Abstract
The role of the tryptophan-metabolizing enzyme indoleamine 2,3-dioxygenase (IDO) in down-regulating human alloresponses has recently been controversially debated. We here demonstrate that human monocyte-derived dendritic cells (mDCs) can be endowed with sustained IDO competence in vitro by 48-hour activation with lipopolysaccharide (LPS) and interferon-gamma (IFN-γ). IFN-γ also amplified proinflammatory cytokine secretion during activation. Yet, on reculture after activation cytokine production ceased, whereas IDO enzymatic activity continued. Manipulation of tryptophan metabolism did not affect proinflammatory cytokine release, suggesting that IFN-γ triggers IDO activity and proinflammatory cytokine release as distinct cellular programs. IDO-competent DCs down-regulated allogeneic T-cell responses, but this IDO-mediated effect was overcome by slightly modifying cell culture conditions. Nevertheless, the CD4+CD25+ T-cell fraction stimulated by IDO-competent DCs displayed substantial suppressor activity. This suppressive activity (1) required allogeneic stimulation for its induction, (2) affected third-party T cells, and (3) was reduced by the IDO inhibitor methyl-thiohydantoin-tryptophan. It became also manifest when DC/T-cell cocultures were initiated with naive (CD4+CD25−CD45RA+) T cells, indicating the differentiation of adaptive regulatory T cells. Together, these findings suggest that IFN-γ triggered IDO competence in human mDCs constitutes a critical factor for endowing allogeneic T cells with regulatory activity.
Introduction
Indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme in tryptophan catabolism, has attracted attention for its proposed role in tolerance induction.1,2 IDO, when expressed in antigen presenting cells (APCs), such as dendritic cells (DCs), establishes a microenvironment that at the DC/T-cell interface is depleted of tryptophan and enriched with tryptophan metabolites. Because of this alteration of the intercellular microenvironment, IDO activity has been suggested to impair T-cell responses.3,4 The mechanisms of IDO-mediated inhibition include that T cells stimulated under tryptophan-depleted conditions are impaired to undergo full cell cycle progression3,5 and are susceptible to apoptotic cell death.6,7 Furthermore, it has been reported that IDO-expressing DCs can expand naturally occurring regulatory T cells.8 In a murine model Fallarino et al9 have shown that IDO activity supported the generation of adaptive regulatory T cells. Likewise, human IDO-expressing tumor cells have been reported to induce CD4+CD25+ regulatory T cells.10 Most recently, the previously recognized immunoregulatory activity of human plasmacytoid DCs has been related to IDO activity.11 Thus, IDO-mediated down-regulation of T-cell responses has been suggested to be involved in a multitude of immunoregulatory processes, for example, pregnancy,12 tumor growth,13 and the induction of tolerance in transplantation (reviewed in Hainz et al14 ).
IDO expression is not a constitutive feature of human DCs in homeostatic immunologic conditions but requires induction. Among the multiple mediators of IDO induction (reviewed in Puccetti15 ), interferon-γ (IFN-γ) plays a prominent role.16 IFN-γ has generally been considered a prototypic proinflammatory cytokine (reviewed in Schoenborn and Wilson17 ); compelling evidence, however, supports the ability of IFN-γ to promote anti-inflammatory responses.18-20 The ability of IFN-γ to induce IDO has been suggested as a critical factor linked to this anti-inflammatory activity.21,22
In our previous studies addressing possible mechanisms of the immunodeficient state after hematopoietic stem cell transplantation,23 we found that monocytes after hematopoietic stem cell transplantation were particularly sensitive to respond to an exposure to IFN-γ with an accelerated release of tryptophan metabolite kynurenine, and, thus, to turn into suppressor cells. This finding suggested the possibility that an augmented IDO activity in recipients of a hematopoietic stem cell transplant might be involved in tolerance induction. On this background we began to test the hypothesis that the targeted induction of IDO enzymatic activity in human APC populations represents a means to achieve T-cell tolerance.
DCs, to prime immune responses, need to be activated. Recent studies have shown that a timely limited exposure of DCs to lipopolysaccharide (LPS) induces the release of proinflammatory cytokines and a T helper type 1 (Th1)–polarized response.24,25 In contrast, prolonged activation results in exhaustion of the DCs to secrete proinflammatory cytokines and supports a Th2-polarized and humoral response. In this context the role of IFN-γ is puzzling. On the one hand, IFN-γ has been reported to boost proinflammatory cytokine release by DCs, thus acting as a proinflammatory compound.26,27 On the other hand, IFN-γ by its capacity to induce IDO might dampen inflammation.
In the present study we demonstrate that abundant and sustained IDO protein expression and enzymatic activity, hereafter termed IDO competence, in LPS-activated human monocyte-derived DCs is in fact dependent on the presence of IFN-γ during activation. The enzymatic activity of IDO, however, persists even when the IFN-γ–triggered capability of DCs to secrete proinflammatory cytokines has ceased. Such IDO competent and cytokine exhausted DCs, set to stimulate allogeneic T cells in vitro, support the de novo differentiation of T cells with explicit regulatory function.
Methods
Samples
All experiments were performed with human blood obtained from healthy volunteers or from blood donors at the blood bank of the General Hospital of Vienna once informed consent was given, in accordance with the Declaration of Helsinki. Approval was obtained from the Children's Cancer Research Institute Institutional Review Board for these studies. Venipunctures were performed by experienced medical staff under standard sterile conditions.
Cell culture medium
DC differentiation and activation was performed in AIM V cell culture medium (Invitrogen) containing 2% human plasma (Octaplas; Octapharm), and 1% vol/vol l-glutamine (PAA Laboratories;), hereafter termed complete medium. T-cell stimulation and suppressor assays were performed in complete medium supplemented with 25 mM Hepes (PAA Laboratories). All cell cultures were maintained in humidified air containing 5% CO2 at 37°C.
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were enriched from whole blood by density centrifugation (Lymphoprep; Nycomed). Monocytes were separated from PBMCs by selective adherence to polystyrene surfaces. Alternatively, the monocyte enrichment was done by counter-flow centrifugal elutriation (Elutra Cell Separation System; Gambro BCT).28 Both techniques typically resulted in a population of greater than 85% CD14+ cells. These monocyte-enriched cell populations were plated in culture flasks (Iwaki; Sterilin) at a density of 0.3 to 0.5 × 106 cells/cm2. Immature DCs were generated by culture in complete medium supplemented with 1000 U/mL GM-CSF (CellGenix) and 400 U/mL IL-4 (CellGenix) for 5 days. For DC activation, immature DCs were exposed to 50 ng/mL LPS (Calbiochem; Escherichia coli O111:B4) with or without 1000 U/mL IFN-γ (Boehringer Ingelheim) for 4 or 48 hours. The quality of DC generation was monitored by visual and flow cytometric evaluation of a typical DC morphology and expression of cell-surface markers, respectively.
Highly enriched T-cell populations were prepared as described.29 In brief, total CD4+ T cells as well as naive CD4+CD45RA+ T cells were isolated from total PBMCs by magnetic cell sorting (Miltenyi Biotec Inc) according to the manufacturer's instructions, typically resulting in a greater than 95% enrichment of the targeted cell population. Purity and viability were monitored by flow cytometry.
Flow cytometry
The following monoclonal antibodies (mAbs) were used: isotype control, IgG1-FITC, IgG2a-PE, IgG1-PE, IgG2a-perCP, IgG1-APC; anti–CD45-perCP (clone 2D1), anti–CD3-APC (clone SK7), anti–CD4-perCP (clone SK3), anti–CD25-PE (clone 2A3), anti–CD86-APC (clone 2331), anti–CD14-perCP-Cy5.5 (clone M5E2), anti–CD45RA-PE (clone HI100), anti–CTLA4-PE-Cy5 (clone BNI3; all from BD Biosciences); anti-HLA class II–FITC (clone CR3/43), anti-HLA class I–PE (clone W6/32; Dako); anti–CD80-PE (clone MAB104; Immunotech; Beckman Coulter). For detection of FOXP3 in T cells we used clone PCH101 (eBioscience) or clone 259D/C7 (BD Biosciences). Both antibodies showed similar results. Flow cytometric analyses to identify or to sort different cell populations were performed with the use of a FACSCalibur or the FACSAria flow cytometer (BD Biosciences). Analyses of list mode data were done with the CellQuest or DivaCell software (BD Biosciences).
T-cell stimulation and mixed lymphocyte reaction
Highly enriched total CD4+ T cells (5 × 105) or CD4+CD45RA+ T cells (5 × 105) were cocultured with allogeneic DCs (5 × 105, unless otherwise indicated) or autologous DCs, (which were pulsed with tetanus toxoid; Calbiochem) for 7 days in 24-well plates (Iwaki). When indicated, DC/T-cell cocultures were supplemented with fresh complete medium on day 4. Proliferative responses were quantified by [3H]thymidine incorporation, as described.23 Alternatively, T cells were stained with carboxyfluorescein succinimidyl ester (CFSE), and proliferation was calculated as the percentage of CFSE-negative cells.30 The competitive IDO inhibitors 1-methyl-DL- tryptophan (1-MT; 300 μM; Sigma-Aldrich) or methyl-thiohydantoin-tryptophan (MTHT; 50 μM; Sigma-Aldrich)31 were used to test for IDO-dependent effects. Of note, the capacity of MTHT to block tryptophan degradation was comparable with that of the more commonly used IDO inhibitor 1-MT (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), and MTHT had no pro-proliferative effect on PBMCs (not shown).
Suppressor assay
At the termination of the DC/T-cell cocultures, the nonadherent cells were recovered by gentle pipetting. The CD4+CD25− and CD4+CD25+ populations were separated by flow cytometry (resulting routinely in ≥ 99% purity). Subsequently, the CD4+CD25− or CD4+CD25+ T cells (0.5 × 105) were cocultured with 0.5 × 105 fresh autologous or third-party CD4+ T cells, as indicated, in 96-well plates (Nunc; Thermo Fischer) and in a final volume of 200 μL medium per well. The fresh T cells were stained with CFSE and were stimulated with immobilized anti-CD3mAb (250 ng/mL; Clone UCHT-1; Sigma-Aldrich). Suppression of proliferation was calculated by comparing the percentage of CFSE-negative fresh T cells in cocultures with CD4+CD25− T cells to cocultures with CD4+CD25+ T cells according to the following formula: percentage of inhibition = [1 − (percentage of CFSE− T cells cocultured with CD4+CD25+ T cells / percentage of CFSE− T cells cocultured with CD4+CD25− T cells)] × 100.
IDO expression and activity
IDO protein expression in DCs was investigated by immunoblot analysis with the use of a mouse monoclonal anti–human IDO antibody, kindly provided by O. Takikawa (National Institute for Longevity Sciences, National Center for Geriatrics and Gerontology, Aichi, Japan).32 IDO enzymatic activity was determined by measuring the levels of tryptophan and kynurenine in the cell culture supernatants by high-pressure liquid chromatography as previously described.23 In brief, tryptophan was detected by its natural fluorescence at 286-nm excitation and 366-nm emission wavelengths. 3-Nitro-l-tyrosine, used as an internal standard, and kynurenine were determined by ultraviolet absorption at 360 nm. An albumin-based external standard mix was prepared and included 50 μM tryptophan (Serva), 10 μM kynurenine (Sigma-Aldrich), and a frozen serum pool. On addition of 25 μL of 2 M trichloroacetic acid (Merck), the reaction vials were immediately vortexed and centrifuged at 12 000g (13 000 rpm) for 6 minutes at room temperature to precipitate protein. The concentration of the components was calculated according to peak heights and was compared with 3-nitro-l-tyrosine as a reference standard.
Cytokine release
For the examination of cytokine secretion, cell culture supernatants were analyzed by the cytokine bead array (FlowCytomix; Bender Medsystems) according to the manufacturer's instructions. In some experiments, to detect a potential effect of tryptophan catabolism on DC cytokine secretion, cultures were supplemented with excess tryptophan (100 μM; Sigma-Aldrich) or a combination of tryptophan catabolites (l-kynurenine, 3-hydroxy-kynurenine, 3-hydroxy-anthranilic acid, quinolinic acid, 25 μM each; all from Sigma-Aldrich).
Statistical analysis
All statistical analyses were performed with the Student t test (paired, 2-tailed). A P value below .05 was considered to indicate statistical significance.
Results
IDO induction in human monocyte-derived DCs
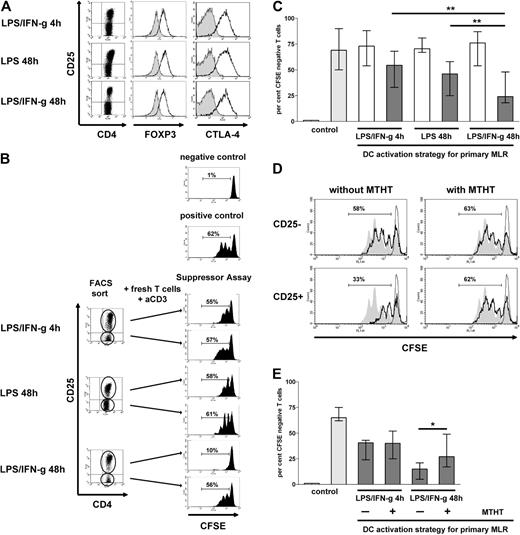
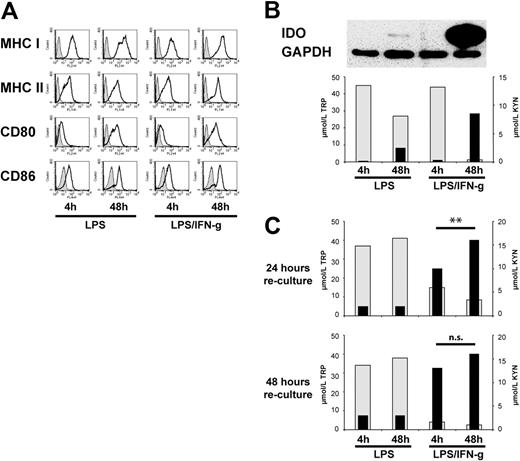
To enlighten the ambivalent effects of IFN-γ in directing opposing types of T-cell responses (“Introduction”), we activated human monocyte-derived DCs with LPS in the presence or absence of IFN-γ for different time periods (4 or 48 hours) and examined their capacity to acquire IDO competence, to secrete cytokines, and to stimulate allogeneic T cells. First, we found that either DC activation strategy induced a similar level of expression of cell-surface markers commonly associated with DC maturation, with the notion that, in line with the aforementioned studies,27 the levels of expression of costimulatory molecules CD80 and CD86 increased with time of activation (Figure 1A).
Characterization of maturation status and IDO competence in human monocyte-derived DCs activated with LPS with or without IFN-γ for different time periods. (A) DCs after activation with LPS only or LPS in combination with IFN-γ for different time periods were examined for the expression of cell-surface molecules of DCs typically associated with maturation (shaded histograms indicate isotype control; open histograms, DCs after activation). One representative of 10 similar experiments is shown. (B top) IDO protein expression in differently activated DCs was examined by immunoblot analysis; (bottom) the concentrations of tryptophan (TRP; ▩; left scale) and kynurenine (KYN; ■; right scale) in cell culture supernatants of the differently activated DCs were determined at the termination of the activation period. Results of 1 of 10 similar experiments are shown. (C) The concentrations of TRP and of KYN were examined as in panel B in cell culture supernatants 24 hours (top) and 48 hours (bottom) after removal of the activating agents and reculture in fresh medium. Results of 1 experiment, representative of 10 experiments, are shown; **P < .01; n.s. indicates not significant.
Characterization of maturation status and IDO competence in human monocyte-derived DCs activated with LPS with or without IFN-γ for different time periods. (A) DCs after activation with LPS only or LPS in combination with IFN-γ for different time periods were examined for the expression of cell-surface molecules of DCs typically associated with maturation (shaded histograms indicate isotype control; open histograms, DCs after activation). One representative of 10 similar experiments is shown. (B top) IDO protein expression in differently activated DCs was examined by immunoblot analysis; (bottom) the concentrations of tryptophan (TRP; ▩; left scale) and kynurenine (KYN; ■; right scale) in cell culture supernatants of the differently activated DCs were determined at the termination of the activation period. Results of 1 of 10 similar experiments are shown. (C) The concentrations of TRP and of KYN were examined as in panel B in cell culture supernatants 24 hours (top) and 48 hours (bottom) after removal of the activating agents and reculture in fresh medium. Results of 1 experiment, representative of 10 experiments, are shown; **P < .01; n.s. indicates not significant.
Remarkably, IDO competence was restricted to long-term LPS/IFN-γ–activated DCs. In fact, only DCs exposed to LPS/IFN-γ for 48 hours expressed abundant amounts of IDO protein (Figure 1B top panel) and depleted the cell culture medium of tryptophan and accumulated high concentrations of kynurenine (Figure 1B bottom panel). In contrast, DCs activated with LPS only, even on a long-term exposure (48 hours), displayed only minor IDO expression and tryptophan-metabolizing capacity.
To assess continuing IDO competence after activation, the differently activated DCs were extensively washed and recultured in fresh complete medium. In these cultures, only DCs that had acquired IDO competence by long-term activation with LPS and IFN-γ rapidly and effectively metabolized tryptophan even after removal of the activation stimuli. Indeed, these recultured DCs induced a tryptophan deplete/kynurenine-enriched cell culture milieu within 24 hours (Figure 1C top panel). Importantly, however, we noticed that DCs having been activated with LPS/IFN-γ short term (4 hours) gradually acquired IDO competence. In fact, 48 hours after the onset of reculture, the cell culture medium containing short-term LPS/IFN-γ–activated DCs was similarly depleted of tryptophan and enriched for kynurenine as the cell culture medium containing long-term (48-hour) LPS/IFN-γ–activated DCs (Figure 1C bottom panel; P = NS), hereafter termed delayed IDO competence. In contrast, DCs activated with LPS only, irrespective of whether they developed IDO competence during activation, failed to display significant IDO competence on reculture (Figure 1C). Of note, the presence of allogeneic T cells did not additionally affect levels of tryptophan depletion or kynurenine accumulation (data not shown), thus excluding that T cells or their cytokine production were required for the maintenance of IDO competence of these DCs.
IFN-γ triggering of cytokine secretion and IDO activity are discrete processes
Because it has been reported that the ability to secrete cytokines, similarly to the induction of IDO competence (Figure 1B), is related to the time of activation24 we addressed the question of whether cytokine production and IDO activity are linked processes. In a subsequent series of experiments we monitored IDO activity and the release of proinflammatory cytokines, IL-12, IL-6, and TNF-α, in DCs activated by LPS with or without IFN-γ in parallel. Several important observations were made. (1) The addition of IFN-γ to LPS stimulated both the production of proinflammatory cytokines26,27 and the IDO activity in a time-dependent manner and lead to accumulation of abundant amounts of cytokines along with abundant IDO enzymatic activity within a 48-hour activation period (Figure 2A). (2) However, when the activating agents were removed and DCs were recultured in fresh medium, their capacity to produce proinflammatory cytokines was markedly reduced, but their capacity to effectively degrade tryptophan and accumulate kynurenine was maintained (Figure 2B). This finding suggests that IFN-γ stimulates proinflammatory cytokine production of DCs and IDO competence in parallel, but the time period of IDO enzymatic activity exceeds that of cytokine secretion.
The IFN-γ–triggered proinflammatory cytokine production and IDO induction in human DCs represent distinct cellular programs. (A) The amount of proinflammatory cytokine release at the end of the activation period was examined in cell culture supernatants of differently activated DCs (left panels). In the same culture supernatants, the concentrations of tryptophan (TRP; ▩) and kynurenine (KYN; ■) were examined (right). The bars indicate the median of 4 experiments. The numbers given in brackets indicate the range. (B) DCs were activated with LPS/IFN-γ for 48 hours to induce IDO competence. Proinflammatory cytokine release was examined in cell culture supernatants after removal of the activating agents and reculture of the activated DCs in fresh medium for a 48-hour period. The values of cytokine release on reculture are expressed as the percentage of the maximum release (= 100%) during the activation period (top). In the same culture supernatants, the concentrations of tryptophan and kynurenine were analyzed (bottom). Results of 1 experiment, representative of 2 experiments, are shown. (C) DCs were activated with LPS/IFN-γ for 48 hours in conditions of external manipulation of tryptophan metabolism, as indicated (TRP met indicates tryptophan metabolites). Proinflammatory cytokine release (left panels) and the concentrations of TRP and KYN (right) were examined. Results of 1 experiment, representative of 2 experiments, are shown.
The IFN-γ–triggered proinflammatory cytokine production and IDO induction in human DCs represent distinct cellular programs. (A) The amount of proinflammatory cytokine release at the end of the activation period was examined in cell culture supernatants of differently activated DCs (left panels). In the same culture supernatants, the concentrations of tryptophan (TRP; ▩) and kynurenine (KYN; ■) were examined (right). The bars indicate the median of 4 experiments. The numbers given in brackets indicate the range. (B) DCs were activated with LPS/IFN-γ for 48 hours to induce IDO competence. Proinflammatory cytokine release was examined in cell culture supernatants after removal of the activating agents and reculture of the activated DCs in fresh medium for a 48-hour period. The values of cytokine release on reculture are expressed as the percentage of the maximum release (= 100%) during the activation period (top). In the same culture supernatants, the concentrations of tryptophan and kynurenine were analyzed (bottom). Results of 1 experiment, representative of 2 experiments, are shown. (C) DCs were activated with LPS/IFN-γ for 48 hours in conditions of external manipulation of tryptophan metabolism, as indicated (TRP met indicates tryptophan metabolites). Proinflammatory cytokine release (left panels) and the concentrations of TRP and KYN (right) were examined. Results of 1 experiment, representative of 2 experiments, are shown.
Next, to examine whether cytokine production is affected by IDO activity, we manipulated tryptophan degradation and kynurenine accumulation during the DC activation period. DCs were activated with LPS/IFN-γ for 48 hours in the presence of excess tryptophan (100 μM) or of exogenous tryptophan metabolites (25 μM each) or blockade of IDO activity by MTHT and 1-MT. Although these manipulations resulted in the expected changes of tryptophan and kynurenine concentrations in cell culture supernatants (Figure 2C right panel), the levels of proinflammatory cytokines remained essentially unaffected (Figure 2C left panels). In addition, a delayed blockade of IDO activity by MTHT did not alter the release of cytokines (data not shown). Taken together, these findings suggest that in monocyte-derived and LPS-activated DCs IFN-γ triggers proinflammatory cytokine secretion and IDO activity as distinct cellular programs.
Inhibition of allogeneic T-cell proliferation by IDO-competent DCs is sensitive to the cell culture condition
Using differently activated DCs as stimulators of allogeneic T cells allowed us to study a possible effect of IDO competence in human DCs on modulating allogeneic T-cell responses.
First, and consistent with previous observations,4,33,34 we found that IDO-competent DCs had a low stimulatory capacity of allogeneic T cells. In fact, when we compared DCs activated with LPS/IFN-γ for 48 hours (IDO competent) to DCs activated with LPS/IFN-γ for 4 hours (delayed IDO competence) as stimulators of a mixed lymphocyte reaction (MLR), the allogeneic T-cell response stimulated by IDO-competent DCs was impaired. The extent of reduction of the proliferative response correlated to the number of IDO-competent DCs added to the allogeneic T cells (Figure 3A), suggesting a suppressive effect on a per cell basis. The inhibition of the alloresponses by IDO-competent DCs was reversible by MTHT-mediated blockade of IDO activity (Figure 3B), thus supporting an IDO-dependent effect.
The reduced capacity of IDO-competent DCs to stimulate allogeneic T cells can be overcome by a slight modification of cell culture conditions. (A) DCs were activated with LPS/IFN-γ for 4 hours (♦) or 48 hours (■) and cocultured in graded amounts with allogeneic T cells (MLR), and their capacity to stimulate an allogeneic T-cell response was determined by [3H]thymidine incorporation. Results of 1 experiment, representative of 2 experiments, are shown. Error bars indicate the SEM. (B) DCs were activated with LPS/IFN-γ for 48 hours and were added in graded amounts to allogeneic T cells as in panel A. MLRs were set up in the presence (▩) or absence (■) of the IDO inhibitor MTHT. The allogeneic T-cell responses were determined as the percentage of CFSE-negative T cells. Results of 1 experiment, representative of 2 experiments, are shown. (C) Differently activated DCs were cocultured with the same amount of allogeneic T cells, and fresh cell culture medium was added to the cell cultures at day 4. The allogeneic T-cell responses were determined as in panel B.
The reduced capacity of IDO-competent DCs to stimulate allogeneic T cells can be overcome by a slight modification of cell culture conditions. (A) DCs were activated with LPS/IFN-γ for 4 hours (♦) or 48 hours (■) and cocultured in graded amounts with allogeneic T cells (MLR), and their capacity to stimulate an allogeneic T-cell response was determined by [3H]thymidine incorporation. Results of 1 experiment, representative of 2 experiments, are shown. Error bars indicate the SEM. (B) DCs were activated with LPS/IFN-γ for 48 hours and were added in graded amounts to allogeneic T cells as in panel A. MLRs were set up in the presence (▩) or absence (■) of the IDO inhibitor MTHT. The allogeneic T-cell responses were determined as the percentage of CFSE-negative T cells. Results of 1 experiment, representative of 2 experiments, are shown. (C) Differently activated DCs were cocultured with the same amount of allogeneic T cells, and fresh cell culture medium was added to the cell cultures at day 4. The allogeneic T-cell responses were determined as in panel B.
Somewhat unexpectedly, however, when we adapted cell culture conditions (see “T-cell stimulation and mixed lymphocyte reaction”) to prevent exhaustion of the cell culture medium and to optimize proliferation of allostimulated T cells, the impairment of the alloresponses by IDO-competent DCs was no longer detectable, even when the DCs were added to allogeneic T cells in equal amounts. Either of the differently activated DC populations induced proliferation of allogeneic T cells to similar extents (Figure 3C). This finding is in agreement with former observations that the capacity of IDO-competent DCs to suppress allogeneic T-cell responses critically depends on cell culture conditions.35,36
IDO-competent DCs, even when not suppressing proliferation, endow allogeneic CD4+ T cells with regulatory activity
When we examined the phenotype of alloreactive T cells (CD4+CD25+CFSE negative) stimulated in cell culture conditions as in Figure 3C, we noticed that the CD4+ T cells stimulated by either of the differently activated DC populations showed similar levels of CD25, FOXP3, and CTLA-4 expression (Figure 4A), compatible with a regulatory phenotype. Yet, true regulatory activity of a CD4+CD25+ T-cell population may not be recognizable by phenotype,37 even when cells express the master regulatory marker FOXP3.38 Thus, to explicitly address a regulatory activity of CD4+CD25+ T cells, we tested the CD4+CD25+ T-cell fractions after stimulation by either of the differently activated DC populations for their suppressive capacity in a conventional suppressor assay. Briefly, after 7 days of MLR, T cells were recovered and were separated by flow cytometry into CD4+CD25− and CD4+CD25+ T-cell fractions and were subsequently added to fresh autologous T cells, which were stained with CFSE and stimulated with immobilized anti-CD3 mAb.
IDO-competent DCs, even when not suppressing the allogeneic T-cell response, induce regulatory activity in allogeneic CD4+ T cells. (A) Differently activated DCs were used as stimulators of an MLR as in Figure 3C. At the end of the coculture T cells were recovered and were examined for CD25, CTLA-4, and FOXP3 expression (shaded histograms indicate CD4+CD25− T cells; open histograms, CD4+CD25+ T cells). Results of 1 experiment, representative of 4 experiments are shown. (B) CD4+ T cells were recovered from MLRs, in which the differently activated DCs were used as stimulators as in panel A, and were sorted by flow cytometry into a CD4+CD25− and a CD4+CD25+ population (left). These separate T-cell populations were subsequently cocultured for 5 days with fresh autologous T cells (1:1; suppressor assay). The latter were stained with CFSE. The proliferative responses of these T cells to stimulation with immobilized anti-CD3 mAb were assessed as the percentage of CFSE-negative cells. One representative experiment is shown. (C) Cumulative results:  indicates the median percentage of CFSE-negative T cells only without (left) or with anti-CD3–mediated stimulation (right);
indicates the median percentage of CFSE-negative T cells only without (left) or with anti-CD3–mediated stimulation (right);  , median percentage of CFSE-negative T cells cocultured with CD4+CD25− T cells retrieved from MLRs stimulated by differently activated DCs, as indicated;
, median percentage of CFSE-negative T cells cocultured with CD4+CD25− T cells retrieved from MLRs stimulated by differently activated DCs, as indicated;  , median percentage of CFSE-negative fresh T cells cocultured with CD4+CD25+ T cells retrieved from the same MLRs. Error bars indicate the range of experimental results of 15 consecutively tested donor/responder pairs. **P < .01. (D) DCs were activated with LPS/IFN-γ for 48 hours and used as stimulators of an MLR as in panel A, in the absence (left) or presence (right) of MTHT. After the MLR, the CD4+ T cells were recovered, sorted, and subjected to suppressor assays as in panel B. Proliferative responses are shown by CFSE dilution of fresh T cells (open histograms with thick black line) on coculture with the sorted autologous CD4+CD25− (top) and CD4+CD25+ (bottom) T cells. Filled gray histograms indicate CFSE dilution of anti-CD3–stimulated fresh T cell only; open histograms with thin line, CFSE dilution of unstimulated fresh T cells only. The numbers indicate the percentage of CFSE-negative cells in suppressor assays. One representative experiment is shown. (E) Cumulative results:
, median percentage of CFSE-negative fresh T cells cocultured with CD4+CD25+ T cells retrieved from the same MLRs. Error bars indicate the range of experimental results of 15 consecutively tested donor/responder pairs. **P < .01. (D) DCs were activated with LPS/IFN-γ for 48 hours and used as stimulators of an MLR as in panel A, in the absence (left) or presence (right) of MTHT. After the MLR, the CD4+ T cells were recovered, sorted, and subjected to suppressor assays as in panel B. Proliferative responses are shown by CFSE dilution of fresh T cells (open histograms with thick black line) on coculture with the sorted autologous CD4+CD25− (top) and CD4+CD25+ (bottom) T cells. Filled gray histograms indicate CFSE dilution of anti-CD3–stimulated fresh T cell only; open histograms with thin line, CFSE dilution of unstimulated fresh T cells only. The numbers indicate the percentage of CFSE-negative cells in suppressor assays. One representative experiment is shown. (E) Cumulative results:  indicates the median percentage of CFSE-negative fresh T cells only in the absence (left) or presence (right) of stimulation with anti-CD3 mAb (controls);
indicates the median percentage of CFSE-negative fresh T cells only in the absence (left) or presence (right) of stimulation with anti-CD3 mAb (controls);  , median percentage of CFSE-negative fresh T cells after coculture with CD4+CD25+ T cells retrieved from MLRs stimulated by DCs activated with LPS/IFN-γ for 4 hours or for 48 hours, as indicated, in the absence or presence of the IDO inhibitor MTHT. Error bars indicate the range of a total of 6 consecutive experiments. *P < .05.
, median percentage of CFSE-negative fresh T cells after coculture with CD4+CD25+ T cells retrieved from MLRs stimulated by DCs activated with LPS/IFN-γ for 4 hours or for 48 hours, as indicated, in the absence or presence of the IDO inhibitor MTHT. Error bars indicate the range of a total of 6 consecutive experiments. *P < .05.
IDO-competent DCs, even when not suppressing the allogeneic T-cell response, induce regulatory activity in allogeneic CD4+ T cells. (A) Differently activated DCs were used as stimulators of an MLR as in Figure 3C. At the end of the coculture T cells were recovered and were examined for CD25, CTLA-4, and FOXP3 expression (shaded histograms indicate CD4+CD25− T cells; open histograms, CD4+CD25+ T cells). Results of 1 experiment, representative of 4 experiments are shown. (B) CD4+ T cells were recovered from MLRs, in which the differently activated DCs were used as stimulators as in panel A, and were sorted by flow cytometry into a CD4+CD25− and a CD4+CD25+ population (left). These separate T-cell populations were subsequently cocultured for 5 days with fresh autologous T cells (1:1; suppressor assay). The latter were stained with CFSE. The proliferative responses of these T cells to stimulation with immobilized anti-CD3 mAb were assessed as the percentage of CFSE-negative cells. One representative experiment is shown. (C) Cumulative results:  indicates the median percentage of CFSE-negative T cells only without (left) or with anti-CD3–mediated stimulation (right);
indicates the median percentage of CFSE-negative T cells only without (left) or with anti-CD3–mediated stimulation (right);  , median percentage of CFSE-negative T cells cocultured with CD4+CD25− T cells retrieved from MLRs stimulated by differently activated DCs, as indicated;
, median percentage of CFSE-negative T cells cocultured with CD4+CD25− T cells retrieved from MLRs stimulated by differently activated DCs, as indicated;  , median percentage of CFSE-negative fresh T cells cocultured with CD4+CD25+ T cells retrieved from the same MLRs. Error bars indicate the range of experimental results of 15 consecutively tested donor/responder pairs. **P < .01. (D) DCs were activated with LPS/IFN-γ for 48 hours and used as stimulators of an MLR as in panel A, in the absence (left) or presence (right) of MTHT. After the MLR, the CD4+ T cells were recovered, sorted, and subjected to suppressor assays as in panel B. Proliferative responses are shown by CFSE dilution of fresh T cells (open histograms with thick black line) on coculture with the sorted autologous CD4+CD25− (top) and CD4+CD25+ (bottom) T cells. Filled gray histograms indicate CFSE dilution of anti-CD3–stimulated fresh T cell only; open histograms with thin line, CFSE dilution of unstimulated fresh T cells only. The numbers indicate the percentage of CFSE-negative cells in suppressor assays. One representative experiment is shown. (E) Cumulative results:
, median percentage of CFSE-negative fresh T cells cocultured with CD4+CD25+ T cells retrieved from the same MLRs. Error bars indicate the range of experimental results of 15 consecutively tested donor/responder pairs. **P < .01. (D) DCs were activated with LPS/IFN-γ for 48 hours and used as stimulators of an MLR as in panel A, in the absence (left) or presence (right) of MTHT. After the MLR, the CD4+ T cells were recovered, sorted, and subjected to suppressor assays as in panel B. Proliferative responses are shown by CFSE dilution of fresh T cells (open histograms with thick black line) on coculture with the sorted autologous CD4+CD25− (top) and CD4+CD25+ (bottom) T cells. Filled gray histograms indicate CFSE dilution of anti-CD3–stimulated fresh T cell only; open histograms with thin line, CFSE dilution of unstimulated fresh T cells only. The numbers indicate the percentage of CFSE-negative cells in suppressor assays. One representative experiment is shown. (E) Cumulative results:  indicates the median percentage of CFSE-negative fresh T cells only in the absence (left) or presence (right) of stimulation with anti-CD3 mAb (controls);
indicates the median percentage of CFSE-negative fresh T cells only in the absence (left) or presence (right) of stimulation with anti-CD3 mAb (controls);  , median percentage of CFSE-negative fresh T cells after coculture with CD4+CD25+ T cells retrieved from MLRs stimulated by DCs activated with LPS/IFN-γ for 4 hours or for 48 hours, as indicated, in the absence or presence of the IDO inhibitor MTHT. Error bars indicate the range of a total of 6 consecutive experiments. *P < .05.
, median percentage of CFSE-negative fresh T cells after coculture with CD4+CD25+ T cells retrieved from MLRs stimulated by DCs activated with LPS/IFN-γ for 4 hours or for 48 hours, as indicated, in the absence or presence of the IDO inhibitor MTHT. Error bars indicate the range of a total of 6 consecutive experiments. *P < .05.
First, as expected, we found that the CD4+CD25− T cells recovered from MLRs stimulated by the differently activated DCs had no suppressive capacity. The percentage of CFSE-negative fresh T cells after coculture with the CD4+CD25− fraction recovered from MLRs stimulated by either of the differently activated DC populations were not different from controls (70% median; Figure 4B-C). However, when we examined the CD4+CD25+ T-cell fractions retrieved from the same MLRs, the differences in their suppressor activity were striking. As depicted from a single experiment (Figure 4B), the CD4+CD25+ T cells recovered from an MLR stimulated by LPS/IFN-γ 4-hour–activated DCs or LPS only 48-hour–activated DCs (displaying delayed or minor IDO competence, respectively) only marginally suppressed the proliferation of fresh CD4+ T cells (Figure 4B top and middle panels). In clear contrast, the CD4+CD25+ T-cell fraction retrieved from an MLR that was stimulated by IDO-competent DCs potently suppressed the anti–CD3-mediated proliferation of fresh CD4+ T cells (Figure 4B bottom panel). This suppressive capacity of CD4+CD25+ T cells stimulated by allogeneic IDO-competent DCs was consistently observed in 15 consecutively tested different stimulator/responder pairs (68% suppression, median; Figure 4C). Suppression was evident when the CD4+CD25+ T cells were added to the fresh T cells in equivalent amounts but only rarely at lower ratios (data not shown). However, the capacity of CD4+CD25+ T cells recovered from MLRs stimulated by IDO-competent DCs to suppress was significantly higher (P < .01) than the only minor suppressor activity of CD4+CD25+ T cells recovered from MLRs stimulated by DCs with delayed or minor IDO competence (25% suppression, median; Figure 4C).
Subsequently, we ascertained that the induction of suppressor T cells was in fact linked to IDO competence in stimulating DCs. In these experiments allogeneic T cells were stimulated by IDO-competent DCs in the presence or absence of the IDO inhibitor MTHT. The presence of MTHT in the MLR reduced the suppressive capability of the CD4+CD25+ T cells as evidenced by a significantly increased proliferative response of the fresh T cells to stimulation with anti-CD3 (1.8-fold, median; P < .05; Figure 4D-E). Notably, the addition of MTHT to MLRs stimulated by short-term LPS/IFN-γ–activated DCs had no effect on the capacity of CD4+CD25+ T cells to alter proliferation of fresh T cells (Figure 4E). These findings strongly support that IDO activity essentially contributes to the induction of regulatory activity in allogeneic T cells.
Next, to explore whether allogeneic stimulation is critical for the induction of regulatory activity in T cells on coculture with IDO-competent DCs, we compared the regulatory activity of T cells stimulated by allogenic and autologous IDO-competent DCs. In the autologous setting IDO-competent DCs presented a nominal antigen (tetanus toxoid). Unexpectedly, the experiments showed (Figure 5) that the CD4+CD25+ T cells on stimulation by autologous IDO-competent DCs were unable to act as regulatory cells in subsequent suppressor assays, in contrast to T cells stimulated by allogeneic IDO-competent DCs. This finding suggests an essential role of allogeneic stimulation39 for the generation of T-cell regulatory activity by IDO-competent DCs.
IDO-competent DCs stimulate T-cell regulatory activity on allogeneic rather than autologous stimulation. DCs were activated with LPS/IFN-γ for 48 hours and used to stimulate an allogeneic or an autologous, TT triggered, response (see “T-cell stimulation and mixed lymphocyte reaction”). CD4+ T cells were recovered from the DC/T-cell cocultures, sorted, and subjected to subsequent suppressor assays as in Figure 4. (Left) The CD4+ T cells were retrieved from DC/T-cell cocultures stimulated by allogeneic DCs. (Right) The CD4+ T cells were retrieved from DC/T-cell cocultures stimulated by autologous, TT pulsed, DCs. Proliferative responses of fresh CFSE-stained and anti-CD3–stimulated T cells are shown by CFSE dilution (open histograms with thick black line) on coculture with the sorted CD4+CD25− (top) and CD4+CD25+ T cells (bottom). Filled gray histograms indicate CFSE dilution of anti-CD3–stimulated fresh T cells only; open histograms with thin line, CFSE dilution of unstimulated fresh T cells only. The numbers indicate the percentage of CFSE-negative cells in suppressor assays. Results of 1 experiment, representative of 3 experiments, are shown.
IDO-competent DCs stimulate T-cell regulatory activity on allogeneic rather than autologous stimulation. DCs were activated with LPS/IFN-γ for 48 hours and used to stimulate an allogeneic or an autologous, TT triggered, response (see “T-cell stimulation and mixed lymphocyte reaction”). CD4+ T cells were recovered from the DC/T-cell cocultures, sorted, and subjected to subsequent suppressor assays as in Figure 4. (Left) The CD4+ T cells were retrieved from DC/T-cell cocultures stimulated by allogeneic DCs. (Right) The CD4+ T cells were retrieved from DC/T-cell cocultures stimulated by autologous, TT pulsed, DCs. Proliferative responses of fresh CFSE-stained and anti-CD3–stimulated T cells are shown by CFSE dilution (open histograms with thick black line) on coculture with the sorted CD4+CD25− (top) and CD4+CD25+ T cells (bottom). Filled gray histograms indicate CFSE dilution of anti-CD3–stimulated fresh T cells only; open histograms with thin line, CFSE dilution of unstimulated fresh T cells only. The numbers indicate the percentage of CFSE-negative cells in suppressor assays. Results of 1 experiment, representative of 3 experiments, are shown.
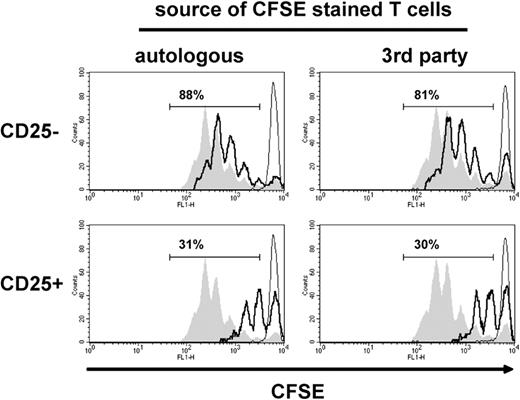
To then determine whether the regulatory activity induced by IDO-competent DCs is restricted to suppressing autologous T cells, we compared the suppressive capacity of CD4+CD25+ T cells retrieved from MLRs stimulated by IDO-competent DCs in suppressor assays containing fresh autologous or third-party T cells. The results showed a similar suppressor activity of CD4+CD25+ T cells on coculture with autologous or third-party T cells (Figure 6), suggesting that the T-cell regulatory activity induced by IDO-competent DCs is not restricted to the antigen through which it was activated40 but is more generally applicable.
CD4+CD25+ T cells stimulated by allogeneic IDO-competent DCs suppress the proliferation of third-party T cells. DCs were activated with LPS/IFN-γ for 48 hours and used as stimulators of an MLR, and the CD4+ T cells retrieved from the MLR were subjected to subsequent suppressor assays as in Figure 4. (Left) CFSE-stained fresh T cells and CD4+ T cells were autologous. (Right) CFSE-stained fresh T cells and CD4+ T cells were allogeneic (third party). Proliferative responses of fresh T cells are shown by CFSE dilution (open histograms with thick black line) on coculture with sorted CD4+CD25− (top) and CD4+CD25+ T cells (bottom). Filled gray histograms indicate CFSE dilution of anti-CD3–stimulated fresh T cell only; open histograms with thin line, CFSE dilution of unstimulated fresh T cells only. The numbers indicate the percentage of CFSE-negative cells in suppressor assays. Results of 1 experiment, representative of 3 experiments, are shown.
CD4+CD25+ T cells stimulated by allogeneic IDO-competent DCs suppress the proliferation of third-party T cells. DCs were activated with LPS/IFN-γ for 48 hours and used as stimulators of an MLR, and the CD4+ T cells retrieved from the MLR were subjected to subsequent suppressor assays as in Figure 4. (Left) CFSE-stained fresh T cells and CD4+ T cells were autologous. (Right) CFSE-stained fresh T cells and CD4+ T cells were allogeneic (third party). Proliferative responses of fresh T cells are shown by CFSE dilution (open histograms with thick black line) on coculture with sorted CD4+CD25− (top) and CD4+CD25+ T cells (bottom). Filled gray histograms indicate CFSE dilution of anti-CD3–stimulated fresh T cell only; open histograms with thin line, CFSE dilution of unstimulated fresh T cells only. The numbers indicate the percentage of CFSE-negative cells in suppressor assays. Results of 1 experiment, representative of 3 experiments, are shown.
Together, these results identify IDO competence in stimulating DCs as a critical factor contributing to the induction of a nonspecific suppressor function in allogeneic T cells. The finding that DCs on short-term activation with LPS/IFN-γ, which develop delayed IDO competence, induced only minor suppressor activity in alloreactive T cells indicates that IDO competence must be present at the initiation of the MLR to induce suppressor function in responder T cells.
IDO-competent DCs induce regulatory CD4+CD25+ T cells from naive CD4+CD25− T cells
Finally, we addressed the question of whether IDO-competent DCs possess the capability to generate CD4+CD25+ suppressor T cells from naive CD4+CD45RA+ T cells. The latter have been shown to be devoid of memory cells and devoid of the naturally occurring CD25+ regulatory T cells (data not shown).41 CD4+CD45RA+ or total CD4+ T cells were cocultured with IDO-competent DCs and after termination of the MLR were assessed for the induction of suppressor activity. Stimulation of CD4+CD45RA+ or CD4+ T cells with allogeneic IDO-competent DCs induced similar proportions of CD4+CD25+ T cells (data not shown). Noticeably, the CD4+CD25+ T cells retrieved from an MLR, in which CD4+CD45RA+ T cells were responders, had equivalent suppressive capacity as did CD4+CD25+ T cells retrieved from an MLR in which responders were total CD4+ T cells (Figure 7; P = NS), indicating that IDO-competent DCs can induce adaptive regulatory T cells.
IDO-competent DCs induce the differentiation of adaptive regulatory T cells. Total CD4+ T cells or naive CD4+CD45RA+ T cells were cocultured with IDO-competent DCs (activated with LPS/IFN-γ for 48 hours) and, after termination of the MLR, were sorted by flow cytometry into a CD4+CD25− and a CD4+CD25+ population. These separate T-cell populations were subsequently cocultured with fresh autologous T cells (1:1) which were stained with CFSE. The bars indicate the median percentage of CFSE-negative cells after 5 days of culture.  indicates the percentage of CFSE-negative fresh T cells only without (left) or with anti-CD3 stimulation (right; controls);
indicates the percentage of CFSE-negative fresh T cells only without (left) or with anti-CD3 stimulation (right; controls);  , percentage of CFSE-negative fresh T cells cocultured with CD4+CD25− T cells retrieved from the MLR;
, percentage of CFSE-negative fresh T cells cocultured with CD4+CD25− T cells retrieved from the MLR;  , percentage of CFSE-negative fresh T cells cocultured with CD4+CD25+ T cells retrieved from the MLR. Error bars indicate the range of experimental results of 4 consecutively tested donor/responder pairs. P = n.s.
, percentage of CFSE-negative fresh T cells cocultured with CD4+CD25+ T cells retrieved from the MLR. Error bars indicate the range of experimental results of 4 consecutively tested donor/responder pairs. P = n.s.
IDO-competent DCs induce the differentiation of adaptive regulatory T cells. Total CD4+ T cells or naive CD4+CD45RA+ T cells were cocultured with IDO-competent DCs (activated with LPS/IFN-γ for 48 hours) and, after termination of the MLR, were sorted by flow cytometry into a CD4+CD25− and a CD4+CD25+ population. These separate T-cell populations were subsequently cocultured with fresh autologous T cells (1:1) which were stained with CFSE. The bars indicate the median percentage of CFSE-negative cells after 5 days of culture.  indicates the percentage of CFSE-negative fresh T cells only without (left) or with anti-CD3 stimulation (right; controls);
indicates the percentage of CFSE-negative fresh T cells only without (left) or with anti-CD3 stimulation (right; controls);  , percentage of CFSE-negative fresh T cells cocultured with CD4+CD25− T cells retrieved from the MLR;
, percentage of CFSE-negative fresh T cells cocultured with CD4+CD25− T cells retrieved from the MLR;  , percentage of CFSE-negative fresh T cells cocultured with CD4+CD25+ T cells retrieved from the MLR. Error bars indicate the range of experimental results of 4 consecutively tested donor/responder pairs. P = n.s.
, percentage of CFSE-negative fresh T cells cocultured with CD4+CD25+ T cells retrieved from the MLR. Error bars indicate the range of experimental results of 4 consecutively tested donor/responder pairs. P = n.s.
Discussion
The essential conclusion of the present study is that IDO competence in human monocyte-derived DCs, even when not causing a quantitative down-regulation of proliferative responses, nevertheless possesses the capacity to support T-cell regulatory activity.
Numerous observations have questioned the relevance of IDO competence in human DCs because of the findings that (1) IDO activity had no down-regulating effect on T-cell proliferation and (2) the commonly used IDO inhibitor 1-MT seemingly had an effect on cell proliferation apart from inhibiting IDO.36,42,43 To this end, our data show that, on a slight modification of the cell culture conditions, the inhibition of proliferative responses of allogeneic T cells by IDO-competent DCs was no longer detectable. Despite different grades of IDO competence either of the examined DC populations induced quantitatively equivalent T-cell responses. Thus, our data support the notion that the T cell–dampening effect of IDO activity in human cells in vitro may critically depend on details of cell culture conditions.4,44 Moreover, they provide sound evidence that alloreactive T cells stimulated by IDO-competent DCs may, even when these T cells retain their ability to proliferate, acquire regulatory activity.
The role of phenotypically mature DCs as powerful stimulators of immune effector responses has lately been revisited, and their ability to induce or activate regulatory T cells and to maintain tolerance has been appreciated.45,46 In a recent report, tolerogenic plasmacytoid DCs when activated through TLR9 ligation have been shown to expand regulatory T cells by the IDO pathway.11 Our data show that, likewise, human monocyte-derived DCs, on activation by TLR4 ligation (LPS) and IFN-γ, acquire sustained IDO competence and can induce T-cell regulatory activity. In our ongoing experiments we found that also a PGE2-based DC activation strategy induced IDO competence and stimulated a regulatory T-cell response (B.J., unpublished observation, January 2009). Collectively, these findings support the view that IDO competence in DCs, regardless of the DC subtype and of the IDO-inducing strategy, constitutes a common and critical factor for enabling DCs to stimulate T-cell regulatory activity. Intriguingly, IDO-competent DCs endowed even naive allogeneic (CD4+CD25−CD45RA+) T cells with the ability to suppress, suggesting that IDO-competent DCs possess the capacity to induce adaptive regulatory T cells.11
Regulatory T cells have in most studies been identified as CD4+CD25+ T cells expressing the transcription factor FOXP3. Substantial evidence now disagrees with the view that the human adaptive regulatory T cells can in fact be identified by the expression of CD25 or FOXP3 or CTLA-4 because these molecules have been reported to be induced to similar levels in recently activated effector T cells.37,38,47 In fact, also in the present study allogeneic CD4+ T cells stimulated by DCs with different degrees of IDO competence showed a largely similar CD4+CD25+FOXP3+CTLA-4+ phenotype, while their capacity to suppress was substantially different. Thus, the suppressive capacity could not be deduced from the marker expression profile of responding T cells.
In our studies, the addition of IFN-γ to LPS as a strategy to activate monocyte-derived DCs in vitro amplified both the production of proinflammatory cytokines and IDO competence. The data further show that DCs after 48 hours of activation with LPS and IFN-γ lose the capacity to secrete proinflammatory cytokines but retain the capability to effectively metabolize tryptophan and accumulate kynurenine (Figure 2B). This divergent balance appears to shape the T-cell response. DCs activated in a short-term strategy (LPS/IFN-γ for 4 hours) have been shown to be able to continue to secrete proinflammatory cytokines on reculture (data not shown)27 and, though gradually developing IDO competence, support a Th1-polarized T-cell response.27 These observations suggest an in vitro model system to enlighten the paradoxical roles of IFN-γ in directing opposing immune reactions2,19,22 : IFN-γ may trigger a signaling program in DCs to stimulate Th1-polarized T-cell responses, but this proinflammatory program is limited by time. IFN-γ concomitantly elicits IDO competence in DCs, which becomes prevalent after the production of proinflammatory cytokines has ceased. Thus, IDO competence may represent a signature of an anti-inflammatory program and may enable APCs to either suppress T-cell responses or induce T-cell regulatory activity.
Our data, by showing that the manipulation of tryptophan catabolism left cytokine production essentially unaffected, support the view that the IFN-γ–triggered induction of proinflammatory cytokines and of IDO competence represent distinct cellular programs. However, to comprehensively address the question of a potentially linked regulation of IDO activity and cytokines, further in-depth studies, including the molecular level, are required. Thus far, this model is compatible with viewing IDO induction as an immunoregulatory feedback mechanism, representing a physiologic limitation of potentially hazardous immune reactions.14,48 The observation that T-cell regulatory activity was induced by allogeneic rather than by autologous IDO-competent DCs is intriguing. One likely possibility to explain this observation may be the lower T-cell precursor frequency specific for cognate antigen (TT) compared with that of T-cell precursors specific for alloantigens. Studying in detail the mechanisms underlying this finding may have important implications for the understanding of IDO-mediated tolerogenesis in vivo.
Irrespective of whether the findings presented herein, including the opposing activities of DCs activated by IFN-γ, translate in vivo, they may be significant for the design of DCs as therapeutic immunomodulators ex vivo.49 Practically, to induce a regulatory T-cell response in vitro, one possible approach is to generate IDO-competent DCs that would be incapable of secreting proinflammatory cytokines and would encounter T cells in a tryptophan-deplete but kynurenine-rich environment. Whether the DC activation strategy used in this study, which is to combine LPS with IFN-γ to generate IDO competence, is the most effective one is currently extensively examined in our laboratory. Furthermore, the implication of our findings on the design of DC vaccination in vivo warrants further investigation.
In conclusion, we propose the following conceptual view for understanding the role of IFN-γ in activating human monocyte-derived DCs: IFN-γ stimulates DCs to release proinflammatory cytokines and to develop IDO competence in parallel but with different time kinetics. As long as proinflammatory cytokine release prevails and IDO competence is not yet fully developed, the DCs may stimulate a proinflammatory T-cell response. However, when cytokine production ceases, the IFN-γ–triggered IDO activity becomes prevalent and ongoing even after activation. The ongoing IDO activity may be particularly relevant when DCs encounter responder T cells. These IDO-competent DCs dampen immune responses. The relevance of IDO competence in dampening immune responses may become evident as the DCs' ability to suppress T-cell responses quantitatively. This effect, however, appears to be sensitive to details of cell culture conditions. Yet, even when the inhibitory effect is not obvious, IDO competence in DCs may nevertheless contribute to modify immune responses by endowing T cells with regulatory activity. Thus, future efforts to elaborate in full the potential of IDO competence and its usefulness in clinical application will have to take into account the various properties of IDO competence in DCs. In addition to the frequently observed suppression of T-cell responses, these properties include the reported capability of IDO-mediated apoptosis that particularly affects activated T cells6 and the capability of DCs to endow T cells with regulatory activity. The appropriate use of the combined effects of IDO competence in APCs may be valuable in the generation of antigen-specific tolerance.50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dieter Printz for excellent assistance in flow cytometric cell sorting; Osamu Takikawa (National Institute for Longevity Sciences, National Center for Geriatrics and Gerontology, Aichi, Japan), who kindly provided the mouse anti–human IDO monoclonal antibody; and Idriss Bennani-Baiti, PhD, Julia Raberger, PhD, and Thomas Wekerle, MD, for carefully reading and discussing the manuscript.
This work was supported by the Austrian Science Fund (project nos. 16764-B13 and 20865-B13).
Authorship
Contribution: B.J. designed and performed the experiments, analyzed data, and wrote the paper; U.H. performed initial experiments and commented on the paper; D.F. performed high-pressure liquid chromatography studies measuring tryptophan and kynurenine concentrations and critically reviewed the manuscript; T.F. designed initial experiments and commented on the paper; and A.H. designed and supervised the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Heitger, Children's Cancer Research Institute, St Anna Kinderkrebsforschung, Transplantation Immunology, Zimmermannplatz 10, A-1090 Vienna, Austria; e-mail: andreas.heitger@ccri.at.


![Figure 3. The reduced capacity of IDO-competent DCs to stimulate allogeneic T cells can be overcome by a slight modification of cell culture conditions. (A) DCs were activated with LPS/IFN-γ for 4 hours (♦) or 48 hours (■) and cocultured in graded amounts with allogeneic T cells (MLR), and their capacity to stimulate an allogeneic T-cell response was determined by [3H]thymidine incorporation. Results of 1 experiment, representative of 2 experiments, are shown. Error bars indicate the SEM. (B) DCs were activated with LPS/IFN-γ for 48 hours and were added in graded amounts to allogeneic T cells as in panel A. MLRs were set up in the presence (▩) or absence (■) of the IDO inhibitor MTHT. The allogeneic T-cell responses were determined as the percentage of CFSE-negative T cells. Results of 1 experiment, representative of 2 experiments, are shown. (C) Differently activated DCs were cocultured with the same amount of allogeneic T cells, and fresh cell culture medium was added to the cell cultures at day 4. The allogeneic T-cell responses were determined as in panel B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/15/10.1182_blood-2008-12-195073/4/m_zh89990941930003.jpeg?Expires=1767765221&Signature=CiL5E4FNENa5C~j68sSbwy0sgTa1RGXSdcJkjV7KSn5hsBCxJv1~0T6vmSSiz9oxut9tSsnQxONFH0piYhBqdIFNtug6PVTsdTPHLjs6g51GW1gw95to0pfGfaTxZ4Xova151-MY3F4Yu-R9WLw5cnfvDeAPeFI6rsZzHvXcERNOBdp527OqYTx0bhYDAfVc9O44kuuPP0FolhB6Ghtb~j9eYaDPkDFACGBT5-2ztzzBtYQtjw8362pqd7DjvbTgyZ6JUB4DNxfLYFrr4tkvtllYPyfP~WNoB1xR8rubXX5qmlaVlFihV4aNmiW8KVIY2-7YQ9--cfCKMNRwyGPunw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)