Abstract
Fine tuning of vascular endothelial growth factor (VEGF) signaling is critical in endothelial cell (EC) differentiation and vascular development. Nevertheless, the system for regulating the sensitivity of VEGF signaling has remained unclear. Previously, we established an embryonic stem cell culture reproducing early vascular development using Flk1 (VEGF receptor-2)+ cells as common progenitors, and demonstrated that cyclic adenosine monophosphate (cAMP) enhanced VEGF-induced EC differentiation. Here we show that protein kinase A (PKA) regulates sensitivity of Flk1+ vascular progenitors to VEGF signaling for efficient EC differentiation. Blockade of PKA perturbed EC differentiation and vascular formation in vitro and ex vivo. Overexpression of constitutive active form of PKA (CA-PKA) potently induced EC differentiation and vascular formation. Expression of Flk1 and Neuropilin-1 (NRP1), which form a selective and sensitive receptor for VEGF165, was increased only in CA-PKA–expressing progenitors, enhancing the sensitivity of the progenitors to VEGF165 by more than 10 times. PKA activation induced the formation of a VEGF165, Flk1, and NRP1 protein complex in vascular progenitors. These data indicate that PKA regulates differentiation potential of vascular progenitors to be endothelial competent via the dual induction of Flk1 and NRP1. This new-mode mechanism regulating “progenitor sensitivity” would provide a novel understanding in vascular development and regeneration.
Introduction
Vascular endothelial growth factor (VEGF) signaling is a key regulator of vascular development during embryogenesis as well as neovascularization in the adult.1-3 Intensity of VEGF signaling is strictly controlled during vascular development through ligand-receptor interaction.4,5 Flk1 (also designated as VEGF receptor-2) is tyrosine-phosphorylated much more efficiently than Flt1 (VEGF receptor-1) upon VEGF binding and is thought to be the major receptor in endothelial cells (ECs) for VEGF-induced responses.6-8 Whereas Flk1-null mice die at embryonic day 8.5 (E8.5) to E9.5 with no organized blood vessels,9 Flt1-null mice die at midgestation with vascular overgrowth and disorganization.10,11 Flt1 tyrosine kinase–deficient homozygous mice, in which VEGF can bind to the cell-surface domain of Flt1 but cannot conduct kinase signaling, developed normal vessels and survived,12 indicating that VEGF signal intensity on Flk1 is regulated by absorption of VEGF to the higher affinity receptor, Flt1. VEGF-A heterozygotes die early in gestation due to failure in vascular system formation.13 On the other hand, 2- to 3-fold overexpression of VEGF-A from its endogenous locus results in aberrant heart development and lethality at E12.5 to E14,14 indicating that strictly balanced VEGF function is important in normal embryogenesis.
Neuropilin-1 (NRP1) is a type 1 membrane protein, which is expressed in particular classes of developing neurons15,16 and functions as a receptor for the class 3 semaphorins mediating semaphorin-elicited inhibitory axon guidance signals to neurons.17,18 NRP1 is also expressed in ECs of blood vessels and endocardial cells of the heart.15,16,19 NRP1, together with Flk1, forms a specific receptor for VEGF165, an isoform of VEGF, and the Flk1-VEGF165-NRP1 complex potently enhances Flk1 signaling.20 Coexpression of NRP1 with Flk1 in cultured ECs enhanced VEGF165 binding to Flk1 and VEGF-elicited mitogenic and chemotactic activities.20 Overexpression of NRP1 in mouse embryos resulted in an excess production of blood vessels and malformed hearts.15 NRP1-null mice die midway through gestation at E10.5 to E12.5 and exhibit defects in the heart, vasculature, and nervous system.16 These findings indicate that NRP1 plays an important role in regulating vascular development, and Flk1/NRP1 system would be important for controlling VEGF signal intensity. However, the regulatory mechanisms of Flk1/NRP1 expression in vascular development are not fully elucidated.
In the early embryo and in differentiating embryonic stem (ES) cells, Flk1 expression marks a common progenitor for both blood and endothelium.21-24 To elucidate the mechanisms underlying vascular development, we have developed a novel ES cell differentiation system that exhibits early vascular development using Flk1+ cells as common progenitors for vascular cells.25 ES cell–derived Flk1+ cells can differentiate into both ECs and mural cells (MCs: vascular smooth muscle cells and pericytes) and form mature vascular-like structures in vitro. We recently reported that adrenomedullin/cyclic adenosine monophosphate (cAMP) pathway enhanced EC differentiation and induced arterial EC appearance from Flk1+ progenitors.26 In the present study, to further elucidate the mechanisms of EC differentiation from vascular progenitor cells, we examined roles of cAMP pathways in EC differentiation. Here we report that protein kinase A (PKA) activation remarkably enhanced EC differentiation and vascular formation from Flk1+ vascular progenitors. PKA markedly increased the sensitivity of vascular progenitors to VEGF through dual up-regulation of Flk1 and NRP1 and played a pivotal role in EC differentiation. This new-mode molecular system regulating “progenitor sensitivity” would offer novel insights for vascular development as well as molecular targets for vascular regeneration strategies.
Methods
Generation of ES cells carrying an inducible expression unit in ROSA locus
Murine ES cell line (EStTA5-4), expressing tetracycline-transactivator protein and containing the puromycin resistance gene,27 was a kind gift from Dr T. Era (Kumamoto University, Kumamoto, Japan). We generated an ES cell line (EStTA-ROSA) by inserting a knockin vector carrying loxP and mutant loxP, loxP511, recombination sites flanking neomycin-resistant and herpes simplex virus thymidine kinase (HSV-TK) genes (a kind gift from Dr K. Tanimoto [University of Tsukuba, Tsukuba, Japan] and Dr P. Soriano [Mt Sinai School of Medicine, New York, NY]) into ROSA locus28 of EStTA5-4 (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Neomycin (200 μg/mL)–resistant colonies were selected and homogenous insertion of the loxP sites into ROSA locus was confirmed by Southern blotting using DIG High Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics; supplemental Figure 1B-C). The probes were generated by polymerase chain reaction (PCR) amplification using the primer pair, 5′ probe: 5′-TTCAACAGGGATATCGCAAGG and 5′-AGCCTGGTAGCAGGAAGATC, and Neo probe: 5′-CTCGACGTTGTCACTGAA and 5′-AAGAACTCGTCAAGAAGGCG.
Generation of ES cells for CA-PKA expression
cDNA for constitutive active form (CA)–PKA (a kind gift from Dr G. S. McKnight, University of Washington, Seattle, WA)29 was introduced into the downstream region of tetracycline responsive element–regulatable cytomegalovirus promoter of Exchange vector (supplemental Figure 1A).
Stable ES cells that express the CA-PKA under the control of the tetracycline responsive element–regulatable cytomegalovirus promoter were generated by introduction of Exchange vectors and pBS185 (Cre expression vector) to EStTA-ROSA cells using mouse ES cells Nucleofector Kit (Amaxa Biosystems). Cells were then plated on 10-cm dishes containing 1 μg/mL doxycycline (Dox+). After 1 day, the medium was changed to Dox+ medium with 200 μg/mL hygromycin. After 10 days, the medium was changed to Dox+ medium with 200 μg/mL hygromycin and 1 μg/mL ganciclovir. Total hygromycin- and ganciclovir-resistant colonies were collected and subjected to further studies.
Antibodies
Monoclonal antibodies for murine Flk1 (AVAS12) and murine vascular endothelial (VE)–cadherin (VECD1, for fluorescence-activated cell sorting [FACS]) were described previously.24 Monoclonal antibodies for murine CD31 (1:500), VE-cadherin (for immunostaining, 1:200), and endothelial nitric oxide synthase (eNOS; 1:200) were purchased from BD Pharmingen. Monoclonal antibodies for murine α-smooth muscle actin (SMA; 1:1000) were from Sigma-Aldrich. Antibodies for SM22α (1:400) and calponin (1:500) were from Abcam. Polyclonal antibodies for murine Claudin-5 (1:100) were from Invitrogen. Polyclonal antibodies for murine VEGF and rat Neuropilin1 were from R&D Systems.
Cell culture
ES cell lines, D3, EStTA-ROSA, and CA-PKA–introduced EStTA-ROSA were maintained as described.26 Induction of differentiation of these ES cell lines was performed using differentiation medium (DM; alpha minimal essential medium [MEM; Gibco] supplemented with 10% fetal calf serum [Japan Bioserum Co Ltd] and 5 × 10−5 M 2-mercaptoethanol [Gibco]) as previously described.25,26 In brief, undifferentiated ES cells were cultured in the absence of leukemia inhibitory factor on collagen type IV–coated dishes (Becton Dickinson) at cell density 0.75 to 1 × 103 cells/cm2 for 96 to 108 hours. Cultured cells were harvested and subjected to magnetic cell sorting (MACS) purification. Purified Flk1+ cells were then plated onto type IV–coated dishes at cell density 0.75 to 1 × 104 cells/cm2 in DM. After 3 days of Flk1+ cell differentiation (Flk-d3), induced ECs were then examined by immunohistochemistry and flow cytometric analysis. Various reagents, human VEGF165, VEGF121 (R&D Systems), 8-bromoadenosine-3′:5′-cyclic monophosphate sodium salt (8bromo-cAMP; Nacalai Tesque), γ-secretase inhibitor, DAPT, PI3K inhibitor, LY294002, GSK3β inhibitor, Bio, Akt inhibitor, TAT-Akt-in, PKA inhibitor, PKI, H89, p38 inhibitor, SB202190, MEK inhibitor, PD98059, PKCαβ inhibitor, PKCη inhibitor, PKCζ inhibitor, H-Ras inhibitor, FTI-277 (Calbiochem), and phospholipase C (PLC) inhibitor, U73122 (Tocris Cookson Inc) were occasionally added to the Flk1+ cell culture. (DAPT, LY294002, Bio, TAT-Akt-in, SB202190, PD98059, PKCαβ inhibitor, PKCη inhibitor, and PKCζ inhibitor did not inhibit cAMP effect.) Human VEGF165 was used as the representative of VEGF isoforms unless stated otherwise. In serum-free culture, a defined medium, SFO3 (Sanko Junyaku; including insulin, transferrin, sodium selenite, and ethanolamine), was used instead of DM.25
Three-dimensional culture
Three-dimensional culture was performed as described previously.25 Briefly, Flk1+ cells (4 × 105 cells/mL) were incubated in DM with VEGF on uncoated Petri dishes for 16 hours to induce aggregation. Aggregates were resuspended in 2 × DM and mixed with an isovolume of collagen I-A gel (3 mg/mL; Nitta Gelatin). We plated 250 to 300 μL of this mixture onto a lucent insert disk, Cell disk (Sumitomo Bakelite), in 24-well dishes. After 30 minutes at 37°C to allow polymerization, we added 500 μL DM. To monitor vascular formation, collagen-embedded Flk1+ cell aggregates were cultured in a temperature- and gas-controlled chamber (37°C, 5% CO2), and phase-contrast images were acquired every 10 minutes with Metamorph software (Molecular Devices) for up to 5 days.
Cell sorting and flow cytometric analysis
After induction of Flk1+ cells, cultured cells were harvested and stained with allophycocyanin (APC)–conjugated anti-Flk1 antibody (AVAS12).24 Flk1+ cells were sorted by auto MACS (Miltenyi Biotec) using anti-APC MicroBeads (Miltenyi Biotec). At Flk-d3, cultured cells were harvested and stained with monoclonal antibodies for phycoerythrin-conjugated CD31 (Mec13.3; BD Pharmingen) together with APC-conjugated VECD1 or biotin-conjugated CXCR4 (BD Pharmingen) followed by streptavidin-conjugated APC (BD Pharmingen) or AVAS12, then subjected to analysis by FACSVantage or FACSAria (Becton Dickinson).
Immunocytochemistry
Immunostaining for cultured cells was carried out as described.25,26 Briefly, 4% paraformaldehyde-fixed cells were blocked by 1% skim milk (BD Biosciences) and incubated overnight with primary antibodies at 4°C. For immunohistochemistry, anti–rat immunoglobulin G (IgG) conjugated with alkaline phosphatase and anti–mouse IgG horseradish peroxidase (Invitrogen) were used as secondary antibodies. For immunofluorescent staining, anti–mouse, –rat, –rabbit, or –goat IgG antibodies conjugated with Alexa488 or Alexa546 (Invitrogen) were used for secondary antibodies. Nuclei were visualized with DAPI (4,6 diamidino-2-phenylindole; Invitrogen). Double staining for NRP1 and CD31 was performed using anti-NRP1 antibody (1:100; R&D Systems) as first antibody, followed by secondary antibody, Alexa Fluor 488–conjugated donkey anti–goat IgG (1:500; Molecular Probes). CD31+ cells were visualized using phycoerythrin-conjugated anti-CD31 antibody (1:300; BD Pharmingen). Stained cells were photographed with inverted fluorescent microscopy, Eclipse TE2000-U (Nikon) and digital camera system AxioCam HRc with the use of AxioVision Software (Carl Zeiss), or confocal fluorescent microscopy (TCS SP2; Leica).
Immunostaining for three-dimensional culture
Immunostaining for vascular structures in type I collagen gel was performed after the whole-mount immunostaining procedure as described.25 In brief, gels were fixed with 4% paraformaldehyde and blocked by 1% skim milk/0.1% Triton X/phosphate-buffered saline solution and incubated with anti-CD31 (BD Pharmingen) and anti–α smooth muscle actin (αSMA; Sigma-Aldrich), or anti-NRP1 (R&D Systems) and anti-Flk1 antibodies (1:200). Alexa Fluor 488–conjugated anti–mouse or anti–goat IgG and Alexa Fluor 567–conjugated anti–rat IgG (1:500; Zymed) were used as secondary antibodies. Alternatively, anti–rat IgG conjugated with alkaline phosphatase and anti–mouse IgG conjugated with horseradish peroxidase (Zymed) were used as secondary antibodies for enzymatic color development.
Cross-section of three-dimensional culture and immunostaining
Gel clots including vascular structure were fixed in 3.7% formaldehyde for 24 hours. Paraffin-embedded gel clots were sectioned at 3-μm thickness. The sections were mounted on glass slides coated with 2% 3-aminopropyl triethoxy silane (Tokyo Kasei). After deparaffinization and washing in distilled water, hematoxylin-eosin or immunohistochemical staining was performed.30 For double immunostaining for CD31 and SMA, CD31 was first stained with whole-mount in-gel staining using anti-CD31 antibody and anti–rat IgG antibody conjugated with horseradish peroxidase. Subsequently, gel clots were subjected to paraffin embedding. Anti-SMA antibody (1:100; DAKO) was applied to sectioned slides overnight at 4°C. They were incubated with biotinylated horse anti–mouse serum diluted to 1:300 followed by streptavidin-alkaline phosphatase.
RNA isolation, RT-PCR, and quantitative RT-PCR
Total RNA was isolated from cells in Dox treatment or Dox-free condition at Flk-d3 using RNeasy (QIAGEN), according to the manufacturer's instructions. Reverse-transcription was performed with the SuperScript III first-strand synthesis system (Invitrogen). Reverse-transcription (RT)–PCR was carried out as described26 using indicated primers (supplemental Table 1). Quantitative RT-PCR was performed using Power SYBR Green PCR Master Mix (Applied Biosystems) and StepOnePlus system (Applied Biosystems). The amount of target RNA was determined from the appropriate standard curve and normalized relative to the amount of Gapdh mRNA. Primer sequences are shown in supplemental Table 2.
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed according to the report by Pan et al.31 In brief, Flk1+ cells were incubated with vehicle, anti-VEGF (5 μg/mL), or anti-NRP1 antibodies (5 μg/mL; R&D Systems) in serum-free medium, SFO3 (Sanko Junyaku),25 for 30 minutes at 37°C. Cells were then cooled on ice for 15 minutes, and VEGF isoforms were added, followed by 30-minute incubation at 4°C. Cells were stimulated for 7 minutes at 37°C and then washed with ice-cold phosphate-buffered saline and lysed in lysis buffer. Cell lysates were subjected to immunoprecipitation using Protein G HP SpinTrap (GE Healthcare) and anti-Flk1 antibody, and immunoblotted with antibodies specific for NRP1 (R&D Systems). Samples were run on sodium dodecyl sulfate/polyacrylamide gel electrophoresis using gradient gel (Atto Co) followed by electrophoretic transfer onto nitrocellulose membranes. After the blots were incubated for 1 hour in blocking agents Blocking One (Nacalai Tesque), they were incubated overnight with the respective NRP1 antibodies (0.5 μg/mL; R&D Systems). Horseradish peroxidase–conjugated anti–goat antibody (Zymed Laboratories) was used as secondary antibody (1:1000). Can Get Signal Immunoreaction Enhancer solution kit (Toyobo) was used for signal enhancement. Immunoreactivity was detected with the enhanced chemiluminescence kit Chemi-Lumi One (Nacalai Tesque).
Ex vivo whole-embryo culture
Embryos were dissected out of the deciduum and placed in 500 μL dimethyl ether containing 50% rat IC serum (Charles River Laboratories), 5 mM nonessential amino acids, 50 mM sodium pyruvate, and 27.5 mM 2-mercaptoethanol, pre-equilibrated at 37°C with 5% CO2. Embryos were cultured at 37°C with 5% CO2 and analyzed. H89 (dissolved in dimethyl sulfoxide) was used at 30 μM. The concentration of dimethyl sulfoxide was set at 0.3% in all cultures.32 Whole-mount staining of embryos and yolk sacs was performed as described previously,33 and microscopy was performed using a microscope (MZ6; Leica) with 5× objectives (Leica 10411589). Images were imported using Adobe Photoshop software, and quantification of whole yolk sacs and CD31-stained areas was performed using ImageJ software (National Institutes of Health). Results of quantification were expressed as ratio of CD31+/whole yolk sac area, which provides an estimate of the proportion of the yolk sacs that were occupied by CD31-stained vascular structures. Animal experiments were done under the approval of the Animal Research Committee of Kyoto University in accordance with the guidelines for animal experiments in the Guide for the Care and Use of Laboratory Animals in Japan.
Statistical analysis
At least 3 independent experiments were performed. Statistical analysis of the data was performed with the Student t test or analysis of variance. P values less than .05 was considered significant. Values are reported as means plus or minus SD.
Results
cAMP/PKA pathway plays a critical role in vascular development
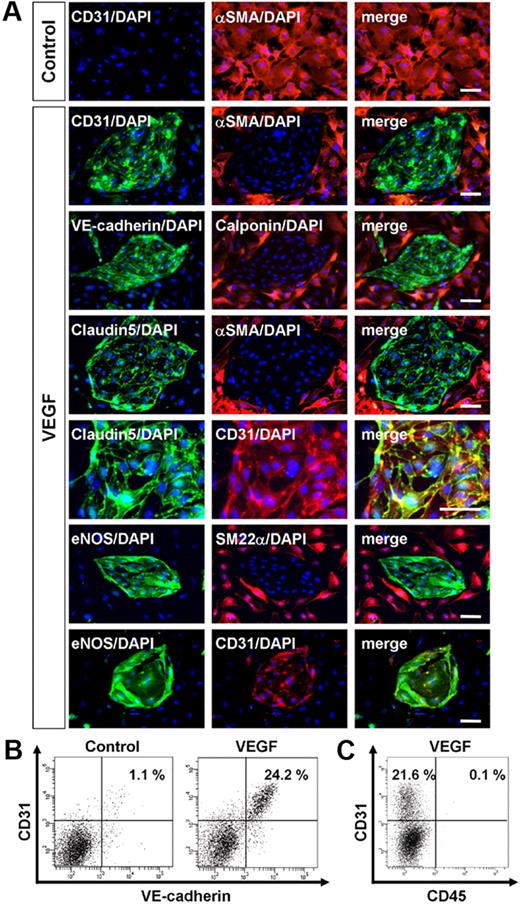
In our ES cell differentiation system, first we induced Flk1+ progenitor cells from undifferentiated ES cells. Flk1+ cells that appeared after 96 to 108 hours of differentiation of undifferentiated ES cells were negative for EC markers, such as CD31 and VE-cadherin.24-26 Then, purified Flk1+ progenitor cells were cultured for further differentiation to vascular cells. As previously reported, whereas no CD31+ ECs appeared when Flk1+ cells were cultured for 3 days with DM (“Methods”) alone, addition of VEGF to the Flk1+ cell culture induced selective appearance of CD31+ ECs and SMA+ MCs (Figure 1A).25,26 Almost all of CD31+ cells were also positive for other EC markers, VE-cadherin, claudin-5, and eNOS (Figure 1A-B).25,26 SMA+ MCs, which were reciprocally negative for EC markers (Figure 1B), expressed other smooth muscle cell markers, SM22α and calponin (Figure 1A). In this culture condition, only these 2 cell types (ie, ECs and MCs), and no blood cells such as CD45+ cells, were specifically induced from Flk1+ cells (Figure 1C).26
Vascular endothelial growth factor induces endothelial cells from vascular progenitors. (A-C) Cells after three-dimensional culture of Flk1+ cells (Flk-d3). (A-B) Exclusive induction of endothelial cells (ECs) and mural cells (MCs) from Flk1+ cells. (A) Expression of EC and MC markers. (Top panels) Double immunostaining of CD31 (green) and αSMA (red) cultured with differentiation medium (DM) alone (control). Note that no CD31+ cells appeared. (Other panels) Vascular endothelial growth factor (VEGF) treatment (50 ng/mL). EC sheets appeared. Double staining with pan-EC markers (CD31, VE-cadherin, Claudin5, or eNOS; green) and MC markers (αSMA, SM22α, or Calponin; red). (Bottom panels) Double staining with eNOS (green) and CD31 (red). ECs and MCs were exclusively induced. Nuclei are stained with DAPI (blue). Scale bar represents 50 μm. (B) Flow cytometry. x-axis: VE-cadherin; y-axis: CD31. Percentages of CD31+/VE-cadherin+ ECs in total Flk1+ cell–derived cells are indicated. (C) Flow cytometry. x-axis: CD45; y-axis: CD31. Percentages of CD31+/CD45− ECs and CD31+/CD45+ blood cells in total Flk1+ cell–derived cells are indicated. Note that almost no CD45+ cells were induced in this culture.
Vascular endothelial growth factor induces endothelial cells from vascular progenitors. (A-C) Cells after three-dimensional culture of Flk1+ cells (Flk-d3). (A-B) Exclusive induction of endothelial cells (ECs) and mural cells (MCs) from Flk1+ cells. (A) Expression of EC and MC markers. (Top panels) Double immunostaining of CD31 (green) and αSMA (red) cultured with differentiation medium (DM) alone (control). Note that no CD31+ cells appeared. (Other panels) Vascular endothelial growth factor (VEGF) treatment (50 ng/mL). EC sheets appeared. Double staining with pan-EC markers (CD31, VE-cadherin, Claudin5, or eNOS; green) and MC markers (αSMA, SM22α, or Calponin; red). (Bottom panels) Double staining with eNOS (green) and CD31 (red). ECs and MCs were exclusively induced. Nuclei are stained with DAPI (blue). Scale bar represents 50 μm. (B) Flow cytometry. x-axis: VE-cadherin; y-axis: CD31. Percentages of CD31+/VE-cadherin+ ECs in total Flk1+ cell–derived cells are indicated. (C) Flow cytometry. x-axis: CD45; y-axis: CD31. Percentages of CD31+/CD45− ECs and CD31+/CD45+ blood cells in total Flk1+ cell–derived cells are indicated. Note that almost no CD45+ cells were induced in this culture.
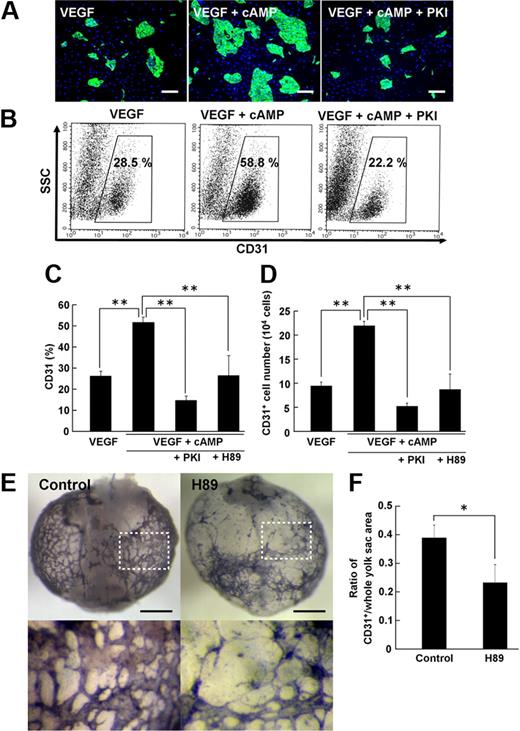
Stimulation of cAMP signaling by addition of 8bromo-cAMP, an analog of cAMP, together with VEGF substantially enhanced CD31+ EC induction from Flk1+ cells (Figure 2A-B). Similar to ECs induced by VEGF alone (Figure 1), CD31+ ECs that appeared by treatment of 8bromo-cAMP and VEGF were also positive for other EC markers, VE-cadherin, eNOS, and claudin5, but not CD45 (supplemental Figure 2). Compared with VEGF alone, VEGF with 8bromo-cAMP induced approximately 2-fold increase in EC population (CD31+ cells: 26.5% ± 2.3% in VEGF alone vs 52.3% ± 2.7% in VEGF with 8bromo-cAMP, n = 16; P < .001; Figure 2C). Total EC numbers induced from the same number of Flk1+ cells were similarly increased approximately 2.3 times by 8bromo-cAMP treatment (CD31+ cells: 9.4 ± 0.8 [× 104] cells in VEGF alone vs 21.8 ± 0.9 [× 104] cells in VEGF with 8bromo-cAMP; n = 4; P < .001; Figure 2D). PKA inhibitors, PKI and H89, but not many other kinase inhibitors (“Methods”), specifically inhibited the cAMP effects on EC induction (Figure 2A-D). These results indicated that the cAMP/PKA pathway specifically enhances the effect of VEGF on EC differentiation from Flk1+ progenitor cells.
Cyclic adenosine monophosphate/protein kinase A pathway plays a critical role in vascular development. (A-D) Enhancement of EC induction by cyclic adenosine monophosphate (cAMP) through protein kinase A (PKA) at Flk-d3. (A) Fluorescent staining for CD31 (green). (Left panel) VEGF treatment alone (50 ng/mL). (Middle panel) VEGF with 8bromo-cAMP (0.5 mM). (Right panel) VEGF with 8bromo-cAMP and PKA inhibitor, PKI (10 μM). Nuclei are stained with DAPI (blue). Scale bars represent 250 μm. (B) Flow cytometry. x-axis: CD31; y-axis: side scatter (SSC). Percentages of CD31+ ECs in total Flk1+ cell–derived cells are indicated. (C-D) Quantitative evaluation of the effect of PKA inhibitors on CD31+ EC induction from Flk1+ cells by FACS. (C) Percentages of CD31+ cell population in total Flk1+ cell–derived cells. VEGF (50 ng/mL; n = 16); VEGF and 8bromo-cAMP (0.5 mM; n = 16); VEGF, cAMP, and PKI (10 μM; n = 7); and VEGF, cAMP, and H89 (10 μM; n = 6) treatment are shown (**P < .01 vs VEGF and 8bromo-cAMP). (D) CD31+ cell number that appeared from 1.5 × 105 Flk1+ cells. VEGF (50 ng/mL; n = 4); VEGF and 8bromo-cAMP (0.5 mM; n = 4); VEGF, cAMP, and PKI (10 μM; n = 4); and VEGF, cAMP, and H89 (10 μM; n = 4) treatment are shown (**P < .01 vs VEGF and 8bromo-cAMP). (E-F) Role of PKA in vascular formation in the embryo. (E) Representative results of ex vivo culture of mouse embryo. Isolated E6.75 concepti were cultured in the absence (control, left panels) or presence (right panels) of H89 (30 μM) for 3 days. Vasculature in yolk sacs of concepti was immunostained for CD31 (purple). Bottom panels correspond to boxed regions in control and H89, respectively. Apparent reduction of CD31+ vascular formation was induced by H89 treatment. Scale bar represents 1 mm. (F) Quantitative evaluation of CD31+ vasculature formation in yolk sacs of concepti. The ratio of CD31+/whole yolk sac area was evaluated (n = 3; *P < .05 vs control).
Cyclic adenosine monophosphate/protein kinase A pathway plays a critical role in vascular development. (A-D) Enhancement of EC induction by cyclic adenosine monophosphate (cAMP) through protein kinase A (PKA) at Flk-d3. (A) Fluorescent staining for CD31 (green). (Left panel) VEGF treatment alone (50 ng/mL). (Middle panel) VEGF with 8bromo-cAMP (0.5 mM). (Right panel) VEGF with 8bromo-cAMP and PKA inhibitor, PKI (10 μM). Nuclei are stained with DAPI (blue). Scale bars represent 250 μm. (B) Flow cytometry. x-axis: CD31; y-axis: side scatter (SSC). Percentages of CD31+ ECs in total Flk1+ cell–derived cells are indicated. (C-D) Quantitative evaluation of the effect of PKA inhibitors on CD31+ EC induction from Flk1+ cells by FACS. (C) Percentages of CD31+ cell population in total Flk1+ cell–derived cells. VEGF (50 ng/mL; n = 16); VEGF and 8bromo-cAMP (0.5 mM; n = 16); VEGF, cAMP, and PKI (10 μM; n = 7); and VEGF, cAMP, and H89 (10 μM; n = 6) treatment are shown (**P < .01 vs VEGF and 8bromo-cAMP). (D) CD31+ cell number that appeared from 1.5 × 105 Flk1+ cells. VEGF (50 ng/mL; n = 4); VEGF and 8bromo-cAMP (0.5 mM; n = 4); VEGF, cAMP, and PKI (10 μM; n = 4); and VEGF, cAMP, and H89 (10 μM; n = 4) treatment are shown (**P < .01 vs VEGF and 8bromo-cAMP). (E-F) Role of PKA in vascular formation in the embryo. (E) Representative results of ex vivo culture of mouse embryo. Isolated E6.75 concepti were cultured in the absence (control, left panels) or presence (right panels) of H89 (30 μM) for 3 days. Vasculature in yolk sacs of concepti was immunostained for CD31 (purple). Bottom panels correspond to boxed regions in control and H89, respectively. Apparent reduction of CD31+ vascular formation was induced by H89 treatment. Scale bar represents 1 mm. (F) Quantitative evaluation of CD31+ vasculature formation in yolk sacs of concepti. The ratio of CD31+/whole yolk sac area was evaluated (n = 3; *P < .05 vs control).
We further examined the role of PKA in vascular development with ex vivo whole-embryo culture assay. Embryonic day 6.75 concepti were picked out from the uteri of pregnant mice and cultured for 3 days, during which CD31+ blood vessels were formed in the yolk sac. Using this system, we could examine early phase of EC differentiation ex vivo. In the presence of H89 during ex vivo culture, formation of blood vessels, which were evaluated by CD31 staining, in yolk sac was markedly disturbed, showing malformation of vascular networks with decrease in the caliber size and CD31+ areas (Figure 2E). Indeed, CD31+ area within whole yolk sac area was significantly decreased in H89-treated embryo to approximately 59% of that in control (n = 3; P = .025; Figure 2F). These results indicate that PKA also plays an important role in early vascular development in vivo.
CA-PKA enhanced EC differentiation and vascular formation from Flk1+ vascular progenitors
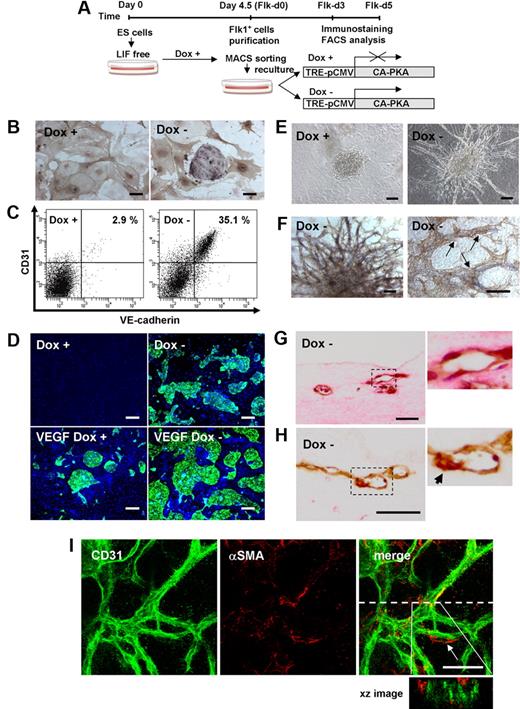
To dissect PKA function in EC differentiation, we generated ES cells expressing CA-PKA by tetracycline-regulatable system (Tet-Off; supplemental Figure 1). Although negative effects of high-dose Dox (∼ 25 μg/mL) on EC differentiation, proliferation, and survival were reported,34 lower concentration of Dox (1 μg/mL) did not affect EC appearance in control ES cells (EStTA-ROSA; supplemental Figure 3). We induced differentiation of ES cells in the presence of Dox for 4.5 days, and purified Flk1+ cells were recultured on type IV collagen–coated dishes with DM alone in the presence or absence of Dox (Figure 3A). In the presence of Dox (Dox+), only SMA+ mural cells, but not ECs were induced (Figure 3B-C), compatible with our previous results25 (Figure 1A-B). Surprisingly, considerable amounts of CD31+ ECs were generated even in the absence of VEGF when CA-PKA expression was induced by the depletion of Dox (Dox−; Figure 3B; supplemental Videos 1-2). Almost all of CA-PKA–induced CD31+ cells on 2-dimensional culture condition were also positive for VE-cadherin, eNOS, and claudin5 (Figure 2C, supplemental Figure 4). CD31−/VE-cadherin− cells observed in CA-PKA–activated condition were positive for SMA, SM22α, and calponin (supplemental Figure 4). When we tested the effects of CA-PKA with VEGF, EC appearance with VEGF in Dox+ condition was further enhanced by expression of CA-PKA (Figure 3D). These results indicate that PKA should enhance EC differentiation from vascular progenitors.
CA-PKA enhances EC differentiation from Flk1+ vascular progenitors. (A) Experimental system for PKA activation. An embryonic stem (ES) cell line expressing constitutive active (CA) form of PKA by tetracycline-inducible expression system (Tet-Off) was established. Doxycycline (Dox) was added during the first 4.5-day culture of ES cell differentiation to Flk1+ cells. Flk1+ cells were sorted by magnetic cell sorting (MACS) and subjected to two-dimensional culture on collagen-coated dishes or three-dimensional culture in collagen gel, and were cultured in the presence or absence of Dox (1 μg/mL). (B-D) Two-dimensional culture with DM, at Flk-d3. (B) Double immunostaining for CD31 (purple) and αSMA (brown). (Left panel) Dox (1 μg/mL) treatment. (Right panel) Dox-free. Culture with DM alone. Scale bar represents 100 μm. (C) Flow cytometry for EC markers, CD31 and VE-cadherin. Percentages of CD31+/VE-cadherin+ ECs in total Flk1+ cell–derived cells are indicated. (D) Fluorescent staining for CD31 (green) and DAPI (blue). (Left panels) Dox (1 μg/mL) treatment. (Right panels) Dox-free. Flk1+ cells stimulated with vehicle (top panels) or VEGF (50 ng/mL; bottom panels). Scale bars represent 250 μm. (E-I) Three-dimensional culture of Flk1+ cell aggregates in type I collagen gel with DM alone. (E) Phase-contrast images after 5-day culture. (Left panel) Dox (1 μg/mL) treatment. (Right panel) Dox-free. Scale bars represent 100 μm. (F) In-gel double immunostaining for CD31 (purple) and αSMA (brown) in Dox-free condition. (Left panel) Gross appearance of vascular structure. (Right panel) Higher magnification view. αSMA+ cells attached to CD31+ EC tube structure are observed (arrows). Scale bars represent 100 μm. (G-H) Cross-section of three-dimensional culture in Dox-free condition. (G) Hematoxylin-eosin staining. (H) Double immunostaining for CD31 (brown) and αSMA (red). Right panels correspond to boxed regions. Scale bars represent 250 μm. αSMA+ cell attached to CD31+ EC tube structure is observed (arrow). (I) Confocal microscopic analysis of vascular structure. Double fluorescent staining for CD31 and αSMA in Dox-free condition. (Left panel) CD31 (green). (Middle panel) αSMA (red). (Right panel) Merged image. αSMA+ cell attached to CD31+ EC tube structure is observed (arrow). CD31+ cells formed true lumen (green) with attached mural cells (red) shown in xz image. Dashed line indicates sliced position. Scale bars represent 100 μm.
CA-PKA enhances EC differentiation from Flk1+ vascular progenitors. (A) Experimental system for PKA activation. An embryonic stem (ES) cell line expressing constitutive active (CA) form of PKA by tetracycline-inducible expression system (Tet-Off) was established. Doxycycline (Dox) was added during the first 4.5-day culture of ES cell differentiation to Flk1+ cells. Flk1+ cells were sorted by magnetic cell sorting (MACS) and subjected to two-dimensional culture on collagen-coated dishes or three-dimensional culture in collagen gel, and were cultured in the presence or absence of Dox (1 μg/mL). (B-D) Two-dimensional culture with DM, at Flk-d3. (B) Double immunostaining for CD31 (purple) and αSMA (brown). (Left panel) Dox (1 μg/mL) treatment. (Right panel) Dox-free. Culture with DM alone. Scale bar represents 100 μm. (C) Flow cytometry for EC markers, CD31 and VE-cadherin. Percentages of CD31+/VE-cadherin+ ECs in total Flk1+ cell–derived cells are indicated. (D) Fluorescent staining for CD31 (green) and DAPI (blue). (Left panels) Dox (1 μg/mL) treatment. (Right panels) Dox-free. Flk1+ cells stimulated with vehicle (top panels) or VEGF (50 ng/mL; bottom panels). Scale bars represent 250 μm. (E-I) Three-dimensional culture of Flk1+ cell aggregates in type I collagen gel with DM alone. (E) Phase-contrast images after 5-day culture. (Left panel) Dox (1 μg/mL) treatment. (Right panel) Dox-free. Scale bars represent 100 μm. (F) In-gel double immunostaining for CD31 (purple) and αSMA (brown) in Dox-free condition. (Left panel) Gross appearance of vascular structure. (Right panel) Higher magnification view. αSMA+ cells attached to CD31+ EC tube structure are observed (arrows). Scale bars represent 100 μm. (G-H) Cross-section of three-dimensional culture in Dox-free condition. (G) Hematoxylin-eosin staining. (H) Double immunostaining for CD31 (brown) and αSMA (red). Right panels correspond to boxed regions. Scale bars represent 250 μm. αSMA+ cell attached to CD31+ EC tube structure is observed (arrow). (I) Confocal microscopic analysis of vascular structure. Double fluorescent staining for CD31 and αSMA in Dox-free condition. (Left panel) CD31 (green). (Middle panel) αSMA (red). (Right panel) Merged image. αSMA+ cell attached to CD31+ EC tube structure is observed (arrow). CD31+ cells formed true lumen (green) with attached mural cells (red) shown in xz image. Dashed line indicates sliced position. Scale bars represent 100 μm.
We further examined vascular formation from Flk1+ cells in three-dimensional culture25 to investigate PKA function in vascular development. When aggregates of Flk1+ cells were cultured in type I collagen gel with DM alone, no sprouting of vessels was observed in Dox+ condition. In contrast, CA-PKA expression (Dox−) induced vascular-like structure formation even in the absence of VEGF (Figure 3E; supplemental Video 3). In-gel immunostaining showed the vascular-like structure consisted of CD31+ ECs with surrounding SMA+ mural cells (Figure 3F). Cross-sections revealed true lumens with CD31+ ECs and attached SMA+ MCs (Figure 3G-H). Confocal microscopic study further showed vascular-like structure formation with EC tube and mural cell attachment (Figure 3I). In addition, PKA activation induced CD45+ blood cells within the vascular lumen (supplemental Figure 5A). Occasionally, beating cardiomyocytes, which were positive for cardiac troponin T, were observed along with vascular structures (supplemental Figure 5B; supplemental Video 4). PKA, thus, should play an important role in vascular development through enhancement of EC differentiation.
Dual up-regulation of Flk1 and NRP1 was induced by PKA
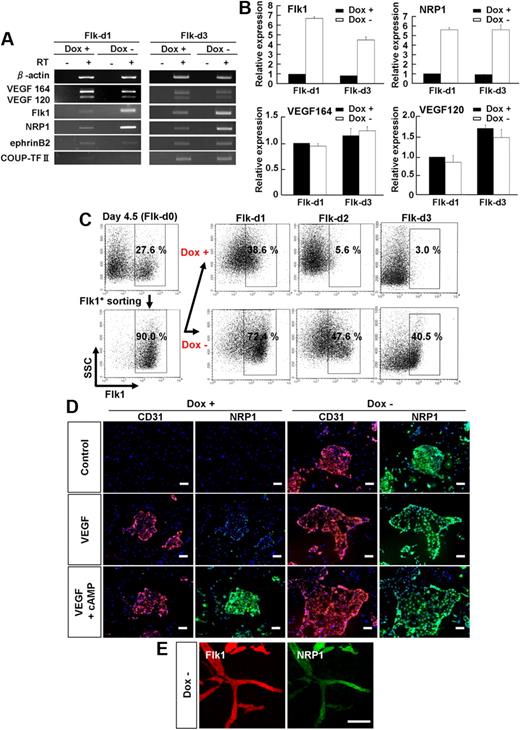
Next, we investigated the molecular mechanism of the PKA effects in EC differentiation and vascular development. First we examined PKA activity in vascular progenitor cells. Whereas PKA activity was significantly increased by addition of 8bromo-cAMP, VEGF treatment did not induce PKA activation (supplemental Figure 6), indicating that PKA pathway did not work downstream of VEGF signaling in vascular progenitor cells. In addition, activation of PKA by induction of CA-PKA in Flk1+ cells did not increase VEGF mRNA expression (Figure 4A-B). These results indicate that PKA signaling did not enhance VEGF signaling through the formation of a positive feedback loop between VEGF and PKA. In contrast, overexpression of CA-PKA up-regulated Flk1 and NRP1 mRNA expression from the early stage of Flk1+ cell culture (ie, at days 1 and 3 after Flk1 sorting; Figure 4A). Arterial EC marker, ephrinB2,35,36 and venous EC marker, COUP-TF II,37 were not affected by CA-PKA expression (Figure 4A). Quantitative RT-PCR analysis revealed that PKA activation induced approximately 5- to 7-fold increase in Flk1 and NRP1 mRNA expression in total cells at Flk-d1 and Flk-d3 (Figure 4B). Similar significant up-regulation of Flk1 and NRP1 mRNA expression was observed in 8bromo-cAMP–treated Flk1+ cells (supplemental Figure 7A). We further confirmed the time course of Flk1 protein expression in the early stage of Flk1+ cell culture by FACS. When purified Flk1+ progenitor cells were cultured in the absence of VEGF, Flk1 expression was rapidly decreased and lost within 3 days, compatible with our previous results25 (Figure 4C top panels). On the other hand, when CA-PKA was induced in purified Flk1+ cells, Flk1 expression was essentially maintained, even in the absence of VEGF. At Flk-d1, almost all Flk1+ cells were still negative for EC markers, CD31 and VE-cadherin (supplemental Figure 8), indicating that PKA should work at the progenitor stage to enhance EC appearance from Flk1+ progenitor cells. As for NRP1 protein expression, whereas clear up-regulation of NRP1 expression was observed only in VEGF with 8bromo-cAMP treatment in Dox+ condition, CA-PKA activation (Dox−) induced NRP1 expression even in the absence of VEGF at Flk-d3 (Figure 4D). Furthermore, Flk1 and NRP1 were also coexpressed in vascular structure in vitro induced with CA-PKA expression (Figure 4E). These results suggest that PKA pathway should enhance EC differentiation and vascular formation through dual induction of Flk1 and NRP1.
Dual up-regulation of Flk1 and NRP1 by PKA activation. (A-D) Two-dimensional culture of Flk1+ cells. (A) RT-PCR showing mRNA expression of VEGF164, VEGF120, Flk1, NRP1, ephrinB2 (arterial marker), and COUP-TF II (venous marker) after 1 (Flk-d1) and 3 (Flk-d3) days of culture of Flk1+ cells with DM alone in the presence or absence of Dox (1 μg/mL). (B) Quantitative RT-PCR showing mRNA expression of Flk1, NRP1, VEGF164, and VEGF120 at Flk-d1 and Flk-d3 in the presence or absence of Dox. mRNA expression at Flk-d1 with Dox was set as 1.0. (C) Time course of Flk1+ cell appearance evaluated by FACS. Flk1+ cell appearance was examined on differentiation day 4.5 before and after sorting, and at Flk-d1, Flk-d2, and Flk-d3 cultured with DM alone in the presence or absence of Dox (1 μg/mL). (Top panels) Dox treatment. (Bottom panels) Dox-free. Percentages of Flk1+ cells are indicated. (D) Double fluorescent staining for CD31 and NRP1 at Flk-d3. (Left 6 panels) Dox treatment. (Right 6 panels) Dox-free. Flk1+ cells stimulated with vehicle (top panels), VEGF (50 ng/mL; middle panels), or VEGF and 8bromo-cAMP (0.5 mM; bottom panels). Scale bars represent 100 μm. (E) Vascular formation from Flk1+ cell aggregates in three-dimensional culture in Dox-free condition at Flk-d5. In-gel double fluorescent staining for Flk1 and NRP1. (Left panel) Flk1 (red). (Right panel) NRP1 (green). Scale bars represent 100 μm.
Dual up-regulation of Flk1 and NRP1 by PKA activation. (A-D) Two-dimensional culture of Flk1+ cells. (A) RT-PCR showing mRNA expression of VEGF164, VEGF120, Flk1, NRP1, ephrinB2 (arterial marker), and COUP-TF II (venous marker) after 1 (Flk-d1) and 3 (Flk-d3) days of culture of Flk1+ cells with DM alone in the presence or absence of Dox (1 μg/mL). (B) Quantitative RT-PCR showing mRNA expression of Flk1, NRP1, VEGF164, and VEGF120 at Flk-d1 and Flk-d3 in the presence or absence of Dox. mRNA expression at Flk-d1 with Dox was set as 1.0. (C) Time course of Flk1+ cell appearance evaluated by FACS. Flk1+ cell appearance was examined on differentiation day 4.5 before and after sorting, and at Flk-d1, Flk-d2, and Flk-d3 cultured with DM alone in the presence or absence of Dox (1 μg/mL). (Top panels) Dox treatment. (Bottom panels) Dox-free. Percentages of Flk1+ cells are indicated. (D) Double fluorescent staining for CD31 and NRP1 at Flk-d3. (Left 6 panels) Dox treatment. (Right 6 panels) Dox-free. Flk1+ cells stimulated with vehicle (top panels), VEGF (50 ng/mL; middle panels), or VEGF and 8bromo-cAMP (0.5 mM; bottom panels). Scale bars represent 100 μm. (E) Vascular formation from Flk1+ cell aggregates in three-dimensional culture in Dox-free condition at Flk-d5. In-gel double fluorescent staining for Flk1 and NRP1. (Left panel) Flk1 (red). (Right panel) NRP1 (green). Scale bars represent 100 μm.
Sensitivity of VEGF signaling is markedly enhanced by PKA
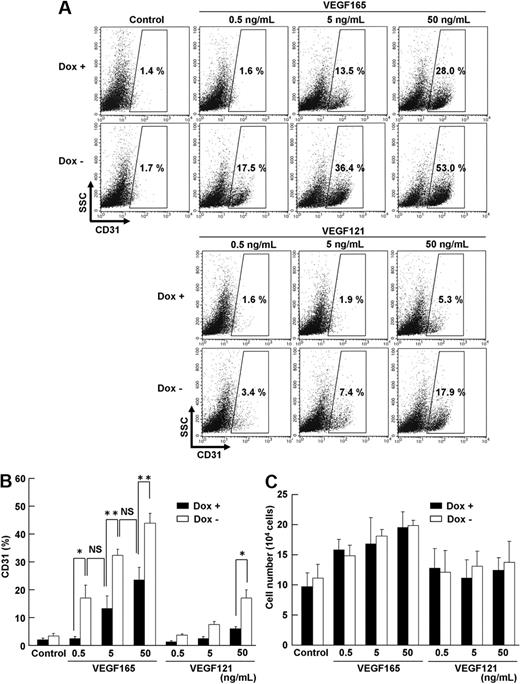
To precisely define the biologic function of up-regulated Flk1 and NRP1 by PKA activation, we tested dose-response effects of VEGF165 and VEGF121 on Flk1+ cells using serum-free culture with a defined medium, SFO3 (including insulin, transferrin, sodium selenite, and ethanolamine).25 In the serum-free condition, CD31+ ECs were not induced from Flk1+ progenitors in the absence of VEGF165. In the control condition (Dox+), 5 to 50 ng/mL VEGF165 induced CD31+ ECs. Surprisingly, although almost no ECs were induced in the absence of VEGF165 even with CA-PKA activation (Dox−), CA-PKA expression induced distinct EC appearance in much lower concentration of VEGF165 (ie, 0.5-5 ng/mL; Figure 5A-B). Similar or higher amounts of ECs were induced by 10 times lower concentration of VEGF165 in Dox− condition compared with those in Dox+ condition (CD31+ cells: 18.1% ± 5.1% [Dox−, 0.5 ng/mL VEGF165] vs 14.2% ± 4.8% [Dox+, 5 ng/mL VEGF165]; 34.6% ± 2.4% [Dox−, 5 ng/mL VEGF165] vs 25.2% ± 4.8% [Dox+, 50 ng/mL VEGF165]; Figure 5B). There was no difference observed in the total cell number that appeared from Flk1+ cells between Dox+ and Dox− treatment (Figure 5C), suggesting that PKA activation should enhance EC differentiation but not proliferation. Furthermore, the potent enhancement of EC differentiation was observed specifically by VEGF165 treatment, and not by VEGF121, which does not bind to NRP1 (Figure 5A).20 Significant increase in EC appearance with 50 ng/mL VEGF121 (Figure 5B) should be induced by binding of VEGF121 to up-regulated Flk1. Similarly, addition of 8bromo-cAMP in Dox+ condition also enhanced response of Flk1+ progenitor differentiation to VEGF (supplemental Figure 9). These results indicate that dual activation of Flk1 and NRP1 by PKA activation markedly enhanced sensitivity of Flk1+ progenitors to VEGF165.
Sensitivity of VEGF signaling is enhanced by PKA. Serum-free culture of Flk1+ cells on two-dimensional condition, at Flk-d3. (A-B) Flow cytometry for CD31 expression in the presence (Dox+; 1 μg/mL) or absence (Dox−) of Dox. x-axis: CD31; y-axis: SSC. Flk1+ cells were incubated with various concentrations of VEGF165 or VEGF121 in serum-free medium, SFO3. Percentages of CD31+ ECs in total Flk1+ cell–derived cells are indicated. (B) Quantitative evaluation of effects of PKA activation on EC differentiation. Percentages of CD31+ EC population in total Flk1+ cell–derived cells are evaluated (n = 3; *P < .05, **P < .01 vs corresponding values; NS indicates not significant). (C) Quantitative evaluation of the number of induced ECs. Total cell number that appeared from 12.5 × 104 of plated Flk1+ cells at Flk-d3 is shown.
Sensitivity of VEGF signaling is enhanced by PKA. Serum-free culture of Flk1+ cells on two-dimensional condition, at Flk-d3. (A-B) Flow cytometry for CD31 expression in the presence (Dox+; 1 μg/mL) or absence (Dox−) of Dox. x-axis: CD31; y-axis: SSC. Flk1+ cells were incubated with various concentrations of VEGF165 or VEGF121 in serum-free medium, SFO3. Percentages of CD31+ ECs in total Flk1+ cell–derived cells are indicated. (B) Quantitative evaluation of effects of PKA activation on EC differentiation. Percentages of CD31+ EC population in total Flk1+ cell–derived cells are evaluated (n = 3; *P < .05, **P < .01 vs corresponding values; NS indicates not significant). (C) Quantitative evaluation of the number of induced ECs. Total cell number that appeared from 12.5 × 104 of plated Flk1+ cells at Flk-d3 is shown.
PKA activation induces Flk1-VEGF-NRP1 complex formation
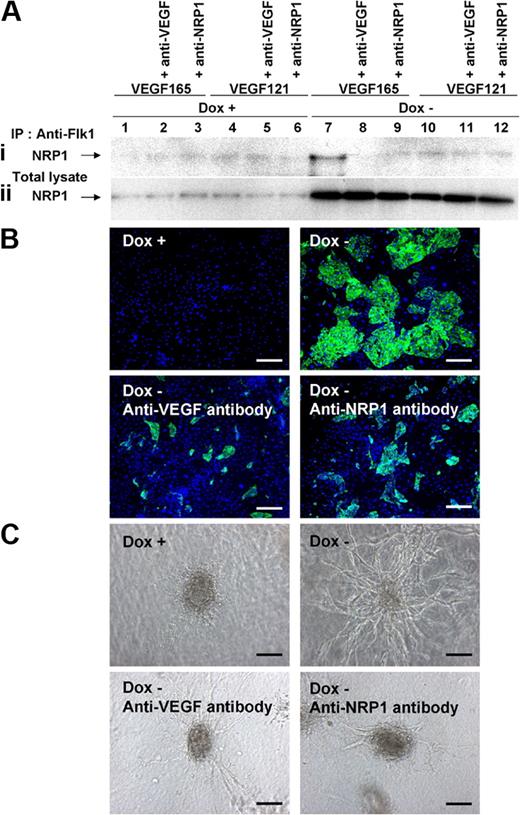
Finally, we confirmed the formation and function of Flk1-VEGF165-NRP1 complex by PKA activation. One day after Flk1+ cell culture in serum-free conditions (Flk-d1), cells were collected and protein interaction of Flk1, NRP1, and VEGF was examined by immunoprecipitation assay.31 Western blot analysis for NRP1 using total cell lysates clearly revealed increase in NRP1 protein by CA-PKA expression (Dox−) at Flk-d1 (Figure 6Aii). Total NRP1 expression was increased approximately 4.3-fold by PKA activation (n = 6; P < .001). In various conditions that we tested, only when added together with CA-PKA expression (Dox−), VEGF165 formed a distinct protein complex with Flk1 and NRP1 (Figure 6Ai lane 7). The protein complex was not formed in the control conditions (Dox+) or Dox− conditions with the addition of VEGF121. Similarly, 8bromo-cAMP treatment also induced formation of a protein complex with Flk1, NRP1, and VEGF165 (supplemental Figure 7B). These results clearly indicated that PKA activation induced both Flk1 and NRP1 expression in vascular progenitors, and VEGF165 in turn specifically induced the protein complex formation of Flk1-VEGF165-NRP1. The formation of Flk1-VEGF165-NRP1 complex was completely blocked by the addition of anti-VEGF or anti-NRP1 neutralizing antibodies (Figure 6Ai lanes 8-9). Parallel to the Flk1-VEGF165-NRP1 complex formation, the CA-PKA–induced EC differentiation as well as vascular formation in three-dimensional culture were drastically inhibited by the addition of anti-VEGF or NRP1 neutralizing antibodies, suggesting a functional significance of the Flk1-VEGF165-NRP1 complex (Figure 6B-C). These results indicate that PKA regulates sensitivity of vascular progenitors to VEGF by dual induction of Flk1 and NRP1, which forms the Flk1-VEGF165-NRP1 complex enhancing VEGF signaling to efficiently induce EC differentiation and vascular formation.
PKA enhanced to form Flk1-VEGF-NRP1 complexes. (A) Immunoprecipitation assay. Formation of Flk1-VEGF-NRP1 complex was examined at Flk-d1 cultured with serum-free medium, SFO3. Immunoblot with anti-NRP1 antibody for cell lysates immunoprecipitated with anti-Flk1 antibody (Ai) and total cell lysates (Aii). (Ai) Note that a distinct band was observed only when VEGF165 was added to PKA-activated (Dox−) condition (lane 7), which was inhibited by addition of anti-VEGF or anti-NRP1 antibodies. (Aii) Total NRP1 expression was markedly increased in PKA-activated condition. (B) Two-dimensional culture of Flk1+ cells with DM, at Flk-d3. Fluorescent staining for CD31 (green). Nuclei are stained with DAPI (blue). (Top left panel) Dox treatment. (Other panels) Dox-free. (Bottom left panel) Dox-free with neutralizing antibody for VEGF. (Bottom right panel) Dox-free with neutralizing antibody for NRP1. Scale bars represent 250 μm. (C) Vascular formation from Flk1+ cell aggregates in three-dimensional culture. (Top left panel) Dox treatment. (Other panels) Dox-free. (Bottom left panel) Dox-free with neutralizing antibody for VEGF. (Bottom right panel) Dox-free with neutralizing antibody for NRP1. Scale bars represent 100 μm.
PKA enhanced to form Flk1-VEGF-NRP1 complexes. (A) Immunoprecipitation assay. Formation of Flk1-VEGF-NRP1 complex was examined at Flk-d1 cultured with serum-free medium, SFO3. Immunoblot with anti-NRP1 antibody for cell lysates immunoprecipitated with anti-Flk1 antibody (Ai) and total cell lysates (Aii). (Ai) Note that a distinct band was observed only when VEGF165 was added to PKA-activated (Dox−) condition (lane 7), which was inhibited by addition of anti-VEGF or anti-NRP1 antibodies. (Aii) Total NRP1 expression was markedly increased in PKA-activated condition. (B) Two-dimensional culture of Flk1+ cells with DM, at Flk-d3. Fluorescent staining for CD31 (green). Nuclei are stained with DAPI (blue). (Top left panel) Dox treatment. (Other panels) Dox-free. (Bottom left panel) Dox-free with neutralizing antibody for VEGF. (Bottom right panel) Dox-free with neutralizing antibody for NRP1. Scale bars represent 250 μm. (C) Vascular formation from Flk1+ cell aggregates in three-dimensional culture. (Top left panel) Dox treatment. (Other panels) Dox-free. (Bottom left panel) Dox-free with neutralizing antibody for VEGF. (Bottom right panel) Dox-free with neutralizing antibody for NRP1. Scale bars represent 100 μm.
Discussion
Here, we showed a novel regulatory mechanism of EC differentiation and vascular formation through the modulation of progenitor properties to be endothelial competent. PKA activation increased both Flk1 and NRP1 expression in vascular progenitors and markedly enhanced the “sensitivity” of the progenitors to VEGF165 by inducing Flk1-VEGF165-NRP1 complex formation. This new-mode regulatory system would provide insights in vascular development and offer options for various therapeutic strategies with vascular manipulation.
Vascular formation is regulated by appropriate intensity, space, and timing of VEGF signaling. This study showed that PKA is involved in vascular formation process through its novel function regulating VEGF signal intensity. Various factors, such as adrenomedullin,38 prostaglandins,39 adiponectin,40 ghrelin,41 klotho,42 and mechanical stress, especially fluid shear stress,43 have been reported to activate PKA in ECs. We previously demonstrated that adrenomedullin could enhance EC differentiation from Flk1+ cells through cAMP signaling.26 Fluid shear stress was reported to enhance EC differentiation from Flk1+ cells by up-regulating VEGF receptors,44 and to induce Flk1 gene expression in EC cell lines.45 These multiple PKA-activating signals should be involved in vascular development to modulate the progenitor sensitivity in vivo.
We previously reported that adrenomedullin/cAMP pathway induced differentiation of arterial ECs.26 We also examined involvement of PKA in arterial-venous specification. Whereas addition of PKI, PKA inhibitor, to Flk1+ cell culture with VEGF and 8bromo-cAMP significantly decreased total CD31+ EC appearance (Figure 2A-D), PKI did not inhibit ephrinB2- or CXCR4-positive arterial EC differentiation (supplemental Figure 10). Moreover, expression of CA-PKA with VEGF did not induce arterial ECs from Flk1+ vascular progenitors (supplemental Figure 11). These results indicated that PKA is not involved in arterial-venous specification. Activation of PKA in Flk1+ cells did not induce prox1- or podoplanin-positive lymphatic ECs (supplemental Figure 11), further suggesting that PKA pathway is involved in common EC differentiation but not in EC specification processes.
Some studies have reported the roles of downstream molecules of Flk1 signaling in EC proliferation and differentiation. Tyrosine residue 1173 of Flk1 (Y1173, corresponding to Y1175 in human Flk1, KDR) is essential for Flk1 function in vasculogenesis.46 Y1175 of KDR is a binding site of PLCγ and is important for VEGF-dependent EC proliferation.47 Furthermore, Ras signaling acting downstream of Flk1 signaling plays a critical role in EC differentiation.32 Indeed, PLC inhibitor, U73122, or H-Ras inhibitor, FTI-277, showed an inhibitory effect on EC differentiation from Flk1+ cells (supplemental Figure 12), indicating that PLC and Ras pathway are both downstream molecules of Flk1 signaling. Enhanced VEGF signaling in vascular progenitors by PKA should be mediated by these molecules to induce basal EC differentiation.
Molecular mechanisms of PKA to induce and/or maintain Flk1 and NRP1 expression in vascular progenitors are largely unknown. NRP1 expression was reported to be up-regulated by cAMP/PKA pathway in olfactory neuron guidance.48 Some other reports have shown that PKA pathway enhances differentiation of neuronal progenitor cells,49 hippocampal progenitor cells,50 and oligodendrocyte progenitor cells.51 Recently, evidence is accumulating to suggest that blood vessels and nerves share a similar molecular machinery to form their networks.52 Blood vessels and nerves may use PKA as common regulatory cues for their differentiation and development. Further elucidation of molecular interaction among PKA, Flk1, and NRP1 should provide a novel molecular framework for tissue development.
Very recently, Cimato et al reported that NRP1 was largely coexpressed with Flk1 to identify endothelial precursors in human and mouse ES cells.53 We confirmed that low-level expression of NRP1 was observed in Flk1+ progenitors and was increased in ECs (supplemental Figure 13). These 2 functional markers for EC progenitors, Flk1 and NRP1, were, thus, commonly regulated by PKA to efficiently enhance their progenitor potentials responding to VEGF signaling.
We have succeeded in uncovering novel roles of PKA in EC differentiation and vascular development using our unique ES cell differentiation system. Elucidation of the new-mode cell fate determination mechanisms by modulation of progenitor potentials would provide novel insights in developmental biology, stem cell biology, and regenerative medicine.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Dr T. Era (Kumamoto University) for EStTA5-4 cells, to G. S. McKnight (University of Washington School of Medicine) for the CA-PKA plasmid, K. Tanimoto (University of Tsukuba) and P. Soriano (Mt Sinai School of Medicine) for the loxP knockin vector for ROSA locus, and Y. Toda for three-dimensional histologic analyses. We thank M. Takahashi for critical reading of the paper.
This study was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan, the Ministry of Health, Labor and Welfare, the New Energy and Industrial Development Organization (NEDO) of Japan, and the Project for Realization of Regenerative Medicine.
Authorship
Contribution: K.Y. performed all experiments and wrote the paper; K.K. and T.W. performed ex vivo whole-embryo experiments; S.K. helped with immunostaining experiments; and J.K.Y. supervised all experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jun K. Yamashita, Laboratory of Stem Cell Differentiation, Stem Cell Research Center, Institute for Frontier Medical Sciences, Kyoto University, 53 Shogoin Kawahara-cho, Sakyo-ku, Kyoto 606-8507 Japan; e-mail: juny@frontier.kyoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal