Abstract
Microenvironmental signals can determine hematopoietic stem cell (HSC) fate choices both directly and through stimulation of niche cells. In the bone marrow, prostaglandin E2 (PGE2) is known to affect both osteoblasts and osteoclasts, whereas in vitro it expands HSCs and affects differentiation of hematopoietic progenitors. We hypothesized that in vivo PGE2 treatment could expand HSCs through effects on both HSCs and their microenvironment. PGE2-treated mice had significantly decreased number of bone trabeculae, suggesting disruption of their microarchitecture. In addition, in vivo PGE2 increased lineage− Sca-1+ c-kit+ bone marrow cells without inhibiting their differentiation. However, detailed immunophenotyping demonstrated a PGE2-dependent increase in short-term HSCs/multipotent progenitors (ST-HSCs/MPPs) only. Bone marrow cells transplanted from PGE2 versus vehicle-treated donors had superior lymphomyeloid reconstitution, which ceased by 16 weeks, also suggesting that ST-HSCs were preferentially expanded. This was confirmed by serial transplantation studies. Thus in vivo PGE2 treatment, probably through a combination of direct and microenvironmental actions, preferentially expands ST-HSCs in the absence of marrow injury, with no negative impact on hematopoietic progenitors or long-term HSCs. These novel effects of PGE2 could be exploited clinically to increase donor ST-HSCs, which are highly proliferative and could accelerate hematopoietic recovery after stem cell transplantation.
Introduction
Hematopoietic stem cells (HSCs) must balance self-renewal and differentiation to replenish the entire hematopoietic system and simultaneously survive throughout the life of a person.1 This essential regulation of stem cells is thought to be determined, at least in part, by the environment, or niche, in which these cells reside.2 We and others first identified osteoblastic cells as a regulatory component in the HSC niche through genetic means,3,4 and several additional potential cellular components have now been identified.5 Thus, microenvironmental signals within the bone marrow, such as in the setting of injury, would be expected to influence HSC behavior both directly as well as indirectly, through their actions on HSC niche cells. One such microenvironmental signal may be prostaglandin E2 (PGE2). Prostaglandins are arachidonic acid derivatives produced through cyclooxygenase 1 and 2 (COX-1 and COX-2) activation in a variety of tissues where they are involved in local physiologic and pathologic processes.6 PGE2 can target both stromal and hematopoietic components of the bone marrow. Specifically, PGE2 or its analogs can stimulate periosteal and trabecular osteoblasts in humans, rats, and mice.7-14 In addition, PGE2 has bone-resorbing effects15 and can either inhibit or stimulate osteoclastic cells, depending on the experimental model used.16-19 Interestingly, parathyroid hormone (PTH), known to indirectly expand HSCs through its effects on osteoblastic cells in the bone marrow, is one of the principal stimulators of PGE2 in osteoblastic cells.20-23
PGE2 has long been known to have both inhibitory and stimulatory effects on differentiation of hematopoietic progenitors and on lineage allocation.24-29 More recently, studies in zebrafish established a role for PGE2 in HSC regulation in vivo in the setting of kidney marrow recovery after myeloablative injury.30 In addition, ex vivo treatment of murine bone marrow cells (BMCs) with a stabilized form of PGE2 increased the frequency of long-term (LT) repopulating HSCs.30,31 However, whether in vivo treatment with PGE2 expands HSCs in mammals is currently unknown.
Emerging data have begun to demonstrate the therapeutic potential of osteoblastic stimulation to achieve in vivo HSC expansion.3,32,33 Because PGE2 and its analogs have been used clinically,34,35 and given the effects of PGE2 on bone and on hematopoietic cells, we hypothesized that in vivo treatment with PGE2 could expand HSCs through its beneficial effects on both osteoblasts and HSCs in the bone marrow. In this study, we modified an in vivo PGE2 regimen previously used to stimulate osteoblasts and tested its effects on both hematopoietic cells and the skeletal structure of treated mice. We demonstrate that PGE2 treatment in vivo (1) alters the bone microarchitecture, (2) has a regulatory effect on HSCs in the absence of injury, (3) preferentially expands short-term (ST) HSCs, and (4) does not impact LT-HSC numbers, lineage distribution, or progenitor differentiation. Taken together, our data suggest that PGE2 treatment (or, potentially, treatment with specific agonists of PGE2 receptors) could be used to preferentially increase donor ST-HSCs, which are highly proliferative,36 to accelerate hematopoietic recovery after stem cell transplantation, and potentially to expand primitive hematopoietic cells in the absence of injury.
Methods
Reagents
PGE2 was purchased from Sigma-Aldrich, and synthetic rat PTH (1-34), was purchased from Bachem.
In vivo PGE2 treatment
Six- to 10-week-old C57bl/6 male mice were injected intraperitoneally twice daily for 16 days with either 6 mg/kg body weight PGE2 or vehicle (4% ethanol in molecular grade water). Hindlimbs were harvested 24 hours after the final injection and either used for bone marrow harvest (left hindlimb) or fixed and analyzed by micro-CT and histology, as described3 (right hindlimb). All experiments were approved by the Institutional Animal Care and Use Committee at the University of Rochester School of Medicine.
Flow cytometric analysis
The bone marrow was flushed from the long bones of the left hindlimb of each mouse. Red blood cells (RBCs) were lysed in 1 mL RBC lysis buffer (156 mM NH4Cl, 127 μM ethylenediaminetetraacetic acid, 12 mM NaHC3) for 5 minutes at room temperature. After RBC lysis, the remaining mononuclear cells were suspended in fluorescence-activated cell sorter buffer (phosphate-buffered saline [PBS] containing 3% heat-inactivated fetal bovine serum [HI-FBS]). A total of 107 cells were then stained with appropriate antibodies (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The cells were washed, and the data were collected on a LSR-II (BD Biosciences) and analyzed using FlowJo software (Tree Star).
CFU-C analysis
Bone marrow cells flushed from the left hindlimbs were resuspended in α-minimum essential medium (Mediatech) supplemented with 2% HI-FBS at a concentration of 2 × 105 cells/mL; 0.4 mL cell-containing media was added to 4 mL methylcellulose containing M3434 media (Stem Cell Technologies), as previously described.3
Peripheral blood cell analysis
Before death, 200 μL blood obtained by mandibular venous plexus sampling was run through a CBC-DIFF Veterinary Hematology System (HESKA) to obtain blood cell counts.
Competitive repopulation assay
Mice expressing the congenic marker CD45.1 were treated with PGE2 or vehicle as previously described. The bone marrow was flushed from the hindlimbs of these mice and mixed with bone marrow cells from a competitor animal expressing CD45.2 at a ratio of 1:2 or 1:1 (donor/competitor). The cell mixture was resuspended in PBS containing 3% HI-FBS. A total of 7.5 × 105 total cells in 100 μL was injected into the tail veins of CD45.2-expressing recipient animals that had received two 500-cGy doses of radiation from an x-ray source separated by 24 hours (the second dose being given 2 hours before injecting the bone marrow cells). To evaluate engraftment, the peripheral blood of recipient animals was obtained by mandibular venous plexus sampling at the times indicated. The blood was incubated in a 2% solution of 5 × 105 molecular-weight dextran to precipitate the RBCs. The resulting supernatant containing the white blood cells was analyzed by flow cytometric analysis using the hematopoietic markers CD45.1, CD45.2, B220, CD3, and CD11b. For some of the experiments, primary recipients were humanely killed at week 36 after transplantation and the bone marrow was analyzed for lineage− Sca-1+ c-kit+ (LSK) content and used for secondary transplantation.
Secondary transplantation
BMCs were harvested from the hindlimbs from primary recipients (CD45.2) of vehicle or PGE2-treated donors (CD45.1) 36 weeks after the primary transplantation. Donor LSKs were enumerated by flow cytometric analysis. BMCs were enumerated and mixed with BMCs from a competitor animal expressing CD45.2 at a ratio of 1:2 (donor/competitor). As for the primary transplantation, the cell mixture was resuspended in PBS containing 3% HI-FBS. A total of 7.5 × 105 total cells in 100 μL were injected into the tail veins of CD45.2-expressing secondary recipient animals that had received two 500-cGy doses of radiation from an x-ray source separated by 24 hours (the second dose being given 2 hours before injecting the bone marrow cells). To evaluate engraftment, the recipient animals were bled by mandibular bleeds at the times indicated. As for the analysis of engraftment after the primary transplantation, the blood was incubated in a 2% solution of 5 × 105 molecular weight dextran to precipitate the RBCs. The resulting supernatant containing the white blood cells was analyzed by flow cytometric analysis using the hematopoietic markers CD45.1, CD45.2, B220, CD3, and CD11b.
CFU-S
The bone marrow from mice treated with PGE2 or vehicle was flushed and resuspended in PBS containing 3% HI-FBS. Recipient mice that had been lethally irradiated (two 500-cGy doses of radiation from an x-ray source separated by 24 hours) were injected with 6 × 104 cells each. After 8, 10, 11, or 12 days, the animals were humanely killed and the spleens were fixed in Telly solution containing 70% ethanol, 2% formaldehyde, and 5% acetic acid for 24 hours. After fixation, the spleens were transferred to 70% ethanol, photographed, and the colonies counted.
Micro-CT analysis
The right hindlimb of each mouse was fixed for 3 days in 10% formalin and then stored in 70% ethanol at 4°C. The limbs were scanned on a Viva CT 40 (Scanco Medical) using a 55-kVp, 145-μA current and a 300-ms integration time. A resolution of 12.5 μm was used. A 2.0875-mm region that was 50 μm above the growth plate in the femur and 50 μm below the growth plate in the tibia was analyzed.
Statistical analysis
For quantitative assays, treatment groups were reported as mean plus or minus SEM and compared using the Student t test. When multiple comparisons were necessary, analysis of variance with Bonferroni posttest was performed using GraphPad Prism, Version 4.01 for Windows (GraphPad Software). Statistical significance was established at P less than or equal to .05.
Results
In vivo PGE2 treatment alters the bone microarchitecture
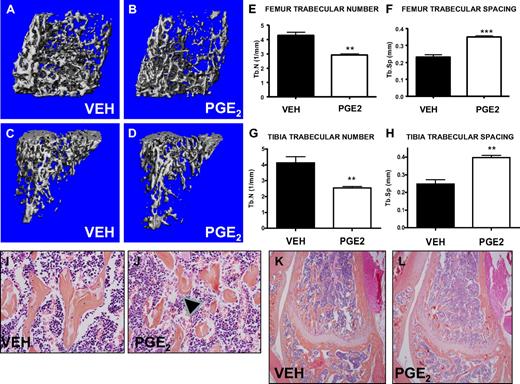
PTH increases trabecular bone volume, and PGE2 treatment has been reported to activate osteoblastic cells, resulting in an anabolic bone effect in rats.9 To activate the bone marrow microenvironment, we treated mice in vivo with either PGE2 or vehicle, modifying a regimen previously found to have bone effects in rats.14 This regimen (6 mg/kg twice daily for 16 days) was well tolerated without morbidity, mortality, or weight changes in the PGE2-treated mice (data not shown). In this model of PGE2 treatment, micro-CT scanning demonstrated significant and consistent changes in trabecular bone microarchitecture, including PGE2-associated decreased numbers of trabeculae in the metaphyseal areas of femora and tibiae and increased trabecular spacing (Figure 1A-H). Histologic analysis of high-power images from the metaphyseal area of the distal femur also demonstrated disrupted trabeculae with increased trabecular spacing in PGE2-treated compared with vehicle-treated mice (Figure 1E-F). Neither histologic images (Figure 1K-L) nor micro-CT evaluation (data not shown) demonstrated any significant gains in trabecular bone volume in mice treated with PGE2 compared with vehicle. These data confirm that PGE2 significantly alters the bone microarchitecture of treated mice.
Systemic PGE2 treatment alters bone marrow microarchitecture. Femora and tibiae of vehicle- (VEH) and PGE2-treated mice were analyzed by micro-CT and histology. Three-dimensional reconstructions of representative scanned femora (A-B) and tibiae (C-D) focusing on trabecular bone. Quantification of the trabecular number (E,G) and trabecular spacing (F,H) (n = 4/group). High- (I-J) and low-power (K-L) representative images of histologic slides stained with hematoxylin and eosin, focusing on the metaphyseal region of the distal femur from vehicle- and PGE2-treated mice. Arrowheads indicate disrupted trabeculae in the PGE2-treated mice (J). **P ≤ .01; ***P ≤ .001.
Systemic PGE2 treatment alters bone marrow microarchitecture. Femora and tibiae of vehicle- (VEH) and PGE2-treated mice were analyzed by micro-CT and histology. Three-dimensional reconstructions of representative scanned femora (A-B) and tibiae (C-D) focusing on trabecular bone. Quantification of the trabecular number (E,G) and trabecular spacing (F,H) (n = 4/group). High- (I-J) and low-power (K-L) representative images of histologic slides stained with hematoxylin and eosin, focusing on the metaphyseal region of the distal femur from vehicle- and PGE2-treated mice. Arrowheads indicate disrupted trabeculae in the PGE2-treated mice (J). **P ≤ .01; ***P ≤ .001.
In vivo PGE2 expands LSK cells without inhibiting differentiation
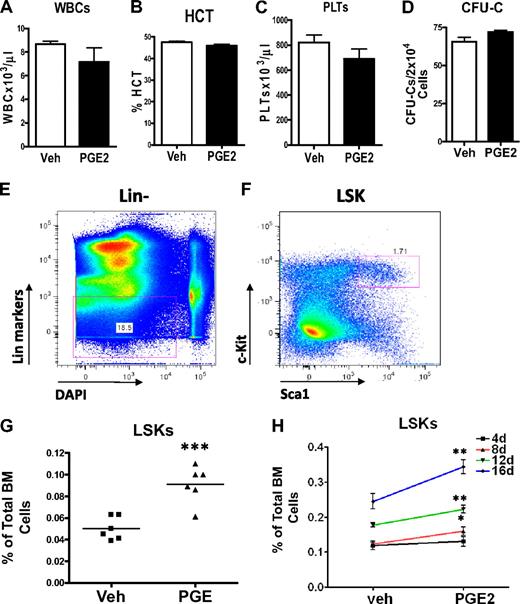
To determine whether systemic PGE2 had hematopoietic effects, we performed peripheral blood cell counts and enumerated bone marrow hematopoietic progenitors using the colony-forming unit (CFU-C) in vitro assay. There was no difference in the hematocrit, the number of platelets, or the total number of white blood cells in the peripheral blood cell counts of PGE2- and vehicle-treated mice at the end of 16 days of treatment (Figure 2A-C), demonstrating that PGE2 treatment did not alter lineage allocation or inhibit hematopoietic differentiation. To ensure that systemic PGE2 did not block differentiation of hematopoietic progenitors, we quantified hematopoietic progenitors using the CFU-C assay. No difference in the number of progenitors was found in the bone marrow comparing PGE2- and vehicle-treated animals (Figure 2D). Finally, to begin to elucidate effects of systemic PGE2 on HSCs, we performed flow cytometric analysis of bone marrow to quantify the HSC-enriched LSK subset (Figure 2E-F). We identified a significant increase in the LSK population after in vivo PGE2 treatment (Figure 2G). This increase in LSK cells was of a similar magnitude as the one induced by PTH.3 The magnitude of the LSK increase was dose dependent (Figure 2H). These data indicate a significant stimulatory effect of systemic PGE2 on LSK cells but no alteration of hematopoietic differentiation.
Systemic PGE2 treatment increases the HSC-enriched LSK population without inhibiting hematopoietic differentiation or affecting lineage distribution. (A-C) Peripheral blood analysis of mice treated systemically with PGE2 or vehicle. (A) The total number of white blood cells present in 1 μL blood. (B) %HCT indicates the percentage of blood volume occupied by red blood cells. (C) The number of platelets present in 1 μL blood. Experiment shown is representative of 3 independent experiments. (D) CFU-C assays on bone marrow cells measure more differentiated hematopoietic progenitor cells with the capacity to form colonies in methylcellulose (n = 4/group). (E-F) Flow cytometric strategy used for quantification of LSK cells in the bone marrow of mice treated with either PGE2 or vehicle. 4,6-Diamidino-2-phenylindole was used as a DNA stain to exclude nonviable cells. (G) Quantification of bone marrow LSKs from vehicle- and PGE2-treated C57/bl6 mice (2 separate experiments are included). Points represent the fold difference between PGE2-treated and VEH-treated animals. (H) LSK quantification from FVB/N mice treated daily for 4, 8, 12, and 16 days with vehicle or PGE2 as indicated in the graph. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Systemic PGE2 treatment increases the HSC-enriched LSK population without inhibiting hematopoietic differentiation or affecting lineage distribution. (A-C) Peripheral blood analysis of mice treated systemically with PGE2 or vehicle. (A) The total number of white blood cells present in 1 μL blood. (B) %HCT indicates the percentage of blood volume occupied by red blood cells. (C) The number of platelets present in 1 μL blood. Experiment shown is representative of 3 independent experiments. (D) CFU-C assays on bone marrow cells measure more differentiated hematopoietic progenitor cells with the capacity to form colonies in methylcellulose (n = 4/group). (E-F) Flow cytometric strategy used for quantification of LSK cells in the bone marrow of mice treated with either PGE2 or vehicle. 4,6-Diamidino-2-phenylindole was used as a DNA stain to exclude nonviable cells. (G) Quantification of bone marrow LSKs from vehicle- and PGE2-treated C57/bl6 mice (2 separate experiments are included). Points represent the fold difference between PGE2-treated and VEH-treated animals. (H) LSK quantification from FVB/N mice treated daily for 4, 8, 12, and 16 days with vehicle or PGE2 as indicated in the graph. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
In vivo PGE2 treatment preferentially expands phenotypic ST-HSC/MPPs
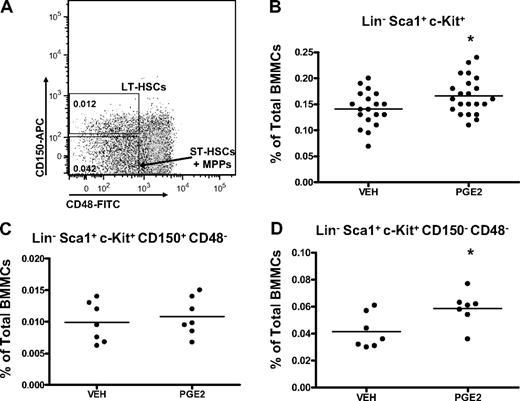
The LSK subset of bone marrow cells is enriched for HSCs.37-41 However, the LSK compartment is heterogeneous and contains at least 3 subpopulations of cells (LT-HSCs, ST-HSCs, and MPPs) with multilineage potential, but with progressively more limited self-renewal. Flow cytometric analysis was next performed to determine whether in vivo PGE2 treatment preferentially expands specific LSK subsets, identified phenotypically by cell-surface markers. HSC subsets can be discriminated with flow cytometry by their differential expression of the SLAM receptors, CD150 and CD48, where, within the LSK subset, LT-HSCs are CD150+ CD48− and ST-HSCs/MPPs are CD150− CD48−.42,43 Using these markers to assess the frequency of the different subpopulations in the bone marrow (Figure 3A), LT-HSCs were not significantly increased with in vivo PGE2 treatment (Figure 3C), whereas ST-HSCs/MPPs were expanded (Figure 3D).
Systemic PGE2 treatment selectively expands CD150+ CD48− LSK cells. (A) Flow cytometric dot plot illustrating gating scheme for subsets of LSK cells using SLAM receptors. Parent population is Lin− Sca1+ c-Kit+. (B-D) Flow cytometric analysis of the frequency of LSK cells (B), LT-HSCs (C), and ST-HSCs/MPPs (D) in the bone marrow after PGE2 or vehicle treatment. *P ≤ .05 (3 separate experiments are included).
Systemic PGE2 treatment selectively expands CD150+ CD48− LSK cells. (A) Flow cytometric dot plot illustrating gating scheme for subsets of LSK cells using SLAM receptors. Parent population is Lin− Sca1+ c-Kit+. (B-D) Flow cytometric analysis of the frequency of LSK cells (B), LT-HSCs (C), and ST-HSCs/MPPs (D) in the bone marrow after PGE2 or vehicle treatment. *P ≤ .05 (3 separate experiments are included).
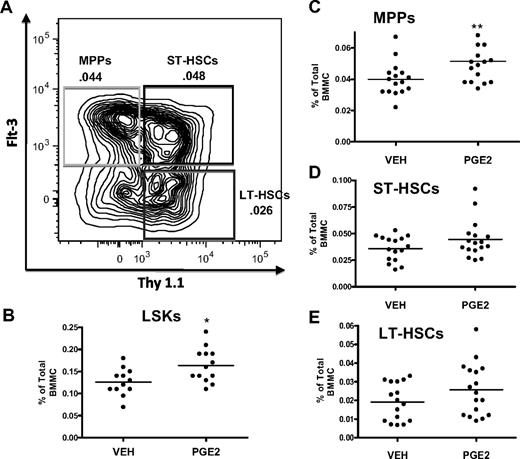
HSC subpopulations can also be separated within the LSK pool based on expression of Flt3 and Thy1.1, where gain of Flt3 and loss of Thy1.1 correlate with increased proliferation36 and decreased self-renewal capability44,45 (Figure 4). This phenotypic analysis of bone marrow cells from mice treated with PGE2 for 16 days demonstrated a significant increase only in the LSK (Figure 4B) and LSK Flt3+ Thy1.1low (Figure 4C) populations, the latter representing mostly MPPs, whereas LSK Flt3− Thy1.1int (Figure 4E), enriched for LT-HSCs and LSK Flt3− Thy1.1int (Figure 4D), enriched for ST-HSCs, were not significantly increased (Figure 4C). Taken together, these data support the notion that in vivo PGE2 treatment increases HSCs that have limited self-renewal capacity.
Systemic PGE2 treatment selectively expands the MPP subset of LSK cells. (A) Flow cytometric dot plot illustrating gating scheme for subsets of LSK cells using Flt3 and Thy1.1 surface markers with a parent population of Lin− Sca1+ c-Kit+. (B-E) Flow cytometric analysis of the frequency of LSK cells (B), MPPs (C), ST-HSCs (D), and LT-HSCs (E) in the bone marrow after PGE2 or vehicle treatment. *P ≤ .05, **P ≤ .01 (3 separate experiments are included).
Systemic PGE2 treatment selectively expands the MPP subset of LSK cells. (A) Flow cytometric dot plot illustrating gating scheme for subsets of LSK cells using Flt3 and Thy1.1 surface markers with a parent population of Lin− Sca1+ c-Kit+. (B-E) Flow cytometric analysis of the frequency of LSK cells (B), MPPs (C), ST-HSCs (D), and LT-HSCs (E) in the bone marrow after PGE2 or vehicle treatment. *P ≤ .05, **P ≤ .01 (3 separate experiments are included).
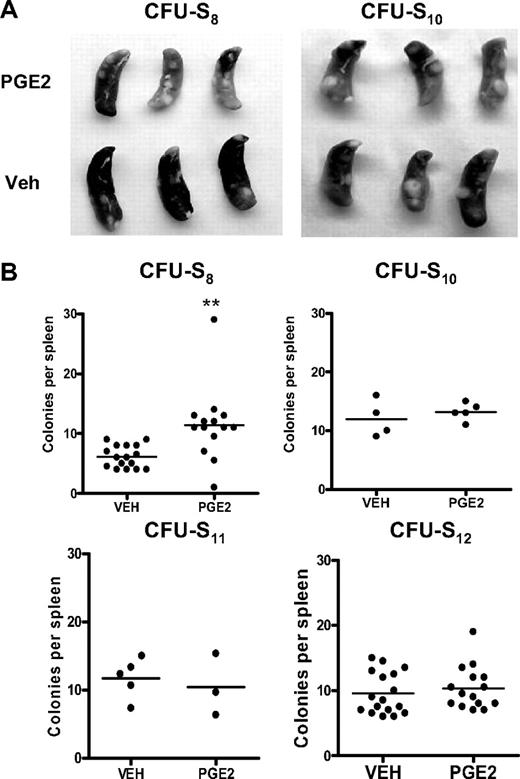
In vivo PGE2 treatment expands early but not late CFU-S
The in vivo CFU-S assay has been used as an alternative method to quantify subpopulations of progenitor/HSC activities,30,43,46 with CFU11-12 being derived from a more primitive progenitor/stem cell population than CFU8.47-50 Donor bone marrow cells from mice treated with PGE2 or vehicle were transplanted into lethally irradiated wild-type recipient mice, and the number of colonies per spleen was quantified at days 8, 10, 11, or 12 after transplantation. Mice transplanted with bone marrow cells from PGE2-treated donors had significantly more spleen colonies 8 days after transplantation compared with mice receiving cells from vehicle-treated mice (Figure 5). However, at days 10 through 12 after transplantation, there was no difference in the number of colonies between mice receiving marrow from PGE2 versus vehicle-treated donors (Figure 5).48 Thus, these data again suggest that in vivo PGE2 treatment selectively expands HSCs of limited self-renewal.
In vivo PGE2 treatment preferentially increases early but not late CFU-S. (A) Representative photographs of spleens removed from mice transplanted with bone marrow from PGE2- or vehicle-treated donor mice at day 8 and day 10 after transplantation. (B) Quantification of splenic colonies in transplanted mice at days 8, 10, 11, and 12 after transplantation. **P ≤ .05 (3 separate experiments are included for days 8 and 12).
In vivo PGE2 treatment preferentially increases early but not late CFU-S. (A) Representative photographs of spleens removed from mice transplanted with bone marrow from PGE2- or vehicle-treated donor mice at day 8 and day 10 after transplantation. (B) Quantification of splenic colonies in transplanted mice at days 8, 10, 11, and 12 after transplantation. **P ≤ .05 (3 separate experiments are included for days 8 and 12).
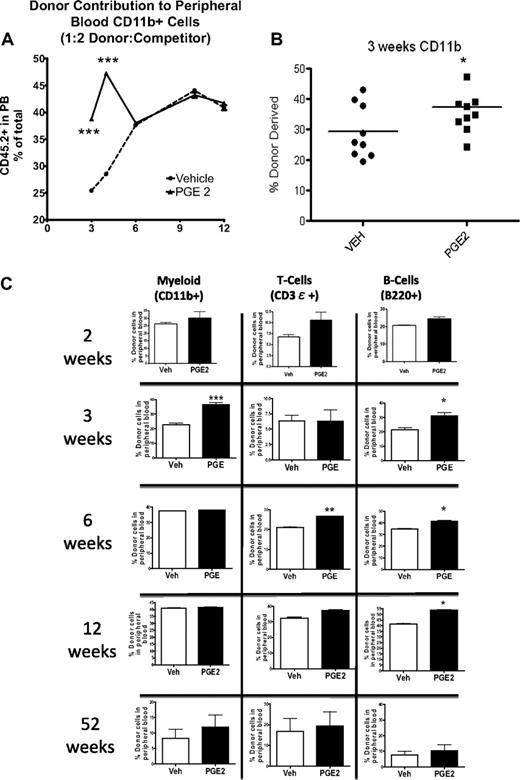
In vivo PGE2 treatment increases short-term but not long-term hematopoietic engraftment
To determine whether the observed increase in LSK subsets was the result of an increase in primitive hematopoietic cells with multilineage reconstitution but limited self-renewal, we performed competitive repopulation assays. At 2, 3, and 4 weeks after transplantation, irrespective of the donor/competitor ratio, the peripheral blood of the recipients that received cells from PGE2-treated donors demonstrated greater reconstitution in the myeloid and B-cell compartments compared with recipients that received cells from vehicle-treated donors as determined by flow cytometric analysis (Figure 6A-B). However, the early increase in engraftment seen in the recipients of PGE2- compared with vehicle-treated donor cells was subsequently lost. This loss was first evident in the myeloid lineage (Figure 6), beginning 6 weeks after transplantation and persisting one year after transplantation (Figure 6C). The enhanced reconstitution of the B-cell compartment in recipients of PGE2-treated cells was maintained through 6 and 12 weeks (Figure 6C) but was also lost by 1 year after transplantation (Figure 6C). Similar kinetics were observed for the loss of the greater reconstitution in the T-cell compartment of recipients of the PGE2-treated cells (Figure 6C). These lineage-dependent differences in duration of PGE2-dependent engraftment superiority are consistent with the kinetics of ST-HSC and MPP progeny, which directly depend on the half-life of the mature cells in each lineage.43 Noncompetitive transplantation in myeloablated recipients also demonstrated a trend toward increased WBC and platelet counts 3 weeks after transplantation, consistent with a PGE2-dependent increase in the less quiescent ST-HSCs/MPPs (supplemental Figure 1). There was no increase in hematocrit (supplemental Figure 1), suggesting that in vivo treatment with PGE2 may not increase erythroid progenitors, as was reported for PGE2 effects in vitro.26,29
Accelerated engraftment from PGE2 versus vehicle-treated BMMCs. Competitive repopulation assays demonstrate superior engraftment of BMMCs from PGE2-treated donor mice at early time points after competitive transplantation. (A) The percentage of CD45.2-expressing cells in the peripheral blood of recipient mice that received CD45.2-expressing donor cells mixed in a 1:2 ratio with CD45.1-expressing competitor cells (n = 4 donors, 8 recipients/group). (B-C) The percentage of CD45.2-expressing myeloid cells (CD11b+), T cells (CD3ϵ+), and B cells (B220+) in the peripheral blood of recipient mice at 3, 6, and 52 weeks (n = 4 donors per transplantation; n = 5-7 recipients/group). Individual data points from each mouse are shown in panel B. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Accelerated engraftment from PGE2 versus vehicle-treated BMMCs. Competitive repopulation assays demonstrate superior engraftment of BMMCs from PGE2-treated donor mice at early time points after competitive transplantation. (A) The percentage of CD45.2-expressing cells in the peripheral blood of recipient mice that received CD45.2-expressing donor cells mixed in a 1:2 ratio with CD45.1-expressing competitor cells (n = 4 donors, 8 recipients/group). (B-C) The percentage of CD45.2-expressing myeloid cells (CD11b+), T cells (CD3ϵ+), and B cells (B220+) in the peripheral blood of recipient mice at 3, 6, and 52 weeks (n = 4 donors per transplantation; n = 5-7 recipients/group). Individual data points from each mouse are shown in panel B. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Several models have demonstrated that increased proliferation of primitive cell types is associated with depletion of the HSC pool.51-54 However, in our experimental model, there was no evidence of decreased PGE2-dependent contribution to engraftment at one year (Figure 6C), suggesting that the accelerated engraftment induced by PGE2 did not compromise long-term repopulation.
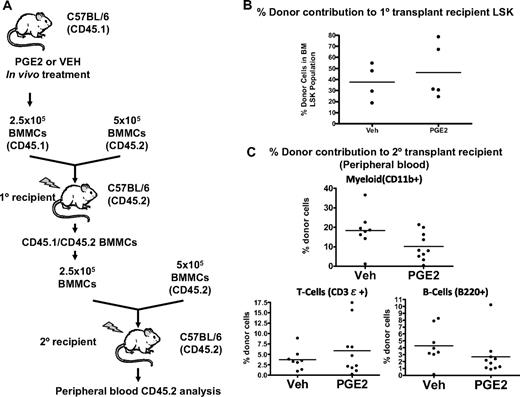
To confirm the transient nature of the increased engraftment of marrow from PGE2-treated mice, we performed secondary transplantation assays 36 weeks after the primary transplantation (Figure 7A). At this time after transplantation, there was no difference in bone marrow donor-derived LSK between primary recipient mice that received marrow from PGE2-treated donors and those that received marrow from vehicle-treated donors (Figure 7B). Engraftment in secondary recipients was measured at 10 weeks, when there was no longer any significant PGE2-dependent increase in lymphomyeloid engraftment (Figure 7C). It is noteworthy that there was no decline in the ability of BMCs derived from PGE2-treated mice to reconstitute bone marrow after secondary transplantation, again suggesting that the accelerated engraftment induced by PGE2 did not compromise long-term repopulation (Figure 7C). Taken together, these data demonstrate a transient increase in engraftment of HSCs from mice treated with PGE2 compared with vehicle, which suggests a PGE2-dependent increase in HSCs with limited self-renewal capacity.
Increased engraftment by in vivo PGE2-treated BMMCs is a transient phenomenon. (A) Serial competitive transplantation scheme. Donor mice (CD45.1) are treated with PGE2 or vehicle, and BMMCs are harvested after treatment. A mixture of CD45.1-expressing donor cells and CD45.2-expressing untreated competitor cells are transplanted into lethally irradiated CD45.2 primary recipient mice. After 36 weeks, BMMCs are harvested from the primary recipient mice and used as donor cells for a second round of competitive transplantation. (B) The percentage of donor-derived (CD45.1) LSK cells in the primary recipient bone marrow 36 weeks after transplantation. (C) Percentage of donor-derived (CD45.1) myeloid cells, T cells, and B cells in the peripheral blood of secondary recipients 10 weeks after transplantation.
Increased engraftment by in vivo PGE2-treated BMMCs is a transient phenomenon. (A) Serial competitive transplantation scheme. Donor mice (CD45.1) are treated with PGE2 or vehicle, and BMMCs are harvested after treatment. A mixture of CD45.1-expressing donor cells and CD45.2-expressing untreated competitor cells are transplanted into lethally irradiated CD45.2 primary recipient mice. After 36 weeks, BMMCs are harvested from the primary recipient mice and used as donor cells for a second round of competitive transplantation. (B) The percentage of donor-derived (CD45.1) LSK cells in the primary recipient bone marrow 36 weeks after transplantation. (C) Percentage of donor-derived (CD45.1) myeloid cells, T cells, and B cells in the peripheral blood of secondary recipients 10 weeks after transplantation.
Discussion
Microenvironmental bone marrow components have been demonstrated to regulate HSC behavior.5 As more of these components are identified, they highlight the complexity of local regulation of HSC cell fate choices and provide additional targets for therapeutic HSC manipulation. Thus, signals in the bone marrow probably influence HSCs both directly as well as through their actions on the HSC niche. Bone marrow injury would be expected to impact HSCs not only directly, but also through its effect on the cellular components of the HSC niche. One of the mediators of injury is PGE2, known to influence not only hematopoietic cells but also osteoblasts and osteoclasts. Here, we demonstrate in mice that in vivo PGE2 administration in the absence of injury is able to activate the bone marrow microenvironment and stimulate cells in the HSC pool.
Current technology enables identification and prospective isolation of HSCs using surface markers and flow cytometric analysis and sorting. The LSK subset of bone marrow cells is enriched for HSCs.37-41 However, the LSK compartment is heterogeneous and contains at least 3 subpopulations of cells with multilineage potential, but with progressively more limited self-renewal. There is significant controversy on the phenotypic markers identifying LT-HSCs versus ST-HSCs and MPPs,37,40,42,46 as well as to whether cells within this compartment can contribute to all hematopoietic progeny.37,43 However, the existence of less quiescent HSCs of limited self-renewal and increased proliferative capacity is well documented in both murine and human hematopoiesis.55,56
Our competitive repopulation data clearly demonstrate that in vivo PGE2 treatment preferentially expands HSCs of limited self-renewal because the PGE2-dependent superior myelogenous and lymphocytic repopulation was lost by 6 and 16 weeks, respectively. Moreover, 36 weeks after primary transplantation, there were no differences in bone marrow LSK cells in primary recipient mice who received bone marrow cells from PGE2 versus vehicle-treated donors, and secondary transplantation no longer identified significant differences in PGE2 versus vehicle-dependent engraftment. In addition, we used 2 different strategies to identify these HSC subsets by flow cytometric analysis; and in each case, PGE2 treatment had a significant and more dramatic effect on ST-HSCs/MPPs.
Another measure of ST-HSC/MPPs activity, CFU-S8 quantification, also supports the claim that in vivo treatment with PGE2 preferentially expanded HSCs of limited self-renewal. Taken together, our data strongly suggest that in vivo PGE2 administration preferentially expands HSCs with limited self-renewal capability. To our knowledge, no other single factor has been reported to preferentially expand ST-HSCs.
The less quiescent subsets of the HSC pool possess higher proliferative capacity36 while being capable of rapidly reconstituting all lineages.43 As such, the expansion of ST-HSC/MPPs could be of great benefit to accelerate recovery after bone marrow injury, as others have suggested.46,49 However, data in zebrafish and in murine repopulation after ex vivo treatment of murine hematopoietic cells with a stable derivative of PGE2, 16-16-dimethyl PGE2, demonstrated increased HSC repopulation ability both in the short and long term.30,31 Interestingly, this enhanced HSC repopulation ability was seen primarily at 6 and 12 weeks after transplantation, with a less dramatic effect seen at 24 weeks, consistent with our preferential effect of PGE2 on ST-HSC/MPPs compared with LT-HSCs.30 Certainly, in our model, there does not appear to be a deleterious effect of PGE2 on LT-HSCs because there were no differences in engraftment after secondary transplantation from PGE2 versus vehicle-treated donors.
In addition to the fact that in our model bone microenvironmental effects may modulate PGE2 action on HSCs, our PGE2 dosing regimen also significantly differed from the experimental regimen by North et al30 and Hoggat et al31 because we administered multiple doses of PGE2 in our in vivo studies and did not use a long-acting PGE2 analog.
It is possible that the preferential effect of PGE2 on HSCs of limited self-renewal defined in our experimental model may be the result of the superior quiescence of LT-HSCs compared with ST-HSC/MPPs57,58 and their relative resistance to injury.59 However, in injury, a direct effect of PGE2 on LT-HSC expansion may be undesirable as it may imperil this essential source of hematopoietic cells. This unfavorable phenomenon has been demonstrated in genetic models in which increased proliferation depletes the HSC pool.51-54 It is indeed possible that the direct effect of PGE2 may be targeted to ST-HSCs, with a secondary effect on LT-HSCs, which becomes apparent only when there is sustained stimulation of PGE2-dependent signaling, as in 16-16-dimethyl PGE2 treatment. Further studies elucidating PGE2 receptor expression and regulation within subsets of HSCs may further address this hypothesis.
LT-HSCs are both required and sufficient to ensure lifelong hematopoietic reconstitution after complete myeloablation. However, rapid and efficient hematopoietic recovery is critical after severe insults to overcome life-threatening cytopenias, and others have suggested that such rapid reconstitution could be most efficiently provided by ST-HSCs because LT-HSCs are quiescent and have delayed production of mature progeny.46 It is intriguing that PGE2 is often locally produced in infection and injury, situations in which rapid stimulation of bone marrow reconstitution would be beneficial.
Our findings suggest that PGE2 can increase ST-HSC/MPPs in the absence of myeloablative injury. This PGE2 effect could be therefore useful in situations of immunosuppression where there has been no injury to the bone marrow, such as hemolytic disorders or treatment with glucocorticoids.
PGE2 has been demonstrated to increase bone; however, in mice these effects have only been seen in the periosteum, where negligible amounts of osteoclastic and hematopoietic cells are present.9,60 The intermittent treatment that increases trabecular bone in rats has not been successfully reproduced in mice. Similarly, we did not measure a net bone gain in the trabecular bone of treated mice. Rather, we demonstrated disruption of the trabecular bone microarchitecture, confirming an effect of PGE2 in the bone microenvironment. We speculate that the lack of a net bone gain may be the result of simultaneous activation of bone formation and bone resorption, which would be consistent with the trabecular changes we observed. Further studies are needed to define more specifically the effects of PGE2 in the bone marrow microenvironment. These microenvironmental effects may also explain how our results on preferential ST-HSC/MPPs expansion of PGE2 in vivo differ from data on HSC treatments ex vivo.30,31
These results also suggest that interactions of a ligand with both HSC and niche cells may result in direct and indirect effects that impact HSC behavior. Our data cannot at this stage determine whether direct and/or microenvironmental effects of PGE2 result in the HSC effects we observed, and we cannot exclude that in vivo PGE2 may have a small effect on LT-HSCs given the precision of the assays we used. However, we think that our data point to aspects of the response of the bone marrow to injury that should be further explored, namely, the concept that the interaction of HSCs with their microenvironment not only provides regulatory signals but that it also must be altered in situations that disrupt the bone marrow, such as injury, infection and, as some groups are beginning to explore, malignancy.
In addition to the potential therapeutic benefit of using PGE2 or analogs to specifically expand ST-HSCs, these data raise the possibility that anti-inflammatory drugs that block PGE2 production may delay or restrict bone marrow recovery after myeloablation. Thus, further studies on the effect of anti-inflammatory drugs in the setting of hematopoietic recovery after chemotherapy and/or stem cell transplantation are warranted.
In contrast to some earlier studies in which PGE2 had inhibitory effects on hematopoiesis,24-28 we did not see any inhibition of differentiation of hematopoietic progenitors, as demonstrated by the lack of inhibitory PGE2 effects on CFU-C, on circulating hematopoietic parameters in PGE2-treated mice as well as at early time points in primary recipients of noncompetitive transplantation. We suspect that these differences may be the result of the PGE2 in vivo regimen we used in these experiments.
In conclusion, we have demonstrated that PGE2 in vivo preferentially expands ST-HSCs without depleting LT-HSCs, a strategy that may be exploited to accelerate recovery of the bone marrow after myeloablative injury. PGE2 may be a local mediator of the effects of PTH on the bone marrow microenvironment that result in ST-HSC expansion, either by acting directly on ST-HSCs or by regulating other HSC niche components. The addition of PGE2 or a PGE2-receptor agonist may therefore potentiate the PTH effect on ST-HSCs. Further studies are now required to elucidate the molecular mechanisms responsible for this novel regulation of ST-HSCs/MPPs by PGE2.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jim Palis for helpful discussion.
This work was supported by the National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Diseases (K08 DK064381, R01 DK 076876; L.M.C.), the Wilmot Cancer Scholar Foundation (L.M.C.), and the Pew Foundation (L.M.C.). R.L.P is a trainee in the Medical Scientist Training Program (National Institutes of Health T32 GM-07356).
National Institutes of Health
Authorship
Contribution: B.J.F., R.L.P., J.M.W., B.J.G., and A.J.O.-S. performed experiments; B.J.F., R.L.P., L.M.C., R.J.O., and C.T.J. analyzed experiments; and B.J.F., R.L.P., and L.M.C. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laura M. Calvi, Endocrine Division, Department of Medicine, University of Rochester School of Medicine, 601 Elmwood Ave, Box 693, Rochester, NY 14642; e-mail: laura_calvi@urmc.rochester.edu.
References
Author notes
B.J.F. and R.L.P. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal