Abstract
Recent studies involving bone marrow mesenchymal stromal cells (MSCs) demonstrated that interferon (IFN)–γ stimulation induces major histocompatibility complex (MHC) class II–mediated antigen presentation in MSCs both in vitro and in vivo. Concordantly, we investigated the ability of MSCs to present extracellular antigen through their MHC class I molecules, a process known as cross-presentation. Using an in vitro antigen presentation assay, we demonstrated that murine MSCs can cross-present soluble ovalbumin (OVA) to naive CD8+ T cells from OT-I mice. Cross-presentation by MSC was proteasome dependent and partly dependent on transporter associated with antigen-processing molecules. Pretreatment of MSC with IFN-γ increased cross-presentation by up-regulating antigen processing and presentation. However, although the transcription of the transporter associated with antigen processing-1 molecules and the immunoproteasome subunit LMP2 induced by IFN-γ was inhibited by transforming growth factor-β, the overall cross-presentation capacity of MSCs remained unchanged after transforming growth factor-β treatment. These observations were validated in vivo by performing an immune reconstitution assay in β2-microglobulin−/− mice and show that OVA cross-presentation by MSCs induces the proliferation of naive OVA-specific CD8+ T cells. In conclusion, we demonstrate that MSCs can cross-present exogenous antigen and induce an effective CD8+ T-cell immune response, a property that could be exploited as a therapeutic cell-based immune biopharmaceutic for the treatment of cancer or infectious diseases.
Introduction
Antigen presentation plays a crucial role in shaping immunity and tolerance. The majority of nucleated cells present endogenous antigenic peptides on major histocompatibility complex (MHC) class I molecules to CD8+ T cells. This presentation is responsible for the recognition and destruction of pathogen-infected or cancer cells by effector cytotoxic T cells (CTLs). T-lymphocyte activation and differentiation into effector cells is dependent on antigen presentation and costimulation by professional antigen-presenting cells (APCs), such as dendritic cells (DCs) and macrophages. DCs and macrophages are able to sample their environment and process exogenous antigens through their MHC class II-mediated antigen presentation machinery for the presentation of antigenic peptides to CD4+ T lymphocytes. In addition, these APCs can process extracellular antigens for MHC class I-mediated presentation to CD8+ T cells, a mechanism known as cross-presentation.1,2 Antigen cross-presentation and subsequent cross-priming are essential to mount effective CTL responses against soluble proteins, antigens captured in immune complexes, cellular pathogens that do not reach the cytosol (eg, Mycobacterium) or do not infect hematopoietic cells (eg, Plasmodium berghei), and tumor antigens from dying tumor cells.1,3,4
In DCs and macrophages, several mechanisms have been described to provide antigen entry for cross-presentation, including phagocytosis of particulate antigens, receptor-mediated endocytosis, fluid-phase endocytosis (macropinocytosis and pinocytosis)5,6 and, recently, seeping through gap junctions.7 However, the exact mechanism by which exogenous antigens reach the cytosol to be processed by the MHC class I antigen machinery is still unclear. In the cytosol, proteins are ubiquitylated and directed to the proteasome for proteolysis. Peptides generated are then translocated into the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP), a heterodimer of TAP1 and TAP2 subunits, where the peptides are further trimmed by ER-associated aminopeptidase into smaller peptides of 8 to 10 amino acids. The peptide-loading complex composed of nascent MHC class I–β2-microglobulin complex associated with tapasin, calreticulin, and ERp57 loads the peptide unto the MHC class I molecule, which can now be transported to the cell surface via the Golgi apparatus where, in association with costimulatory molecules, can induce the differentiation of naive CD8+ T cells into activated CTLs.3,8
Although cross-presentation is a characteristic of DCs9 and macrophages,10 cross-presentation can be performed, although less efficiently, by other cell types such as neutrophils,11 B cells,12 and endothelial cells.13 Bone marrow mesenchymal stromal cells (MSCs), which are nonhematopoietic progenitor cells, possess, along with their ability to differentiate into several mesenchymal tissue lineages, the capacity to behave as APCs. More precisely, once stimulated with interferon (IFN)–γ, these cells can uptake, process, and present exogenous antigens through their MHC class II molecules, leading to the activation of naive CD4+ T cells in vitro and in vivo.14,15
To unravel the distinct APC properties of MSCs, their ability to perform cross-presentation of soluble exogenous proteins was tested, as well as modulation of these functions by IFN-γ and transforming growth factor (TGF)–β. Unstimulated MSCs cross-presented soluble chicken white egg ovalbumin (OVA), leading to the activation of OVA-specific naive CD8+ T cells in vitro, a response that was increased after IFN-γ stimulation. In vivo cross-presentation by MSCs was confirmed upon adoptive transfers into β2-microglobulin−/− mice deficient for MHC class I molecules and therefore cross-presentation. Concomitant treatment with TGF-β did not alter the basal or IFN-γ–up-regulated ability of MSC to cross-present, although it inhibited IFN-γ–induced up-regulation of Tap1 and Lmp2 expression. Overall, this study demonstrated that MSCs can cross-present an exogenous antigen and induce CD8+ T-cell response both in vitro and in vivo.
Methods
Reagents
Recombinant mouse (rm) IFN-γ was from Invitrogen, recombinant human (rh) TGF-β and rm-GM-CSF (granulocyte monocyte colony stimulating factor) were from R&D Systems, and rmIL-4 was from eBioscience. Lactacystin (L6785), chicken egg white OVA, and lipopolysaccharide (LPS) from Escherichia coli O26:B6 were from Sigma-Aldrich.
Cell isolation and culture
All animal protocols were approved by the McGill University Animal Care Committee. Mouse MSCs were obtained from femur and tibia marrow plugs of female C57BL/6 or B6.129S2-Tap1tm1Arp/J (Tap1 KO) mice (The Jackson Laboratory), as previously described.16 In brief, bone marrow cells from femurs and tibias were extracted by flushing and plated in MSC medium (ie, high-glucose Dulbecco modified Eagle medium, 10% fetal bovine serum [FBS], and 100 U/mL penicillin/streptomycin [P/S]). Floating cells were removed by adding fresh medium every 3 to 4 days until the culture reached 80% confluence. MSC populations were tested for the absence of CD31+, CD45+, or CD11b+ cells; for the expression CD34, CD44, CD73, and CD105; and for the ability to differentiate into adipocytes, osteocytes, and chondrocytes, as described.16 OVA-specific (amino acid 257-264, SIINFEKL epitope) CD8+ T cells (OT-I T lymphocytes) were isolated from C57BL/6-Tg (TcraTcrb)1100Mjb (OT-I) mice (The Jackson Laboratory) mice by a CD8+ T cell–negative selection (StemCell Technologies) on their splenocytes (99% CD8+ T cells).
Bone marrow–derived DCs were obtained by culturing 106 bone marrow cells from C57Bl/6 mice per well in 6-well plate with 3 mL of DC differentiation medium (ie, RPMI, 10% FBS, 100 U/mL P/S, 200 U/mL rm-granulocyte monocyte colony stimulating factor, and 200 U/mL rmIL-4). On day 3, half the medium was replaced, and DC maturation was induced on day 6 by adding 1 μg/mL LPS for 24 hours. The next day, floating and loosely adherent cells were collected and tested for CD11c expression by flow cytometry (65% CD11c+ cells). Peritoneal macrophages were collected from C57Bl/6 or B6.129-B2mtm1JaeN12 (β2-microglobulin−/− (Taconic) mice by lavaging the peritoneal cavity. Cells were plated in serum-free medium, and 2 hours later nonadherent cells were removed by gentle washing with PBS. Macrophages were then cultured in RPMI, 10% FBS, and 100 U/mL P/S and tested for Mac-3 expression by flow cytometry the next day (72% and 36% Mac-3+ cells for wild-type (WT) and β2-microglobulin−/− mice, respectively).
Flow cytometry analysis
The following antibodies specific for mouse molecules were used: biotin-coupled anti-CD31 (clone 390) and -CD34 (clone RAM34), phycoerythrin-conjugated anti-CD44 (clone IM7), -CD45 (clone 30-F11), -CD73 (clone TY123), -CD105 (cloneMJ7/16, eBioscience), -CD8a (clone 53-6.7), -CD11b (clone M1/70), -CD11c (clone HL3), -H2-Kb (clone AF6-88.5), and -Mac-3 (clone M3/84). Biotin-coupled antibodies were revealed with phycoerythrin-conjugated streptavidin. Except where indicated, antibodies and reagents were from BD Biosciences.
Real-time RT-PCR
DNA-free total RNA was prepared with use of the RNeasy kit with DNase digestion (QIAGEN Inc). RNA (2 μg) was reverse transcribed (RT) with the MuLV reverse transcriptase (Applied Biosystems) by use of the random hexamers in the presence of RNase inhibitor. An aliquot of 1/50th of the resulting cDNA was used for polymerase chain reaaction (PCR) amplification in a 10-μL reaction volume. Quantitative PCR assays were performed in duplicate on an ABI 7500 Fast Real-Time PCR system thermal cycler and SYBR Green Mastermix (Applied Biosystems). Mouse primer sequences (5′-3′ forward, reverse) were Lmp2: GAGGACTTGTTAGCGCATCTCA, CATATACCTGTCCCCCCTCACA (63 bp), Tap1: GGAGACAGGGTTTTTCCTGAAGA, TGTCCTCAGTCACCCGAGATG (61 bp), and 18S: TTACCAAAAGTGGCCCACTA, GAAAGATGGTGAACTATGCC (200 bp). Data were analyzed by use of the relative quantification method (based on the relative expression of target gene mRNA vs 18S RNA mRNA levels as a reference). Specificity of PCR amplification was tested by melting curves analysis. The absence of genomic DNA contamination was demonstrated by analysis of PCR performed with total RNA and with each of the primer sets.
Immunoblot assay
Immunoblot analyses were performed as described.17 Primary antibodies were specific for: Tap1 (clone M-18, Santa Cruz Biotechnology), LMP2 (Abcam), α-Tubulin (clone TU-02, Santa Cruz Biotechnology), and β-Tubulin (Cell Signaling Technology).
MHC class I–mediated antigen presentation assay
Confluent MSC cultures were treated or not with IFN-γ (10 ng/mL) for 1 hour before the addition of OVA (1 mg/mL) for 18 hours. Cells were lifted by incubation with trypsin, washed, and seeded in a flat bottom 96-well plate at 2 × 104 cells per well. Kb-restricted OVA-specific (amino acid 257-264, SIINFEKL epitope) CD8+ T cells were purified by negative selection by the use of the mouse CD8+ T-cell enrichment kit (StemCell Technologies) from the spleen of OT-I transgenic mice and were added to the wells (105 cells) in a total volume of 200 μL. After 48 hours, levels of IL-2 produced by the activated CD8+ T cells were quantified by enzyme-linked immunosorbent assay (ELISA; eBioscience) performed on supernatants. Flow cytometry analysis of purified CD8+ T cells demonstrated greater than 95% purity (data not shown). Controls consisting of OT-1 CD8+ T cells cultured with IFN-γ and OVA reported only marginal interleukin-2 (IL-2) production. To inhibit proteasome activity, lactacystin (1-3 μmol/L) was added 1 hour after the addition of IFN-γ and 1 hour before the addition of OVA. To determine the toxicity effect of lactacystin, the mitochondrial activity of 48 hour–treated MSCs was tested by the use of a MTT assay (Promega).
Immune reconstitution assay
β2-microglobulin−/− mice were injected intravenously with 3 × 106 OT-1 CD8+ T cells previously labeled with 1 μmol/L CFSE. At 3 hours later, mice either intraperitoneally received 4 × 106 OVA-primed IFN-γ–stimulated MSCs, 4 × 106 OVA-primed DCs, or PBS. Five days later, cells from the lymph nodes were retrieved, stained with anti-CD8 antibody, and analyzed by flow cytometry for the expression level of carboxyfluorescein succinimidyl ester (CFSE) in the CD8+ population.
Results
MSCs cross-present soluble OVA to CD8+ T cells in vitro
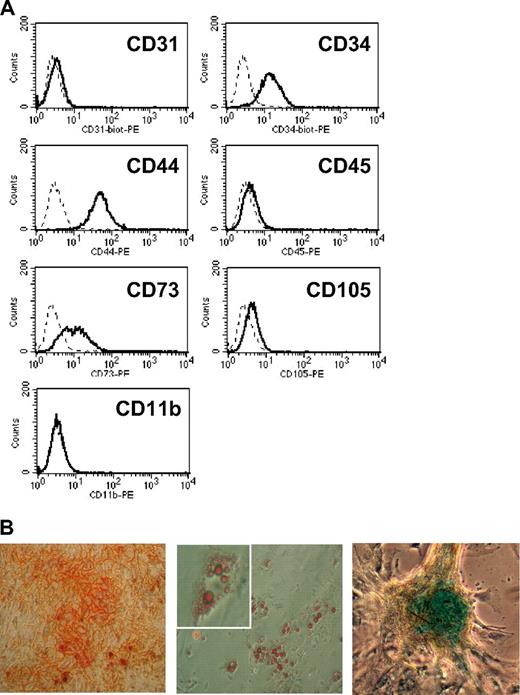
Previous studies, including ours, demonstrated that IFN-γ–activated mouse or human MSCs were able to uptake, process, and present soluble antigens to CD4+ T cells.15,17,18 Here, we tested whether mouse MSCs were able to perform cross-presentation of soluble OVA and activate naive OVA-specific CD8+ T cells from OT-I mice that possess T-cell receptors that specifically recognize OVA peptide SIINFEKL loaded unto MHC class I molecules. Early-passage (< 10 passages) C57bl/6 mouse MSCs were generated, and a complete phenotypic analysis of MSC cultures ruled out the presence of hematopoietic cells (CD45+), endothelial cells (CD31+), and/or macrophages (CD11b+; Figure 1A). In addition, the mesenchymal plasticity of the MSCs was confirmed after differentiation into osteocytes, adipocytes, or chondrocytes in specific differentiation mediums (Figure 1B). Cross-presentation was tested by the use of an APC assay in which MSCs were treated or untreated with 10 ng/mL rmIFN-γ for 19 hours, primed with increasing doses of OVA (0.3, 1, 3 mg/mL) for 18 hours, and cocultured with OT-1 CD8+ T cells. The level of IL-2 produced by activated OT-I CD8+ T responder cells was measured 48 hours later by ELISA.
Phenotypic analysis of C57Bl/6 MSCs. (A) Mesemchymal stromal cells (MSCs) were analyzed by flow cytometry analysis for surface expression of CD31, CD34, CD44, CD45, CD73, CD105, and CD11b. The dotted line and the solid line represent the isotype and the specific antibody, respectively. (B) MSCs were tested for their differentiation potential into osteocytes, adipocytes, or chondrocytes in the presence of bone, fat, or cartilage differentiation medium, respectively. The left panel shows a light microscopy picture of Alizarin Red S–stained osteocyte-differentiated MSC at a magnification of ×50, the middle panel shows Oil red–stained lipid droplets in adipocyte-differentiated MSC at ×50 and ×100, and the right panel shows a Alcian blue–stained-chondrocyte-differentiated MSC as ×50.
Phenotypic analysis of C57Bl/6 MSCs. (A) Mesemchymal stromal cells (MSCs) were analyzed by flow cytometry analysis for surface expression of CD31, CD34, CD44, CD45, CD73, CD105, and CD11b. The dotted line and the solid line represent the isotype and the specific antibody, respectively. (B) MSCs were tested for their differentiation potential into osteocytes, adipocytes, or chondrocytes in the presence of bone, fat, or cartilage differentiation medium, respectively. The left panel shows a light microscopy picture of Alizarin Red S–stained osteocyte-differentiated MSC at a magnification of ×50, the middle panel shows Oil red–stained lipid droplets in adipocyte-differentiated MSC at ×50 and ×100, and the right panel shows a Alcian blue–stained-chondrocyte-differentiated MSC as ×50.
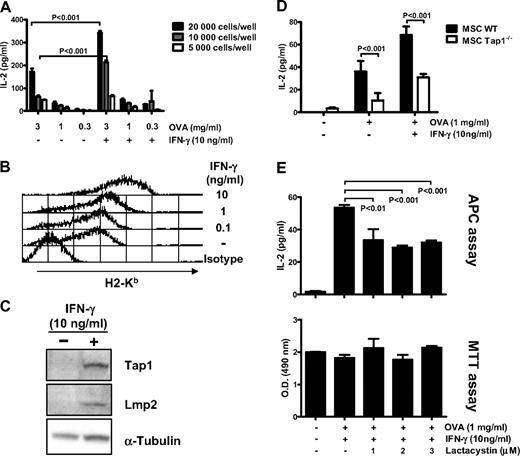
Results showed that unstimulated MSCs pulsed with OVA were able to induce the production of IL-2 by OT-I CD8+ T cells in a dose-dependent manner (Figure 2A). Furthermore, prestimulation of MSCs with IFN-γ increased the production of IL-2 and thus the activation of CD8+ T cells by 2-fold and 3.4-fold with 2 × 104 and 104 MSCs treated with 3 mg/mL OVA, respectively (P < .001). IFN-γ stimulation also increased the expression of surface MHC class I molecules in a dose-dependent manner (Figure 2B), as well as of Tap1 molecules involved in the translocation of proteasome-cleaved peptide into the ER and the subunit LMP2 express after IFN-γ stimulation along with the subunits LMP7 and MECL, which bind to the proteasome to form the immunoproteasome (Figure 2C). These results demonstrate that MSCs cross-present exogenous OVA to naive CD8+ T cells in vitro and that activation with IFN-γ increases this process.
MSCs can cross-present soluble antigen in vitro. (A) Untreated or IFN-γ–stimulated MSCs were primed with the indicated concentration of OVA for 18 hours and cocultured at various density with primary OT-I CD8+ T cells for 48 additional hours. Levels IL-2 produced by activated CD8+ T cells were quantified by ELISA. (B) Flow cytometry analysis of surface expression of MHC class I (H2-Kb) on MSC treated with different doses of IFN-γ for 48 hours. (C) Western blot analysis of Tap1 and LMP2 protein in MSCs treated or untreated with 10 ng/mL of IFN-γ for 48 hours. Loading control was performed on α-tubulin. (D) Untreated or IFN-γ–stimulated MSC WT and MSC Tap1−/− were primed with 1 mg/mL of OVA for 18 hours and cocultured at 10000 cells/well with primary OT-I CD8+ T cells for 48 additional hours. Analysis of the assay was performed as described previously in panel A. (E) IFN-γ–stimulated MSCs were treated with the indicated concentration of lactacystin, primed with OVA (1 mg/mL) for 18 hours, and cocultured at 10000 cells/well with OT-I CD8+ T cells for 48 hours. Analysis of the assay was performed as described previously in panel A (top). Cell viability of the lactacystin-treated MSCs was assayed by a MTT cell viability assay measured by tetrazolium reduction to a 490-nm absorbing formazan compound (bottom panel). The results of the APC assay and MTT assay show means of triplicates ± SD of 1 of 3 representative experiments.
MSCs can cross-present soluble antigen in vitro. (A) Untreated or IFN-γ–stimulated MSCs were primed with the indicated concentration of OVA for 18 hours and cocultured at various density with primary OT-I CD8+ T cells for 48 additional hours. Levels IL-2 produced by activated CD8+ T cells were quantified by ELISA. (B) Flow cytometry analysis of surface expression of MHC class I (H2-Kb) on MSC treated with different doses of IFN-γ for 48 hours. (C) Western blot analysis of Tap1 and LMP2 protein in MSCs treated or untreated with 10 ng/mL of IFN-γ for 48 hours. Loading control was performed on α-tubulin. (D) Untreated or IFN-γ–stimulated MSC WT and MSC Tap1−/− were primed with 1 mg/mL of OVA for 18 hours and cocultured at 10000 cells/well with primary OT-I CD8+ T cells for 48 additional hours. Analysis of the assay was performed as described previously in panel A. (E) IFN-γ–stimulated MSCs were treated with the indicated concentration of lactacystin, primed with OVA (1 mg/mL) for 18 hours, and cocultured at 10000 cells/well with OT-I CD8+ T cells for 48 hours. Analysis of the assay was performed as described previously in panel A (top). Cell viability of the lactacystin-treated MSCs was assayed by a MTT cell viability assay measured by tetrazolium reduction to a 490-nm absorbing formazan compound (bottom panel). The results of the APC assay and MTT assay show means of triplicates ± SD of 1 of 3 representative experiments.
Next, we tested whether the TAP complex and the proteasome, both involved in the MHC class I antigen presentation pathway, were required for cross-presentation in MSCs using knock-out or knock-down cells. First, MSCs were isolated from Tap1-deficient mice and were used in APC assay. Compared with WT MSCs, a partial reduction in OT-1 CD8+ T-cell activation was observed after culture with OVA-pulsed untreated or IFN-γ–stimulated Tap1−/− MSCs (3.6- or 2.2-fold reductions, respectively, P < .001; Figure 2D). Second, IFN-γ–stimulated MSCs were treated with increasing doses of lactacystin, a known inhibitor of the proteasome.19 The level of IL-2 production is consistent with a reduction of OT-1 CD8+ T-cell activation after culture with lactacystin pretreated, OVA-pulsed MSCs (1.6- to 1.8-fold reduction, P < .01, Figure 2E). Of note, tested doses of lactacystin did not appear to affect MSCs viability, as assayed by measuring levels of mitochondrial activity (MTT assays, Figure 2E). Overall, cross-presentation by MSC was partially independent of the TAP complex and the immunoproteasome.
TGF-β and antigen cross-presentation by MSCs
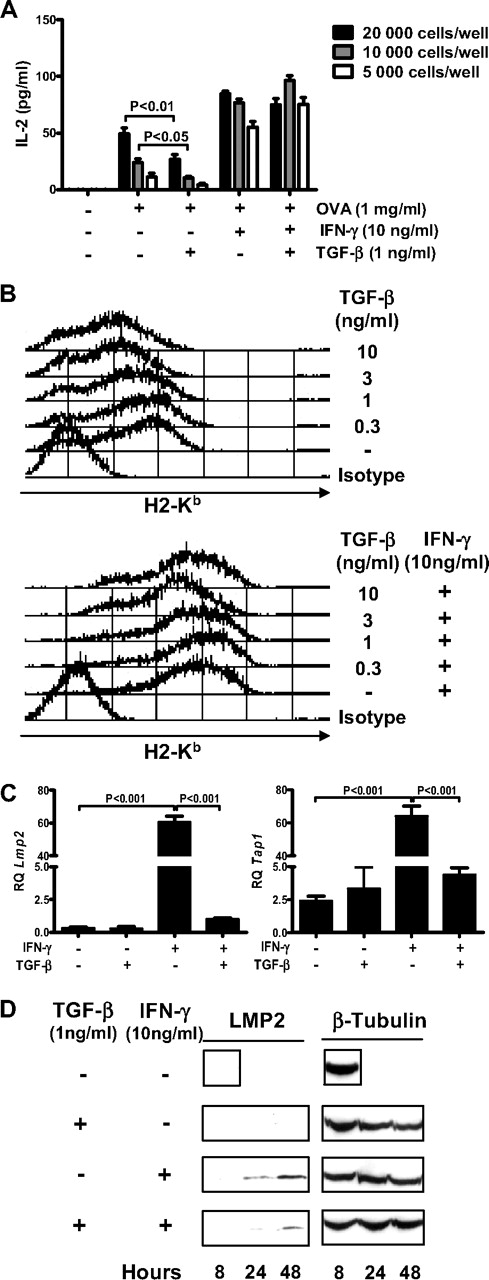
The authors of several studies20,21 described TGF-β as an inhibitor of antigen presentation in DC. In MSCs, we previously observed that TGF-β down-regulated IFN-γ–induced MHC class II expression and antigen presentation.17 Here, we observed that exposure of MSCs to TGF-β for 24 hours before the addition of antigen only marginally down-regulated OVA presentation to OT-I CD8+ T lymphocytes (Figure 3A). In addition, TGF-β, when added 1 hour before IFN-γ priming, did not down-regulate antigen cross-presentation induced by IFN-γ in MSCs (Figure 3A). Flow cytometry analysis of the surface expression of MHC class I H2-Kb molecules on MSCs treated with IFN-γ, TGF-β, or a combination of both revealed that the basal expression of H2-Kb is down-regulated in a dose-dependent manner after TGF-β treatment of cells (Figure 3B top panel). However, the IFN-γ–induced expression levels of H2-Kb molecules were unchanged in the presence of TGF-β, independently of the dose of TGF-β used (Figure 3B bottom panel). However, expression analysis of Tap1 and Lmp2 mRNA levels reported that TGF-β blocked the transcriptional activation of both genes induced by IFN-γ but not their basal expression (Figure 3C). Protein level analysis of LMP2 by Western blot also confirms the inhibition of IFN-γ–induced LMP2 expression in TGF-β–treated MSCs (Figure 3D). Overall, although TGF-β inhibited the up-regulation by IFN-γ of the expression of MHC class I machinery components, it had no significant effect on antigen cross-presentation by IFN-γ–activated MSCs to naive CD8+ T cells.
TGF-β does not interfere with IFN-γ–induced antigen cross-presentation by MSCs. (A) TGF-β– or IFN-γ–treated MSCs primed with OVA (1 mg/mL) for 18 hours were cocultured at the indicated density with OT-I CD8+ T cells for 48 hours. Analysis of the assay was performed as described previously in Figure 2A. The results show means of triplicates ± SD of 1 of 3 representative experiments. (B) Flow cytometry analysis of surface expression of MHC class I (H2-Kb) on MSC treated with different doses of TGF-β only (top panel) or with different doses of TGF-β in combination with IFN-γ (10 ng/mL; bottom panel). (C) Quantitative RT-PCR analysis of Tap1 (left panel) and Lmp2 (right panel) mRNA levels in MSC treated with TGF-β (1 ng/mL), IFN-γ (10 ng/mL), or the combination of both for 48 hours. Relative quantification (RQ) was calculated by normalizing to 18S mRNA levels. Figure shows means of triplicates ± SD. (D) Western blot analysis of LMP2 on MSCs treated or untreated with TGF-β (1 ng/mL) and IFN-γ (10 ng/mL) for 8, 24, and 48 hours (right panel). Loading control was performed on β-tubulin.
TGF-β does not interfere with IFN-γ–induced antigen cross-presentation by MSCs. (A) TGF-β– or IFN-γ–treated MSCs primed with OVA (1 mg/mL) for 18 hours were cocultured at the indicated density with OT-I CD8+ T cells for 48 hours. Analysis of the assay was performed as described previously in Figure 2A. The results show means of triplicates ± SD of 1 of 3 representative experiments. (B) Flow cytometry analysis of surface expression of MHC class I (H2-Kb) on MSC treated with different doses of TGF-β only (top panel) or with different doses of TGF-β in combination with IFN-γ (10 ng/mL; bottom panel). (C) Quantitative RT-PCR analysis of Tap1 (left panel) and Lmp2 (right panel) mRNA levels in MSC treated with TGF-β (1 ng/mL), IFN-γ (10 ng/mL), or the combination of both for 48 hours. Relative quantification (RQ) was calculated by normalizing to 18S mRNA levels. Figure shows means of triplicates ± SD. (D) Western blot analysis of LMP2 on MSCs treated or untreated with TGF-β (1 ng/mL) and IFN-γ (10 ng/mL) for 8, 24, and 48 hours (right panel). Loading control was performed on β-tubulin.
OVA-primed MSCs induce CD8+ T-cell proliferation in vivo
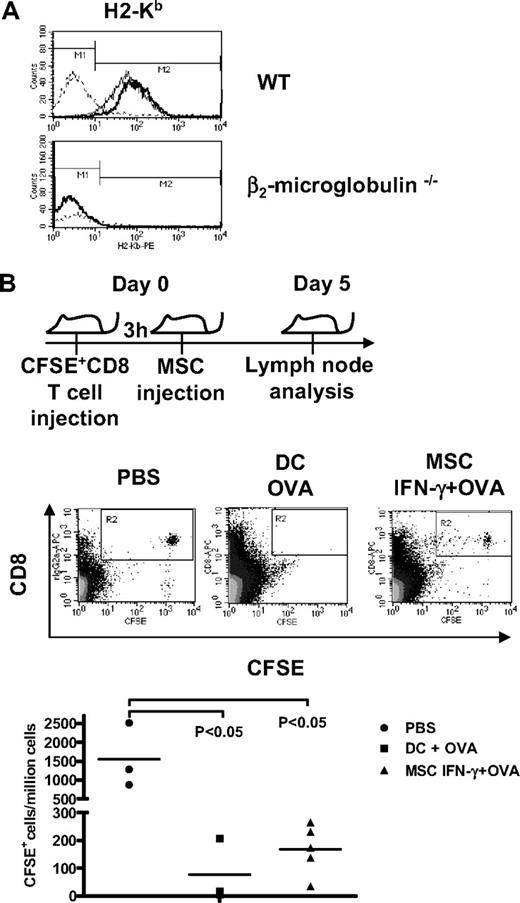
To test whether IFN-γ–stimulated MSCs pulsed with OVA in vitro could activate naive CD8+ T cells in vivo, we performed adoptive cell transfers into β2-microglobulin−/− mice. These mice are deficient for endogenous MHC class I-mediated antigen presentation and exhibit depletion of CD8+ T cells and NK1.1+-cell populations because of their dependence on MHC class I molecules for development. β2-microglobulin−/− mice were given intravenous CFSE-labeled OT-I CD8+ T cells, and 3 hours later, untreated MSCs, IFN-γ–primed OVA-pulsed MSCs, or OVA-pulsed DCs were injected in the abdominal cavity. After 5 days, lymph nodes were retrieved, dissociated to single-cell suspension, and analyzed by flow cytometry for CFSE+ cells. The fate of transferred CFSE-labeled CD8+ T cells was assessed by counting the number of CFSE-positive CD8+ T cells per million lymph node-derived cells. Because endogenous APCs in these mice do not express MHC class I molecules, as shown by the absence of MHC class I molecules in peritoneal macrophages (Figure 4A), OT-I CD8+ T-cell proliferation—and loss of CFSE labeling—can be attributed to cross-presentation, activation, and proliferation of OT-1 cells by MHC class I+ APCs. Analysis of CFSE levels in CD8+ T cells in lymph nodes (Figure 4B) showed that injection of OVA-pulsed IFN-γ–stimulated MSCs induced significant CD8+ T-cell proliferation in vivo (average of 172 CFSE+ cells/million cells, P < .05) compared with PBS control (1557 CFSE+ cells/million cells) or untreated MSCs (data not shown). The MSC-induced activation was comparable in intensity with that achieved by LPS-activated DCs (average of 75 CFSE+ cells/million cells, P < .05).
OVA cross-presentation by MSCs induces the proliferation of OT-I CD8+ T cells in vitro and in vivo. (A) Flow cytometry analysis of surface expression of H2-Kb on untreated or IFN-γ–stimulated peritoneal macrophage collected form WT and β2-microglobulin−/− mice. Dotted line, solid line, and bold line represent the isotype, untreated cells, and IFN-γ–treated cells, respectively. (B) β2-microglobulin−/− mice were injected with 3 × 106 CFSE+-OT-1 CD8+ T cells intravenously followed 3 hours later by an intraperitoneal injection of 4 × 106 OVA-primed IFN-γ–stimulated MSCs, 4 × 106 OVA-pulsed LPS-stimulated DCs, or PBS intraperitoneally. At 5 days later, lymph nodes were retrieved, and CD8+ T-cell proliferation was assayed by flow cytometry analysis of the CFSE level.
OVA cross-presentation by MSCs induces the proliferation of OT-I CD8+ T cells in vitro and in vivo. (A) Flow cytometry analysis of surface expression of H2-Kb on untreated or IFN-γ–stimulated peritoneal macrophage collected form WT and β2-microglobulin−/− mice. Dotted line, solid line, and bold line represent the isotype, untreated cells, and IFN-γ–treated cells, respectively. (B) β2-microglobulin−/− mice were injected with 3 × 106 CFSE+-OT-1 CD8+ T cells intravenously followed 3 hours later by an intraperitoneal injection of 4 × 106 OVA-primed IFN-γ–stimulated MSCs, 4 × 106 OVA-pulsed LPS-stimulated DCs, or PBS intraperitoneally. At 5 days later, lymph nodes were retrieved, and CD8+ T-cell proliferation was assayed by flow cytometry analysis of the CFSE level.
Discussion
Because human and mouse MSCs up-regulate MHC class II and acquire APC function upon activation with IFN-γ,14,15,17,18 we investigated whether MHC class I–mediated antigen cross-presentation also is operative in these cells. A previous study15 from our laboratory on the APC-like feature of MSC suggested the possibility for cross-presentation to occur in MSCs. In an in vivo model of MSC-based tumor immunization, in which naive C57Bl/6 mice were immunized against OVA-expressing EG.7 lymphoma cells with OVA-pulsed IFN-γ–stimulated MSC, showed complete protection against tumor cell challenge, and immunized mice developed an OVA-specific CD8+ T-cell cytotoxic immune response,15 suggesting that antigen-pulsed MSCs may have cross-primed CD8+ T cells in vivo. Here, using both in vitro and in vivo assays in which OVA-specific CD8+ T cells from OT-1 mice were used as responder cells, we were able to show that murine MSCs could cross-present soluble exogenous OVA and activate naive CD8+ T cells. In addition, we showed that IFN-γ priming of MSC before the addition of OVA boosted antigen cross-presentation and therefore CD8+ T-cell activation. This increase in T-cell activation can directly be attributed to the up-regulation of components of the MSC class I antigen presentation machinery.
MSC surface expression of MHC class I molecules in addition to the expression of Tap1 and Lmp2 showed a net increase after IFN-γ stimulation. In the immune reconstitution assay, β2-microglobulin−/− mice, deficient for MHC class I molecules, were used to quantify in vivo cross-presentation by MSC while excluding any potential cross-presentation by host APC. The injection of OVA-pulsed IFN-γ–stimulated MSCs in these mice resulted in the activation and proliferation of previously injected CFSE-labeled OVA-specific CD8+ T cells from OT-1 mice, therefore confirming direct cross-presentation by MSC in vivo. Our previous study on mouse MSC clonal subsets reported that although approximately half of them constitutively expressed CD80, the majority lacked expression of costimulatory molecules CD40, CD86, and CD54 even after activation with IFN-γ,15 which could hamper the cross-priming capacity of MSC compared with professional APC such as DC. When flow cytometry analysis for costimulatory molecules was performed, the MSCs used only express low levels of CD80, which was up-regulated after the exposure to 10 ng/mL of IFN-γ for 48 hours. CD86, ICOS-L, BFDC, LFA-1, CD70, OX40L, and ICAM-1 were all negative (data not shown).
Although we did not compare alternate routes of antigen cellular entry, results obtained gave hints on antigen cross-presentation pathways in MSC. APC assay by the use of Tap1−/− and proteasome-inhibited MSC displayed a decrease in CD8+ T-cell activation, demonstrating that cross-presentation in MSC is partly dependent on the proteasome and the TAP complex. This observation suggested that the antigen is not entirely required to access the ER to be loaded onto MHC class I molecules. Alternative routes for the antigen cross-processing bypassing the Tap complex and the ER have been suggested in DCs. Houde and colleagues proposed a model of cross presentation in which peptide loading onto MHC class I molecules occurs in the phagosome instead of the ER. Their observations showed that the ER donates part of its membrane to the nascent phagosome, thus providing the essential machinery of the MHC class I pathway.22,23 The Sec61 protein, which forms a pore complex involved in the retranslocation of misfolded protein from the ER to the cytosol (ER-associated degradation, ie, ERAD), is also introduced into the phagosome, providing a means by which the protein can exit the phagosome to access the proteasome. The peptide generated can afterward return into the phagosome by the Sec61 pore complex to be loaded onto the MHC class I molecule. In addition, MSCs were shown to phagocyte large particles.14
Although RT-PCR analysis of the transcription level of Tap1 and Lmp2 genes and Western blot analysis of LMP2 revealed that TGF-β hinders their up-regulation by IFN-γ, TGF-β treatment of MSC had practically no effect on the ability of MSCs to activate naive CD8+ T cells in the presence of IFN-γ. There is thus an absence of correlation between the effect of TGF-β on the processing machinery and cross-presentation by MSCs. We found that the basal expression of the TAP1 molecules, involved in the translocation of proteasome-cleaved peptide into the ER, was unaffected by TGF-β and therefore still operational in the presence of TGF-β. LMP2, along with LMP7 and MECL, are IFN-γ–inducible subunits that are incorporated into an alternative form of the proteasome, described as the immunoproteasome, which increases the spectrum of generated antigenic peptide rather than their production.24 Because the T-cell receptor of the CD8+ T cells isolated from the OT-1 mice recognize the OVA-immunodominant epitope (amino acid 257-264; SIINFEKL), the formation of the immunoproteasome is unlikely to play a significant role in promoting the cross-presentation potential of MSCs in this type of assay. Overall, these results demonstrate that the regulation of the MHC class I machinery by TGF-β and IFN-γ is not preeminent in MSC. However, the surface expression of MHC class I molecules is essential to cross-presentation. Flow cytometry analysis of TGF-β–treated MSCs revealed that TGF-β down-regulated the basal expression of MHC class I. Conformingly, cross-presentation by these cells also was diminished. However, when the levels of MHC class I remained the same, as for the case of TGF-β– and IFN-γ–treated MSCs, so did CD8+T-cell activation. Further investigations will be needed to test the regulation of the repertoire of antigenic peptides cross-presented by MSC and its regulation by IFN-γ and TGF-β, which plays a crucial role in the broadness of the immune response to dominant and subdominant epitopes.
Marrow-resident MSC have long been regarded as part of the hematopoietic niche and as a mesenchymal progenitor reservoir for local and possibly distant tissue repair response.25,26 More recently, we have also found that MSCs can meaningfully interact with lymphoid cells via their secretome,27 which may represent an extension of their role in supporting/regulating the marrow hematolymphoid niche. The ability to cross-present antigen further extends the observation that MSCs also can adopt an APC-like function when activated with IFN-γ.15,17 MSCs also express an array of Toll-like receptors and respond to their cognate ligands by adopting a resolutely proinflammatory phenotype.28 Like the central nervous system, the hematopoietic marrow is devoid of an organized lymphatic system. Therefore, one may speculate that MSCs, especially those positioned as marrow vascular pericytes, may play a role in the immune surveillance and defense of this most unique organ system. In conclusion, the demonstration here made that MSCs can cross-present exogenous antigens strongly supports the notion that MSCs are in essence the combat engineers of the marrow space: at default, poised to support hematopoiesis and mesenchymal repair but also equipped to fight and defend when so prompted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Moïra François is supported by a Canadian Institute for Health Research Scholarship and Jacques Galipeau is a Fonds de la recherche en santé du Québec chercheur-boursier sénior. This work was supported by Terry Fox New Frontiers Program Project Grant No. 018005.
Authorship
Contribution: M.F. designed the research plan, performed experiments, and wrote paper; R.R.-M. designed experiments; S.S.-M. and M.-N.B. performed experiments; J.L.B. provided experimental design guidance; and J.G. designed the research plan.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Jacques Galipeau, Sir Mortimer B. Davis Jewish General Hospital & Lady Davis Institute for Medical Research, 3755 Cote Sainte-Catherine Rd, Montreal, QC, Canada H3T 1E2; e-mail: jacques.galipeau@mcgill.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal