Down syndrome (DS) persons are born with various hematopoietic abnormalities, ranging from relatively benign, such as neutrophilia and macrocytosis, to a more severe transient myeloproliferative disorder (TMD). In most cases, these abnormalities resolve in the first few months to years of life. However, sometimes the TMD represents a premalignant disease that develops into acute megakaryocytic leukemia (AMKL), usually in association with acquired GATA1 mutations. To gain insight into the mechanisms responsible for these abnormalities, we analyzed the hematopoietic development of the Ts1Cje mouse model of DS. Our analyses identified defects in mature blood cells, including macrocytosis and anemia, as well as abnormalities in fetal liver and bone marrow stem and progenitor cell function. Despite these defects, the Ts1Cje mice do not develop disease resembling either TMD or AMKL, and this was not altered by a loss of function allele of Gata1. Thus, loss of Gata1 and partial trisomy of chromosome 21 orthologs, when combined, do not appear to be sufficient to induce TMD or AMKL-like phenotypes in mice.
Introduction
Down syndrome (DS) results from trisomy of chromosome 21 and is the most common and best-characterized chromosomal disorder in humans. The frequency of DS is approximately 1 in 700 to 800 live births. Affected persons display a wide variety of phenotypic and physiologic abnormalities, including mental retardation, facial dysmorphia, and cardiac anomalies. DS persons are also afflicted with a variety of hematopoietic defects encompassing all lineages of the hematopoietic system, including thrombocytopenia, neutrophilia, and macrocytosis.1 These defects generally resolve in the first year or 2 of life; however, macrocytosis persists in approximately two-thirds of DS persons.2
Approximately 10% of DS neonates are born with a transient myeloproliferative disease (TMD), which in most cases resolves within the first 3 months of life. TMD is characterized by uncontrolled proliferation of immature megakaryoblasts, which accumulate in the peripheral blood, fetal liver (FL), and bone marrow (BM).3 It is thought to be a disorder of FL hematopoiesis because human fetuses, as young as 25 weeks, have been diagnosed with a congenital leukemia, suggestive of TMD.4 The spontaneous remission of most DS TMD patients coincides with the transition from FL to BM hematopoiesis.5,6 This suggests that the FL environment may be contributing to maintenance of the disease.
In approximately 30% of DS TMD cases, the disorder represents a preleukemic disease that develops into acute megakaryocytic leukemia (AMKL).7,8 This is a direct progression from TMD to AMKL, as the TMD blast cells cannot to be distinguished phenotypically from the AMKL blast cells of the same person.9 The global transcriptional pattern of the 2 cell types is also remarkably similar, although they can be distinguished using hierarchical clustering.10,11
In addition to a 500-fold increased risk of AMKL, DS persons also have a 20-fold increased lifetime risk of acute lymphocytic leukemia (ALL).12 It is not known why this leukemia predisposition occurs; however, acquired trisomy 21 is a common chromosomal aberration in sporadic acute myeloid leukemia (AML) and ALL.13 Thus, it is probable that leukemia predisposition results from altered expression of one or more key genes on this chromosome.
One of the most intriguing discoveries in DS-TMD and AMKL has been the recent observation that acquired exon 2 GATA1 mutations are present in almost all DS derived TMD and AMKL samples analyzed.14,,,,–19 More intriguingly, these mutations do not appear to play a role in non-DS AMKL, with the exception of one case of identical twins both with acquired trisomy 21 and GATA1 exon 2 mutations.17 This suggests that the oncogenic function of an exon 2 mutation in GATA1 is dependent on trisomy 21.
Mutations in the second exon of GATA1 prevent expression of the wild-type full-length isoform of the protein and result in the production of a shorter isoform, GATA1s.18,20 The GATA1s isoform, whose function is not currently well understood, lacks the N-terminal activation domain of GATA1 but is still able to efficiently bind both DNA and its cofactor FOG1.18 Gata1ΔN mice, which express endogenous levels of Gata1s from the Gata1 locus, display normal adult hematopoiesis; however, FL hematopoiesis is perturbed. The most striking FL abnormality is a transient hyperproliferation of megakaryocyte progenitors from E9.5 to E16.5.21 Studies on a mouse expressing a Gata1s-like protein, lacking the activation domain (ΔNt-GATA1), have demonstrated that, if expressed in abundance, this isoform can compensate for loss of full-length Gata1.22 Interestingly, a germline mutation in exon 2 of GATA1 in humans leads to exclusive production of GATA1s in affected males, and X-linked macrocytic anemia with normal platelet counts and neutropenia. This further indicates that GATA1s cannot sustain normal hematopoiesis if expressed at endogenous levels.23 However, the discrepancy between the effects of GATA1s on adult hematopoiesis in the mouse compared with the human suggests that there may be differences between the roles of this isoform in the 2 organisms. Further evidence to support this suggestion is the observation that a point mutation in exon 2 does not result in expression of Gata1s in the mouse.24
In human DS, it has been suggested that there is a specific FL progenitor cell that is especially sensitive to the effect that GATA1s has on proliferation, and this effect may be enhanced by trisomy 21.21 This suggestion stems from the observations of transient FL-derived megakaryocyte hyperproliferation in the Gata1ΔN mice,21 and the presence of GATA1 mutations in prenatal TMD.25 This may also explain the common resolution of disease in DS when hematopoiesis switches from FL to BM.
Several mouse models have been generated to study the pathogenesis of DS. These models use the fact that the majority of genes on human chromosome 21 are syntenic to mouse chromosome 16 (with some genes also syntenic to mouse chromosomes 17 and 10). However, little study has been performed on the hematopoietic development of these mouse models. The most comprehensive study to date analyzed the hematopoietic development of the Ts65Dn mouse model. This mouse is trisomic for a 15.6-Mb region on the distal end of chromosome 16, which contains 104 conserved genes26 (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This study demonstrated that the Ts65Dn mice display several peripheral blood anomalies, including macrocytosis and increasing thrombocytosis. They also develop a myeloproliferative disease (MPD) similar to the human disease, with increased numbers of Kit+ progenitor cells in the BM, extramedullary hematopoiesis in the spleen, myelofibrosis, and an increased number of morphologically abnormal megakaryocyte progenitors.26 These data suggest that trisomy for a subset of genes on human chromosome 21 can lead to abnormal hematopoietic development, including a myeloproliferative disorder resembling TMD.
In the study presented here, we have analyzed the hematopoietic development of another DS mouse model, the Ts1Cje mouse, which is trisomic for approximately two-thirds of the triplicated genes in the Ts65Dn mouse model (Table S1). This mouse model was derived during a gene-targeting event for Sod1 and resulted from translocation of the distal end of chromosome 16 to chromosome 12.27 Studies on this mouse model to date have identified several neurologic and skeletal abnormalities, such as a reduced cerebellar volume,28 learning and behavioral abnormalities,27 and craniofacial abnormalities.29 Currently, there are no published data regarding the hematopoietic development of the Ts1Cje mouse model.
Our data demonstrate several overlapping phenotypes between the 2 mouse models, suggesting that only two-thirds of the triplicated genes in the Ts65Dn mouse are required for these phenotypes. However, the Ts1Cje mice do not display increasing thrombocytosis or the development of the MPD that is observed in the Ts65Dn mice. This implicates those additional triplicated genes in the Ts65Dn mice in development of the more severe hematopoietic phenotypes of DS.
A unique aspect to the study presented here is the analysis of prenatal hematopoiesis in the Ts1Cje mice. In agreement with the idea that the FL is particularly sensitive to the effects of trisomy 21, we have observed that FL hematopoiesis is perturbed in the Ts1Cje mice. Similarly, in agreement with the observed resolution of the majority of hematopoietic defects shortly after birth in DS, postnatal BM function in the Ts1Cje mice was largely normal. However, we identified some abnormalities that persisted in BM-derived hematopoiesis, especially in the development of red blood cells, which may explain the persistence of macrocytosis throughout life in many DS persons.
Methods
Mice
Ts1Cje mice are trisomic for the distal end of chromosome 16, stretching from Sod1 to Mx1, which contains approximately 97 known genes orthologous to human chromosome 21.27 Ts1Cje mice were originally obtained from The Jackson Laboratory (Bar Harbor, ME), and subsequently bred onto a C57BL/6 background. For experimentation, these Ts1Cje mice were bred with C57BL/6 disomic controls to generate either Ts1Cje trisomic or C57BL/6 disomic control offspring. Mice were genotyped using a duplex polymerase chain reaction amplification of the neomycin-resistance sequence and App as an internal control, primer sequences have been previously described.28,30 All animal work has been approved by the Walter and Eliza Hall Institute of Medical Research Ethics Committee.
Gata1Plt13 mice on a C57BL/6 × Balb/c F1 background were generated through an N-ethyl-N-nitrosourea (ENU) mutagenesis screen at the Walter and Eliza Hall Institute, and were genotyped using an allelic discrimination single nucleotide polymorphism–based assay as described in Majewski et al.24
Peripheral blood cell counts
Up to 200 μL of blood was extracted from the retro-orbital sinus using glass capillaries lined with heparin and stored in ethylenediaminetetraacetic acid tubes for analysis. Cell counts were performed using an automated ADVIA cell counter (ADVIA 120 Haematologic System; Bayer Diagnostics, Tarrytown, NY).
Histologic sections
Sternum and spleen were fixed in 10% buffered formalin and processed by the Walter and Eliza Hall Institute histology department. Paraffin sections were stained with hematoxylin and eosin and analyzed under ×100 or ×200 original magnification using a Zeiss Axiophot microscope with inbuilt camera (Carl Zeiss, Thornwood, NY). A 10×/0.30 air objective was used. Images were acquired using a Zeiss Axiocam camera and Axiovision software version 3.1. Images were put together using Adobe Photoshop CS3 (Adobe Systems, San Jose, CA).
Flow cytometry
Live nucleated cells were counted using eosin exclusion, and cells were washed and stained in balanced salt solution (150 mM NaCl, 3.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 7.4 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-NaOH, 1.2 mM KH2PO4, 0.8 mM K2HPO4, pH 7.2) with 10% (vol/vol) fetal calf serum (FCS). Peripheral blood was first treated with a hypotonic lysis solution to remove red cells. Lin−Sca1+Kit+ (LSK) cells were stained with a cocktail of lineage antibodies (anti-CD2, -CD3, -CD5, -CD8, -Mac-1, -Ter119, -B220, and -Gr-1) and an anti–rat IgG–fluorescein isothiocyanate (FITC) secondary antibody (BD Biosciences PharMingen, San Diego, CA). Lineage-stained cells were then stained with anti–Sca-1-phycoerythrin (PE) and anti–kit-allophycocyanin (BD Biosciences PharMingen). To distinguish the different myeloid progenitor compartments, cells were stained with lineage markers as for LSK cells, anti–rat IgG-FITC, anti–Sca-1-FITC, anti–kit-allophycocyanin, anti–CD34-biotin, anti–FcRα-PeCy7, and streptavidin-PE (BD Biosciences PharMingen). Erythroblast populations were distinguished by staining cells with anti–Ter119-PE, anti–CD71-biotin, and streptavidin-FITC (BD Biosciences PharMingen). All antibody incubations were performed for 25 minutes on ice, and all washes performed in ice-cold balanced salt solution/10% FCS.
Dead cells were stained by adding 0.1% (wt/vol) propidium iodide or 2% (vol/vol) Fluorogold (Invitrogen, Carlsbad, CA), and cells were analyzed on a LSR FACScan from BD Biosciences (San Jose, CA; http://www.bd.com/). Data were analyzed using FlowJo software version 6.4.7 (TreeStar, Ashland, OR).
Colony-forming unit spleen assay
Six-week-old recipient C57BL/6/Ly5.1 congenic recipient mice were irradiated with 2 rounds of 5.5-Gy γ-irradiation, 3 hours apart. After a 2-hour recovery, 106 E12.5 FL cells from either disomic or Ts1Cje mice were injected into the tail vein. Recipient mice were then maintained on neomycin drinking water for 12 days (1.1 g/L neomycin sulfate; Sigma-Aldrich, St Louis, MO), then killed and their spleens removed, weighed, and fixed in Bouin fixative for 48 hours. Colonies were counted using a dissection microscope. The spleen comparison image was taken using a Zeiss Axiocam camera with Axiovision software version 3.1.
Semisolid agar cultures
FL cells were resuspended in Dulbecco modified Eagle medium with 10% FCS, and 104 nucleated cells were added to a cytokine mix in 1 mL semisolid medium (DME with 20% bovine calf serum and 0.3% Bacto-Agar from BD Biosciences). The cytokine mix consisted of interleukin-3 (IL-3), stem cell factor (SCF), and erythropoietin (EPO) at the following concentrations: IL-3, 10 ng/mL; SCF, 100 ng/mL; and EPO, 4 IU/mL.
Cultures were incubated at 37°C in 10% CO2 for 7 days, fixed in 1 mL 2.5% glutaraldehyde solution, and then intact cultures were floated onto slides and left to dry for 1 to 2 days. After this they were stained for acetylcholinesterase activity and with luxol fast blue and hematoxylin. Definitive colony counts were then performed under microscopic examination (original magnification ×40) using a Zeiss Axioplan 2 microscope. Images were acquired using a Zeiss Axiocam camera and Axiovision software version 3.1.
Reconstitution assays
Disomic C57BL/6/Ly5.1 congenic recipient mice were given 2 rounds of 5.5-Gy γ-irradiation to ablate all recipient hematopoietic cells, and allowed to recover over 3 hours. Test cells (from disomic or Ts1Cje Ly5.2 mice), either in the presence or absence of an equal number of competitor cells (Ly5.1), were injected, via the tail vein, into the lethally irradiated recipient mice. The recipient mice were maintained on neomycin drinking water (1.1 g/L neomycin sulfate; Sigma-Aldrich) and allowed to recover for 6 to 8 weeks, before being used for analysis. Test cells were prepared from either the bone marrow or fetal liver and injected into recipient mice without additional treatment.
Results
The multiple hematopoietic defects in human DS neonates are apparent immediately after birth and have even been observed in human fetuses as young as 25 weeks gestation.4 This suggests that the genetic/physiologic mechanisms responsible begin to exert an effect during embryogenesis. In the mouse, like the human, the FL represents the major organ of hematopoietic development during the fetal period. To investigate fetal hematopoietic development in the Ts1Cje mice, the FL was analyzed at embryonic day 12.5 (E12.5). This time point represents the early stages of hematopoietic progenitor cell development and differentiation within the FL of the mouse. Blood from 1-, 3-, 6-, and 10-month-old mice and BM and spleen from 3- to 4-week-old mice were also used in these analyses, given that human DS persons usually resolve their hematopoietic defects shortly after birth.
Ts1Cje FL displays perturbations in the hematopoietic stem and progenitor cell compartment
Flow cytometric analysis identified no significant alteration in the number or proportion of LSK cells, which are enriched for hematopoietic stem cells (HSCs), in Ts1Cje FL (Figure 1A). However, Ts1Cje FL appears to be completely unable to reconstitute the hematopoietic system of lethally irradiated recipient mice. A noncompetitive transplant assay demonstrated that Ts1Cje FL cells (2 × 106 cells) were unable to radioprotect recipient mice, with all 10 recipients of these cells dying within 6 weeks of transplantation. In contrast, only 1 of 10 recipients receiving disomic donor FL cells died within this same time frame (data not shown). In a competitive transplant assay, disomic C57BL/6/Ly5.1 congenic competitor cells (106 cells; Ly5.1) were injected, along with Ts1Cje or disomic test cells (106 cells; Ly5.2), into lethally irradiated 6-week-old disomic C57BL/6/Ly5.1 congenic recipients. After 8 weeks of reconstitution, the proportion of test-derived (Ly5.2) cells was assessed in the peripheral blood of recipient mice. This analysis demonstrated that Ts1Cje FL donor cells contributed to less than 2% of the peripheral blood of reconstituted mice (Figure 1B). Thus, it appears that Ts1Cje HSCs derived from the FL are functionally impaired in their ability to regenerate the hematopoietic system of irradiated mice.
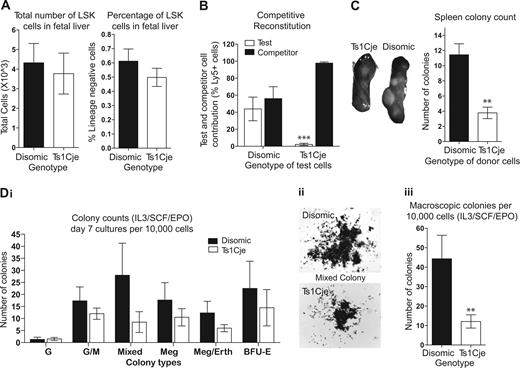
Fetal liver abnormalities are observed in Ts1Cje mice. (A) The proportion of HSCs in the Ts1Cje FL is slightly reduced. n = 5 disomic and 4 Ts1Cje embryos. (B) Ts1Cje FL HSCs cannot compete with disomic competitor cells, showing less than 2% contribution in the peripheral blood. n = 2 disomic and 6 Ts1Cje embryos, donor cells from each embryo injected into 2 recipient mice each. (C) CFU-S day 12 colonies. Ts1Cje FL-derived HSCs show a reduced capacity to form spleen colonies in recipient mice. Recipient mice receiving Ts1Cje FL donor cells had smaller spleens and fewer visible colonies than those mice receiving disomic FL donor cells. Cells from 3 donors of each genotype were injected into 3 recipients each. (D) Ts1Cje FL progenitor cells have an impaired ability to form colonies under IL-3/SCF/EPO stimulation: (i) Ts1Cje cultures had reduced numbers of each type of colony (G) granulocyte, (GM) granulocyte/macrophage, (Mixed) mix of different cell types, (Meg) megakaryocytic, (Meg/Eryth) megakaryocytic, and (BFU-E) erythroid; (ii) colonies formed in Ts1Cje cultures were significantly smaller than disomic cultures (original magnification ×20); (iii) fewer colonies were visible macroscopically (typically defined by the presence of > 1000 cells). n = 3 disomic and 4 Ts1Cje embryos. **P < .01; ***P < .001. Error bars represent SEM.
Fetal liver abnormalities are observed in Ts1Cje mice. (A) The proportion of HSCs in the Ts1Cje FL is slightly reduced. n = 5 disomic and 4 Ts1Cje embryos. (B) Ts1Cje FL HSCs cannot compete with disomic competitor cells, showing less than 2% contribution in the peripheral blood. n = 2 disomic and 6 Ts1Cje embryos, donor cells from each embryo injected into 2 recipient mice each. (C) CFU-S day 12 colonies. Ts1Cje FL-derived HSCs show a reduced capacity to form spleen colonies in recipient mice. Recipient mice receiving Ts1Cje FL donor cells had smaller spleens and fewer visible colonies than those mice receiving disomic FL donor cells. Cells from 3 donors of each genotype were injected into 3 recipients each. (D) Ts1Cje FL progenitor cells have an impaired ability to form colonies under IL-3/SCF/EPO stimulation: (i) Ts1Cje cultures had reduced numbers of each type of colony (G) granulocyte, (GM) granulocyte/macrophage, (Mixed) mix of different cell types, (Meg) megakaryocytic, (Meg/Eryth) megakaryocytic, and (BFU-E) erythroid; (ii) colonies formed in Ts1Cje cultures were significantly smaller than disomic cultures (original magnification ×20); (iii) fewer colonies were visible macroscopically (typically defined by the presence of > 1000 cells). n = 3 disomic and 4 Ts1Cje embryos. **P < .01; ***P < .001. Error bars represent SEM.
Colony-forming unit spleen day 12 (CFU-S12) assays were performed to look more closely at the function of short-term repopulating HSCs and multipotent progenitors. In mice receiving Ts1Cje donor FL cells, both the spleen weight and visible colony number were significantly reduced, compared with those mice receiving disomic FL cells (Figure 1C).
Semisolid agar cultures using IL-3/SCF/EPO stimulation of 104 nucleated FL cells identified defects in the ability of the more committed progenitor cells in the Ts1Cje FL to form colonies. The cultures generated from Ts1Cje FL cells demonstrated an impaired ability of progenitor cells to generate myeloid colonies under IL-3/SCF/EPO stimulation. The Ts1Cje FL cells produced a reduced number of each colony type, and colony size was also significantly smaller than disomic cultures (Figure 1D).
Ts1Cje mice display several peripheral blood abnormalities; however, BM and spleen morphology appears normal
Analysis of the peripheral blood of 1-, 3-, 6-, and 10-month-old Ts1Cje trisomic mice and their littermate disomic controls demonstrated that white cell and platelet counts were normal. However, analysis of the red cell compartment revealed a defect at all ages analyzed (Figure 2A). Ts1Cje mice had a reduction in the total number of red blood cells, a significant increase in mean corpuscular hemoglobin, and a significant increase in mean corpuscular volume, suggestive of macrocytosis and anemia (Figure 2A; Table S2).
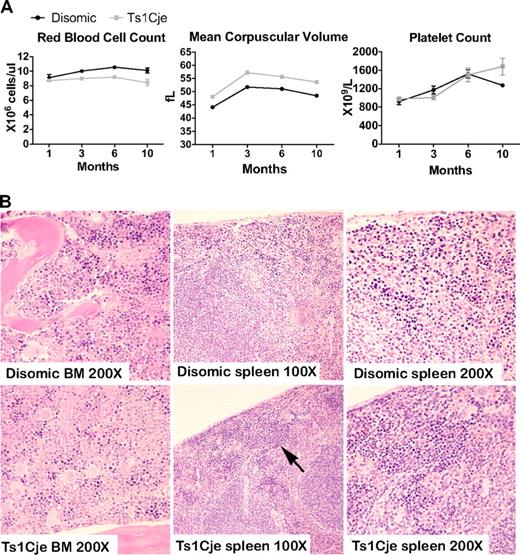
Phenotypic analysis of hematopoiesis in Ts1Cje postnatal mice detects peripheral blood abnormalities. (A) Ts1Cje mice at 1, 3, 6, and 10 months display abnormalities in the red cell lineage. (For each genotype at each age, n > 7, except 10 months, when n = 4). Platelet counts, however, are normal at all 4 ages and P < .001 for red blood cell count at 3 and 6 months, and for mean corpuscular volume at all 4 ages. (B) Sternum and spleen sections from 2-month-old Ts1Cje and disomic control mice. Slightly increased numbers of nucleated erythrocytes (arrow) were observed in the Ts1Cje spleen, and reduced numbers were present in the BM. BM images: original magnification ×200; spleen, original magnification ×100 and ×200. Sections are representative of 3 mice per genotype.
Phenotypic analysis of hematopoiesis in Ts1Cje postnatal mice detects peripheral blood abnormalities. (A) Ts1Cje mice at 1, 3, 6, and 10 months display abnormalities in the red cell lineage. (For each genotype at each age, n > 7, except 10 months, when n = 4). Platelet counts, however, are normal at all 4 ages and P < .001 for red blood cell count at 3 and 6 months, and for mean corpuscular volume at all 4 ages. (B) Sternum and spleen sections from 2-month-old Ts1Cje and disomic control mice. Slightly increased numbers of nucleated erythrocytes (arrow) were observed in the Ts1Cje spleen, and reduced numbers were present in the BM. BM images: original magnification ×200; spleen, original magnification ×100 and ×200. Sections are representative of 3 mice per genotype.
Reconstitution assays demonstrated that these peripheral blood abnormalities were intrinsic to the BM. Six-week-old disomic C57BL/6/Ly5.1 congenic lethally irradiated recipient mice were injected via the tail vein with either 3 × 106 Ts1Cje or disomic BM cells (Ly5.2). Six to 8 weeks after reconstitution, the peripheral blood of the recipient mice was analyzed. All recipient mice that received Ts1Cje BM cells recapitulated the macrocytosis and mild anemia of the donor mice. Interestingly, these recipient mice also displayed a significant neutrophilia that was not consistently observed in the donor mice. The recipient mice that received the disomic BM cells did not display any of these abnormalities (Figure S1).
Histologic examination of BM and spleen of 2-month-old Ts1Cje mice did not identify any significant abnormalities (Figure 2B). A slight increase in the number of nucleated erythrocytes in the spleen and a reduction in the BM were apparent, which is interesting given the macrocytosis and anemia observed in the peripheral blood analyses (Figure 2B). Spleen weight was not significantly different (data not shown).
Ts1Cje mice display mild perturbations in the hematopoietic stem and progenitor cell compartment in the BM
Flow cytometric analysis did not identify any difference in the percentage of LSK cells in the postnatal Ts1Cje BM; however, a significant reduction in the total number of these cells was observed (Figure 3A). When BM cellularity was analyzed, it was found that there was a significantly reduced number of cells in the Ts1Cje BM, and this probably explained the reduction in the total number of LSK cells observed (Figure 3B). Unlike the FL HSCs, BM HSCs were able to reconstitute the hematopoietic system of lethally irradiated recipient mice as efficiently as wild-type mice, with an average of 45% reconstitution from the Ts1Cje test cells (Figure 3C).
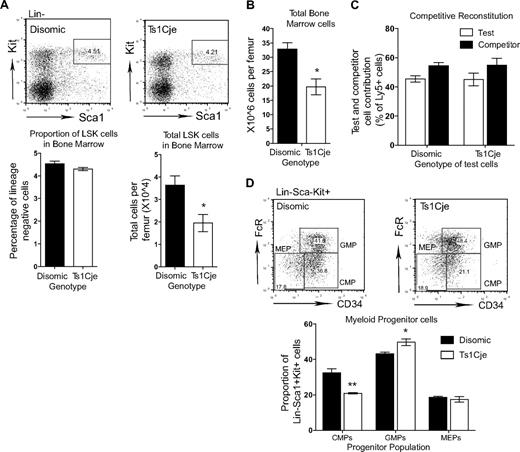
Hematopoietic abnormalities are seen in the Ts1Cje bone marrow. (A) The number of LSK cells in the Ts1Cje BM is reduced (n = 3 mice of each genotype). (B) Ts1Cje BM has a reduced cellularity compared with disomic controls (n = 3 mice of each genotype). (C) Ts1Cje BM HSCs are able to reconstitute hematopoiesis as efficiently as disomic competitor cells. BM from 3 mice of each genotype was transplanted into 5 recipients per donor mouse. (D) Myeloid progenitor cells in the Ts1Cje BM show a reduced proportion of CMPs and a concomitant increase in GMPs. MEPs are not affected (n = 3 mice of each genotype). *P < .05; **P < .01. Error bars represent SEM.
Hematopoietic abnormalities are seen in the Ts1Cje bone marrow. (A) The number of LSK cells in the Ts1Cje BM is reduced (n = 3 mice of each genotype). (B) Ts1Cje BM has a reduced cellularity compared with disomic controls (n = 3 mice of each genotype). (C) Ts1Cje BM HSCs are able to reconstitute hematopoiesis as efficiently as disomic competitor cells. BM from 3 mice of each genotype was transplanted into 5 recipients per donor mouse. (D) Myeloid progenitor cells in the Ts1Cje BM show a reduced proportion of CMPs and a concomitant increase in GMPs. MEPs are not affected (n = 3 mice of each genotype). *P < .05; **P < .01. Error bars represent SEM.
Flow cytometric analysis of the common myeloid progenitors (CMPs; LKSca1−FcRα−CD34+), the granulocyte-macrophage progenitors (GMPs; LKSca1−FcRα+CD34+), and the megakaryocyte erythroid progenitors (MEPs; LKSca1−FcRα+CD34−) identified a slight skewing in the proportion of these different progenitor populations. A significant reduction in the proportion of CMPs in the Ts1Cje BM was observed (Figure 3D), with a concomitant increase in the proportion of GMPs. The proportion of MEPs, however, appeared normal. The observed decrease in CMPs and increase in GMPs suggest that differentiation toward the granulocytic lineage might be enhanced in the Ts1Cje BM.
Ts1Cje mice, unlike Ts65Dn mice, do not display thrombocytosis or myeloproliferative disease at older ages
Kirsammer et al identified an MPD in the Ts65Dn mice, which resembled the TMD observed in human DS.26 Key features of the disease in these mice were increasing thrombocytosis, an accumulation of morphologically altered megakaryocytes in the BM and spleen, as well as granulocyte hyperplasia and fibrosis in the BM (the hematopoietic phenotype of the Ts65Dn mice is summarized in Table S3). To investigate whether the Ts1Cje mice also develop signs of an MPD, mice were aged to 2 years and BM and spleen sections were analyzed.
Peripheral blood from Ts1Cje mice up to 10 months of age was analyzed and demonstrated that platelet levels remained relatively normal (Figure 2A). The life span was not altered between the Ts1Cje mice and age-matched disomic controls (Figure 4A), and no abnormalities were observed in spleen and BM sections from 24-month-old Ts1Cje mice (Figure 4B,C). These data therefore suggest that the genes triplicated in the Ts1Cje segment are not sufficient for development of the MPD found in the Ts65Dn mice.
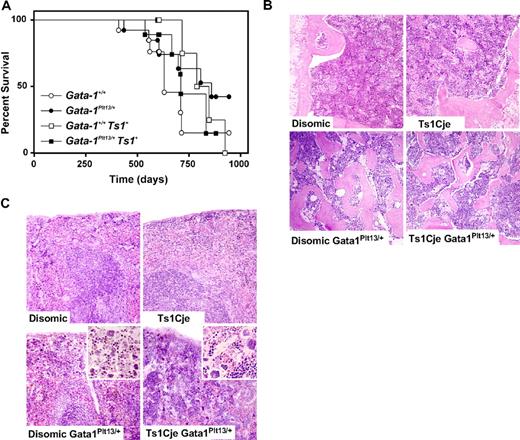
Ts1Cje mice, with or without Gata1 mutation, do not develop a myeloproliferative disorder. (A) Disease incidence was monitored in a cohort of Ts1Cje mice, and in Ts1Cje mice that carried a loss of function exon 2 mutation in Gata1 (Gata1Plt13), over a 2-year period (n = 9-13 mice of each genotype). Mice were killed when they exhibited signs of illness, and an autopsy and histologic analysis were performed. Ts1Cje mice had a similar life expectancy to disomic mice (○, □). The presence of the Gata1Plt13 mutation did not significantly alter the survival curve of Ts1Cje mice, nor did it alter the disease spectrum (●, ■). (B) BM sections (original magnification ×100) from 2-year-old Ts1Cje mice. The Gata1Plt13 mutation normally leads to increased numbers of megakaryocytes and bone overgrowth in the BM. Crossing this mutation onto the Ts1Cje background did not alter this phenotype. (C) Spleen sections (original magnification ×100) from aged mice also did not show any signs of myelofibrosis in Ts1Cje mice. The Gata1Plt13 mutation normally leads to increased numbers of megakaryocytes in the spleen, and this frequency was not altered by crossing to the Ts1Cje mice (insets; original magnification ×200).
Ts1Cje mice, with or without Gata1 mutation, do not develop a myeloproliferative disorder. (A) Disease incidence was monitored in a cohort of Ts1Cje mice, and in Ts1Cje mice that carried a loss of function exon 2 mutation in Gata1 (Gata1Plt13), over a 2-year period (n = 9-13 mice of each genotype). Mice were killed when they exhibited signs of illness, and an autopsy and histologic analysis were performed. Ts1Cje mice had a similar life expectancy to disomic mice (○, □). The presence of the Gata1Plt13 mutation did not significantly alter the survival curve of Ts1Cje mice, nor did it alter the disease spectrum (●, ■). (B) BM sections (original magnification ×100) from 2-year-old Ts1Cje mice. The Gata1Plt13 mutation normally leads to increased numbers of megakaryocytes and bone overgrowth in the BM. Crossing this mutation onto the Ts1Cje background did not alter this phenotype. (C) Spleen sections (original magnification ×100) from aged mice also did not show any signs of myelofibrosis in Ts1Cje mice. The Gata1Plt13 mutation normally leads to increased numbers of megakaryocytes in the spleen, and this frequency was not altered by crossing to the Ts1Cje mice (insets; original magnification ×200).
Given the identified key role of exon 2 GATA1 mutations in the development of TMD and AMKL in human DS, we decided to investigate whether an analogous murine mutation may induce MPD in the context of partial trisomy in the Ts1Cje mice. Ts1Cje mice were crossed to Gata1Plt13/+ mice, which carry a point mutation in exon 2 of Gata1 leading to disruption of the start codon and loss of translation of full-length protein.24 Interestingly, this mutation does not lead to production of the shorter isoform, Gata1s, in contrast to what is observed in humans.24
Female mice that carry the Gata1Plt13 mutation normally develop thrombocytopenia and show an accumulation of megakaryocytes in the spleen and BM, a reduction of megakaryocyte progenitors in the BM, extramedullary hematopoiesis in the spleen, and a reduced life span.24 When these mice were crossed onto the Ts1Cje background, no change in this phenotype was observed. The Ts1Cje/Gata1Plt13/+ mice had increased megakaryocytes in the spleen and BM and a reduced life span compared with Ts1Cje/Gata1+/+ mice (Figure 4). However, these were not significantly different from disomic/Gata1Plt13/+mice, and neither group of mice showed any signs of obvious myelofibrosis in the spleen (Figure 4C). Both the Ts1Cje/Gata1Plt13/+ and the disomic/Gata1Plt13/+ groups of mice displayed a similar low frequency of lung adenoma, reticulum cell sarcoma, lymphoid leukemia, and polycystic kidneys, and in both groups there was a 60% incidence of bone overgrowth (Table S4). Bone overgrowth in mice with low expression of Gata1 has been described previously.31
These data suggest that the combination of partial trisomy 16 and Gata1 exon 2 mutation in the mouse does not cooperate to induce MPD formation, unlike what is seen in human DS. However, given that the Gata1Plt13 mutation does not lead to production of the Gata1s protein, our data may instead suggest that loss of full-length Gata1 protein, without the added effect of Gata1s expression, is not sufficient to contribute to DS MPD and leukemogenesis. It should also be noted, however, that both the 24-month-old Ts1Cje mice and the Gata1Plt13/+ mice were on a mixed (F1) C57BL/6 × Balb/c background. This is in contrast to the Ts1Cje mice used for all other analyses, which were maintained on a pure C57BL/6 background. However, the peripheral blood defects, including macrocytosis and anemia, that were observed on the C57BL/6 background were still evident on the mixed background at 7 weeks of age (Figure S2). Indeed, these defects were even more prominent in the Ts1Cje/Gata1Plt13/+ mice, indicating a possible additive effect.
Erythrocyte development/maturation is perturbed in Ts1Cje BM
To further investigate the underlying cause of the macrocytosis and reduced red blood cell count, a detailed analysis of erythrocyte development was performed. During erythrocyte maturation, the early erythroblast progenitor cell, the proerythroblast, undergoes progressive differentiation through several different erythroblast stages. From least differentiated to most differentiated, these populations are the proerythroblasts, the basophilic erythroblasts, the chromatophilic and orthochromatophilic erythroblasts, and reticulocytes.32
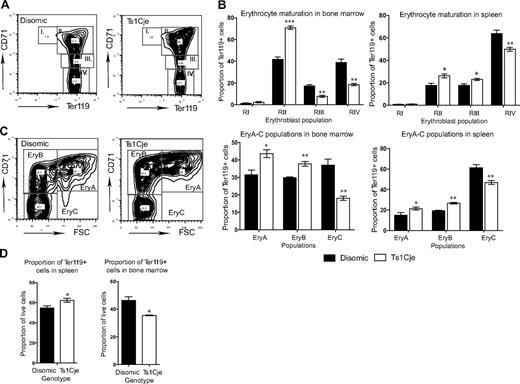
Socolovsky et al determined that up-regulation of the glycophorin protein Ter119 and down-regulation of the transferrin receptor CD71 occurs on the surface of erythroblasts as they mature.33 Thus, the different erythroblast populations are readily distinguishable by flow cytometric analysis of Ter119 and CD71 cell-surface markers (Figure 5A).
Ts1Cje mice display erythroid abnormalities. (A) Flow cytometric analysis of Ter119 and CD71 cell-surface markers allows identification of different erythroblast populations. Regions I and II, proerythroblasts and early basophilic; regions III and IV, late basophilic and chromatophilic erythroblasts. (B) Erythrocyte maturation was perturbed in the Ts1Cje 3-week-old BM and spleen with an increase in the proportion of early erythroblasts and a concomitant decrease in the proportion of late erythroblasts n = 3 mice of each genotype for BM and n = 4 of each genotype for spleen. (C) Further stratifying the Ter119+ population into Ery.A, Ery.B, and Ery.C populations demonstrates a significant increase in the proportion of Ery.A and Ery.B cells and a concomitant decrease in the proportion of Ery.C cells in both the Ts1Cje BM and spleen. n = 3 mice of each genotype for BM and n = 4 of each genotype for spleen. (D) The overall proportion of Ter119+ cells in the Ts1Cje BM is decreased, whereas it is increased in the spleen. n = 3 mice of each genotype for BM and n = 4 of each genotype for spleen. *P < .05; **P < .01; ***P < .001. Error bars represent SEM.
Ts1Cje mice display erythroid abnormalities. (A) Flow cytometric analysis of Ter119 and CD71 cell-surface markers allows identification of different erythroblast populations. Regions I and II, proerythroblasts and early basophilic; regions III and IV, late basophilic and chromatophilic erythroblasts. (B) Erythrocyte maturation was perturbed in the Ts1Cje 3-week-old BM and spleen with an increase in the proportion of early erythroblasts and a concomitant decrease in the proportion of late erythroblasts n = 3 mice of each genotype for BM and n = 4 of each genotype for spleen. (C) Further stratifying the Ter119+ population into Ery.A, Ery.B, and Ery.C populations demonstrates a significant increase in the proportion of Ery.A and Ery.B cells and a concomitant decrease in the proportion of Ery.C cells in both the Ts1Cje BM and spleen. n = 3 mice of each genotype for BM and n = 4 of each genotype for spleen. (D) The overall proportion of Ter119+ cells in the Ts1Cje BM is decreased, whereas it is increased in the spleen. n = 3 mice of each genotype for BM and n = 4 of each genotype for spleen. *P < .05; **P < .01; ***P < .001. Error bars represent SEM.
Erythrocyte maturation was perturbed in both the 3-week-old Ts1Cje BM and spleen, with a significant increase in the proportion of early erythroblasts and a decrease in the proportion of late basophilic and chromatophilic erythroblasts (Figure 5A,B). This defect was not observed in the FL (data not shown). These Ter119+ erythroblast populations can be further stratified into Ery.A, Ery.B, and Ery.C populations according to their forward scatter and CD71 profile (Figure 5C).34 This analysis identified, in agreement with the previous data, that there is an increase in the proportion of the less mature erythroblasts (Ery.A and Ery.B) and a concomitant decrease in the proportion of the more mature erythroblasts (Ery.C) in both the BM and spleen (Figure 5C). Interestingly, despite this apparent perturbed maturation, there is no significant difference in the number and proportion of reticulocytes in the peripheral blood of 3- and 4-month-old disomic and Ts1Cje mice (data not shown).
In concordance with the increased number of nucleated erythrocytes observed histologically in the Ts1Cje spleen, the proportion of splenic Ter119+ cells (populations II-IV) was significantly increased (Figure 5D). In contrast, there was a significant decrease in the proportion of these cells in the Ts1Cje BM, again suggesting a perturbation in erythrocyte development (Figure 5D). There was no significant difference in the proportion of Ter119+ cells in FL (data not shown). Because the spleen is responsible for increasing erythropoiesis under conditions such as anemia, the increase in Ter119+ erythroid cells observed in the spleen, which was not seen in the other organs, may be the result of the slight anemia in the Ts1Cje mice and may help explain the presence of a normal number of reticulocytes in the peripheral blood.
Discussion
In this study, analyses were performed on both prenatal and postnatal hematopoiesis in Ts1Cje mice to gain insight into why DS persons are born with a variety of hematopoietic abnormalities and why these usually resolve shortly after birth. Our analyses of FL hematopoiesis identified several defects in the HSC and myeloid progenitor cell compartments, most notably a reduced number of myeloid colony-forming cells with limited ability to form large colonies, as well as a complete lack of HSC reconstitution ability. This demonstrates that trisomy of orthologous genes on chromosome 21 is sufficient to impair hematopoiesis in the FL. In addition, the functional defects we observed in the earliest progenitor cell, the HSC, may explain why perinatal abnormalities in DS persons encompass multiple hematopoietic lineages.
Despite these FL HSC and progenitor defects, Ts1Cje mice survive to term and display only mild postnatal blood cell defects at 1 month of age or older. This indicates that BM hematopoiesis is able to sustain adult hematopoiesis adequately, which agrees with the resolution of most human DS hematopoietic defects after birth. However, investigation into Ts1Cje BM hematopoiesis identified a reduction in BM cellularity and LSK cell number, and a skewing in the proportion of CMPs and GMPs, suggesting that BM function is slightly perturbed. The apparent disparity in the effect of trisomy of chromosome 21 orthologs on FL and BM function in the Ts1Cje mice further demonstrates the differences between prenatal and postnatal hematopoiesis. It also suggests that the FL is particularly sensitive to trisomy 21, as alluded to by Li et al.21
These analyses have demonstrated that triplication of a particular set of chromosome 21 orthologs in the Ts1Cje mice is sufficient to lead to aberrant hematopoiesis. Interestingly, despite the defects we observed in the FL, the Ts1Cje mice do not show any sign of thrombocytosis or MPD development, in contrast to what has been observed in the Ts65Dn mice (Table S3). This would suggest that different chromosome 21 genes are responsible for the various hematopoietic abnormalities of DS and that those orthologs within the Ts65Dn triplicated segment, but not within the smaller Ts1Cje triplicated segment, are responsible for development of the more severe phenotypes (Table S1 outlines the triplicated genes that are distinct in the Ts65Dn mice). However, it cannot be excluded that the different genetic backgrounds of the Ts1Cje mice used in this study and the Ts65Dn mice used by Kirsammer et al26 may be playing a role in the phenotypic diversity.
Intriguingly, if genes within the Ts1Cje triplicated region are not responsible for the TMD and AMKL in human DS, this would rule out involvement of 2 of the probable candidate genes in the DS trisomic region. These 2 genes, the integral hematopoietic transcription factors Runx1 and Erg, are both triplicated in the Ts1Cje and Ts65Dn models. Kirsammer et al26 demonstrated that Runx1 was indeed not responsible for the MPD by generating Ts65Dn mice that were functionally disomic for Runx1. They observed that there was no difference in the MPD phenotype between these mice and the original Ts65Dn mice.26 A similar experiment with Erg is required to definitively address its role in the TMD and AMKL of DS.
Our data also suggest that a murine mutation that leads to loss of Gata1 expression does not cooperate with trisomy of orthologous chromosome 21 genes, unlike what is seen in human DS. This is in agreement with the data of Kirsammer et al, in which the authors were unable to detect any Gata1 exon 2 mutation in Ts65Dn mice, despite the development of MPD.26 Thus, it appears as though the seemingly essential role of GATA1 in DS TMD and AMKL is not recapitulated in the mouse, at least via exon 2 mutations. This may not be surprising given that mouse studies have shown the effect of Gata1s in mice to be different from that in humans. Indeed, Gata1s does not seem to be normally expressed in mice and, unlike humans, it is not induced on loss of expression of the full-length isoform.24 Our data, therefore, may indicate that it is the production of the dominantly acting GATA1s protein in human DS that is particularly leukemogenic, and this cannot be addressed through studies of Gata1 mutant mice that do not express Gata1s. Additional experiments looking specifically at the effect of Gata1s expression in a partially trisomic mouse model of DS will provide further insight into this issue.
The major peripheral blood abnormality observed in the Ts1Cje mice is a BM intrinsic macrocytosis and mild anemia, which is still present at 6 months of age. This correlates well with the macrocytosis that persists throughout life in more than 60% of DS persons. Although this phenotype was also observed in the Ts65Dn mice, it appears that the gene(s) responsible lie within the smaller segment of trisomy in the Ts1Cje mice. Analyses of erythrocyte maturation in the Ts1Cje BM and spleen demonstrated that there is a significant block in maturation leading to an increased proportion of early to late erythroblasts. The underlying cause for this perturbed maturation is unknown; however, it is probable that additional studies on this mouse model will provide significant understanding of the mechanisms and genes that are involved in the macrocytosis observed in DS.
The combined results from this study support a role for the Ts1Cje mouse as a useful model for investigation into the mechanisms, processes, and genes underlying the hematopoietic defects in human DS, particularly macrocytosis. The abnormalities observed in the FL HSCs and progenitor cells correlate well with the developmental time point of hematopoietic defects in human DS persons. This further highlights the importance of understanding how trisomy 21 impacts on FL hematopoiesis and why it does not have the same detrimental effects in the BM. Identifying the molecular perturbations responsible for the FL and BM abnormalities in the Ts1Cje mice may provide new insights into the hematopoietic defects that affect persons with DS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Melanie Pritchard (Monash Institute of Medical Research, Clayton, Australia) for supplying the Ts1Cje mice; Sandra Mitsuda, Ladina di Rago, Jason Corbin, Manuela Hancock, and Margareta Go for their excellent technical assistance; and Melissa Smith, Liana Mackiewicz, Rebecca Towns, and Gabriela Panoschi for their excellent skills in animal husbandry.
This work was supported by grants from the APEX Foundation for Research into Intellectual Disability (Sydney, Australia), the National Health and Medical Research Council of Australia (Canberra ACT, Australia; program grants 461219 [W.S.A.], 257501 [H.S.S.], and 219176 [H.S.S.], fellowships 305503 [W.S.A.], 461300 [D.J.H.], 171601 [H.S.S.], and 461204 [H.S.S.], a Dora Lush Postgraduate Award [C.L.C.]), the Leukaemia Foundation of Australia (Victoria, Australia; postdoctoral fellowship; C.L.C.), the Australian Government (Canberra, Australia; Australian Postgraduate Award; I.J.M.), the Cancer Council of Australia (Sydney, Australia; postdoctoral fellowship; I.J.M.), the Cancer Council of Victoria (Victoria, Australia; D.M.), the Australian Cancer Research Foundation (Sydney, Australia), and MuriGen Pty Ltd (Victoria, Australia).
Authorship
Contribution: C.L.C. designed the research and performed the experimentation, data analysis, and interpretation; I.J.M., D.M., D.J.H., and W.S.A. contributed to the research; C.A.H. and H.S.S. contributed to the experimental design; and C.L.C. wrote the paper. All authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for Dr Hewitt is Department of Pathology, Peter MacCallum Cancer Centre, Melbourne, Australia. The current addresses for Dr Scott are Division of Molecular Pathology, The Institute of Medical and Veterinary Science and The Centre for Cancer Biology, The Hanson Institute, SA, Australia, and The Adelaide Cancer Research Institute, The School of Medicine, University of Adelaide, SA, Australia.
Correspondence: Catherine Carmichael, Molecular Medicine, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Melbourne 3050, VIC, Australia; e-mail: carmichael@wehi.edu.au; or Hamish Scott at the current addresses: Division of Molecular Pathology, The Institute of Medical and Veterinary Science and The Centre for Cancer Biology, The Hanson Institute, Box 14 Rundle Mall Post Office, Adelaide, SA 5000 and The Adelaide Cancer Research Institute, The School of Medicine, University of Adelaide, SA 5005, Australia; e-mail: hamish.scott@imvs.sa.gov.au.