMR (P = .004). Furthermore, the risk of eventually developing an event increases in parallel as patients take longer to achieve a CCyR (P = .16). Thus, for instance, a patient who has not achieved a CCyR at 6 months has a 57% probability of still eventually achieving a CCyR over time with continuation of therapy. However, the risk of events at a later time point for such patients is 34%. After 12 months of continuous imatinib therapy without a CCyR, the risk of developing an event increases to 38%, whereas the probability of achieving a CCyR decreases to 42%.
Outcome of patients in CCyR and not in CCyR at specific time points during imatinib therapy
| Months on treatment . | No. (%) in CCyR . | No. (%) not in CCyR . | Percentage eventually achieving outcome if not in CCyR at specified time . | ||
|---|---|---|---|---|---|
| CCyR . | MMR . | Event . | |||
| 3 | 143 (56) | 109 (43) | 75 | 62 | 23 |
| 6 | 190 (79) | 47 (20) | 57 | 43 | 34 |
| 12 | 200 (85) | 26 (12) | 42 | 31 | 38 |
| P | .002 | .004 | .16 | ||
| Months on treatment . | No. (%) in CCyR . | No. (%) not in CCyR . | Percentage eventually achieving outcome if not in CCyR at specified time . | ||
|---|---|---|---|---|---|
| CCyR . | MMR . | Event . | |||
| 3 | 143 (56) | 109 (43) | 75 | 62 | 23 |
| 6 | 190 (79) | 47 (20) | 57 | 43 | 34 |
| 12 | 200 (85) | 26 (12) | 42 | 31 | 38 |
| P | .002 | .004 | .16 | ||
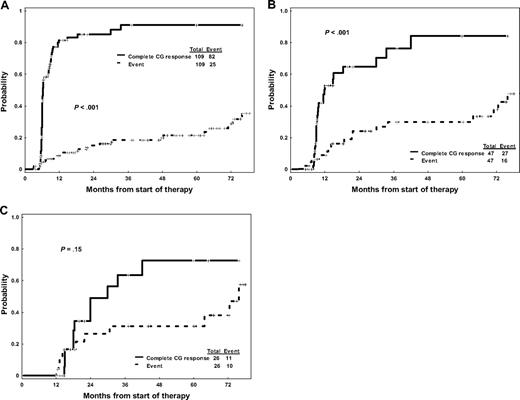
When paired together at successive time points, the competing probabilities of developing an event and achieving CCyR for patients not in CCyR at the different time points are significantly different at 3 months (P < .001) and 6 months (P < .001): at these time points, the probability of achieving a CCyR is still significantly higher than the risk of eventually developing an event (Figure 2A,B). However, by 12 months, the probabilities of improving the cytogenetic response and of developing an event become much more similar (P = .15; Figure 2C). Among patients who do achieve a CCyR, no significant differences were observed in duration of CCyR (P = .90) or EFS (P = .63) regardless of whether the CCyR was obtained before 6 months, between 6 and 12 months, or after 12 months. A similar trend was observed for TFS at 3 and 6 months (but not at 12 months), although this was not statistically significant as the number of patients who transformed during therapy was small. The exceedingly low mortality rate observed in our patient cohort precluded a similar analysis for overall survival. Overall, these results suggest that, although delayed responses may indeed occur and potentially result in favorable outcomes, patients not achieving an early cytogenetic response may progress before they achieve a meaningful response.
Probability of achieving a complete cytogenetic response versus developing an event for patients not in complete cytogenetic response. (A) After 3 months of imatinib therapy. (B) After 6 months of imatinib therapy. (C) After 12 months of imatinib therapy.
Probability of achieving a complete cytogenetic response versus developing an event for patients not in complete cytogenetic response. (A) After 3 months of imatinib therapy. (B) After 6 months of imatinib therapy. (C) After 12 months of imatinib therapy.
Because lack of CCyR at early time points correlates with the probability of eventually achieving CCyR and the probability of eventually failing therapy, we then analyzed the factors associated with achievement of early responses. By univariate analysis, variables associated with a lower probability of achieving a CCyR at 3 months included low hemoglobin, high peripheral blood and bone marrow blast percentage, splenomegaly, and imatinib therapy at standard dose (vs 800 mg/day; Table 3). In a multivariable analysis, low hemoglobin, higher peripheral blood blast percentage, and treatment with standard dose imatinib remained as predictors of a decreased probability of achieving a CCyR by 3 months of therapy (Table 4). The mean (± SD) hemoglobin level and peripheral blood blast percentage among patients with CCyR at 3 months was 12.6 (± 1.56) and 0.4 (± 0.63), respectively, whereas in patients who failed to achieve a CCyR at 3 months was 11.9 (± 0.59) and 1.1 (± 2.06), respectively. Notably, peripheral blood blast percentage was also a predictor of CCyR at 6 months but not at 12 months by multivariate analysis.
Univariate analysis for the probability of achieving a complete cytogenetic response on imatinib by 3 months of therapy
| Parameter . | Effect . | CCyR within 3 mo, median (range) or no. (%) . | P . | |
|---|---|---|---|---|
| Yes (n = 143) . | No (n = 109) . | |||
| Age, y | NS | 48 (15-79) | 48 (17-84) | .84 |
| Lower hemoglobin, g/dL | Adverse | 12.7 (8.8-16.7) | 12 (6.2-15.1) | < .001 |
| Higher WBC, ×109/L | Adverse (trend) | 24.5 (3.5-239.5) | 35.8 (2.2-283) | .06 |
| Platelets, ×109/L | NS | 372 (107-1476) | 356 (100-1413) | .71 |
| PB basophils, % | NS | 3 (0-19) | 4 (0-19) | .98 |
| Higher PB blasts, % | Adverse | 0 (0-3) | 0 (0-12) | < .001 |
| BM basophils, % | NS | 2 (0-8) | 3 (0-15) | .1 |
| Higher BM blasts, % | Adverse | 1 (0-13) | 2 (0-14) | .04 |
| Presence of splenomegaly | Adverse | 24 (17) | 44 (41) | < .001 |
| Presence of clonal evolution | NS | 4 (3) | 3 (3) | .99 |
| Longer CML duration, mo | Adverse | 0 (0-6) | 1 (0-12) | .06 |
| Dose (400 vs 800 mg/day) | Adverse | 18 (13) | 31 (29) | .001 |
| More than 90% Ph+ cells at start of therapy | Adverse | 130 (91) | 104 (96) | .09 |
| Sokal risk score | ||||
| Low | NS | 105 (73) | 57 (53) | .83 |
| Intermediate | NS | 33 (23) | 34 (31) | .83 |
| High | NS | 5 (4) | 17 (16) | .83 |
| Parameter . | Effect . | CCyR within 3 mo, median (range) or no. (%) . | P . | |
|---|---|---|---|---|
| Yes (n = 143) . | No (n = 109) . | |||
| Age, y | NS | 48 (15-79) | 48 (17-84) | .84 |
| Lower hemoglobin, g/dL | Adverse | 12.7 (8.8-16.7) | 12 (6.2-15.1) | < .001 |
| Higher WBC, ×109/L | Adverse (trend) | 24.5 (3.5-239.5) | 35.8 (2.2-283) | .06 |
| Platelets, ×109/L | NS | 372 (107-1476) | 356 (100-1413) | .71 |
| PB basophils, % | NS | 3 (0-19) | 4 (0-19) | .98 |
| Higher PB blasts, % | Adverse | 0 (0-3) | 0 (0-12) | < .001 |
| BM basophils, % | NS | 2 (0-8) | 3 (0-15) | .1 |
| Higher BM blasts, % | Adverse | 1 (0-13) | 2 (0-14) | .04 |
| Presence of splenomegaly | Adverse | 24 (17) | 44 (41) | < .001 |
| Presence of clonal evolution | NS | 4 (3) | 3 (3) | .99 |
| Longer CML duration, mo | Adverse | 0 (0-6) | 1 (0-12) | .06 |
| Dose (400 vs 800 mg/day) | Adverse | 18 (13) | 31 (29) | .001 |
| More than 90% Ph+ cells at start of therapy | Adverse | 130 (91) | 104 (96) | .09 |
| Sokal risk score | ||||
| Low | NS | 105 (73) | 57 (53) | .83 |
| Intermediate | NS | 33 (23) | 34 (31) | .83 |
| High | NS | 5 (4) | 17 (16) | .83 |
NS indicates not significant.
Multivariate analysis for the probability of achieving a complete cytogenetic response on imatinib by 3 months of therapy
| Factor . | Estimate of coefficient . | Estimate of odds ratio . | P . |
|---|---|---|---|
| Dose 400 mg/day (baseline, 800 mg/day) | −1.395 | 0.25 | < .001 |
| Higher PB blasts, % | −0.59 | 0.55 | < .001 |
| Lower hemoglobin | 0.29 | 1.34 | .001 |
| Factor . | Estimate of coefficient . | Estimate of odds ratio . | P . |
|---|---|---|---|
| Dose 400 mg/day (baseline, 800 mg/day) | −1.395 | 0.25 | < .001 |
| Higher PB blasts, % | −0.59 | 0.55 | < .001 |
| Lower hemoglobin | 0.29 | 1.34 | .001 |
Risk of progression according to molecular response
Because the achievement of an MMR has been associated with improved probability of durable cytogenetic remission and EFS,6,8 we analyzed the long-term outcome of patients not in MMR according to the BCR-ABL1 transcript levels at different time points during the first 12 months of imatinib therapy. To this end, we divided patients in groups according to the level of BCR-ABL1/ABL1 ratio and compared their probability of eventually developing an event according to the molecular response achieved at different time points (Table 5). By 3 months, patients with a BCR-ABL1/ABL1 transcript ratio higher than 10% have a probability of eventually achieving a CCyR of 67% and only a 54% probability of eventually achieving an MMR, which is significantly lower than the probability for patients with transcript levels lower than or equal to 10% at the same time point. Interestingly, patients with BCR-ABL1/ABL1 transcript ratio from greater than 1% to 10% define a group with intermediate characteristics between patients with BCR-ABL1/ABL1 ratio higher than 10% and those with a ratio lower than or equal to 1%. Although most of these patients will eventually achieve a CCyR (92% of those not yet in CCyR) at some point over the course of therapy, their probability of achieving an MMR is only 53% and their risk of developing an event at a later time point during therapy is almost 3-fold (11%) that of patients with BCR-ABL1/ABL1 transcript ratio lower than or equal to 1% (probability of developing an event, 4%). Therefore, the outcome of this group of patients resembles more that of patients with transcript levels higher than 10% than that of patients with lower transcript values. As time goes by, the outcome of patients with transcript levels higher than 10% deteriorates further, with decreasing probabilities of eventually achieving MMR and increasing risk of developing an event. Thus, after 6 months of therapy, approximately 75% of patients have BCR-ABL1/ABL1 transcript levels lower than or equal to 1%, but the differences among groups becomes more evident with a 23% event rate among patients with BCR-ABL1/ABL1 ratio higher than 10%. Finally, although by 12 months most patients have low transcript levels, those with a ratio higher than 10% have clearly a lower probability of improving their cytogenetic response (45%) and achieving an MMR (7%) and higher risk of developing an event (50%).
Probability of achieving an MMR and the risk of developing an event according to the reduction of BCR-ABL1 transcripts at fixed time points
| BCR-ABL1/ABL1 transcript ratio . | Percentage probability of outcome according to transcript ratio at specified time points (median months to outcome) . | |||||
|---|---|---|---|---|---|---|
| MMR (BCR-ABL1/ABL1< 0.05%) . | Event . | |||||
| 3 mo . | 6 mo . | 12 mo . | 3 mo . | 6 mo . | 12 mo . | |
| ≤ 0.1% | 100 (3) | 96 (6) | 97 (12) | 4 (13) | 1 (38) | 3 (40) |
| > 0.1% to 1% | 84 (6) | 69 (12) | 61 (18) | 3 (46) | 7 (30) | 2 (48) |
| > 1% to 10% | 53 (17) | 44 (18) | 20 (33) | 11 (21) | 9 (34) | 8 (47) |
| > 10% | 33 (15) | 15 (18) | 7 (46) | 13 (47) | 23 (14) | 50 (19) |
| BCR-ABL1/ABL1 transcript ratio . | Percentage probability of outcome according to transcript ratio at specified time points (median months to outcome) . | |||||
|---|---|---|---|---|---|---|
| MMR (BCR-ABL1/ABL1< 0.05%) . | Event . | |||||
| 3 mo . | 6 mo . | 12 mo . | 3 mo . | 6 mo . | 12 mo . | |
| ≤ 0.1% | 100 (3) | 96 (6) | 97 (12) | 4 (13) | 1 (38) | 3 (40) |
| > 0.1% to 1% | 84 (6) | 69 (12) | 61 (18) | 3 (46) | 7 (30) | 2 (48) |
| > 1% to 10% | 53 (17) | 44 (18) | 20 (33) | 11 (21) | 9 (34) | 8 (47) |
| > 10% | 33 (15) | 15 (18) | 7 (46) | 13 (47) | 23 (14) | 50 (19) |
Discussion
The depth of response obtained by patients with CML during imatinib therapy is critical from a prognostic standpoint. Patients who achieved a CCyR at 12, 18, or 24 months in the International Randomized Study of Interferon versus STI571 (IRIS) study had improved PFS compared with those who obtained only a PCyR at each of those time points. Moreover, the prognostic impact of the presence of detectable levels of BCR-ABL1 transcripts has been firmly established after therapy with IFN-α and stem cell transplantation.11-13 Patients achieving a CCyR and a more than 3-log reduction in BCR-ABL1 transcript levels have a PFS of 100% after 60 months of imatinib therapy.2 In contrast, when a CCyR is accompanied by less than 3-log reduction in levels of BCR-ABL1 transcripts, the PFS is 98%, and it decreases to 88% for patients not achieving a CCyR (P < .001).2 Initial reports suggested that the time to response correlated with outcome, where patients who achieved CCyR or even MCyR early during the course of therapy had the best long-term outcome (ie, EFS and PFS). However, the 5-year update of the IRIS study indicates that PFS is better for patients who achieve a CCyR response regardless of whether this is obtained at 12, 18, or 24 months, thus making the time to cytogenetic response a less relevant endpoint.2 Similarly, a recent analysis of patients treated with imatinib after failure of IFN-α therapy suggests that patients who obtained a CCyR after more than 12 months of imatinib therapy had PFS and estimated 4-year overall survival rates similar to those who achieved a cytogenetic response within 12 months.7
It has been clearly established that response is the most important prognostic factor for long-term outcome in CML.14 However, although the long-term prognosis of patients with CML achieving a CCyR on imatinib is remarkably favorable, an analysis investigating the prognostic impact of the time to response should consider not only the favorable consequences of achieving the desired response, but also the risks any patient faces while this response is yet to be achieved. Although some patients can certainly improve their response over time with continuous imatinib therapy, any given patient not in CCyR constantly faces the dual and often competing possibilities of either achieving a CCyR or eventually progressing. Thus, in the present study, rather than focusing only on the patients who do achieve a CCyR at different time points, we analyzed the outcome of patients with CML CP not in CCyR at different time points during imatinib therapy. Our results suggest that failure to achieve CCyR within the first 12 months of imatinib therapy is associated with higher rates of disease progression and that this risk is discernible early during imatinib therapy (eg, 3 months into the therapy). Furthermore, the risk of progression can be inferred from the depth of molecular response achieved after 3 months on imatinib. Indeed, patients having a BCR-ABL1/ABL1 ratio higher than 1% at that time point have a risk of progression significantly higher than those with lower levels. Therefore, a BCR-ABL1/ABL1 ratio of 1% could be considered the optimal response at the 3-month landmark. Caution, however, must be exercised regarding the extrapolation of these values to those obtained in other laboratories given the current lack of interlaboratory standardization. Efforts aimed at setting an international standard scale for comparison of BCR-ABL1 mRNA levels derived from different laboratories are under way.15 Nevertheless, we believe that, even if the actual values here presented may not be translatable to those obtained in other laboratories, the prognostic significance of early responses remains valid.
Because achieving a CCyR is associated with a favorable long-term prognosis,2 the identification early into the course of therapy of patients at high risk of not achieving a CCyR is critical. This is particularly relevant because continuing imatinib therapy at any given time point in patients not in CCyR will eventually result in CCyR only in a fraction of them. Our data show that the probability of eventually achieving a CCyR or an MMR steadily declines after 3, 6, and 12 months on imatinib whereas the risk of progression increases as it takes longer for patients to achieve a CCyR. Collectively, these results support the notion that attaining early CCyR reduces the probability of progression at later time points. A recent analysis of the IRIS trial suggested that, among patients achieving CCyR, EFS is equivalent regardless of the time at which CCyR is achieved.16 However, the interpretation of this study is hampered by the fact that, unlike our study, patients who received fewer than 12 months of imatinib therapy were excluded from the analysis. In addition, looking only at patients who did achieve a response at certain time points does not account for the fact that patients who have not achieved a remission at any given time may eventually improve their response, but they can also fail and progress. Indeed, the probability of achieving a CCyR decreased over time in that analysis. Moreover, albeit not statistically significant, the duration of cytogenetic response was higher for patients who achieved a CCyR at earlier time points.16
By monitoring the depth of the molecular responses obtained at successive time points, we found that the risk of progression increased and the probability of achieving CCyR and MMR diminished gradually over time for patients with increasing levels of BCR-ABL1 transcripts. In simple terms, the deeper the molecular response, and the earlier these responses are achieved, the higher the probability of achieving an MMR. Attaining a suboptimal molecular response early in the course of therapy not only may limit the probabilities of achieving an MMR but, more important, is associated with an increased risk of progression as well.
Considering these results, one could speculate that treatment approaches that increase the number of patients able to achieve CCyR and possibly more profound reductions in transcript levels early during therapy would be desirable. Among the options that have been suggested to have this effect are higher doses of imatinib,4 or the second-generation TKIs dasatinib17 and nilotinib18,19 in the frontline setting. The reported rates of CCyR at 6 months are 82% with high-dose imatinib, 94% with dasatinib,17 and 100% with nilotinib.18 Comparison with historical controls suggests that early responses with high-dose imatinib might result in an improvement in EFS and TFS over what is expected with standard-dose imatinib. Once the studies using nilotinib or dasatinib as frontline therapy reach reasonable accrual and follow-up, it will be interesting to carry out a similar analysis to the one herein described to see whether our conclusions hold true in that setting.
Alternatively, one could argue that responders, regardless of when responses are achieved, are patients who have a disease that is intrinsically more sensitive to therapy and that the percentage of patients who are indeed resistant will not be significantly changed by forcing late responders to become early responders. Ongoing, randomized clinical trials comparing treatment options resulting in earlier responses with standard therapy with imatinib may help answer this question. In this regard, it is pertinent to highlight the fact that the majority of patients treated with imatinib at our institution receive high-dose imatinib (ie, 800 mg/day) as initial therapy. It is possible that this may have affected the percentage of patients expected to have an early response. Whether the quality of the response or the significance of not achieving an early response is affected by the use of high-dose versus standard-dose imatinib is currently unknown. Initial results from the ongoing Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study, in which patients with newly diagnosed CML are randomized to receive imatinib at either 400 mg/day or 800 mg/day as initial therapy, suggest that, although there is an improvement in the rate of CCyR and MMR at earlier time points, these differences narrow and become not statistically significant by 12 months from start of therapy.20 Further follow-up will be required to assess whether this higher rate of early responses translates into improved long-term benefit.
In conclusion, our results suggest that, although the long-term outcome of patients who achieve a CCyR may be similar regardless of the time at which that is achieved, patients who fail to attain a CCyR within the first 12 months of imatinib therapy have higher rates of disease progression and a lower probability of achieving an MMR. The depth of molecular response at 3 months appears to identify a subset of patients with a higher risk of progression, suggesting that alternative therapies associated with higher rates of early molecular response might be desirable.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
A.Q.-C. is a recipient of an American Society of Clinical Oncology Young Investigator Award 2006-2008. J.C., H.K., and S.O. received research funding from Bristol-Myers Squibb (Princeton, NJ). J.C. and H.K. received research funding from Novartis (Basel, Switzerland).
Authorship
Contribution: A.Q.-C. and J.C. designed and performed research, analyzed data, and wrote the paper; H.K., D.J., J.S., S.K., and S.O. analyzed data; and G.B. and D.T. performed research.
Conflict-of-interest disclosure: J.C. and H.K. received grant support from Bristol-Myers Squibb and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Jorge Cortes, University of Texas M. D. Anderson Cancer Center, Department of Leukemia, Unit 428, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: jcortes@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal