To the editor:
Recently, Blood published on the decisive pathogenic role of the zinc-finger transcription factor GATA1 in the development of transient myeloproliferative disorders (TMDs) and acute megakaryoblastic leukemias (AMKLs) in the setting of Down syndrome.1,2
Importantly, all mutations so far described cluster in exon 2 and encode a functionally impaired, shorter GATA1 protein, which finally enhances proliferation and stops maturation of hematopoiesis.1,3,4
In our daily routine we diagnosed an intrauterine TMD on a placental specimen submitted for diagnosis after emergency Cesarean section of a preterm boy (32nd gestational week) due to pathologic cardiotocography in the setting of a clinically known Down syndrome. The patient showed characteristics of peripheral blood at the time of birth consistent with TMD, including hyperleukocytosis of 343.80 × 109/L with 78.5% atypical cells, erythrocytes of 2.76 × 1012/L, hemoglobin of 92 g/L, and platelets of 738 × 109/L. Atypical cells were characterized as megakaryoblasts by immunohistochemistry on the placental paraffin specimens. We analyzed formalin-fixed, paraffin-embedded blood clots from the umbilical vein, containing high numbers of blasts, for GATA1 exon 2 mutations. The entire region of exon 2 was sequenced using consecutive first and nested PCR. A new point mutation leading to the formation of a premature stop at codon 2 (E2Term) was detected (Figure 1). This mutation results in the earliest known premature stop codon in the sequence of GATA1 compared with the previously reported mutations in the literature.3,4 Because our mutated sequence of GATA1 encodes only for a single methionine amino acid, and because of its position on chromosome X, with no alternatively available allele for GATA1 transcription, this mutation must lead to complete loss of GATA1 protein in TMD blasts. On the protein level, using immunohistochemistry (ab28839, Abcam, rabbit polyclonal) the blasts did not express GATA1, whereas nuclear staining was present in a few maternal blood cells in the intervillous space.
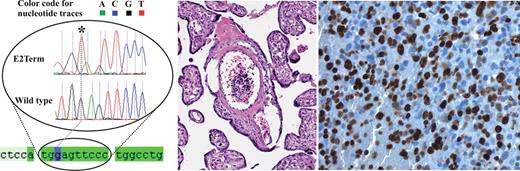
Alignment of the selected region of the GATA1 sequence for control cells and blasts cells harboring the E2Term mutation leading to a stop codon in codon 2. (Left) The star indicates the position of a homozygous replacement of the first nucleotide of codon 2 (labeled G in violet) by a T. Below, a part of the GATA1 sequence mRNA is imprinted as light green and the coding region as dark green. (Middle and right) Placental specimen with high numbers of blasts in the fetal vessels in the placental villi with massively elevated proliferation rate in the MIB1 staining, diagnostic for TMD.
Alignment of the selected region of the GATA1 sequence for control cells and blasts cells harboring the E2Term mutation leading to a stop codon in codon 2. (Left) The star indicates the position of a homozygous replacement of the first nucleotide of codon 2 (labeled G in violet) by a T. Below, a part of the GATA1 sequence mRNA is imprinted as light green and the coding region as dark green. (Middle and right) Placental specimen with high numbers of blasts in the fetal vessels in the placental villi with massively elevated proliferation rate in the MIB1 staining, diagnostic for TMD.
GATA1 is essential in hematopoiesis and performs several different tasks in erythro- and megakaryopoiesis as well as eosinophil and mast cell differentiation.5-8 Complete loss of GATA1 expression in knockout mice leads to early death in embryogenesis due to severe anemia.9 Based on the published data, a remaining GATA1 protein fragment (GATA1s) is required for the development of TMD.10 Since in our case GATA1 expression is completely lost, this might point toward a different pathway in leukemogenesis. The entire GATA1 loss might have been accompanied by loss of important transdifferentiation signals in erythro- and megakaryopoesis.
Our reported newborn boy suffered from disseminated intravascular coagulation, hepatopathy, hypoalbuminemia, hypercholesterinemia, persisting pulmonary hypertension, extremely high LDH (14 833 U/L), hyperuricaemia as well as renal (hyperkalemia up to 10 mM) and liver failure. He died 2 days after delivery because of multiorgan failure, after it had been decided to withdraw intensive care because of a large intracranial hemorrhage. This poor outcome might point to a more aggressive behavior of TMD with more severe neurologic and hemostaseologic complications, which may be linked to complete GATA1 protein loss.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisabeth Bruder, University of Basel, Schoenbeinstrasse 40, Basel 4031, Switzerland; e-mail: elisabeth.bruder@unibas.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal