Abstract
Adult bone marrow (BM) contributes to neovascularization in some but not all settings, and reasons for these discordant results have remained unexplored. We conducted novel comparative studies in which multiple neovascularization models were established in single mice to reduce variations in experimental methodology. In different combinations, BM contribution was detected in ischemic retinas and, to a lesser extent, Lewis lung carcinoma cells, whereas B16 melanomas showed little to no BM contribution. Using this spectrum of BM contribution, we demonstrate the necessity for site-specific expression of stromal-derived factor-1α (SDF-1α) and its mobilizing effects on BM. Blocking SDF-1α activity with neutralizing antibodies abrogated BM-derived neovascularization in lung cancer and retinopathy. Furthermore, secondary transplantation of single hematopoietic stem cells (HSCs) showed that HSCs are a long-term source of neovasculogenesis and that CD133+CXCR4+ myeloid progenitor cells directly participate in new blood vessel formation in response to SDF-1α. The varied BM contribution seen in different model systems is suggestive of redundant mechanisms governing postnatal neovasculogenesis and provides an explanation for contradictory results observed in the field.
Introduction
The mechanisms governing bone marrow (BM)–derived contribution to tissue neovascularization and the origin of marrow cells participating in this process are undefined, and remain a root of controversy in the field. Although initially thought to arise from local angiogenic events, recent studies purport that BM-derived cells including endothelial progenitor cells, hemangiocytes, and hemangioblasts contribute directly to vessel formation in different models of neovascularization.1-10 However, contradictory results relegate BM involvement to paracrine mechanisms rather than direct vessel contribution through the action of cells such as tie-2 expressing monocytes (TEMs), tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and recruited blood circulating cells (RBCCs).11-19 Moreover, it was recently reported that BM-derived endothelial progenitor cells expressing vascular endothelial growth factor (VEGF) receptor-2 (VEGFR-2+) are not mobilized from BM in a mouse model of cancer.14
Several reports indicate the importance of timing and environment on BM-derived neovascularization in cancer.2,5,20,21 These results coupled with different model systems and experimental techniques may explain confounding results.
Therefore, we reasoned that we could test various situations of neovessel formation using a novel technique in which multiple neovascularization models were established in individual mice. This technique reduced experimental variations and allowed direct comparative analyses among models.
Our data suggest that neovascularization can occur through multiple redundant mechanisms dictated by the local microenvironment. BM-derived cells can participate in neovascularization in some but not all settings. BM contribution is dependent on site-specific expression of stromal cell derived factor-1α (SDF-1α), its mobilizing effects on BM, and its capacity to promote homing of those mobilized cells to specific tissues. Furthermore, we show that SDF-1α activity can be significantly inhibited by therapeutic intervention, thereby reducing BM contribution to neovascularization. We also confirm the adult hematopoietic stem cell (HSC) as a long-term source of cells for neovascularization and show that CD133+CXCR4+ myeloid progenitor cells enrich for an “effector” population directly participating in neovascularization. Our results demonstrate that, in such an important process as neovasculogenesis, nature has developed redundant mechanisms to ensure a viable and versatile vascular system. In this light, the divergent observations in the field may all be correct in that they describe different aspects of this redundant system.
Methods
Animals
Wild-type C57BL/6 mice were purchased from Charles River Laboratories. C57BL/6 mice that ubiquitously express DsRed.MST under the control of the chicken β-actin promoter and cytomegalovirus enhancer were obtained from The Jackson Laboratory. The green fluorescent protein–positive (GFP+) mice are from STOCK Tg(GFPU)5Nagy/J mice (The Jackson Laboratory). All experimental procedures performed on animals were approved by the University of Florida institutional review board and Animal Care and Use Committee. Generation of radiation chimeras, retinal injury, and fluorescence-activated cell sorting (FACS) analyses were performed as previously reported3,22 and as described in supplemental methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Tumor inoculation
C57BL/6 chimeric mice were injected with 2 × 106 Lewis lung carcinoma (LLC) cells (ATCC) and/or melanoma cells (B16; ATCC) intramuscularly in hind limbs. Tumors were harvested for analysis once they reached a volume of between 500 and 600 mm3. In mice where retinal injury and LLC tumor models were combined, the injury was first established followed by LLC inoculation at day 28.
Isolation and infusion of CD133+/CXCR4+ cells
Peripheral blood from GFP+ or DsRed+ transgenic mice was isolated and the mononuclear cell fraction was collected with Ficoll Paque (Amersham Biosciences) centrifugation purification. The mononuclear cells were washed in 5× volumes of phosphate-buffered saline (PBS). The mononuclear layer was then resuspended in 100 μL of PBS and stained with monoclonal antibodies: rat anti–mouse monoclonal antibodies directed against CD133 (clone 13A4; fluorescein isothiocyanate [FITC] conjugate) and CD184/CXCR4 (clone 2B11), which was detected with an allophycocyanin-conjugated goat anti–rat immunoglobulin G (IgG) antibody (BD Pharmingen). The cells were sorted using the FACSVantage SE for CD133+CXCR4+ (GFP+ or DsRed+) cells. One day after vessel photocoagulation, mice were anesthetized and 106 CD133+/CXCR4+ cells were infused into the retro-orbital sinus.
Administration of SDF-1α and CXCR4 antibodies in the retinal injury model
Immediately after laser photocoagulation, mice were anesthetized and SDF-1α–neutralizing antibody (MAB310; R&D Systems) or CXCR4-neutralizing antibody (2B11; BD Pharmingen) was injected intravitreally (2 μL total volume) to achieve a final concentration of 1 μg/μL for the anti–SDF-1α antibody and 10 μg/μL for the CXCR4 antibody. Cohorts were given weekly booster injections for 4 weeks.
Anti–SDF-1α treatment in the tumor model
To test the effects of SDF-1α on BM activity in cancer, we made slight modifications to the transplant model. After transplantation and peripheral blood analysis at 3 months to confirm hematopoietic chimerism, mice received injections of 2 × 106 LLC cells or B16 cells intramuscularly in hind limbs. For each tumor type, one group of mice served as a control group receiving intratumoral injections of immunoglobulin isotype antibodies, whereas the other group received 25 μg of anti–SDF-1α antibodies (R&D Systems) in 20 μL of PBS intratumorally each day. Tumor diameter measurements were taken at different time points over a 2-week period using calipers to calculate tumor volumes.
To test the direct cytotoxicity of anti–SDF-1α antibodies on LLC cells, we established cultures of LLCs in the presence of increasing concentrations of anti–SDF-1α antibodies. Cultures were initiated with 105 LLC cells. Cells were grown in Dulbecco modified Eagle media supplemented with 10% fetal bovine serum at 37°C in an atmosphere of 5% CO2 in air. Cells were exposed to 0, 12.5, 25, and 50 μg of anti–SDF-1α antibodies per milliliter. We used concentrations equal to and above the levels used in the in vivo animal experiments. Cell numbers were evaluated at different time points over a 10-day period.
Isolation of tissues for SDF-1α ELISA
The tissues that were collected for the detection of SDF-1α by enzyme-linked immunosorbent assay (ELISA) included blood serum and vitreous fluid. Samples were collected as outlined in supplemental methods.
Tissue analysis
Tissues were analyzed using a Leica TCS SP2 laser scanning spectral confocal microscope or a Leica DM 2500 immunofluorescence microscope (Microsystems). Objectives used were 20×/0.70, 40×/0.75 or 63×/1.20 (water-corrected objective). Images were obtained with an Optronics camera using MagnaFire 2.1C software. Slides were mounted with Vectashield containing 4′-6-diamidino-2-phenylindole (DAPI; Vector Laboratories).
Statistics
Statistical differences between different experimental groups were determined by 1-way analysis of variance and Student t test. The reported values represent the mean plus or minus SEM. A P value less than .05 was considered significant.
Results
BM contribution to neovascularization is dependent on model system
To study mechanisms responsible for governing BM contribution in postnatal neovasculogenesis, models were chosen based on observed differences in their levels of BM contribution during neovasculogenesis. As one model system, we used a unique murine model of proliferative retinopathy previously established in our laboratory.3,22,23 Using this retinal injury model, we are able to consistently demonstrate robust generation of new blood vessels composed largely of BM-derived cells. In addition, we tested several tumor neovascularization models (lung cancer [LLC], melanoma [B16], pancreatic [PAN-02], lymphoma [EL4]) that showed a gradation of levels of BM cell migration to the tumor mass as well as integration into tumor-associated vasculature.
To test BM contribution in different neovascularization models, we developed a novel technique in which multiple neovascularization models were established in individual mice. For these studies, irradiated C57BL/6 mice received a transplant of BM-derived cells from mouse donors ubiquitously expressing green fluorescent protein (GFP) or DsRed. Mice showing stable BM engraftment 3 months after transplantation were subjected to a combination of either LLC and B16 tumors or retinal injury and LLC tumors. Use of this technique allowed us to track the fate of BM-derived cells in different models in single mice with similar engraftment chimerism, age, treatment, and housing environment, thus controlling for potential experimental variables that may confound overall data output and interpretation.2,5,20,21
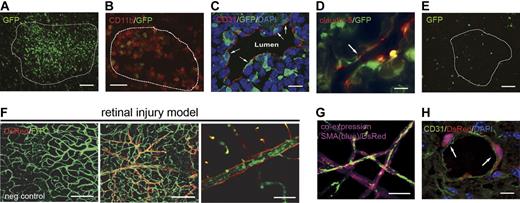
LLC lung cancers and B16 melanomas were inoculated in mice that received a transplant of GFP+ BM (n = 8) with LLCs in left hind limbs and B16 cells in contralateral right hind limbs. Tumors were harvested at the same time once they both achieved a total volume between 500 and 600 mm3. In our system, the growth kinetics of these tumors were similar, making it possible to obtain tumors of similar age and size (data not shown). Analysis of GFP+ BM contribution in each paired tumor set showed pronounced and preferential BM recruitment to LLC tumors in comparison with B16 tumors. In all LLC tumors analyzed, recruitment of BM cells was observed, with the majority of cells found throughout the tumor mass (Figure 1A). Immunofluorescent staining showed that these cells were mainly CD11b+ myelomonocytic cells, which are known to promote neovascularization through paracrine mechanisms (Figure 1B).24 Confocal microscopy of LLC sections for BM-derived cells coexpressing platelet endothelial cell adhesion molecule 1 (CD31) revealed BM-derived cells with elongated endothelial phenotype lining lumens of tumor-associated vasculature (Figure 1C; supplemental Video 1). Physical integration into LLC endothelia was detected by staining for claudin-5, a tight-junction protein associated with endothelial cells (Figure 1D).25 Primarily BM-derived vessels were not observed as in the retinal injury model, however the percentage of LLC vasculature containing at least 1 BM-derived, integrated endothelial cell was 17% (± 4%; control in Figure 4C). Of particular importance was the restricted expression of marrow-derived CD31 and claudin-5 to cells lining the lumens of tumor-associated vasculature. Rarely were these phenotypes observed on cells found throughout the tumor mass, which were solely CD11b+ (nonluminal GFP+ cells in Figure 1C-D). Conversely, analysis of B16 tumors revealed significantly less BM recruitment to the tumor mass with no observable contribution to tumor-associated vasculature (Figure 1E).
Differential BM contribution results from activation of redundant mechanisms of postnatal neovasculogenesis. Combinations of models of adult neovasculogenesis were established in single mice and result in different levels of BM contribution. (A-B) Lewis lung carcinoma cell (LLC)–based tumors showed GFP+ bone marrow (BM) contribution throughout the tumor mass (A; scale bar represents 100 μm) mainly from CD11b+ cells (B; n = 8; scale bar represents 50 μm). Tumors are outlined with dashed lines. (C-D) Within tumor-associated vasculature, CD31 (C) and claudin-5 (D) staining showed integration from GFP+ BM cells (scale bars represent 20 μm). (E) B16 tumors had low levels of GFP+ BM contribution in comparison with all other models and no contribution within tumor-associated vasculature (n = 8; scale bar represents 100 μm). (F) The most robust contribution was seen in the retinal injury model. This model uses vascular endothelial growth factor (VEGF) overexpression by a recombinant adeno-associated virus type 2 that overexpresses the murine 188 isoform of VEGF-A (rAAV2 VEGF-A 188) and laser-induced ischemic injury to promote robust BM-derived neovascularization. DsRed+ BM-derived blood vessels are shown (middle panel) along with a negative control (left panel; scale bars represent 100 μm). All animals were perfused with FITC-dextran to show functional vasculature (right panel; n = 5; scale bar represents 50 μm). Similar results were observed with GFP+ BM (data not shown). (G) Retinas were costained with α-SMA to confirm endothelial phenotype (n = 5; scale bar represents 50 μm). (H) LLC tumors established in mice that received a transplant of DsRed+ BM along with retinal injury also showed BM integration into tumor vasculature (n = 5; scale bar represents 20 μm). Confocal microscopy with 0.5-micron Z-step analysis was necessary to identify nucleated cells coexpressing donor GFP or DsRed and endothelial proteins (CD31).
Differential BM contribution results from activation of redundant mechanisms of postnatal neovasculogenesis. Combinations of models of adult neovasculogenesis were established in single mice and result in different levels of BM contribution. (A-B) Lewis lung carcinoma cell (LLC)–based tumors showed GFP+ bone marrow (BM) contribution throughout the tumor mass (A; scale bar represents 100 μm) mainly from CD11b+ cells (B; n = 8; scale bar represents 50 μm). Tumors are outlined with dashed lines. (C-D) Within tumor-associated vasculature, CD31 (C) and claudin-5 (D) staining showed integration from GFP+ BM cells (scale bars represent 20 μm). (E) B16 tumors had low levels of GFP+ BM contribution in comparison with all other models and no contribution within tumor-associated vasculature (n = 8; scale bar represents 100 μm). (F) The most robust contribution was seen in the retinal injury model. This model uses vascular endothelial growth factor (VEGF) overexpression by a recombinant adeno-associated virus type 2 that overexpresses the murine 188 isoform of VEGF-A (rAAV2 VEGF-A 188) and laser-induced ischemic injury to promote robust BM-derived neovascularization. DsRed+ BM-derived blood vessels are shown (middle panel) along with a negative control (left panel; scale bars represent 100 μm). All animals were perfused with FITC-dextran to show functional vasculature (right panel; n = 5; scale bar represents 50 μm). Similar results were observed with GFP+ BM (data not shown). (G) Retinas were costained with α-SMA to confirm endothelial phenotype (n = 5; scale bar represents 50 μm). (H) LLC tumors established in mice that received a transplant of DsRed+ BM along with retinal injury also showed BM integration into tumor vasculature (n = 5; scale bar represents 20 μm). Confocal microscopy with 0.5-micron Z-step analysis was necessary to identify nucleated cells coexpressing donor GFP or DsRed and endothelial proteins (CD31).
The combination of retinal injury and LLC inoculation was performed in mice that received a transplant of DSRed+ BM (n = 5). We observed primarily DsRed+ BM-derived vessels and perivascular cells that coexpressed α-smooth muscle actin (αSMA) in the injured retina, and luminal cells that we previously reported express the endothelial type markers factor VIII, CD31, and MECA-32 (Figure 1F-G).3,22,23 BM contribution to the LLC tumor mass was similar to that observed when LLC tumors were inoculated in combination with B16 tumors. As before, individual BM cells were integrated within tumor-associated vasculature without entire BM-derived vessels observed (Figure 1H). Given that LLC tumors exhibited similar levels of BM-derived cells whether in combination with the retinal injury or B16 melanoma suggests that site-specific factors regulate BM contribution to neovascularization. No difference in BM contribution was seen when GFP+ or DsRed+ chimeric mice were used. Taken together, these comparative biology results demonstrated a gradation in BM contribution in different model systems with the retinal injury model showing the highest BM contribution followed by LLC lung cancers and the least in B16 melanomas. Furthermore, BM contribution is lineage-wide, comprising cell populations known to participate in neovascularization including angiogenic monocytes (CD11b+) and endothelial cells (CD31+, claudin-5+). Based on these results, we used these models in concert to define global mechanisms facilitating BM contribution during neovascularization.
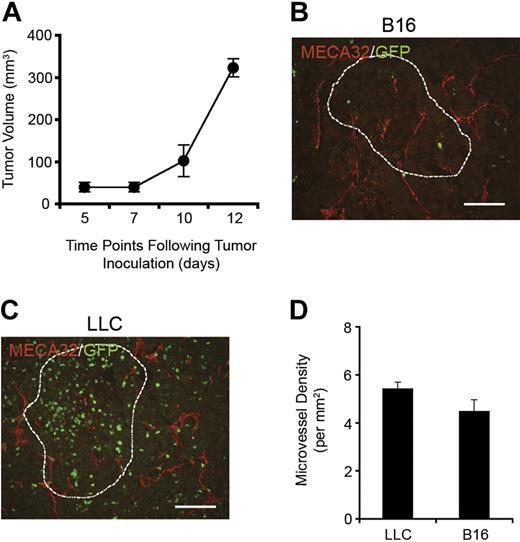
B16 tumors undergo neovascularization even without observable BM contribution
BM contribution to neovasculogenesis can be attributed to direct integration into blood vessels or via paracrine mechanisms. However, even without apparent gross BM migration to melanomas, tumor volume measurements over a 12-day period showed that B16 tumors were still capable of robust growth (Figure 2A). Based on this observation, we hypothesized that for these tumors to grow they must undergo neovascularization. When B16 tumors were stained for MECA32 to visualize blood vessels and analyzed with fluorescent microscopy, we observed newly generated blood vessels throughout the tumor mass (Figure 2B). A similar MECA32 staining pattern was observed in LLC tumors from contralateral limbs (Figure 2C). When microvessel density (MVD) was quantified, we found that both tumor types had statistically similar MVDs even with observed differences in BM contribution (Figure 2D). Given the lack of GFP+ BM-derived cells in B16 melanomas, the data strongly suggest that neovascularization occurs through redundant mechanisms that do not involve BM either via direct integration or paracrine regulation. These results likely explain why VEGFR2+ cells are not mobilized and do not contribute to melanoma progression.14
B16 melanoma neovessels in the absence of BM contribution. (A) B16 tumors were capable of robust growth even without BM-derived neovascularization (n = 8). (B-C) MECA-32 staining demonstrated the presence of new blood vessels within B16 (B) and LLC tumors (C; scale bars represent 100 μm). (D) By quantifying MVD, it was found that B16 and LLC tumors contained microvessels at similar densities. LLC and B16 tumors are outlined with dashed lines.
B16 melanoma neovessels in the absence of BM contribution. (A) B16 tumors were capable of robust growth even without BM-derived neovascularization (n = 8). (B-C) MECA-32 staining demonstrated the presence of new blood vessels within B16 (B) and LLC tumors (C; scale bars represent 100 μm). (D) By quantifying MVD, it was found that B16 and LLC tumors contained microvessels at similar densities. LLC and B16 tumors are outlined with dashed lines.
Endogenous SDF-1α expression is necessary for BM contribution in neovasculogenesis
To begin to define redundant mechanisms (BM contributing or non-BM contributing) during postnatal neovascularization, we first analyzed the role of leukocyte trafficking factors known to modulate BM migration and homing. Specifically, we focused on the local endogenous production of SDF-1α in each of our models given its strong chemotactic effects on BM cells.22,26
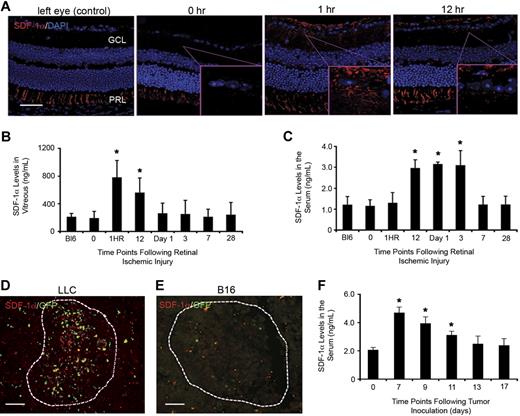
After laser-induced retinal ischemic injury, retinas showed immediate up-regulation of SDF-1α in the ganglion cell layer (GCL)—the initiating site for proliferative neovascularization induced by this model. SDF-1α protein expression was increased in as little as 1 hour after ischemic injury and continued up to 12 hours after laser injury (Figure 3A). Unmanipulated control eyes and 0-hour eyes (before laser treatment) showed nondetectable levels of SDF-1α expression during the same time period. SDF-1α is constitutively expressed in the retinal-pigmented epithelium, thus serving as an internal positive control for SDF-1α staining (photoreceptor layer [PRL]).27 Kinetic ELISA analysis of SDF-1α in the vitreal space showed significant increases in SDF-1α levels from 1 to 12 hours after laser injury before returning to background levels (Figure 3B). Analysis of blood serum demonstrated increased levels of SDF-1α at 12 hours that continued until day 3 (Figure 3C).
Endogenously produced SDF-1α is a trigger for BM contribution to sites of postnatal neovasculogenesis. (A) Eyes were harvested and embedded in paraffin at different time points after retinal injury. Tissues were sectioned (5 μm) and staining was performed for SDF-1α and DAPI (4,6 diamidino-2-phenylindole). As expected, consistent expression of SDF-1α in the PRL was observed. Nontreated left eyes served as controls. SDF-1α expression is observed in the GCL at 1 and 12 hours after laser injury (n = 5; scale bar represents 100 μm). (B-C) SDF-1α ELISA of samples from the vitreous and blood serum after retinal ischemic injury. Vitreous fluid and blood serum were obtained from mice at different time points after retinal injury. ELISA analysis showed a direct correlation to the staining results with significant increases in SDF-1α levels observed at 1 hour and 12 hours in the vitreous (B) and blood serum (C), respectively (n = 5; *P < .05). (D-E) SDF-1α expression was also observed in LLC tumors (D) with nondetectable levels seen in B16 tumors (E). Tumors are outlined by dashed lines (n = 8; scale bars represent 100 μm). (F) ELISA analysis of SDF-1α in the serum of mice inoculated with both LLC and B16 tumors showed a significant increase in serum levels 7 days after tumor inoculation, with levels returning to background by day 13 (n = 5; *P < .05).
Endogenously produced SDF-1α is a trigger for BM contribution to sites of postnatal neovasculogenesis. (A) Eyes were harvested and embedded in paraffin at different time points after retinal injury. Tissues were sectioned (5 μm) and staining was performed for SDF-1α and DAPI (4,6 diamidino-2-phenylindole). As expected, consistent expression of SDF-1α in the PRL was observed. Nontreated left eyes served as controls. SDF-1α expression is observed in the GCL at 1 and 12 hours after laser injury (n = 5; scale bar represents 100 μm). (B-C) SDF-1α ELISA of samples from the vitreous and blood serum after retinal ischemic injury. Vitreous fluid and blood serum were obtained from mice at different time points after retinal injury. ELISA analysis showed a direct correlation to the staining results with significant increases in SDF-1α levels observed at 1 hour and 12 hours in the vitreous (B) and blood serum (C), respectively (n = 5; *P < .05). (D-E) SDF-1α expression was also observed in LLC tumors (D) with nondetectable levels seen in B16 tumors (E). Tumors are outlined by dashed lines (n = 8; scale bars represent 100 μm). (F) ELISA analysis of SDF-1α in the serum of mice inoculated with both LLC and B16 tumors showed a significant increase in serum levels 7 days after tumor inoculation, with levels returning to background by day 13 (n = 5; *P < .05).
LLC tumors, which consistently show BM contribution but at lower levels than the retinal injury model, also demonstrated marked SDF-1α expression within the tumor mass (Figure 3D). In contrast, B16 melanomas, showing little to no BM contribution, demonstrated nondetectable levels of SDF-1α expression (Figure 3E). Analysis of serum SDF-1α levels in mice inoculated with both LLC and B16 tumors showed an increase from days 7 to 11 after inoculation, and then returning to background levels by day 13 (Figure 3F). Importantly, in these individual mice harboring both tumors, B16 melanomas were systemically exposed to elevated serum SDF-1α levels brought about by the LLC tumors and still showed little to no BM homing to the tumor sites. The data further highlights the importance of site-specific SDF-1α expression as a key aspect of BM-derived neovascularization. Subsequent analysis of supernatants derived from in vitro–cultured LLC and B16 melanoma cells showed nondetectable levels of SDF-1α protein, suggesting that endogenous SDF-1α is locally generated by sources within the tumor microenvironment.10,28 Together, these results point to a time-dependent cascade between site-specific SDF-1α expression and serum levels, suggestive of an endogenous SDF-1α accumulation that promotes mobilization of BM cells to the peripheral blood and subsequent migration to sites of neovascularization.3,19,28
Blocking SDF-1α inhibits BM contribution in neovasculogenesis and tumor growth
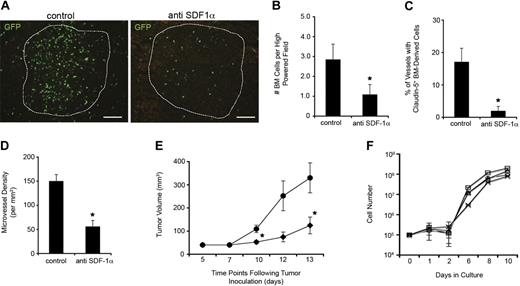
To further confirm the link between SDF-1α production and BM contribution, we performed antibody-blocking studies to prevent functional SDF-1α activity. The antibodies were administered directly at sites of neovascularization given our data showing that site-specific, endogenous chemokine production is a requirement for BM contribution. We chose direct tumoral injection versus systemic injection to achieve a high concentration of the antibody in the local tumor microenvironment with lower systemic concentrations/effects. We reasoned local administration would better block SDF-1α–dependent homing to the site with fewer effects on mobilization of the marrow. We previously demonstrated that treatment with anti–SDF-1α–neutralizing antibodies in the vitreous 1 day after ischemic retinal injury blocked recruitment and BM-derived neovascularization.22 Using similar concentrations of anti–SDF-1α antibodies with validated activity, injection in LLC tumors resulted in significantly lower BM recruitment within the tumor mass (Figure 4A-B) as well as integrated within blood vessels (Figure 4C). MVD was also significantly lower in anti–SDF-1α–treated LLC tumors (Figure 4D). The overall effect of anti–SDF-1α treatment on tumor size, which is a direct correlation to the extent of tumor neovascularization, was also assessed over a 2-week period and showed that anti–SDF-1α treatment generated significantly smaller tumors in comparison with controls (Figure 4E). Similar blocking studies were performed in mice inoculated with both LLC and B16 tumors. The results of these experiments showed no effects of anti–SDF-1α treatment on either MVD or tumor size in B16 tumors consistent with the lack of SDF-1α activity in B16 tumor neovascularization (n = 6; data not shown).
Blocking SDF-1α activity inhibits BM contribution to tumor neovascularization. After LLC inoculation, mice were treated with intratumoral anti–SDF-1α antibodies to block BM contribution (n = 5). (A-B) Tumors treated with anti–SDF-1α contained significantly lower numbers of BM-derived cells throughout the tumor mass seen visually (A; scale bars represent 100 μm) and by counting GFP+ cell numbers in standardized field sizes (B; *P < .05). (C-D) Treated mice also had significantly fewer cells integrated within blood vessel walls (C) and decreased MVD (D) compared with control tumors (*P < .05). (E) Animals treated with anti–SDF-1α antibodies (♦) also generated significantly smaller tumors in comparison with controls (●; *P < .05). (F) The growth kinetics of LLCs in culture with various doses of anti–SDF-1α antibodies (0 to 50 μg/mL) were similar over the range of concentrations tested. No cytotoxicity was observed at 0 (○), 12.5 (□), 25 (▵), and 50 μg/mL (x) of the anti–SDF-1α antibodies in growth media (n = 4).
Blocking SDF-1α activity inhibits BM contribution to tumor neovascularization. After LLC inoculation, mice were treated with intratumoral anti–SDF-1α antibodies to block BM contribution (n = 5). (A-B) Tumors treated with anti–SDF-1α contained significantly lower numbers of BM-derived cells throughout the tumor mass seen visually (A; scale bars represent 100 μm) and by counting GFP+ cell numbers in standardized field sizes (B; *P < .05). (C-D) Treated mice also had significantly fewer cells integrated within blood vessel walls (C) and decreased MVD (D) compared with control tumors (*P < .05). (E) Animals treated with anti–SDF-1α antibodies (♦) also generated significantly smaller tumors in comparison with controls (●; *P < .05). (F) The growth kinetics of LLCs in culture with various doses of anti–SDF-1α antibodies (0 to 50 μg/mL) were similar over the range of concentrations tested. No cytotoxicity was observed at 0 (○), 12.5 (□), 25 (▵), and 50 μg/mL (x) of the anti–SDF-1α antibodies in growth media (n = 4).
One possibility explaining the observed effects of anti–SDF-1α treatment is a direct cytotoxic effect of the antibodies on growing cancer cells. To address this possibility, we established in vitro cultures of LLCs in the presence of escalating concentrations of anti–SDF-1α antibodies. We used concentrations equal to and above the levels used in the in vivo animal experiments. In vitro cell growth was similar between all cultures, regardless of the presence of anti–SDF-1α and regardless of antibody concentration (Figure 4F). Thus, it is unlikely that a direct cytotoxic effect was the cause of tumor inhibition, and more likely that alternative effects such as impaired neovasculogenesis explain cancer inhibition from anti–SDF-1α treatment. Together, the data strongly implicate SDF-1α as a major effector in the mechanism driving BM-derived neovasculogenesis.
HSCs are a source of BM cells that participate in long-term neovasculogenesis
To better elucidate mechanisms of long-term BM contribution to postnatal neovascularization, we next set out to identify cell populations that participate in this process. Our group previously demonstrated that the adult HSC is capable of repairing blood vessels in ischemic retinas.3,29 It is unknown whether HSCs can also serve as a source of blood vessels in cancer. Thus, we performed studies to test the adult HSC using our lung cancer model given evidence of marrow-derived cancer neovasculogenesis. The classic definitive assay for HSC function in murine models is single-cell transplantation, demonstrating durable multilineage reconstitution followed by serial transplantation to demonstrate self-renewal. Therefore, we analyzed HSC-derived contribution to LLC lung cancers in mice that had received serial transplants from donors initially engrafted with single HSCs.
Irradiated C57BL/6 recipients received a transplant of single HSCs from mouse donors ubiquitously expressing GFP. Single GFP+ HSCs were initially enriched as a Sca-1+ c-kit+ lin− (SKL) population by FACS followed by individual selection with micromanipulators before transplantation. Of 80 single HSC transplant recipients, 3 demonstrated long-term, multilineage GFP+ hematopoietic chimerism. BM from the 3 engrafted mice was then isolated and serially transplanted into 20 secondary recipients. Six months after secondary transplantation, multilineage engraftment was established in 9 of the 20 mice (Figure 5A). These engrafted mice were then inoculated with LLC cancer cells and tumors allowed to grow to 500 mm3 to 600 mm3 in volume. All LLC tumors demonstrated HSC-derived contribution to the tumor mass (Figure 5B). HSC-derived contribution to cancer neovessels was 5% (± 2%; Figure 5C). The degree of BM-derived cells in tumor vasculature was significantly lower in comparison with mice that received a transplant of whole BM (P < .05; vs control in Figure 4C), which is attributed to lower levels of hematopoietic engraftment seen in mice that underwent serially transplantation (data not shown). These results show that the adult HSC is a source of cells that contribute to long-term cancer neovasculogenesis.
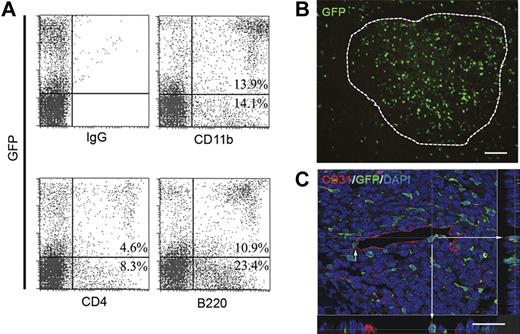
Progeny of single HSCs participate in neovascularization. (A) Mice that received a transplant of GFP+ BM cells from primary donors initially engrafted with single HSCs demonstrate multilineage engraftment. Shown are representative flow cytometry plots demonstrating both myeloid (CD11b) and lymphoid (CD4 and B220) cell populations in engrafted secondary mice. Also shown is a representative isotype control. (B) In these mice, LLC tumors showed substantial HSC-derived GFP+ cell contribution throughout the tumor mass (n = 9; scale bar represents 100 μm). (C) Confocal micrograph of HSC-derived GFP+ cell in blood vessel wall coexpressing CD31. Orthogonal views created from confocal sections verify luminal expression of CD31 on HSC-derived cells.
Progeny of single HSCs participate in neovascularization. (A) Mice that received a transplant of GFP+ BM cells from primary donors initially engrafted with single HSCs demonstrate multilineage engraftment. Shown are representative flow cytometry plots demonstrating both myeloid (CD11b) and lymphoid (CD4 and B220) cell populations in engrafted secondary mice. Also shown is a representative isotype control. (B) In these mice, LLC tumors showed substantial HSC-derived GFP+ cell contribution throughout the tumor mass (n = 9; scale bar represents 100 μm). (C) Confocal micrograph of HSC-derived GFP+ cell in blood vessel wall coexpressing CD31. Orthogonal views created from confocal sections verify luminal expression of CD31 on HSC-derived cells.
CD133+CXCR4+ effector cells contribute to robust neovasculogenesis
To define an “effector” cell population responsible for directly participating in HSC-derived neovascularization, we hypothesized that progenitor cells responsive to SDF-1α are likely to contribute to neovasculogenesis. Thus, we focused on the vasculogenic activity of cells expressing CXCR4. We also focused on cells expressing CD133, given reports of CD133 expression on progenitor cells from a variety of tissues and our own evidence that these progenitor cells participate in the repair of retinal pigmented epithelium after injury.30 Using competitive repopulation assays, we determined that CD133+CXCR4+ cells are short-term repopulating hematopoietic progenitor cells (supplemental Figure 1). Furthermore, having shown that the progeny of SKL HSCs participates in long-term neovacularization (Figure 5)3 and that SKL HSCs do not directly participate in this process (data not shown), we hypothesized that marrow-derived hematopoietic progenitor cells coexpressing CD133 and CXCR4 contribute directly to neovascularization (Figure 6A). For this study, we sought for the first time to define the vasculogenic potential of this progenitor cell population as a means to define a new marrow-derived vasculogenic effector cell population. FACS analysis of BM-derived CD133+CXCR4+ cells revealed expression of a plurality of markers that encompass different, phenotypically defined BM cells known to participate in neovascularization (Figure 6B).31,32 Interestingly, CD133+CXCR4+ cells resided mainly in the granulocyte-monocyte progenitor cell population and not in populations expressing markers associated with long-term HSCs or lymphoid progenitors (supplemental Figure 2).
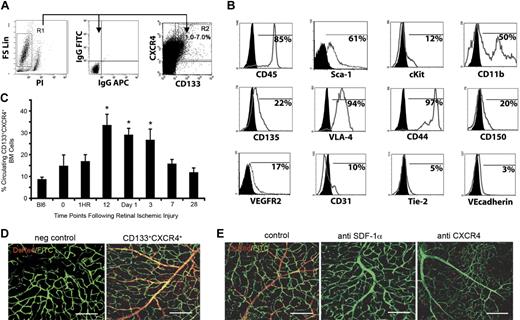
BM-derived CD133+CXCR4+ cells enrich for neovascularization potential. The retinal injury model that shows the most robust BM contribution was used to identify BM-derived cells with direct neovasculogenic potential. (A) BM isolated from wild-type C57Bl/6 mice was analyzed for CD133 and CXCR4 expression. (B) Flow cytometric analysis of gated CD133+CXCR4+ cells shows an expression pattern that includes markers known to be found on BM-derived cells that participate in neovasculogenesis. CD133+CXCR4+ cells express endothelial cell surface markers such as VEGFR2, CD31, VE-cadherin, and tie 2. They also express CD45, CD117 (c-kit), Sca-1, VLA-4, CD11b, CD44, CD150, and CD135 (flt-3). (C) Kinetic analysis of CD133+CXCR4+ cell mobilization into the peripheral blood of mice after retinal injury showed an increase in cell number that correlated with SDF-1α levels in blood serum (n = 6; *P < .05). (D) Retinal flat mounts of injured eyes after induction of retinal ischemic injury that were adoptively transplanted with 106 BM-derived CD133+CXCR4+DsRed+ cells showed contribution to retinal neovascularization (n = 6). Untreated left eye (negative control) is also shown (scale bars represent 100 μm). Similar results were observed with GFP+ BM (data not shown; E) CD133+CXCR4+DsRed+ cell transplanted eyes that underwent induction of retinal ischemic injury with the added step of intravitreal injection with PBS containing an anti–SDF-1α– or anti–CXCR4-neutralizing antibody to a final concentration of 1 μg/mL are shown. Note the absence of newly formed vessels from CD133+CXCR4+DsRed+ BM cells under these conditions. As a control, treated eyes that underwent induction of retinal ischemic injury were treated with PBS containing an isotype-matched control antibody (n = 6; scale bars represent 100 μm). All animals were perfused with FITC-dextran to show functional vasculature.
BM-derived CD133+CXCR4+ cells enrich for neovascularization potential. The retinal injury model that shows the most robust BM contribution was used to identify BM-derived cells with direct neovasculogenic potential. (A) BM isolated from wild-type C57Bl/6 mice was analyzed for CD133 and CXCR4 expression. (B) Flow cytometric analysis of gated CD133+CXCR4+ cells shows an expression pattern that includes markers known to be found on BM-derived cells that participate in neovasculogenesis. CD133+CXCR4+ cells express endothelial cell surface markers such as VEGFR2, CD31, VE-cadherin, and tie 2. They also express CD45, CD117 (c-kit), Sca-1, VLA-4, CD11b, CD44, CD150, and CD135 (flt-3). (C) Kinetic analysis of CD133+CXCR4+ cell mobilization into the peripheral blood of mice after retinal injury showed an increase in cell number that correlated with SDF-1α levels in blood serum (n = 6; *P < .05). (D) Retinal flat mounts of injured eyes after induction of retinal ischemic injury that were adoptively transplanted with 106 BM-derived CD133+CXCR4+DsRed+ cells showed contribution to retinal neovascularization (n = 6). Untreated left eye (negative control) is also shown (scale bars represent 100 μm). Similar results were observed with GFP+ BM (data not shown; E) CD133+CXCR4+DsRed+ cell transplanted eyes that underwent induction of retinal ischemic injury with the added step of intravitreal injection with PBS containing an anti–SDF-1α– or anti–CXCR4-neutralizing antibody to a final concentration of 1 μg/mL are shown. Note the absence of newly formed vessels from CD133+CXCR4+DsRed+ BM cells under these conditions. As a control, treated eyes that underwent induction of retinal ischemic injury were treated with PBS containing an isotype-matched control antibody (n = 6; scale bars represent 100 μm). All animals were perfused with FITC-dextran to show functional vasculature.
To test whether BM-derived CD133+CXCR4+ cells directly participate in postnatal neovascularization, we used our retinal ischemia model, given that it has the most robust marrow-derived neovasculogenesis. We first measured CD133+CXCR4+ levels in peripheral blood after retinal ischemic injury. We observed a sustained increase in the percentage of circulating CD133+CXCR4+ cells from 12 hours to 3 days in injured mice (Figure 6C). Importantly, there was a distinct positive correlation between percentage of circulating CD133+CXCR4+ cells and level of SDF-1α in blood serum (Figure 3C). Timing of the highest levels of CD133+CXCR4+ cell mobilization coincided with timing of the highest levels of serum SDF-1α levels.
To determine whether BM-derived CD133+CXCR4+ cells actively participate in short-term effector cell neovascularization, we adoptively transferred 106 CD133+CXCR4+DsRed+ donor cells intravenously 1 day after retinal injury (n = 6). Four weeks after ischemia, injured right eyes and control left eyes were enucleated and retinas were flat-mounted. We observed extensive contribution of CD133+CXCR4+DsRed+ donor cells to sites of neovascularization with entire vessels made up of donor cells (Figure 6D). All left eye controls showed no contribution from CD133+CXCR4+DsRed+ donor cells. These test retinas were indistinguishable from the standard retinal neovascularization model control.3,22,23
Based on our data showing that SDF-1α plays a major role in recruitment of BM-derived cells to sites of neovascularization, we performed studies in which neutralizing antibodies to either SDF-1α or CXCR4 were concurrently injected into the vitreous of separate cohorts of mice receiving CD133+CXCR4+DsRed+ progenitor cells (n = 6). In eyes that received anti–SDF-1α or anti-CXCR4 antibodies, there was maximal blockage of recruitment and incorporation at sites of vascular injury (Figure 6E). Interestingly, in eyes receiving anti-CXCR4 antibody, there appeared to be some recruitment to sites of injury but no incorporation of CD133+CXCR4+DsRed+ cells into new vessels. Together, these data suggest that BM-derived CD133+CXCR4+ cells are recruited to sites of neovascularization, directly participate in vessel formation, and respond to modulation of the SDF-1α/CXCR4 axis.
Discussion
Opposing conclusions have been made about BM contribution to postnatal neovasculogenesis based on data indicating that BM can1-10 and cannot11-19 generate blood vessel endothelial cells. Different model systems used in these studies may explain these confounding conclusions. For example, differences among previous studies using cancer as the model system include cancer type, carcinomas versus adenomas, sites of tumor growth, numbers and type of transplanted cells, and methods of detection. Although the heterogeneity of BM contribution has been acknowledged, the reasons have remained largely unexplored.
To control for variability in experimental parameters, we used a novel technique in which combinations of neovascularization models were established in individual mice to conduct a comparative analysis of adult BM activity in settings of ischemic injury and cancer. The data indicate a spectrum of BM involvement in neovasculogenesis that is dependent on model system with SDF-1α as a key permissive trigger. We also demonstrate that where BM does contribute to neovascularization, single HSCs are a source of cells providing long-term neovascularization and that myeloid progenitor cells expressing CXCR4 and CD133 exhibit potential to directly participate in blood vessel formation. Our study provides an explanation for contradictory results observed in the field, thus establishing a unified understanding of BM contribution to postnatal neovasculogenesis, and identifies populations of BM cells that participate in this process.
In our own experience, we noted that certain conditions elicit BM contribution to neovessels (ie, retinal ischemia, LLC lung cancer), whereas other conditions (ie, B16 melanoma) induce little to no BM contribution. By combining neovascularization models in single mice, we conducted head-to-head comparisons of BM contribution with neovessel growth, while controlling for all other experimental variables. We report a wide range of BM contribution among neovascularization model systems, with the retinal injury model inducing the highest levels, followed by LLC lung cancers, and then B16 melanomas. In search of mechanisms evoking BM contribution, we report that neovascularization conditions with BM-derived neovessels exhibited both increased SDF-1α serum levels and site-specific SDF-1α expression, whereas conditions with no BM-derived neovessels (ie, B16 melanomas) exhibited no site-specific SDF-1α expression. Moreover, even though B16 melanomas grew in an environment of elevated serum SDF-1α levels, induced by contralateral LLC tumors, they still contained little to no BM contribution. The data indicate that SDF-1α is a key permissive trigger and that microenvironmental chemokine production and activity are critical for the multistep process of BM-derived neovasculogenesis.
Previous investigators have also noted little to no BM-derived endothelial cells in B16 melanomas, further supporting our findings of a diminished role of BM in this tumor model.14 However, we also show that B16 tumors contained blood vessels at a density similar to that of LLC tumors, indicating the ability of melanomas to generate new blood vessels even in the absence of BM-derived cells. These results clearly demonstrate that neovascularization must occur through redundant mechanisms. We also report that LLC lung cancers, although showing BM contribution to neovessels, did not generate entire blood vessels compared with our retinal injury model. These data suggest that LLC lung cancers use a combination of mechanisms (BM derived and non–BM derived) to achieve neovascularization. This also may explain many of the contradictory conclusions described in the literature. Specifically, studies using LLC cancers have demonstrated opposing results, with some investigators demonstrating a reliance of BM-derived cells in tumor neovascularization,4 whereas other investigators have shown neovascularization in LLC lung cancers mediated via local angiogenic events.12 Previously, it was concluded that these discrepancies might be due to differences in experimental techniques. Based on our data, it may also be that the different techniques triggered a dominance of one of the redundant mechanisms over the other that could have influenced conclusions about BM contribution in this model system.
Given the hemangioblast potential of the adult HSC,3 we also questioned whether the HSC is a source of cells that participate in tumor neovascularization. To rigorously address HSC contribution to cancer neovessels, we used engrafted secondary mice that resulted from a combination of single HSC transplantation in primary mice followed by serial transplantation. The model was intended to address the limitations of previous studies and rigorously test the HSC as a clonal source of cells that play a role in tumor neovascularization. The data indicate that a clonal, self-renewing HSC is capable of generating cells that actively participate in blood vessel formation within LLC tumors. Moreover, the level of contribution was commensurate with hematopoietic engraftment, much like in whole BM experiments. This study also identifies a BM-derived HSC progeny subset that enriches for vasculogenic potential. Specifically, we observed that BM-derived CD133+CXCR4+ cells exhibit neovascular potential in the retinal injury model where BM contribution was most pronounced. We show significant mobilization of this subset in response to SDF-1α signaling and direct contribution to neovessels after adoptive transplantation of these cells after retinal injury. Importantly, phenotypic analysis of this progenitor population shows that it comprises many of the known angiogenic cell types identified in BM, including those of the hematopoietic and endothelial lineages.
Given our findings of redundant and diverse mechanisms governing adult neovasculogenesis, we speculate that therapies aimed at abrogating new blood vessel formation must simultaneously target mechanisms that use BM as well as non-BM constituents. The data presented herein support this concept. In the retinal injury model, treatment with anti–SDF-1α antibodies alone was able to abrogate BM-derived neovascularization, suggesting a profound reliance of this model system on BM contribution. In the LLC model, although we were able to significantly inhibit BM-derived neovasculogenesis, tumors were still able to proliferate and grow, albeit at a significantly slower rate in comparison with control tumors. A multitarget, antivascular approach is likely needed for conditions such as LLC lung cancers, which can draw upon BM-derived and non–BM-derived neovascularization. Finally, blocking SDF-1α activity in B16 melanomas had no effect on tumor neovascularization or growth due to the naturally nonessential role of BM in this tumor type. In our studies, we directly blocked SDF-1α activity with neutralizing antibodies at a fixed dose; however, determining responses over a range of anti–SDF-1α concentrations may provide additional information especially as it relates to future therapeutic applications. The varied responses to anti–SDF-1α treatments in our studies further characterize the diverse mechanisms in which neovascularization can occur, provide some explanation of the varied responses to current antivascular therapies, and highlight the potential need to apply combination therapies that suppress both BM-derived and non–BM-derived neovasculogenesis to achieve effective antivascular responses.33 In fact, reports have already described the diversity of endothelial cells by recognizing the need to use multiantigen staining techniques for robust endothelial cell identification and imaging.34 Clinically, targeting blood vessels for therapeutic purposes will likely require similar multiantigen strategies.
The controversy that exists over the role of BM during postnatal neovascularization stems from an ideology that categorizes this process into an all or nothing phenomenon. However, as with most biologic processes governing life, redundancy is built into this system where several processes are capable of achieving the desired outcome. Here we propose a unified understanding of postnatal neovasculogenesis where previously divergent results now converge to describe the interrelated components of active redundant processes. Our coordinated use of neovascularization model systems demonstrate a spectrum of BM contribution, making it possible for us to postulate mechanisms outlined in Figure 7. Based on our experience with these diversified models, we demonstrate that endogenous SDF-1α is a key permissive trigger for BM contribution. Moreover, this activity with vasculopathic consequences can be targeted in a therapeutic manner. At sites lacking endogenous SDF-1α, BM contribution was minimal and neovascularization was achieved via activation of alternative mechanisms from non-BM sources. The unified understanding and methods presented in this paper permit clarification of the role of BM in postnatal vasculogenesis and facilitate discussion of best methods to target vasculopathic conditions such as ischemia and cancer.
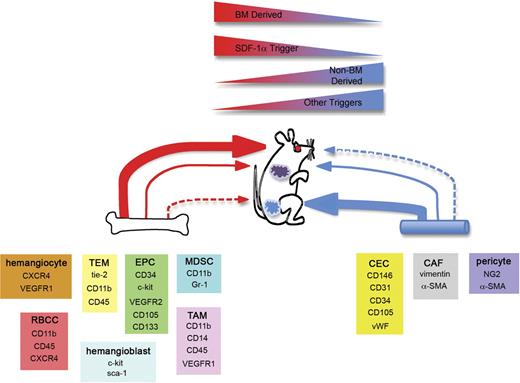
Redundant mechanisms of postnatal neovascularization. Based on the studies outlined herein, we postulate that redundant mechanisms exist to achieve postnatal neovascularization. Within BM and other tissues including blood vessels, reside cells that are capable of participating in new blood vessel formation. Examples of these cell types and their phenotypes are provided. The extent of contribution is dependent on the model system. Some models including injured retinas (red circle) demonstrate robust BM contribution resulting in entire BM-derived blood vessels. Other models like LLC tumors (purple tumor) will incorporate BM cells into new vasculature, but the microenvironment of the tumor may also use other means of tumor neovessel formation. In this setting, redundant mechanisms act in concert to achieve new blood vessel growth. Finally, models such as B16 tumors (blue tumor) will have little to no BM contribution and rely mainly on non–BM-derived cells to generate new vessels. At sites of neovascularization, SDF-1α acts as a regulatory molecule necessary for BM recruitment and participation. Active sites that do not express SDF-1α are much less prone to BM involvement and undergo neovascularization via a non–BM-derived mechanism.
Redundant mechanisms of postnatal neovascularization. Based on the studies outlined herein, we postulate that redundant mechanisms exist to achieve postnatal neovascularization. Within BM and other tissues including blood vessels, reside cells that are capable of participating in new blood vessel formation. Examples of these cell types and their phenotypes are provided. The extent of contribution is dependent on the model system. Some models including injured retinas (red circle) demonstrate robust BM contribution resulting in entire BM-derived blood vessels. Other models like LLC tumors (purple tumor) will incorporate BM cells into new vasculature, but the microenvironment of the tumor may also use other means of tumor neovessel formation. In this setting, redundant mechanisms act in concert to achieve new blood vessel growth. Finally, models such as B16 tumors (blue tumor) will have little to no BM contribution and rely mainly on non–BM-derived cells to generate new vessels. At sites of neovascularization, SDF-1α acts as a regulatory molecule necessary for BM recruitment and participation. Active sites that do not express SDF-1α are much less prone to BM involvement and undergo neovascularization via a non–BM-derived mechanism.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Robert Mames, Wenyin Shi, Doug Smith, and Neal Benson for technical assistance.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; K08 DK067359; C.R.C.); Florida Department of Health James & Esther King Biomedical Research Program (C.R.C.); National Heart, Lung, and Blood Institute (NHLBI; R01 HL070738 and R01 HL075258; E.W.S); as well as National Cancer Institute (NCI) Public Health Service (PHS) grants (CA084408 and CA089655; D.W.S.).
National Institutes of Health
Authorship
Contribution: G.J.M. and J.M.B. contributed to conception of hypotheses, experimental design, execution of experiments, data analysis, and paper preparation; K.H., M.J., D.F., S.M.G., A.K.S., A.B., K.J.R., and J.O. contributed to execution of experiments; D.W.S. contributed to conception of hypotheses and experimental design; and E.W.S. and C.R.C. contributed to conception of hypotheses, experimental design, data analysis, and paper preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edward W. Scott, 1600 SW Archer Rd, Box 100232, Gainesville, FL 32610-0232; e-mail: escott@ufl.edu; or Christopher R. Cogle, 1600 SW Archer Rd, Box 100278, Gainesville, FL 32610-0278; e-mail: c@ufl.edu.
References
Author notes
G.J.M. and J.M.B. contributed equally to this work.