Abstract
Approximately 5% to 10% of diffuse large B-cell lymphomas (DLBCLs) harbor an MYC oncogene rearrangement (MYC+). The prognostic significance of MYC+ DLBCL was determined in an unselected population of patients with newly diagnosed DLBCL treated with rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy (R-CHOP). Using a Vysis break-apart fluorescence in situ hybridization probe, 12 of 135 (8.8%) cases of MYC+ DLBCL were identified that had no defining high-risk features. MYC+ DLBCL was associated with an inferior 5-year progression-free survival (66% vs 31%, P = .006) and overall survival (72% vs 33%, P = .016). Multivariate analysis confirmed the prognostic importance of MYC for both progression-free survival (hazard ratio = 3.28; 95% confidence interval, 1.49-7.21, P = .003) and overall survival (hazard ratio = 2.98; 95% confidence interval, 1.28-6.95, P = .011). Cases of MYC+ DLBCL also had a higher risk of central nervous system relapse (P = .023), independent of other risk factors. The diagnosis of MYC+ DLBCL is likely underappreciated; and given the lack of defining risk factors, fluorescence in situ hybridization for MYC rearrangements should be performed in all patients with DLBCL. In the R-CHOP treatment era, MYC+ DLBCLs have an inferior prognosis. Treatment regimens similar to those used in Burkitt lymphoma may be more appropriate in this patient population and need to be prospectively tested.
Introduction
Diffuse large B-cell lymphomas (DLBCLs) are recognized to be a heterogeneous group of diseases with clinical, morphologic, immunohistochemical, and molecular subtypes defined in the updated World Health Organization (WHO) classification.1 Further, a new category has been created defined as “borderline cases,” which are considered B-cell lymphomas, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma.2 Morphologically, these tumors typically have a mixture of medium- to large-sized cells, a high proliferation rate, and 35% to 50% of cases have an 8q24/MYC translocation.2 However, approximately 5% to 10% of DLBCLs with typical morphology also harbor an MYC rearrangement (herein after referred to as MYC+), and these cases are considered in the category of DLBCL, not otherwise specified, in the updated WHO classification.3
There is very little information regarding the prognostic importance of an isolated MYC rearrangement in DLBCL using modern diagnostic criteria. A recent study suggested that MYC gene rearrangements identified by fluorescence in situ hybridization (FISH) in pathologically defined DLBCL patients treated with cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP)–like chemotherapy are associated with an inferior prognosis.4 However, it is unclear whether there are identifiable clinical or pathologic characteristics that suggest that a case may harbor an MYC rearrangement to prompt evaluation. Further, prior studies evaluating the prognostic implications of MYC in DLBCL have been performed before the use of rituximab. With studies showing improved outcome using rituximab in combination with CHOP (R-CHOP) or CHOP-like therapies in the treatment of DLBCL,5-8 the importance of MYC rearrangement status in this population must be reestablished.
The purpose of this study was 2-fold: (1) to screen an unselected series of patients with DLBCL for MYC rearrangements to determine the frequency of this occurrence and whether there were any pathologic or clinical defining features in the MYC+ group and (2) to assess the prognostic impact of MYC gene rearrangements in DLBCL patients treated with R-CHOP chemotherapy.
Methods
Patient identification
The British Columbia Cancer Agency (BCCA) Lymphoid Cancer Database was screened to identify adult patients (> 15 years of age) with newly diagnosed DLBCL treated with curative intent with CHOP in combination with rituximab (R-CHOP). Patients who were HIV+ were excluded. Those cases with available paraffin-embedded tissue blocks from the diagnostic biopsy were used to construct a tissue microarray (TMA). For this analysis, only those cases considered to be DLBCL, not otherwise specified, by the recently updated WHO classification of lymphomas recently published were included.3 Clinical information, including baseline characteristics for the calculation of the International Prognostic Index (IPI), was determined.9 This study was approved by the BCCA Research Ethics Board.
TMA
Three independent TMAs were constructed using duplicate 0.6-mm cores from formalin-fixed, paraffin-embedded tissue derived from a total of 137 samples of newly diagnosed cases of DLBCL according to the WHO classification.3
Immunohistochemistry
Immunohistochemistry (IHC) was performed on archived paraffin-embedded tissue using CD20 (L26; Dako North America), CD3 (Dako North America), CD10 (clone 56C6; Vector Laboratories), Ki-67 (Dako North America), BCL6 (clone PG-B6p; Dako North America), MUM1 (clone MUM1p),10 and BCL2 (clone 124; Dako North America). The TMAs were stained using automated IHC on a Ventana Benchmark using standard protocols. The Ki-67 antibody MIB1 was used to determine the proliferation rate, and more than 80% was defined as “high proliferation” as previously described.11 For all other immunostains, a cutoff of more than 30% was used in accordance with other studies.12
Determination of DLBCL cell of origin subtypes
For 76 patients, frozen tissue was available for gene expression profiling (GEP) analysis. RNA was extracted using the ALL PREP kit (QIAGEN), and after reverse transcription it was hybridized to the U133-2 Plus arrays (Affymetrix) according to the manufacturer's protocol. CEL files were normalized using robust multichip analysis.13 The cell of origin (COO) phenotype (germinal center B-cell type [GCB], activated B-cell type [ABC], or unclassified) was determined using the 185 gene list and model scores determined by the Bayesian formula as previously described.14 For the remaining patients, the COO was determined to be GCB or non-GCB using IHC according to the Hans algorithm.12 Cases that were unclassified by the Bayesian formula were assigned using the Hans criteria.
Cytogenetic analysis
All cases were screened for an MYC rearrangement using a commercial Vysis dual-color FISH break-apart probe (Abbott Molecular) applied to the TMA. Cases harboring an MYC rearrangement were also screened for the presence of a t(14;18) rearrangement using the LSI IGH/BCL2 dual-color, dual-fusion translocation probe (Abbott Molecular) on isolated cells from the original paraffin-embedded tissue block and/or frozen or methanol/acetic acid-fixed cell pellets if available.
Statistical analysis
Progression-free survival (PFS) was determined from the date of the pathologic lymphoma diagnosis to the date of relapse, progression or death resulting from lymphoma, or treatment toxicity. Overall survival (OS) was determined from the date of diagnosis to the date of death of any cause. The time to central nervous system (CNS) relapse was defined from the date of diagnosis to the date of documented relapse in the CNS. The χ2 test was used to compare baseline characteristics between MYC+ and MYC− cases. Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test.15 The Cox proportional hazards model was used, including factors with a P less than .1 in univariate analysis, to determine the impact of multiple factors on PFS, OS, and time to CNS relapse.16 All statistical analyses were performed using SPSS software, Version 11.5.
Results
A total of 137 R-CHOP–treated cases of DLBCLs had tissue available for the TMA (61% nodal and 39% extranodal biopsy specimens), and FISH analysis was successful in 135 cases at defining the presence or absence of an MYC rearrangement, with 2 technical failures. In total, 12 of 135 (8.8%) cases of DLBCL were positive for an MYC rearrangement in this series. Three of the MYC+ cases had a concurrent t(14;18) chromosome, so-called dual translocations or “double-hit.”
Patients who had MYC+ DLBCL were predominantly male (75%) with a median age of 69 years (range, 22-85 years) with no particular high risk defining clinical features other than a higher incidence of testicular involvement at disease presentation, compared with MYC− cases (Table 1). Half were early stage and were in a favorable risk group by the IPI (Table 1). There was no difference in the frequency of extranodal versus nodal primary biopsy sites in the MYC+ and MYC− groups (P = .301).
Characteristics of MYC+ and MYC− DLBCL patients
| Feature . | MYC+, n (%) (n = 12) . | MYC−, n (%) (n = 123) . | P . |
|---|---|---|---|
| Median age, y | 68 | 61 | — |
| Age > 60 y | 8 (67) | 68 (55) | .448 |
| Male sex | 9 (75) | 73 (59) | .289 |
| Stage 3 or 4 | 6 (50) | 75 (61) | .459 |
| B symptoms | |||
| Extranodal any site | 8 (67) | 72 (58) | .584 |
| Extranodal > 1 | 4 (33) | 25 (20) | .295 |
| Bone marrow | 0 | 11 (9) | .280 |
| Testicular | 2 (17) | 3 (2) | .013 |
| Sinus | 0 | 2 (1.6) | .280 |
| Gastrointestinal | 2 (17) | 19 (15) | .911 |
| Kidney | 0 | 2 (1.6) | .656 |
| Liver | 0 | 7 (6) | .396 |
| Bulky disease* | 4 (33) | 31 (26) | .575 |
| PS > 2* | 5 (42) | 42 (36) | .677 |
| LDH abnormal* | 9 (75) | 54 (50) | .100 |
| LDH > 2× ULN | 3 (25) | 28 (26) | .930 |
| IPI 0-2 vs 3-5 | 6 (50) | 48 (39) | .459 |
| Ki-67† | |||
| More than 80% | 7 (58) | 27 (22.5) | .007 |
| More than 90% | 6 (50) | 9 (7.5) | < .001 |
| More than 95% | 4 (12) | 8 (6.7) | .002 |
| BCL2 protein+‡ | 8 (67) | 86 (70) | .782 |
| GCB phenotype§ | 7 (58) | 61 (51) | .640 |
| Feature . | MYC+, n (%) (n = 12) . | MYC−, n (%) (n = 123) . | P . |
|---|---|---|---|
| Median age, y | 68 | 61 | — |
| Age > 60 y | 8 (67) | 68 (55) | .448 |
| Male sex | 9 (75) | 73 (59) | .289 |
| Stage 3 or 4 | 6 (50) | 75 (61) | .459 |
| B symptoms | |||
| Extranodal any site | 8 (67) | 72 (58) | .584 |
| Extranodal > 1 | 4 (33) | 25 (20) | .295 |
| Bone marrow | 0 | 11 (9) | .280 |
| Testicular | 2 (17) | 3 (2) | .013 |
| Sinus | 0 | 2 (1.6) | .280 |
| Gastrointestinal | 2 (17) | 19 (15) | .911 |
| Kidney | 0 | 2 (1.6) | .656 |
| Liver | 0 | 7 (6) | .396 |
| Bulky disease* | 4 (33) | 31 (26) | .575 |
| PS > 2* | 5 (42) | 42 (36) | .677 |
| LDH abnormal* | 9 (75) | 54 (50) | .100 |
| LDH > 2× ULN | 3 (25) | 28 (26) | .930 |
| IPI 0-2 vs 3-5 | 6 (50) | 48 (39) | .459 |
| Ki-67† | |||
| More than 80% | 7 (58) | 27 (22.5) | .007 |
| More than 90% | 6 (50) | 9 (7.5) | < .001 |
| More than 95% | 4 (12) | 8 (6.7) | .002 |
| BCL2 protein+‡ | 8 (67) | 86 (70) | .782 |
| GCB phenotype§ | 7 (58) | 61 (51) | .640 |
PS indicates Performance Status; LDH, lactic dehydrogenase; and ULN, upper limit of normal.
Missing data: LDH, n = 15; bulky disease, n = 3; PS, n = 5.
Failed in 3 MYC− cases.
Results using the Dako antibody; not available in 1 MYC− case.
Not available in 4 MYC− cases.
IHC and cell of origin
MYC+ cases were more likely to have a high proliferation rate (Table 1). Eight cases (67%) of the MYC+ DLBCL were BCL2+ using the commercially available Dako antibody with no difference in frequency observed compared with the MYC− cases (Table 1). Of interest, 2 out of 3 of the MYC+ cases that were also t(14;18)+ were BCL2 protein− by IHC using the Dako antibody. Given this finding, these 2 cases were subsequently analyzed using an alternate antibody, clone E17 (Epitomics), which targets amino acids 60 to 80 compared with the Dako BCL2 clone 124, which targets amino acids 41 to 54. Using the E17 antibody, there was nearly 100% staining for BCL2. In one of these cases, subsequent sequencing of the BCL2 gene detected mutations in the flexible loop domain but not the BH3 domain,17 thus interfering with binding of the Dako antibody. However, the clinical significance of this finding is unknown.
The updated WHO classification of lymphomas recognizes 2 molecular subtypes of DLBCL based on the COO phenotype as GCB and ABC3,14 or by IHC as GCB and non-GCB. The COO phenotype was available for 131 cases (97%; 4 missing in the MYC− group); 62 were assigned by GEP14 and 69, including 14 unclassified cases by GEP, using IHC by the Hans algorithm.12 Of note, there were 58 cases in which we had both GEP and IHC results that were obtained in a blinded fashion, to evaluate the concordance of the 2 approaches at assigning the COO status. Of the 46 cases with a definitive COO assignment by GEP (12 were unclassified), 35 were concordant with the Hans COO designation for an overall agreement rate of 85%, consistent with prior studies.12
For the purpose of the COO assignment in our study, 2 groups were considered: GCB and non-GCB (the latter including those assigned as ABC by GEP). Cases that were unclassifiable by GEP were assigned by the Hans criteria. A GCB phenotype was observed in 68 (51%) cases. There was no difference in the frequency of GCB versus non-GCB between the MYC+ and MYC− cases (Table 1). Not surprisingly, the 3 cases with dual translocations had a GCB phenotype.
Impact on survival of MYC rearrangements in R-CHOP–treated patients
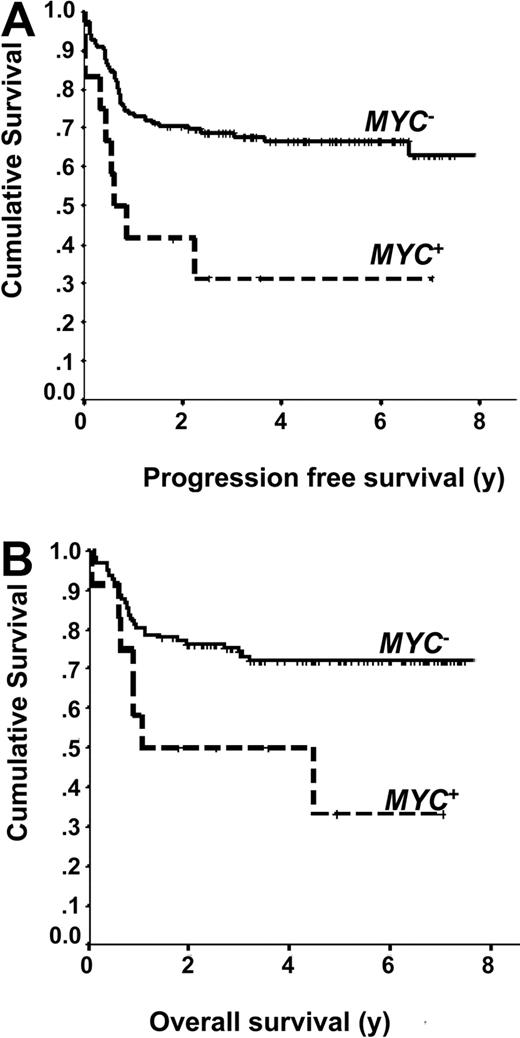
The 5-year PFS (66% vs 31%, P = .006) and OS (72% vs 33%, P = .016) were inferior in cases of DLBCL treated with R-CHOP that harbored an MYC rearrangement (Figure 1). Within the favorable GCB subgroup (n = 68), cases that harbored an MYC translocation had an inferior PFS (P = .049) and OS (P = .014). However, within the non-GCB subgroup (n = 63), there was an inferior PFS (P = .033) but not OS (P = .303). In univariate analysis, in addition to the presence of an MYC rearrangement, the IPI, a non-GCB phenotype, extranodal involvement and bone marrow involvement with DLBCL were all associated with an inferior PFS (Table 2). Similar results were observed for OS, although only a trend for an inferior outcome was observed with a non-GCB phenotype. BCL2 protein expression and a high proliferation rate, including variable cutoffs for Ki-67 (≥ 80%, ≥ 90%, or ≥ 95%) were not prognostic for PFS or OS (Table 2). Multivariate analysis using a Cox proportional hazard model confirmed that the presence of an MYC rearrangement remained a significant factor for both PFS (hazard ratio [HR] = 3.28; 95% confidence interval [CI], 1.49-7.21, P = .003) and OS (HR = 2.98; 95% confidence interval, 1.28-6.95, P = .011; Table 3). Of note, if the MYC+ cases with a dual translocation are removed and the analysis is repeated, MYC+ status retains prognostic significance for both PFS (P = .009) and OS (P = .036; results not shown).
Outcomes of patients with MYC+ DLBCL treated with R-CHOP. (A) Progression-free survival of MYC+ and MYC− DLBCL. (B) Overall survival of MYC+ and MYC− DLBCL.
Outcomes of patients with MYC+ DLBCL treated with R-CHOP. (A) Progression-free survival of MYC+ and MYC− DLBCL. (B) Overall survival of MYC+ and MYC− DLBCL.
Univariate analysis of risk factors for PFS and OS for DLBCL patients treated with R-CHOP
| Risk factor . | PFS P . | OS P . |
|---|---|---|
| MYC+ | .006 | .016 |
| IPI ≥ 3 | < .001 | < .001 |
| Non-GCB phenotype | .041 | .058 |
| BCL2 protein* | .313 | .492 |
| Ki-67 | ||
| More than 80% | .582 | .418 |
| More than 90% | .759 | .751 |
| More than 95% | .351 | .642 |
| Extranodal sites, any | .014 | .034 |
| Extranodal sites > 1 | .013 | .040 |
| Bone marrow DLBCL+ | < .001 | < .001 |
| Testicular | .124 | .263 |
| Bulky disease | .334 | .841 |
| Risk factor . | PFS P . | OS P . |
|---|---|---|
| MYC+ | .006 | .016 |
| IPI ≥ 3 | < .001 | < .001 |
| Non-GCB phenotype | .041 | .058 |
| BCL2 protein* | .313 | .492 |
| Ki-67 | ||
| More than 80% | .582 | .418 |
| More than 90% | .759 | .751 |
| More than 95% | .351 | .642 |
| Extranodal sites, any | .014 | .034 |
| Extranodal sites > 1 | .013 | .040 |
| Bone marrow DLBCL+ | < .001 | < .001 |
| Testicular | .124 | .263 |
| Bulky disease | .334 | .841 |
Results using the Dako antibody; not available in 1 MYC− case.
Multivariate analysis of risk factors of PFS and OS of DLBCL patients treated with R-CHOP
| Risk factor . | PFS . | OS . | ||
|---|---|---|---|---|
| Hazard ratio (CI) . | P . | Hazard ratio (CI) . | P . | |
| MYC+ | 3.28 (1.49-7.21) | .003 | 2.98 (1.28-6.95) | .011 |
| IPI ≥ 3 | 2.69 (1.48-4.86) | .001 | 3.29 (1.68-6.46) | .001 |
| Non-GCB phenotype* | 1.86 (1.04-3.34) | .038 | — | NS |
| Bone marrow DLBCL+ | 3.74 (1.67-8.36) | .001 | 4.06 (1.72-9.58) | .001 |
| Risk factor . | PFS . | OS . | ||
|---|---|---|---|---|
| Hazard ratio (CI) . | P . | Hazard ratio (CI) . | P . | |
| MYC+ | 3.28 (1.49-7.21) | .003 | 2.98 (1.28-6.95) | .011 |
| IPI ≥ 3 | 2.69 (1.48-4.86) | .001 | 3.29 (1.68-6.46) | .001 |
| Non-GCB phenotype* | 1.86 (1.04-3.34) | .038 | — | NS |
| Bone marrow DLBCL+ | 3.74 (1.67-8.36) | .001 | 4.06 (1.72-9.58) | .001 |
NS indicates not significant; and —, not applicable.
Not available in 4 MYC− cases.
Risk of CNS relapse in R-CHOP–treated patients with an MYC rearrangement
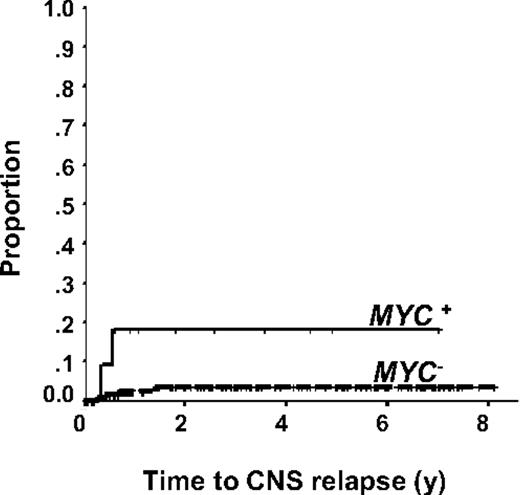
Given that CNS relapse is a known consequence in Burkitt lymphoma, we evaluated whether there was an increased risk of CNS relapse in cases of DLBCL that harbor an MYC rearrangement. Only one patient with testicular involvement received intrathecal prophylaxis at the time of primary therapy; otherwise, CNS prophylaxis was not used in the primary therapy. In total, there were 6 CNS relapses (2 of 12, 17% MYC+ vs 4 of 123, 3% MYC−). Neither of the cases of CNS relapse in the MYC+ group had testicular involvement. Using the time to CNS relapse as the endpoint, the presence of an MYC rearrangement (P = .018) was predictive of a CNS relapse in R-CHOP–treated patients (Figure 2). In multivariate analysis, the presence of an MYC rearrangement (HR = 8.0; 95% CI, 1.33-48.03, P = .023) and kidney involvement (HR = 25.28; 95% CI, 2.60-245.86, P = .005) remained significant in the Cox proportional hazards model after adjusting for the IPI, extranodal sites more than 1, or other high-risk extranodal sites (bone marrow, testicular involvement, and sinus involvement). Of interest, a high proliferation rate was not predictive of an increased risk of CNS relapse (results not shown).
Discussion
A limited number of studies have evaluated the prognostic importance of MYC status in DLBCL patients treated with CHOP-like regimens.4,18-20 In the large GEP effort by the Molecular Mechanisms in Malignant Lymphomas Network project, MYC+ aggressive lymphomas with a “nonmolecular Burkitt lymphoma” or “intermediate” gene expression signature profile had an inferior prognosis compared with those that were MYC−. However, not all of these cases were morphologically DLBCL, and most were treated with CHOP-like chemotherapy without rituximab.19 Interestingly, 11 cases of DLBCL in this study were determined to have a molecular signature of Burkitt lymphoma, but only 8 of these cases harbored an MYC translocation, suggesting that other genetic alterations can lead to MYC deregulation, such as copy number changes. The outcome of these patients is not reported; thus, it is unknown whether they may have benefitted with a more dose-intensive approach. The German High-Grade Non-Hodgkin Lymphoma Study Group recently evaluated 177 patients who were treated in the BH121 and BH222 clinical trials, which compared CHOP-like regimens in patients with aggressive B-cell lymphomas.4 Confining the analysis to DLBCL, cases were selected if they had tissue available for the construction of a TMA to assess for the presence of an MYC gene rearrangement. In this comprehensive analysis, the presence of an MYC rearrangement was associated with an inferior OS (P = .047), and there was a trend to a reduction in EFS (P = .062).4 It is unclear from this analysis what proportion of cases had a concurrent t(14;18) translocation. In a study by Niitsu et al, 11% of cases of DLBCL were found to harbor an MYC translocation; however, the cases evaluated were those that had abnormal karyotypes, which may have been selected based on high-risk features, which is also reflected by the high proportion of cases with a concurrent BCL2 translocation.20 The presence of an MYC translocation was associated with an inferior prognosis; however, this was largely driven by the presence of a concurrent t(14;18). In contrast, one study found that the presence of an MYC rearrangement did not predict for a worse outcome in DLBCL; however, the treatment received was not detailed, and it is possible that patients may have received more dose-intensive regimens.23
Importantly, all of the prior studies were performed before the routine use of rituximab-containing anthracycline-based regimens, which is the accepted standard of care in DLBCL based on established superiority in several randomized controlled trials.5,7,8,24 The present study is the first published to evaluate whether the presence of an MYC gene rearrangement is still of clinical relevance in patients treated in the R-CHOP era. In this “unselected” population of DLBCL, the frequency of MYC rearrangements was 8.8%, which is comparable with the German study (7.9%). Similar to the German study, we did not identify any high-risk clinical features at presentation in the MYC+ group; and contrary to prior studies, there was no difference in the frequency of extranodal disease in MYC+ patients.23 Although there was a tendency for the tumors in our study to have a high Ki-67 score, a range was seen, and this feature cannot be relied on to identify patients at a higher risk of harboring an MYC rearrangement. Furthermore, our results are in contrast to the German High-Grade Non-Hodgkin Lymphoma Study Group study in which no correlation between a high proliferation rate and the presence of an MYC rearrangement was found, which may reflect the high variability and poor reproducibility of this IHC marker.25 Nevertheless, a high proliferation rate was also not associated with outcome in this analysis. In contrast, the presence of an MYC rearrangement retained prognostic significance in R-CHOP–treated patients in multivariate analysis for both PFS and OS; thus, it is independent of clinical risk factors and COO phenotype.
CNS relapse is a known risk in patients with Burkitt lymphoma; and as a result, chemoprophylaxis is incorporated into treatment regimens for this disease. Although there was a small number of CNS relapse cases overall, we did find that there was an increased risk of CNS relapse in cases of DLBCL treated with R-CHOP that harbored an MYC rearrangement adjusting for other high-risk factors. Given the overall poor outcome of patients with secondary CNS disease, these results raise the question as to whether this population should be treated with Burkitt lymphoma–type regimens, which integrates intensive CNS prophylaxis. This approach has been explored in patients with aggressive B-cell lymphomas with a high Ki-67 fraction (≥ 95%) who received dose-modified CODOX (cyclophosphamide, doxorubicin, vincristine, methotrexate)–M/IVAC (ifosfamide, Ara-C, carboplatin). The authors concluded that the regimen did not improve outcome in patients with DLBCL26 ; however, the overall 2-year PFS for the DLBCL patients in this study was 55%, which appears to be more favorable than estimates with CHOP-like chemotherapy. Furthermore, this study did not provide information on the outcome of patients with MYC+DLBCL in isolation and was performed in the prerituximab treatment era. Thus, the efficacy of this regimen and other dose-intensive therapies, in combination with rituximab, in MYC+ DLBCL requires further study.
In conclusion, MYC gene rearrangements define a small group of patients with DLBCL who are less probable to be cured with R-CHOP and may have an increased risk of CNS relapse. There are no identifiable clinical, histologic, or immunophenotypic features signaling that a case of DLBCL may harbor an MYC rearrangement. Thus, all patients with DLBCL should undergo FISH or karyotype analysis for assessment of MYC rearrangement status in addition to analysis for the t(14;18) and BCL2 protein expression. MYC+ DLBCL may represent a distinct DLBCL subtype, and regimens more in line with those used in Burkitt lymphoma may be more appropriate and require further study in this patient population.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ryan Woods (Surveillance and Outcome Unit, British Columbia Cancer Agency) for statistical guidance as well as Jane Donaldson and Suman Singh for database management.
N.A.J. is a research fellow of the Terry Fox Foundation through an award from the National Cancer Institute of Canada (019005) and the Michael Smith Foundation for Health Research (ST-PDF-01 793). J.M.C., D.E.H., and R.D.G. are supported by a National Cancer Institute of Canada Terry Fox Program Project (award 019001).
Authorship
Contribution: K.J.S. designed and performed the research, contributed to the data collection, analyzed the data, and wrote the manuscript; N.A.J. contributed to the data collection and analyzed the data; R.D.G. performed the pathologic review, designed the research, contributed to the data collection, and analyzed the data; D.E.H. performed the FISH experiments, contributed to the data collection, and analyzed the data; S.B.-N. performed the FISH experiments; P.F. performed the pathologic review and analyzed the data; and L.H.S. and J.M.C. contributed to the data collection and analyzed the data.
Conflict-of-interest disclosure: K.J.S. has received honoraria from Roche. L.H.S. and R.D.G. have received honoraria and research funding from Roche and both serve as consultants for Roche. J.M.C. has received research funding from Roche. The remaining authors declare no competing financial interests.
Correspondence: Kerry J. Savage, British Columbia Cancer Agency, 600 West 10th Ave, Vancouver, BC, Canada V5Z 4E6; e-mail: ksavage@bccancer.bc.ca.