Abstract
Decreased activity of osteoblasts (OBs) contributes to osteolytic lesions in multiple myeloma (MM). The production of the soluble Wnt inhibitor Dickkopf-1 (DKK1) by MM cells inhibits OB activity, and its serum level correlates with focal bone lesions in MM. Therefore, we have evaluated bone anabolic effects of a DKK1 neutralizing antibody (BHQ880) in MM. In vitro BHQ880 increased OB differentiation, neutralized the negative effect of MM cells on osteoblastogenesis, and reduced IL-6 secretion. In a severe combined immunodeficiency (SCID)–hu murine model of human MM, BHQ880 treatment led to a significant increase in OB number, serum human osteocalcin level, and trabecular bone. Although BHQ880 had no direct effect on MM cell growth, it significantly inhibited growth of MM cells in the presence of bone marrow stromal cells (BMSCs) in vitro. This effect was associated with inhibition of BMSC/MM cell adhesion and production of IL-6. In addition, BHQ880 up-regulated β-catenin level while down-regulating nuclear factor-κB (NF-κB) activity in BMSC. Interestingly, we also observed in vivo inhibition of MM cell growth by BHQ880 treatment in the SCID-hu murine model. These results confirm DKK1 as an important therapeutic target in myeloma and provide the rationale for clinical evaluation of BHQ880 to improve bone disease and to inhibit MM growth.
Introduction
A cardinal clinical feature of multiple myeloma (MM) is the presence of osteolytic bone lesions. Myeloma cells disrupt the delicate balance between bone formation and bone resorption.1,2 Various clinical observations3 and experimental studies4,5 have linked the level of MM bone disease with disease burden. Increased osteoclastic activity and its molecular basis have long been considered a primary pathogenic event in MM bone disease. However, a molecular basis for the well recognized lack of osteoblast (OB) function, specifically DKK-1, in the MM bone disease has only recently been described.6,7 Canonical Wnt pathway plays an important role in controlling proliferation, differentiation, and survival of OB.8-11 Previous studies have reported high expression levels of the canonical Wnt inhibitor DKK1 and osteolytic bone lesions in various tumor types including breast,12,13 neuroblastoma,14 esophageal, and lung cancer,15 and conversely enhanced OB activity and osteoblastic bone lesions associated with decreased DKK1 levels in prostate and colon cancers.16-18 In MM, high serum DKK1 levels were correlated with focal bone lesions.19 The DKK1 produced by MM cells can inhibit the differentiation of OB precursor cells19 and bone formation in vitro20 through a DKK1-mediated attenuation of Wnt3a-induced stabilization of β-catenin.21 These findings confirm DKK1 as an important regulator of bone formation in the bone microenvironment. The importance of DKK1 secretion in diseases associated with bone destruction is reinforced by a recent study showing that DKK1 mediates the bone destructive effects of rheumatoid arthritis and that a neutralizing antibody to DKK1 could inhibit the bone destructive process in that disease.22
There is also emerging evidence that the cellular bone compartment affects MM cell growth and progression. This is supported by the observation that osteoclasts can support long-term survival and proliferation of primary MM cells,23,24 and OB may impede MM cell growth.7,25 Thus, targeting these cellular elements may also favorably affect disease control. Therefore, we have evaluated DKK1 as a therapeutic target in MM in the context of the bone marrow (BM) microenvironment, analyzing the effect of a human DKK1 neutralizing antibody (BHQ880). We show that this clinically applicable antibody increases OB function and number and also has anti-MM effect when evaluated in the presence of the BM milieu.
Methods
Reagents
BHQ880 is a phage-derived DKK1 neutralizing human immunoglobulin G1 (IgG1) antibody (provided by Novartis, Cambridge, MA). BHQ880 has a high affinity for and can neutralize both human DKK1 and murine DKK1. IgG1 isotype antibody was used as control.
Cells
Bone marrow mononuclear cells (BMMNCs) and primary MM cells were isolated using Ficoll-Hypaque density gradient sedimentation from BM aspirates from MM patients after informed consent, in accordance with the Declaration of Helsinki, and Institutional Review Board (IRB; Dana-Farber Cancer Institute, Boston, MA) approval. BMMNCs were cultured in RPMI 1640 supplemented with 20% fetal bovine serum (FBS; 4-8 weeks) to establish bone marrow stromal cells (BMSCs). MM cells were separated from BM samples by antibody-mediated positive selection using anti-CD138 magnetic-activated cell separation microbeads (Miltenyi Biotech). A purity of 95% CD138+ cells was obtained. The interleukin-6 (IL-6)-dependent MM cell lines INA6 and XG1 were cultured in RPMI 1640 (Mediatech) supplemented with 10% FBS and 1 ng/mL rhIL-6 (R&D Systems). MM1S, OPM1, OPM2, and U266 MM cell lines were cultured in RPMI 1640 supplemented with 10% FBS.
OB calcium deposition assay
Human osteoprogenitor cells (pre-OB) were obtained from BM adherent cells from MM patient after 3 days attachment period. The pre-OB cells were stimulated with osteoblastic differentiation media containing 2.16 mg/mL β-glycerolphosphate, 0.05 mg/mL ascorbic acid, and 10 nM dexamethasone (Sigma-Aldrich) for 3 weeks with or without INA6 cells, in the presence or absence of 1 μg/mL BHQ880. At the end of the culture period, cells were fixed in 10% formaldehyde and stained with Alizarin Red for 30 minutes to assess the mineralizing activity. The amount of calcium deposition was quantified by enzyme-linked immunosorbent assay (ELISA) extracting the alizarin red with 10% acetic acid, according to the manufacturer's instructions (Millipore).
Cell proliferation assay
MM cell proliferation was measured by [3H]-thymidine (Perkin-Elmer) incorporation assay. MM cells (104 cells/well) were cultured in 96-well plates (Costar) at 37°C for 24, 48, and 72 hours, in the presence or absence of BHQ880 monoclonal antibody (mAb). Cells were pulsed with [3H]-thymidine (0.5 microCi/well) for 6 hours, harvested, and radioactivity was counted using the LKB Betaplate scintillation counter (Wallac). In coculture experiments, MM cells (2 × 104 cells/well) were incubated in BMSC-coated 96-well plates (Costar) at 37°C for 24, 48, and 72 hours, in the presence or absence of BHQ880 mAb. All experiments were carried out in triplicates.
Cytokine measurements
Levels of huIL-6 and hDKK1 proteins in cell culture supernatants were measured by ELISA (Quantikine; R&D Systems), according to the manufacturer's instructions.
Adhesion assay
Cell adhesion assay was done as described previously.26 In brief, MM cells and patient MM CD138+ cells (5 × 106/mL) were labeled with calcein AM (Molecular Probes) for 30 minutes at 37°C, washed, and resuspended in culture medium. Cells were added to BMSC-coated 96-well plates, treated with different concentrations of BHQ880 and incubated at 37°C for 4 hours. Unbound cells were removed by 4 washes with RPMI 1640. The absorbance of each well was measured using 492/520 nm filter set with a fluorescence plate reader (Wallac VICTOR2; Perkin-Elmer).
Immunoblotting, electrophoretic mobility shift assay, and nuclear factor-κB p65 ELISA
BMSC were cultured with isotype control or BHQ880 (1 μg/mL) for 24 hours. Cells were harvested and subjected to cell lysis. The 30 μg cytoplasmic fraction was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using “Precast Gel” (Bio-Rad Laboratories), transferred to a nitrocellulose membrane (Bio-Rad Laboratories), and immunoblotted with antibodies specific for β-catenin (BD Transduction Laboratories, BD Biosciences) and phospho-β-catenin (Ser552; Cell Signaling). After incubating with secondary antibody, membranes were developed by enhanced chemiluminescence (GE Healthcare). Nuclear factor-κB (NF-κB) activity of BMSC in the presence or absence of BHQ880 was assessed by electrophoretic mobility shift assay (EMSA) using nuclear extracts and 32P-labeled NF-κB binding consensus oligonucleotide probe (5′-GGGGACTTTCCC-3′; Santa Cruz Biotechnology), as in our previous studies,27,28 or Oct-1 binding sites (Santa Cruz Biotechnology). A total of 15 μg nuclear proteins from each treatment were analyzed for NF-κB activity using the Transcription Factor ELISA kit (Panomics). NF-κB p65 antibody was used as primary antibody and anti–rabbit IgG horseradish peroxidase was used as secondary antibody. The absorbance was measured at a wavelength of 450 nm on a spectrophotometer.29
Severe combined immunodeficient-hu mouse model
Six- to 8-week-old male CB-17 severe combined immunodeficient (SCID) mice (Taconic) were housed and monitored in our Animal Research Facility. All experimental procedures and protocols had been approved by the Institutional Animal Care and Use Committee (VA Boston Healthcare System, Boston, MA). Human fetal bone grafts were subcutaneously implanted into SCID mice (SCID-hu), as previously described.30 Four weeks after bone implantation, 2.5 × 106 INA-6 MM cells were injected directly into the human bone implant. Mouse sera were serially monitored for shuIL-6R, huDKK1, and huOsteocalcin levels by ELISA (R&D Systems). Mice were injected with IgG1 isotype control mAb (200 μg per mouse 3×/week) or BHQ880 (200 μg per mouse 3×/week) for 4 consecutive weeks.
Histologic analysis
One month after treatment with BHQ880, bone chips were retrieved, fixed in freshly prepared 4% paraformaldehyde (PFA) overnight, washed with 70% ethanol, and analyzed by bone histology. Specimens fixed in 4% PFA were demineralized with 14% ethylenediaminetetraacetic acid (EDTA) in phosphate-buffered saline (PBS). The specimens were processed and embedded in paraffin, and 5-μm sections were prepared for histologic analysis. Immunohistochemical staining was performed as previously reported31 using a goat polyclonal antibody to human alkaline phosphatase (AP; sc-23 430; Santa Cruz Biotechnology). The sections were developed with diaminobenzidine tetrahydrochloride (DAB) substrate (Vector Laboratories), counterstained with hematoxylin, and then sealed with Permount (Fisher Scientific Company). Sections were observed and photographed with a Nikon transmitted light microscope (Nikon).
Statistical analysis
All values are expressed as mean plus or minus the standard deviation (SD). The statistical significance of differences between treatments was analyzed using the Student t test; differences were considered significant when P was less than or equal to .05.
Results
BHQ880 reverses the inhibitory effect of MM cells on osteoblastogenesis
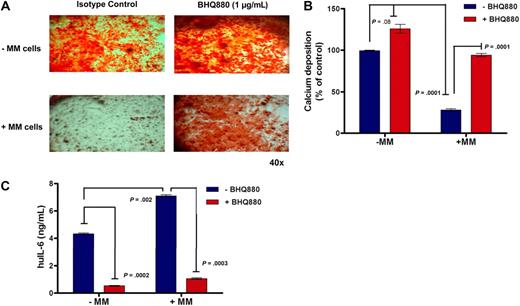
We first analyzed the effect of BHQ880 on OB differentiation. Human pre-OBs were stimulated with OB differentiation media and treated for 3 weeks with different concentrations of BHQ880 in the presence or absence of myeloma cells. At the end of the culture period, the cells were evaluated for OB differentiation with Alizarin Red staining. BHQ880 was able to increase differentiation of pre-OB in a dose-dependent manner (data not shown), with a significant increase in the presence of BHQ880 at 1 μg/mL (Figure 1A top panel). Importantly, the presence of MM cells significantly reduced OB differentiation, whereas treatment with BHQ880 was able to restore OB activity, neutralizing the negative effect of MM cells on osteoblastogenesis (Figure 1A bottom panel). We quantified the amount of calcium deposition in this assay using an osteogenesis assay kit and confirmed that the decrease in calcium deposition in the presence of myeloma cells is reversed by BHQ880 (Figure 1B). The calcium deposition in myeloma/stromal coculture treated with BHQ880 reaches the same level as stroma without myeloma cells, suggesting that BHQ880 restores calcium deposition to normal stromal level. However, in the stromal culture without myeloma, BHQ880 is able to further increase calcium deposition. Because OB produces IL-6, we measured IL-6 levels in the supernatants from OB cultures in presence or absence of MM cells and with and without BHQ880 treatment. As seen in Figure 1C, BHQ880 was able to inhibit IL-6 production by OBs. Importantly, coculture with MM cells significantly increased IL-6 production which was down-regulated by BHQ880 treatment (Figure 1C). BHQ880 was able to inhibit IL-6 production by both immature and mature OB (data not shown). BHQ880 had no significant effect on osteoclast formation in an in vitro assay (data not shown).
BHQ880 reverses the inhibitory effect of MM cells on osteoblastogenesis. (A) BMSC obtained from BM aspirate of MM patients were stimulated with OB differentiation media for 3 weeks in the presence of IgG1 isotype control antibody or 1 μg/mL BHQ880 and in the absence (top panel) or in the presence (bottom panel) of INA-6 MM cells. At the end of the culture period, the cells were fixed in 10% formaldehyde and stained with Alizarin Red for 30 minutes. (B) Calcium deposition was quantified in these cultures using osteogenesis assay kit. The amount of calcium deposits in differentiated BMSC treated with BHQ880 (red column) is expressed as percent of calcium deposition compared with differentiated BMSC treated with isotype control antibody (blue column) in the absence and presence of MM cells. (C) Supernatants from these assays were analyzed for huIL-6 level by ELISA. IL-6 production was suppressed after treatment with BHQ880 in supernatants from both cultures with and without MM cells.
BHQ880 reverses the inhibitory effect of MM cells on osteoblastogenesis. (A) BMSC obtained from BM aspirate of MM patients were stimulated with OB differentiation media for 3 weeks in the presence of IgG1 isotype control antibody or 1 μg/mL BHQ880 and in the absence (top panel) or in the presence (bottom panel) of INA-6 MM cells. At the end of the culture period, the cells were fixed in 10% formaldehyde and stained with Alizarin Red for 30 minutes. (B) Calcium deposition was quantified in these cultures using osteogenesis assay kit. The amount of calcium deposits in differentiated BMSC treated with BHQ880 (red column) is expressed as percent of calcium deposition compared with differentiated BMSC treated with isotype control antibody (blue column) in the absence and presence of MM cells. (C) Supernatants from these assays were analyzed for huIL-6 level by ELISA. IL-6 production was suppressed after treatment with BHQ880 in supernatants from both cultures with and without MM cells.
BHQ880 overcomes the growth-promoting effect of BMSC on MM cells
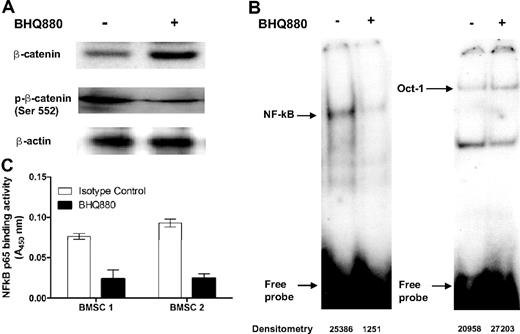
We next examined the effect of BHQ880 on INA6 cell growth by incubating MM cells in presence of BHQ880 at various concentrations for 24 to 48 hours. As seen in Figure 2A, BHQ880 had no significant direct effect on INA6 cell growth. However, in the presence of BMSC, BHQ880 was able to inhibit MM cells growth in a dose-dependent manner (Figure 2A right panel). As INA-6 cells produce modest levels of DKK1, we next examined the effect of BHQ880 in a panel of MM cell lines (Figure 2B) and primary tumor cells (Figure 2C) not producing DKK1. Although BHQ880 had no direct effect on these cells (data not shown), as seen in Figure 2B and C, BHQ880 was able to overcome the growth promoting effects of BMSC on MM cell lines and primary cells. Because IL-6 is the major growth and survival factor produced by BMSC in MM microenvironment, we evaluated IL-6 levels in the BMSC culture supernatants with and without BHQ880. Similar to its effects on IL-6 production by OB, BHQ880 significantly inhibited IL-6 secretion from BMSCs both with and without MM cells (Figure 3A). To evaluate if the effect of BHQ880 on MM cell proliferation seen in the presence of BMSC was due to the reduction of IL-6 level in the BM milieu, we next examined whether addition of IL-6 could reverse BHQ880-induced MM cell growth inhibition. As seen in Figure 3B, although majority of growth inhibitory effect of BHQ880 was overcome by addition of IL-6, other elements may play a role in the overall effect of this molecule on myeloma cell growth. In addition, we observed that BHQ880 also inhibited adhesion of both MM cell lines and primary cells to BMSC (Figure 3C), supporting the idea that the effect of BHQ880 on MM cell growth in the context of the BM microenvironment is related to several components. To further examine the molecular mechanisms whereby BHQ880 induced MM cell growth inhibition in the context of the BM microenvironment, we have focused on molecular changes in stromal cells after BHQ880 treatment without the presence of myeloma cells. We evaluated effect of BHQ880 on β-catenin as well as the NF-κB pathway, which is the prime mechanism mediating IL-6 secretion by the BMSC. We cultured BMSC with isotype control antibody or BHQ880 (1 μg/mL) for 24 hours. Cytoplasmic fractions were subjected to Western blot analysis to assess total β-catenin (active) as well as phosphorylated β-catenin, while the nuclear fraction was evaluated by EMSA to evaluate NF-κB activity. As seen in Figure 4A, BHQ880 up-regulated total protein levels of β-catenin while down-regulating phosphorylated β-catenin levels. NF-κB binding activity was significantly lower in cells treated with BHQ880 compared with cells treated with isotype control, whereas no difference was observed in the control (Oct-1) DNA binding (Figure 4B). We also further confirmed the effect of BHQ880 on NF-κB activity using an ELISA-based assay specific for NF-κB p65 binding activity29 (Figure 4C).
BHQ880 overcomes the growth promoting effect of BMSC on both IL-6-dependent and IL-6-independent MM cells. (A) INA-6 MM cells were cultured without and with BMSC in the presence of different doses of BHQ880 for 24 to 48 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and is presented as percentage change from control. Data represent mean ± SD of 4 independent experiments performed in triplicates. (B) MM1S, OPM1, OPM2, U266, XG1 MM cell lines, and (C) primary cells from 5 MM patients were cultured without (−) and with (+) BMSC in the presence of BHQ880 for 48 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and presented as change from control cells cultured in the absence of BMSC (−). Data represent mean ± SD of 4 independent experiments performed in triplicate.
BHQ880 overcomes the growth promoting effect of BMSC on both IL-6-dependent and IL-6-independent MM cells. (A) INA-6 MM cells were cultured without and with BMSC in the presence of different doses of BHQ880 for 24 to 48 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and is presented as percentage change from control. Data represent mean ± SD of 4 independent experiments performed in triplicates. (B) MM1S, OPM1, OPM2, U266, XG1 MM cell lines, and (C) primary cells from 5 MM patients were cultured without (−) and with (+) BMSC in the presence of BHQ880 for 48 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and presented as change from control cells cultured in the absence of BMSC (−). Data represent mean ± SD of 4 independent experiments performed in triplicate.
BHQ880 inhibits IL-6 production and MM cell adhesion to BMSC from MM patients. (A) Both BMSC from MM patient alone and cocultured with INA-6 cells were treated with isotype control antibody (□) or 1 μg/mL BHQ880 (■) for 24 hours. Culture supernatant was then analyzed for huIL-6 level by ELISA. (B) INA-6 and MM1S MM cells were cultured in the presence of BMSC and increasing concentrations of rIL-6 (0, 0.5, and 1 ng/mL) with and without BHQ880 for 24 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and is presented as percentage change from control. (C) Serum-starved MM cell lines and CD138+ patient MM cells were labeled with calcein AM, washed, and added to BMSC-coated plates for 4 hours with isotype control or increasing amounts of BHQ880 (0.1 and 1 μg/mL). Adhesion was evaluated by measuring the absorbance using 492/520 nm filter set with a fluorescence plate reader. Data represent mean ± SD of 4 independent experiments performed in triplicate.
BHQ880 inhibits IL-6 production and MM cell adhesion to BMSC from MM patients. (A) Both BMSC from MM patient alone and cocultured with INA-6 cells were treated with isotype control antibody (□) or 1 μg/mL BHQ880 (■) for 24 hours. Culture supernatant was then analyzed for huIL-6 level by ELISA. (B) INA-6 and MM1S MM cells were cultured in the presence of BMSC and increasing concentrations of rIL-6 (0, 0.5, and 1 ng/mL) with and without BHQ880 for 24 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and is presented as percentage change from control. (C) Serum-starved MM cell lines and CD138+ patient MM cells were labeled with calcein AM, washed, and added to BMSC-coated plates for 4 hours with isotype control or increasing amounts of BHQ880 (0.1 and 1 μg/mL). Adhesion was evaluated by measuring the absorbance using 492/520 nm filter set with a fluorescence plate reader. Data represent mean ± SD of 4 independent experiments performed in triplicate.
BHQ880 inhibits IL-6 production and MM cell adhesion and modulates β-catenin and NF-κB pathways in BMSC from MM patients. BMSC were incubated for 24 hours with 1 μg/mL isotype control or BHQ880. Cells were harvested and subjected to cell lysis. (A) Cytoplasmic fraction was subjected to immunoblotting using anti-phospho β-catenin antibody and anti–β-catenin antibody. (B) Nuclear fraction was subjected to EMSA for NF-κB binding activity. Oct-1 DNA binding activity by EMSA was used as internal control. (C) Nuclear proteins (15 μg) were analyzed for NF-κB activity using the Transcription Factor ELISA kit, which measures DNA binding activity. Absorbance was obtained with a spectrophotometer at 450 nm and is presented as optical density (OD).
BHQ880 inhibits IL-6 production and MM cell adhesion and modulates β-catenin and NF-κB pathways in BMSC from MM patients. BMSC were incubated for 24 hours with 1 μg/mL isotype control or BHQ880. Cells were harvested and subjected to cell lysis. (A) Cytoplasmic fraction was subjected to immunoblotting using anti-phospho β-catenin antibody and anti–β-catenin antibody. (B) Nuclear fraction was subjected to EMSA for NF-κB binding activity. Oct-1 DNA binding activity by EMSA was used as internal control. (C) Nuclear proteins (15 μg) were analyzed for NF-κB activity using the Transcription Factor ELISA kit, which measures DNA binding activity. Absorbance was obtained with a spectrophotometer at 450 nm and is presented as optical density (OD).
BHQ880 promotes osteoblastogenesis and bone formation in vivo
To evaluate the in vivo effect of BHQ880 on human bone as well as myeloma cells, we used a SCID-hu mouse model where myeloma cells are injected in the implanted human bone in mice and sIL-6R levels are measured in murine blood as a marker of myeloma tumor burden. In this model associated with increase in serum levels of huIL-6sR reflecting myeloma cell growth, we also observed an increased level of soluble hu-DKK1 in murine blood (Figure 5A). Human bone in mice develops osteoporosis and bone lesions with growth of myeloma cells in the bone.32 To evaluate the in vivo effect of BHQ880 on osteoblastogenesis, SCID-hu mice were injected with BHQ880 3 times a week for 4 weeks after first detection of shuIL-6R in mice. The control mice were injected with IgG1 isotype control antibody. We observed a significant increase in number of OB by alkaline phosphatase (ALP) staining in the human bone from BHQ880-treated mice compared with control (54 ± 4.5 versus 11 ± 5.7 OB/mm, P = 0.01; Figure 5B). We also examined serum level of human osteocalcin in the murine sera as a marker of OB activity. We observed a significantly increased serum osteocalcin level in BHQ880-treated mice compared with isotype control antibody-treated mice (Figure 5C). We did not observe a significant difference in osteoclast cell number as assessed by tartrate-resistant acid phosphatase (TRAP) staining in bones from control and BHQ880-treated mice (data not shown). Histologic analysis of the bone by hematoxylin and eosin (H&E) staining showed increased trabecular bone (Figure 5D).
BHQ880 improves bone disease in a murine model of human MM. SCID-hu mice were injected with INA-6 cells into the implanted bone and treated with isotype control (n = 7) or BHQ880 (n = 7) after first detection of tumor. (A) After injection of INA-6 MM cells directly into the human bone implant, serum from SCID-hu mice were monitored for production of DKK1 (blue column) and shuIL-6R (green column) by ELISA. (B) One month after treatment, bone chips were retrieved and decalcified, and sections from control and BHQ880-treated bones were immunohistochemically stained for ALP (top), and the number of OB was measured per field in isotype control and BHQ880-treated bones (bottom). Sections were observed and photographed with a Nikon transmitted light microscope. Original magnification ×200. Data are expressed as ± SD. (C) Serum level of human osteocalcin in murine blood was evaluated at the end of the treatment period by ELISA. Data are expressed as mean ± SD. (D) Sections from control and BHQ880-treated bones were stained by H&E showing increased bone tissue and decreased MM cell number in the BHQ880-treated compared with isotype control-treated samples. Sections were observed and photographed with a Nikon transmitted light microscope. Original magnification ×100.
BHQ880 improves bone disease in a murine model of human MM. SCID-hu mice were injected with INA-6 cells into the implanted bone and treated with isotype control (n = 7) or BHQ880 (n = 7) after first detection of tumor. (A) After injection of INA-6 MM cells directly into the human bone implant, serum from SCID-hu mice were monitored for production of DKK1 (blue column) and shuIL-6R (green column) by ELISA. (B) One month after treatment, bone chips were retrieved and decalcified, and sections from control and BHQ880-treated bones were immunohistochemically stained for ALP (top), and the number of OB was measured per field in isotype control and BHQ880-treated bones (bottom). Sections were observed and photographed with a Nikon transmitted light microscope. Original magnification ×200. Data are expressed as ± SD. (C) Serum level of human osteocalcin in murine blood was evaluated at the end of the treatment period by ELISA. Data are expressed as mean ± SD. (D) Sections from control and BHQ880-treated bones were stained by H&E showing increased bone tissue and decreased MM cell number in the BHQ880-treated compared with isotype control-treated samples. Sections were observed and photographed with a Nikon transmitted light microscope. Original magnification ×100.
BHQ880 inhibits myeloma cell growth in SCID-hu mice
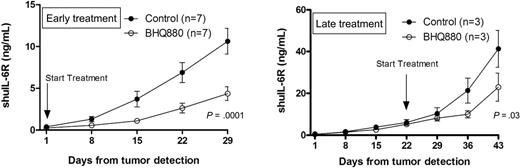
We finally investigated the effect of BHQ880 treatment on MM cell growth in vivo. We have used 2 models of disease state: the minimal disease model allows evaluation of treatment before extensive myeloma cell growth, and the high-tumor-burden model allows investigation of effect after extensive MM cell growth. In the minimal disease model, we started BHQ880 treatment in mice after first detection of shuIL-6R in murine blood (shuIL6R = 0.24 ± 41.03 ng/mL; Figure 6A), whereas in the high-tumor-burden model, BHQ880 treatment was started 3 weeks after first detection of shuIL-6R in murine blood (shuIL6 = 5.13 ± 0.4 ng/mL; Figure 6B). Mice were injected with isotype control mAb (200 μg per mouse 3 × /week) or BHQ880 (200 μg per mouse 3 × /week). In both models, we observed significant antitumor activity of BHQ880 as measured by shuIL-6R levels in murine blood, suggesting MM cell growth inhibitory effects of BHQ880. We also confirmed the antimyeloma effect by histologic analysis by H&E staining, showing decreased number of MM cell in the bone from BHQ880-treated mice compared with the control mAb (Figure 5D).
BHQ880 inhibits myeloma cell growth in SCID-hu mice. (A) In the early treatment model, mice were injected with isotype control (200 μg per mouse 3×/week; n = 7) and BHQ880 (200 μg per mouse 3×/week; n = 7) at the first detection of tumor. Serum samples were collected weekly, and the level of shuIL-6R was measured by ELISA. (B) In the late treatment model, mice were injected with isotype control (200 μg per mouse 3×/week; n = 3) and BHQ880 (200 μg per mouse 3×/week; n = 3) 3 weeks after first detection of tumor. Serum samples were collected weekly, and the level of shuIL-6R was measured by ELISA. Baseline values before treatment were not significantly different among groups.
BHQ880 inhibits myeloma cell growth in SCID-hu mice. (A) In the early treatment model, mice were injected with isotype control (200 μg per mouse 3×/week; n = 7) and BHQ880 (200 μg per mouse 3×/week; n = 7) at the first detection of tumor. Serum samples were collected weekly, and the level of shuIL-6R was measured by ELISA. (B) In the late treatment model, mice were injected with isotype control (200 μg per mouse 3×/week; n = 3) and BHQ880 (200 μg per mouse 3×/week; n = 3) 3 weeks after first detection of tumor. Serum samples were collected weekly, and the level of shuIL-6R was measured by ELISA. Baseline values before treatment were not significantly different among groups.
Discussion
Osteolytic bone lesions are a hallmark of bone disease in myeloma. Targeting osteoclasts through bisphosphonates has been the mainstay of the therapeutic intervention so far. However, the lack of bone formation, mainly due to suppressed OB function, has not been therapeutically targeted. This is partly due to the lack of information, until recently, regarding the molecular mechanisms responsible for suppressed OB function. Tian et al were the first to report production of DKK1 by myeloma cells and associate it with occurrence of bone disease as well as OB number and function.19 More recently, Kaiser et al demonstrated in 184 previously untreated MM patients a statistically significant correlation between serum DKK1 levels and the extent of myeloma bone disease.33 The serum concentration of DKK1 has been also shown to decrease with response to therapy.34 Such association with DKK1 and bone disease has also been established in other malignancies including breast, esophageal, lung, prostate, and colon cancers,12,13,15-18 making DKK1 an important target to improve bone disease in cancer in general and myeloma in particularly, where suppression of OB function is perhaps the greatest.
Here we have evaluated in vitro and in vivo effects of an anti-DKK1 neutralizing antibody in myeloma bone disease. We first analyzed the effects of BHQ880 on OB differentiation. As seen in Figure 1A and B, we observed an increase in basal OB function with BHQ880. We also observed, in this culture system, that MM cells suppress OB function and consequent calcium deposition. Interestingly, BHQ880 was able to neutralize these effects of myeloma cells on OB function and improve calcium deposition to normal stromal level. Although immature OB produce a significant amount of IL-6, upon differentiation, IL-6 production decreases substantially.35 DKK1 produced by MM cells leads to inhibition of OB differentiation and thereby augments IL-6 production, which is one of the key antiapoptotic and survival factor in MM.36 We and others have observed that supernatants from cultures of undifferentiated BMSCs contain high levels of IL-6.35 We observed in our experiments that osteoblastic differentiation media as well as BHQ880 decreased IL-6 production by BMSC. Combination of BHQ880 and the OB differentiation media had the greatest effect. Moreover, increased IL-6 production triggered by adhesion of MM cells to BMSC was also reduced by BHQ880 treatment (Figure 1C). These data suggest that DKK1 might indirectly contribute to MM cell growth and/or survival via IL-6 production by BMSC, and some of the anti-MM effects of BHQ880, observed in vitro as well as in vivo in the murine model, may be related to the decline in IL-6 levels.
To evaluate the in vivo effect of BHQ880 on osteoblastogenesis and MM bone disease, we used the SCID-hu mouse model of human MM, in which a fetal human bone is implanted in SCID mice and injected with IL-6 dependent and DKK 1 producing INA-6 MM cells.30 In this model, we found an increased level of soluble hu-DKK1 in murine blood, associated with increasing MM tumor burden. In this model, BHQ880 treatment for 1 month led to a significant increase in OB number and serum human osteocalcin levels compared with mice injected with isotype control antibody. These effects also correlated with increased trabecular bone (Figure 5). By microcomputed tomography (microCT) analysis, we also observed increased bone formation in the murine bone in mice treated with BHQ880 (data not shown). It is unclear if this effect represents effect of BHQ880 on myeloma related changes in murine bone. Importantly in myeloma, as the disease affects all bones, the concern for osteosclerotic bone changes is less significant. Moreover, we have also evaluated effect of BHQ880 on human bone implanted in mice without myeloma. In this system, we have not observed a significant effect of BHQ880 treatment on bone (data not shown).
Of note, there was no significant difference in osteoclast number between the control and BHQ880-treated cohort, as assessed by TRAP staining (data not shown). The role and effect of DKK1 on osteoclastogenesis remains unclear. In various studies anti-DKK1 antibody has provided varying effects. This has been dependent on the antibody type as well as the models used. In our model system, we have not observed effect of BHQ880 on the osteoclast number. These results are similar to the recent report by Heath and colleagues,37 in a 5T2MM murine model, showing that BHQ880 inhibited 5T2MM-induced suppression of OB numbers with no effect on osteoclast numbers; while Yaccoby et al have shown effect on osteoclastogenesis.38
The effect of BHQ880 on myeloma cell growth and survival is intriguing. The lack of a significant direct effect of the BHQ880 on MM cells suggests that the antibody does not bind to MM cells, or that DKK1 does not directly regulate MM cell growth, or that DKK1 binding to LRP5/6 expressed by MM cells and its inhibition by BHQ880 does not affect Wnt signaling in myeloma. BHQ880 significantly inhibited MM cell growth only in the presence of BMSC. Although the large part of the anti-MM effect of BHQ880 was associated with reduction of IL-6 production by BMSC, we also believe that the inhibition of BMSC/MM cell adhesion may play a role in the overall effect of BHQ800 on MM cell growth. Our examination of the molecular mechanisms whereby BHQ880 inhibits MM cell growth in the BM milieu, confirmed activation of β-catenin and down-regulation of NF-κB activity in BMSC. NF-κB signaling is increased in primary BMSC from MM patients39 and is considered the prime mechanism mediating IL-6 secretion by the BMSC.28 As β-catenin is a major substrate of GSK-3b,40,41 and GSK-3β can modulate NF-κB activity,42 it raises an interesting possibility that β-catenin might serve as a mediator for the cross-regulation between Wnt and NF-κB pathways. Although the data presented in Figure 1C represents decrease in IL-6 levels at 3 weeks, we have observed significant decrease in IL-6 level on day 3 (data not shown). We believe that while part of the decrease in IL-6 level may be related with inhibition of NF-κB at an early time point when we do not see OB differentiation (data not shown), the decline in IL-6 observed at 3 weeks after BHQ880 treatment may be partly related with the OB differentiation process.
It is well established that the Wnt signaling pathway plays a key role in bone formation,8 and we have observed its activation in the BMSC with BHQ880 treatment. However its precise role in growth and survival of myeloma cells remains unclear.43-45 Activation of the Wnt signaling pathway through β-catenin activation has been linked to several forms of cancer, including hematologic malignancies,46 and expression of β-catenin has been demonstrated in myeloma cell lines and primary MM cells from patients.20,45 The observation that neutralizing DKK1, a Wnt inhibitor, and therefore enhancing Wnt signaling, has negative effects on MM tumor growth might be considered counterintuitive. However, in line with the observed lack of direct effect of BHQ880 on MM cells, we have also not observed any direct effect of the antibody on Wnt signaling in MM.
We have observed a clear anti-MM response after BHQ880 treatment in the SCID-hu murine model of human MM (Figure 6). The effect was obvious in both low as well as high tumor burden disease. In both models, BHQ880 was effective in controlling MM growth detected by both measurement of soluble huIL-6R and by histology of the implanted bone. To confirm in vivo the ability of BHQ880 to induce MM cell growth inhibition only in the context of the BM microenvironment, we have used our plasmacytoma xenograft mouse model47 in which OPM2 MM cell line was injected subcutaneously in SCID mice. In this model, no significant difference in tumor size between the control and BHQ880-treated cohort mice was observed confirming the lack of direct effect of the antibody on MM tumor growth (data not shown).
Yaccoby et al have previously shown that daily injections of a neutralizing DKK1 antibody in the area surrounding myelomatous bone, ameliorated bone turnover and reduced tumor burden in the SCID-rab model.38 In our study, we have treated mice with systemic administration of the antibody. Consistent with our data, Qiang et al have also shown that activation of canonical Wnt signaling by Wnt3a in the SCID-hu model demonstrated an anti-MM effect on MM cell lines and primary MM samples when growth was restricted to implanted human bones in SCID mice and not when the tumor was growing subcutaneously.48
These data also confirm the need to study MM in vivo using animal models that take into consideration the influences of the human bone milieu.
Production of DKK1 is also modulated by several drugs used to treat MM including dexamethasone49 and the immunomodulatory agents, thalidomide and lenalidomide.50,51 The treatment of OB with dexamethasone results in a time- and dose-dependent increase in DKK1 expression, suggesting that dexamethasone also blocks bone formation through a mechanism affecting osteoblastogenesis providing partial explanation for osteoporosis observed with long term glucocorticoid use.49 Thus, combining current treatments with anti-DKK1 antibody may abrogate dexamethasone-induced osteoporosis and stimulate bone formation.
In conclusion, our study demonstrates a striking direct effect of BHQ880 on the bone microenvironment in MM by increasing bone formation and indirectly inhibiting tumor growth. This is the first study using clinical grade humanized anti-DKK1 antibody, demonstrating its in vitro effect on myeloma cells in the presence of human stromal cell compartment as well as direct effect on stromal cell confirming the role of the microenvironment in DKK1 related effects. In addition, the in vivo study using the SCID-hu murine model confirms anti-myeloma effects of BHQ880 as well as its bone-related effects in a human setting. With identification of emerging problems, such as osteonecrosis of the jaw, with bisphosphonates, which mainly affects the catabolic part of the bone turnover, BHQ880 through its anabolic effect on bone formation may provide an alternative to treat MM bone disease along with additional anti-tumor effects.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the Department of Veterans Affairs Merit Review Awards, the National Institutes of Health (grant nos. RO1-124929 [N.C.M.], P50-100007, and PO1-78378 [N.C.M. and K.C.A.]), and Associazione Italiana per la Ricerca sul Cancro (AIRC).
National Institutes of Health
Authorship
Contribution: M.F. developed the experimental plan, carried out in vitro and in vivo experiments, analyzed the data, and prepared the manuscript; P.T. assisted in data interpretation, analysis, and preparation of manuscript; T.H. carried out EMSA assay and helped in the analysis and interpretation of data; S.V. performed the OB differentiation in vitro assay; P.N. participated in cell culture studies; Z.S. carried out immunohistochemistry analyses; N.P. performed microCT analyses; Y.T. carried out the osteoclast differentiation in vitro assay; S.A.E. and D.R.S. provided the reagents and helped in the analysis and interpretation of data; D.C., C.M., R.P., N.R., and K.C.A. helped in the analysis and interpretation of data; and N.C.M. envisioned the study, and participated in its design and coordination, and helped to draft the manuscript. All authors have read and approved the final manuscript.
Conflict-of-interest disclosure: S.A.E. and D.R.S. are employees of Novartis Pharmaceuticals. C.M., K.C.A., and N.C.M. have been consultants for Novartis. The remaining authors declare no competing financial interests.
Correspondence: Nikhil C. Munshi, Dana-Farber Cancer Institute, 44 Binney St, M1B28, Boston, MA 02115; e-mail: Nikhil_Munshi@DFCI.Harvard.edu.

![Figure 2. BHQ880 overcomes the growth promoting effect of BMSC on both IL-6-dependent and IL-6-independent MM cells. (A) INA-6 MM cells were cultured without and with BMSC in the presence of different doses of BHQ880 for 24 to 48 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and is presented as percentage change from control. Data represent mean ± SD of 4 independent experiments performed in triplicates. (B) MM1S, OPM1, OPM2, U266, XG1 MM cell lines, and (C) primary cells from 5 MM patients were cultured without (−) and with (+) BMSC in the presence of BHQ880 for 48 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and presented as change from control cells cultured in the absence of BMSC (−). Data represent mean ± SD of 4 independent experiments performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/2/10.1182_blood-2008-11-191577/4/m_zh89990938350002.jpeg?Expires=1769114471&Signature=lxJteJOq~CCUdtZ-OjAD5kkuFrRqFmIPTfQcCFEkbw~rVmYGgq5F3621q~a7ai2ISRZz0kbxjCzZLUvtjmaPvBc2t71JutMaeZmpbXJi0icnY3nL7E12U~VVaWbRoERLsrED14NIcTLLCLQWptjZcxYJBIf83YYLoT7mOB7zEdmVUYQKowaxIXld4~Fvlp9LRA8TElwR4wBa0ecnCANJ~SW~S8O7wlfsuGfHmKYJM41vllf6GqXoOSqph56M-Gohu902IKViXTy0NmrepE5AJGSqRYvDu9q1xRlAkEEd9E8D0fqFqmvO5jTN9-KFrdTCdyWwm5kn9WrseZYccwFxtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. BHQ880 inhibits IL-6 production and MM cell adhesion to BMSC from MM patients. (A) Both BMSC from MM patient alone and cocultured with INA-6 cells were treated with isotype control antibody (□) or 1 μg/mL BHQ880 (■) for 24 hours. Culture supernatant was then analyzed for huIL-6 level by ELISA. (B) INA-6 and MM1S MM cells were cultured in the presence of BMSC and increasing concentrations of rIL-6 (0, 0.5, and 1 ng/mL) with and without BHQ880 for 24 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and is presented as percentage change from control. (C) Serum-starved MM cell lines and CD138+ patient MM cells were labeled with calcein AM, washed, and added to BMSC-coated plates for 4 hours with isotype control or increasing amounts of BHQ880 (0.1 and 1 μg/mL). Adhesion was evaluated by measuring the absorbance using 492/520 nm filter set with a fluorescence plate reader. Data represent mean ± SD of 4 independent experiments performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/2/10.1182_blood-2008-11-191577/4/m_zh89990938350003.jpeg?Expires=1769114471&Signature=fZ1su-a2J9Q0Fpcsp6poynlZrW1YBaiPhKIyebm1-PQ2i75TLz9rOdm9RS8Vp9mt9baYk-t2wH9jtUXFrikh7OOOTbgXd-VN~Ny7zDihC1U7TV~7MDQHYktsQt4ktmru-Jr8fM8wMiPlpeCmS9Fx3trvSvNqoeyn8OSSWiGwu0urr8RAGrFzW74ZV-dmlsVXCvM8pKzaiU8SVNLYxi25k8QmKEEhzh53lIt99uxDzlv8eX39jj6VBSunEP2vjvOR1xjf~SIB1wz6oKGw3qym0WjLCd~7hmI1cAl5rjdY2F5bcBwyNjkxg22Qv-8ku0Ft9psmbWVdkijSxHCH62MoAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal