Abstract
Chronic blood transfusion is increasingly indicated in patients with sickle cell disease. Measuring resulting iron overload remains a challenge. Children without viral hepatitis enrolled in 2 trials for stroke prevention were examined for iron overload (STOP and STOP2; n = 271). Most received desferrioxamine chelation. Serum ferritin (SF) changes appeared nonlinear compared with prechelation estimated transfusion iron load (TIL) or with liver iron concentrations (LICs). Averaged correlation coefficient between SF and TIL (patients/observations, 26 of 164) was r = 0.70; between SF and LIC (patients/observations, 33 of 47) was r = 0.55. In mixed models, SF was associated with LIC (P = .006), alanine transaminase (P = .025), and weight (P = .026). Most patients with SF between 750 and 1500 ng/mL had a TIL between 25 and 100 mg/kg (72.8% ± 5.9%; patients/observations, 24 of 50) or an LIC between 2.5 and 10 mg/g dry liver weight (75% ± 0%; patients/observations, 8 of 9). Most patients with SF of 3000 ng/mL or greater had a TIL of 100 mg/kg or greater (95.3% ± 6.7%; patients/observations, 7 of 16) or an LIC of 10 mg/g dry liver weight or greater (87.7% ± 4.3%; patients/observations, 11 of 18). Although SF changes are nonlinear, levels less than 1500 ng/mL indicated mostly acceptable iron overload; levels of 3000 ng/mL or greater were specific for significant iron overload and were associated with liver injury. However, to determine accurately iron overload in patients with intermediately elevated SF levels, other methods are required. These trials are registered at www.clinicaltrials.gov as #NCT00000592 and #NCT00006182.
Introduction
Increasing numbers of patients with sickle cell disease (SCD) are receiving chronic blood transfusions for the prevention or management of disease-related complications. As a result, these patients require treatment with either chelator drugs1 or exchange transfusion2 to prevent tissue injury from iron overload. Serum ferritin (SF) is a noninvasive measure widely used to monitor iron load.1-3 However, the relationship between SF to other iron load measures varies among studies.4-12 SF levels increase in inflammatory states; thus, levels can be variable over relatively short time frames.13 In patients with thalassemia cured with bone marrow transplantation,10 liver iron concentrations (LICs) obtained from liver biopsy correlated more closely with iron stores measured by phlebotomy than SF. To better characterize measures of iron overload in children with SCD, this study examined patients enrolled in 2 clinical trials in which blood transfusion was evaluated for stroke prevention.14,15 The relation of SF to iron load estimated from transfusion history and LICs was examined.
Methods
Study population
STOP was a prospective randomized clinical trial of children with SCD who were identified as having risk of stroke by transcranial Doppler ultrasound.14 The children were randomly assigned to either chronic blood transfusions or observation and followed to assess the occurrence of stroke. In the follow-up study by the same investigators, STOP2, patients who were initially at risk for stroke and whose transcranial Doppler velocities normalized after at least 30 months on chronic transfusion were randomly assigned either to continue or cease transfusion therapy.15
During the course of both trials, enrolled patients were evaluated every 3 months, with history review, physical examination, and laboratory tests. Both quarterly tests and annual viral hepatitis serology were performed at the trial core laboratory at Medical College of Georgia (MCG, Augusta, GA) on randomized patients, as described in detail elsewhere.11 Briefly, serum chemistries, including alanine transaminase (ALT), were measured by DuPont RXL Chemistry Analyzer and SF by Abbott AXSYM system immunoassay. Only blood chemistry measures assayed at the core laboratory were examined in the current study (collected only after patient randomization). To reduce possible acute-phase reactant effect,13 laboratory measures obtained within 2 weeks before or after a documented infection or SCD-related complication or within 2 weeks after a surgical procedure were excluded. Transfusion intervals in patients who were enrolled in both trials were assumed to be the same between studies as during study periods. Exchange transfusion use was only documented after randomization during the course of the trials. Prior to participation in STOP and STOP2 clinical trials, patient informed consent was obtained in accordance with the Declaration of Helsinki.
Transfusion iron load
SF levels were compared with 2 benchmark measures of iron load: transfusion iron load (TIL) and LIC. TIL was estimated from the cumulative blood volume received in patients who only received simple transfusions,14 before start of chelation therapy. Transfusion volume data from the onset of the stroke prevention program were available only in the first STOP trial. Patients documented with more than 10 transfusions before randomization or who received exchange transfusion(s) were excluded from this analysis. For the comparison between SF and TIL, data were censored if transfused blood volume information was missing on more than one consecutive transfusion; otherwise, missing transfusion volume was assumed to be equal to average patient transfusion dose. To calculate the amount of iron received, blood unit hematocrit was estimated to be 60%, based on blood preservatives data recorded during the study, and 1 mL of red blood cells was estimated to contain 1 mg of iron.16 Patient weights at each monthly transfusion and quarterly visit were recorded. Weights in between visits were estimated in relation to time elapsed from actual measurements (weighted averages). To calculate TIL, cumulative iron load received was divided by the estimated weight on the date of TIL assessment.
Liver iron concentration
LIC measurements were not mandated but were instead performed at the discretion of investigators, as clinically indicated.17 As with all procedures, liver biopsy dates were recorded prospectively during the course of the trials. Permission for the current secondary analysis of STOP and STOP2 data, which included retrospective collection of additional LIC-related data after the trials were completed, was obtained from the STOP2 Steering Committee, Data Safety Monitoring Board, and Institutional Review Boards at Morehouse School of Medicine, MCG, and participating sites. Additional data collected included: LIC results, pathology reports, biopsy complications, and start dates of transfusion, exchange transfusion, and chelation therapy. Possible liver biopsy complications were also identified by reviewing trial data. Only LICs assayed by inductively coupled plasma-mass spectrometry (ICPMS)18 were considered for analysis of iron measures. For the purpose of this study, an acceptable iron load range was defined as TIL of 25 or more to less than 100 mg/kg or LIC of 2.5 or more to less than 10 mg/g dry weight. Liver fibrosis, as described in clinical pathology reports, was grouped into 3 severity levels: no fibrosis (stage 019 ), mild fibrosis (stage 119 : minimal fibrosis or fibrous expansion of some portal areas, with or without short fibrous septa), or moderate to severe fibrosis (stages 2-619 : fibrous expansion of most portal areas to definitive cirrhosis).
Statistical considerations
STOP, STOP2, and LIC datasets were merged. Data software included Excel 2000 (Microsoft Corporation) and SAS version 9.1 (SAS Institute). Differences in proportions were assessed by 2-tailed Fisher exact method, differences in continuous variables by Wilcoxon rank test, and differences in variances distribution by F test.20 Relation of SF to TIL or LICs was estimated by Pearson correlation coefficient, sensitivity, specificity, and receiver operating characteristic (ROC) area under the curve (AUC).21 To account for repeated measures in individual patients, data from a group of patients under consideration were sampled randomly 1000 times (one measure per patient). Each set of sampled measures was analyzed separately, after which results were averaged.22 Factors that may affect SF or ALT change were examined using mixed models.23
Results
Study population
Of the 277 enrolled patients who had clinical trial study visits and documented viral hepatitis serology, 6 (2%) were excluded (hepatitis C, n = 4; hepatitis B, n = 1; hepatitis B and C, n = 1). Of the remaining 271 patients, 163 were randomized subjects (86 in STOP only, 38 in STOP2 only, 39 in both) and 108 were STOP2 patients observed on transfusion that were not randomized in either study. A total of 54% of patients (n = 89) were female, 1% (n = 2) were diagnosed with hemoglobin S beta-0 thalassemia, the remainder with hemoglobin SS. A total of 24% of patients (n = 39) participated in both STOP and STOP2. The average age at start of transfusion therapy was (± SD) 8.5 plus or minus 3.4 years. For the most part (97% of the time; median, 28 days), transfusions were administered within less than 60 days of each other. The average volume of simple transfusion was 11.0 plus or minus 3.6 mL/kg. Chelation therapy was documented in 60% (n = 98) of patients, starting on average after 33 plus or minus 20 transfusions; 1% (n = 2) initially received deferasirox, the remainder desferrioxamine. Chelation therapy was administered on average 60% plus or minus 27% of the time while patients where on transfusion, according to quarterly visit records. A total of 11% (n = 18) of patients had a splenectomy; 50% (n = 9) of these occurred after start of transfusion therapy. Exchange transfusion use was reported in 42% (n = 68); 54% (n = 37) received 10 or more exchanges.
LIC
LIC information was obtained in 103 liver biopsies from 60 patients enrolled in STOP/STOP2. These included 83% (77 of 93) of patients initially identified from the database as having had a liver biopsy. The remainder were either liver biopsies done during the trials but not initially identified (n = 12), or biopsies done within 10 plus or minus 9 months outside of trial active observation periods (n = 14). Complications included pain after biopsy (n = 2, 1 requiring hospitalization for 5 days) and a liver abscess requiring surgical drainage 5 months after biopsy in 1 patient. LIC was assayed by ICPMS (n = 85), colorimetric methods (n = 8), atomic absorption spectroscopy (n = 7), or undetermined methods (n = 3). Only LIC measured by ICPMS in patients without viral hepatitis were further considered for analysis (n = 83). A total of 17% (n = 14) were obtained from 1998 to 2000; the remainder from 2001 to 2006. A total of 64% (n = 53) were from 3 centers with previously published LIC experience.9,12,17 A total of 23% (n = 19) of patients had a second analyzable LIC, 11% (n = 9) a third, and 1% (n = 1) a fourth one. The mean age at first LIC was 11 plus or minus 4 years (median, 11 years; range, 4-19 years). Mean first LIC was 13.6 plus or minus 8.5 mg/g dry weight (median, 12.1 mg/g dry weight; range, 1.8-41.3 mg/g dry weight). In patients with multiple LICs, last LIC was on average 15.9 plus or minus 10.1 mg/g dry weight (median, 12.8 mg/g dry weight; range, 4.3-37.1 mg/g dry weight).
Iron measure comparisons
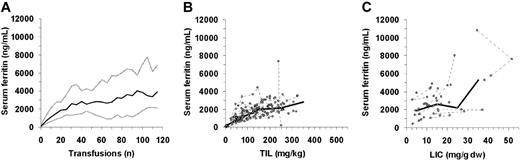
Median SF change with increasing number of transfusions, TIL, and LICs are presented in Figure 1 and Table 1. TIL data included 164 observations in 26 patients, after an average of 22 plus or minus 15 transfusions (range, 1-64 transfusions; volume data were missing in 3%). LICs that had corresponding SF measures included 47 observations in 33 patients, after an average of 69 plus or minus 30 transfusions (range, 14-126 transfusions). SF levels were obtained on average 31 plus or minus 30 days (range, 0-117 days) from the time of LIC.
SF changes with increasing iron load. (A) SF changes in relation to number of transfusions in all randomized STOP and STOP2 patients. SF changes in relation to (B) TIL or (C) LIC. Thick lines represent median change; gray lines, 10th and 90th percentiles; and dashed lines, change in individual patients.
SF changes with increasing iron load. (A) SF changes in relation to number of transfusions in all randomized STOP and STOP2 patients. SF changes in relation to (B) TIL or (C) LIC. Thick lines represent median change; gray lines, 10th and 90th percentiles; and dashed lines, change in individual patients.
Iron load measures and corresponding serum ferritin levels
| Iron load . | Patients, n . | Serum ferritin, ng/mL . | ||||
|---|---|---|---|---|---|---|
| Mean . | (SD) . | Median . | Minimum . | Maximum . | ||
| Pretransfusion | 95 | 139 | (126) | 97 | 10 | 628 |
| TIL, mg/kg | ||||||
| 50 | 26 | 914 | (581) | 838 | 29 | 2549 |
| 150 | 20 | 2066 | (762) | 1996 | 1019 | 4401 |
| 250 | 9 | 2259 | (991) | 2165 | 173 | 3669 |
| 350 | 3 | 2697 | (912) | 2816 | 1732 | 3544 |
| LIC, mg/g dw | ||||||
| 5 | 13 | 1889 | (980) | 1888 | 393 | 3837 |
| 15 | 16 | 2852 | (1128) | 2586 | 1060 | 4847 |
| 25 | 7 | 3276 | (2366) | 2179 | 1360 | 8016 |
| 35 | 3 | 6009 | (4474) | 5287 | 1940 | 10800 |
| Iron load . | Patients, n . | Serum ferritin, ng/mL . | ||||
|---|---|---|---|---|---|---|
| Mean . | (SD) . | Median . | Minimum . | Maximum . | ||
| Pretransfusion | 95 | 139 | (126) | 97 | 10 | 628 |
| TIL, mg/kg | ||||||
| 50 | 26 | 914 | (581) | 838 | 29 | 2549 |
| 150 | 20 | 2066 | (762) | 1996 | 1019 | 4401 |
| 250 | 9 | 2259 | (991) | 2165 | 173 | 3669 |
| 350 | 3 | 2697 | (912) | 2816 | 1732 | 3544 |
| LIC, mg/g dw | ||||||
| 5 | 13 | 1889 | (980) | 1888 | 393 | 3837 |
| 15 | 16 | 2852 | (1128) | 2586 | 1060 | 4847 |
| 25 | 7 | 3276 | (2366) | 2179 | 1360 | 8016 |
| 35 | 3 | 6009 | (4474) | 5287 | 1940 | 10800 |
Closest serum ferritin obtained before the start of transfusion, or closest to reference TIL value indicated (± 50 mg/kg) or LIC (± 5 mg/g dw). Serum ferritin variance was significantly greater for LICs, compared with TILs at equivalent iron load (distribution ratio F test, P < .005).
dw indicates dry liver weight.
Averaged correlation between SF and TIL was r = 0.70 plus or minus 0.09; when TILs less than 50 mg/kg were excluded, r = 0.47 plus or minus 0.14. Averaged correlation between SF and LICs was r = 0.55 plus or minus 0.06. SF ROC AUC are presented in Figure 2. Predictive value of SF ranges compared with iron load ranges is presented in Table 2. All SF measures of 4000 ng/mL or more were associated with LICs of 10 mg/g or more dry weight (8 patients, 11 observations).
SF receiver operating characteristic area under the curve. SF ROC AUC for iron load determined by (A) TIL, (B) TIL limited to measures more than 50 mg/kg, or (C) LIC. Circles represent averages of 1000 random data sampling; bars, SD. ROC AUC represents the area under the curve of sensitivity plotted against 1 − specificity for all possible SF values for a given iron load level. The closer ROC AUC equals 1, the better SF is, as a discriminator of iron; the closer ROC AUC equals 0.5, the closer SF is to random.21
SF receiver operating characteristic area under the curve. SF ROC AUC for iron load determined by (A) TIL, (B) TIL limited to measures more than 50 mg/kg, or (C) LIC. Circles represent averages of 1000 random data sampling; bars, SD. ROC AUC represents the area under the curve of sensitivity plotted against 1 − specificity for all possible SF values for a given iron load level. The closer ROC AUC equals 1, the better SF is, as a discriminator of iron; the closer ROC AUC equals 0.5, the closer SF is to random.21
Percentage of patients within given serum ferritin ranges with low, moderate, and increased iron load
| Serum ferritin, ng/mL . | Transfusion iron load, mg/kg, mean percentage (SD) . | Liver iron concentration, mg/g dw, mean percentage (SD) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Patients/observations, n . | %Less than 25 . | %25 to less than 100 . | %100 or more . | Patients/observations, n . | %Less than 2.5 . | %2.5 to less than 10 . | %10 or more . | |
| Less than 750 | 14/24 | 51.2 (7.9) | 46.4 (8.0) | 2.4 (3.4) | 1/1 | — | 100 | 0 |
| 750 to less than 1500 | 24/50 | 4.2 | 72.9 (5.9) | 22.9 (5.9) | 8/9 | — | 75.0 | 25.0 |
| 1500 to less than 2250 | 19/51 | 0 | 29.0 (5.2) | 71.0 (5.2) | 8/10 | — | 37.5 | 62.5 |
| 2250 to less than 3000 | 13/23 | 0 | 11.5 (3.8) | 88.5 (3.8) | 9/9 | — | 22.2 | 77.8 |
| 3000 or more | 7/16 | 0 | 4.5 (6.6) | 95.5 (6.6) | 11/18 | — | 12.1(4.3) | 87.9 (4.3) |
| 1000 plus or minus 250 | 24/38 | 4.2 | 83.0 (3.9) | 12.9 (3.9) | 4/4 | — | 75.0 | 25.0 |
| Serum ferritin, ng/mL . | Transfusion iron load, mg/kg, mean percentage (SD) . | Liver iron concentration, mg/g dw, mean percentage (SD) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Patients/observations, n . | %Less than 25 . | %25 to less than 100 . | %100 or more . | Patients/observations, n . | %Less than 2.5 . | %2.5 to less than 10 . | %10 or more . | |
| Less than 750 | 14/24 | 51.2 (7.9) | 46.4 (8.0) | 2.4 (3.4) | 1/1 | — | 100 | 0 |
| 750 to less than 1500 | 24/50 | 4.2 | 72.9 (5.9) | 22.9 (5.9) | 8/9 | — | 75.0 | 25.0 |
| 1500 to less than 2250 | 19/51 | 0 | 29.0 (5.2) | 71.0 (5.2) | 8/10 | — | 37.5 | 62.5 |
| 2250 to less than 3000 | 13/23 | 0 | 11.5 (3.8) | 88.5 (3.8) | 9/9 | — | 22.2 | 77.8 |
| 3000 or more | 7/16 | 0 | 4.5 (6.6) | 95.5 (6.6) | 11/18 | — | 12.1(4.3) | 87.9 (4.3) |
| 1000 plus or minus 250 | 24/38 | 4.2 | 83.0 (3.9) | 12.9 (3.9) | 4/4 | — | 75.0 | 25.0 |
Averages from 1000 random data samplings (1 observation within serum ferritin range per patient selected at each sampling; same patient may be represented in > 1 serum ferritin range). All LICs in this analysis were more than 2.5 mg/g dw.
SD indicates sampling standard deviation; dw, dry liver weight; and —, not applicable.
The relation between TIL and LIC obtained within a month of start of chelation therapy was estimated in 15 patients on simple transfusion (STOP2 transfusion volume data in 8 patients who were never randomized, and in 5 patients before randomization, was estimated to be the same as the average volume administered in the study). Correlation coefficient was r = 0.64. Linear regression relation10 was expressed as: TIL (mg/kg) = 9.0 × LIC (mg/g dry weight), assuming that the amount of blood received before the trials was negligible.
To examine whether trends in SF levels can be used as a qualitative measure of increasing or decreasing iron overload, SF linear regression trends over time were compared with LIC pair trends (all SF measures within 4 months before first or after last LIC were included). Fifteen LIC pairs in 10 patients were examined. These were obtained on average 1.6 plus or minus 1.3 years apart (range, 0.5-4.7 years). SF and LIC trends were significantly more probable in the same direction (87%, increasing n = 8, decreasing n = 5; P = .007), than in the opposite direction (13%, increasing SF and decreasing LIC, n = 2).
Liver injury and SF
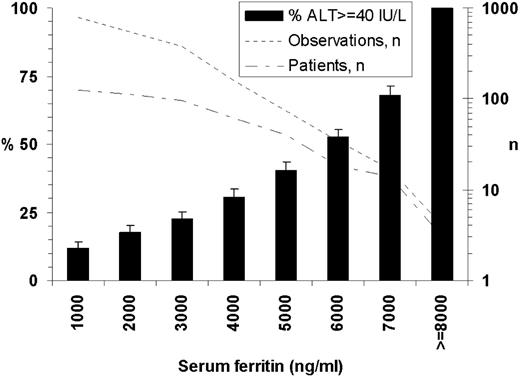
Of patients on transfusions on whom ALT levels were available, 57% (85 of 150) had at least one ALT measure of 40 IU/L or more. For these, peak ALT elevation was on average 73 plus or minus 52 IU/L (range, 40-329 IU/L) and occurred on average after 43 plus or minus 32 transfusions. The relation between SF and ALT is presented in Figure 3. The averaged correlation of SF with ALT within the first 50 transfusions was r = 0.09 plus or minus 0.10; and after 50 transfusions, r = 0.53 plus or minus 0.08. Multivariate mixed models that examined variables that affected SF or ALT changes are presented in Table 3.
Percentage of patients within given SF who have increased ALT levels. SF was plus or minus 1000 ng/mL level indicated (except for last level indicated left of chart). Percentages are averages from 1000 random data samplings; bars represent sampling SD: one observation within SF range per patient selected at each sampling; the same patient may be presented in more than 1 stratum.
Percentage of patients within given SF who have increased ALT levels. SF was plus or minus 1000 ng/mL level indicated (except for last level indicated left of chart). Percentages are averages from 1000 random data samplings; bars represent sampling SD: one observation within SF range per patient selected at each sampling; the same patient may be presented in more than 1 stratum.
Multivariate mixed models examining serum ferritin or ALT changes during chronic transfusion
| Responding/exposure variables . | Iron load measure method . | |||
|---|---|---|---|---|
| TIL . | LIC . | |||
| F . | P . | F . | P . | |
| Serum ferritin | ||||
| Iron load | 147.79 | < .001 | 15.66 | .006 |
| ALT | 0.37 | .542 | 8.02 | .025 |
| Sex | 0.41 | .523 | 1.32 | .288 |
| Splenectomy | 0.48 | .490 | 0.14 | .715 |
| Weight | 0.51 | .478 | 7.97 | .026 |
| ALT | ||||
| Iron load | 13.62 | < .001 | 10.43 | .012 |
| Sex | 0.03 | .858 | 4.28 | .072 |
| Splenectomy | 0.03 | .859 | 0.26 | .622 |
| Weight | 2.10 | .149 | 0.15 | .709 |
| Responding/exposure variables . | Iron load measure method . | |||
|---|---|---|---|---|
| TIL . | LIC . | |||
| F . | P . | F . | P . | |
| Serum ferritin | ||||
| Iron load | 147.79 | < .001 | 15.66 | .006 |
| ALT | 0.37 | .542 | 8.02 | .025 |
| Sex | 0.41 | .523 | 1.32 | .288 |
| Splenectomy | 0.48 | .490 | 0.14 | .715 |
| Weight | 0.51 | .478 | 7.97 | .026 |
| ALT | ||||
| Iron load | 13.62 | < .001 | 10.43 | .012 |
| Sex | 0.03 | .858 | 4.28 | .072 |
| Splenectomy | 0.03 | .859 | 0.26 | .622 |
| Weight | 2.10 | .149 | 0.15 | .709 |
Splenectomy before the start of transfusion therapy, weight at start of transfusion therapy. Model degrees of freedom (numerator/denominator) TIL, 1 of 136; LIC, 1 of 7. When lactose dehydrogenase, white cell count, or hemoglobin S concentration was included in models, these variables did not reach significance (data not shown).
The presence or absence of liver fibrosis was described in 90% of biopsies (n = 75; trichrome stain documented in 69). In these, fibrosis was absent in 56% (n = 42), mild in 37% (n = 28), and moderate to severe in 7% (n = 5). When liver biopsies with the highest degree of fibrosis per patient were selected, average SF in patients without or with mild liver fibrosis was 2415 plus or minus 1097 ng/mL (n = 34); in patients with moderate to severe fibrosis, average SF was 3571 plus or minus 2158 ng/mL (n = 4, P = .234). Respective values for LIC were 14.0 plus or minus 8.2 mg/g dry weight (n = 45) and 25.5 plus or minus 15.6 mg/g dry weight (n = 4, P = .097). In univariate mixed models, the degree of fibrosis was associated with ALT (P = .018) and approached significance with γ-glutamyl transferase levels (P = .051) and with number of transfusions (P = .081).
Discussion
The natural history of iron overload was examined in chronically transfused children with SCD and without viral hepatitis, who were observed for up to 10 years in 2 consecutive stroke prevention trials. These trials were conducted at a time when most patients received chelation as injectable desferrioxamine, which can be difficult to administer.1 This allowed an analysis over a wide range of iron overload. SF level changes appeared nonlinear compared with increasing iron load iron measured by TIL or LIC. After an initial rapid rise, SF rate of change seemed to slow after reaching approximately 1500 to 2500 ng/mL, despite evidence of increasing iron load. After further iron overload, patients developed high levels of SF (≥ 3000 ng/mL). SF measures above this level appeared to be associated with both increased LIC and liver injury, as estimated by ALT levels.
Intracellular ferritin is a hollow protein shell made of 24 heavy (H) or light (L) subunits that stores iron.24 Synthesis is differentially regulated at a posttranscriptional level, mediated by iron-binding proteins.25 L-subunits contain iron storage-facilitating ferroxidase enzymatic activity24 and are approximately 3 to 4 times more abundant than H-subunits in the liver of normal persons.26 Cardiac ferritin is composed principally of H-subunits.27 During iron overload, liver ferritin content increases more than 4-fold and is further enriched in L-subunits.26 However, liver hemosiderin, which is formed from degraded ferritin in the lysome, is constituted predominantly of H-subunits.26 L-subunits are actively secreted in response to iron and inflammatory cytokines28 by an unknown mechanism. In patients with thalassemia major on chronic tranfusion,5 glycosylated SF levels reached a plateau after 100 units, consistent with a rate-limited active secretion process. Rapid SF level changes at low iron load and slower rate of change at moderate iron load levels were also described in patients with hemochromatosis undergoing phlebotomy,4 and were noted in a previous analysis of STOP data.11 After a flattened response, a near-exponential increase in SF was observed at high LIC levels in patients with a variety of hematologic conditions or hemachromatosis.4 In patients with thalassemia major, unglycosylated (and thus presumably not actively secreted) SF continued to rise with further transfusions5 ; the authors proposed that SF changes beyond the flattened phase were the result of leakage from damaged hepatocytes. An association between ALT and SF levels was observed in an iron overload rat model29 and in patients on chronic transfusion for thalassemia major5,30 or acquired anemias.31 It can also occur in patients with acute liver injury without iron overload.4
In the current study, most patients developed elevated ALT levels during the course of transfusion therapy. However, peak ALT elevations and degree of liver fibrosis were mostly mild, consistent with previous observations.9 ALT level changes were significantly associated with iron load estimated by both TIL and LIC. A modest but significant association between fibrosis and LIC noted in a previous report9 was not evident in the present study, probably because only a small number of patients with moderate to severe fibrosis were observed. The association between ALT and SF was evident in patients with iron load assessed by LIC, and in general in patients who received more than 50 transfusions, suggesting that in children with SCD, this phenomenon occurs after prolonged exposure to chronic transfusion.
Weight at the start of transfusion therapy was also associated with increased SF levels in the multivariate analysis. SF changes were not significantly associated with other variables, such as sex4 or splenectomy. Possible other reasons for low SF levels, such as low ascorbic acid levels32 or asymptomatic mutations of the ferritin L-subunit gene (FTL),33 were not examined.
The utility of SF as a measure of iron load has been questioned.3,12 Poor predictability of SF can be in part attributed to when data are sampled in relation to degree of iron load. Indeed, SF levels correlate poorly with iron load when data points are obtained in the flattened part of the response curve. This effect can be simulated with TIL, by removing data points with TIL less than 50 mg/kg, as illustrated in Figure 2. An analysis comparing SF ranges with iron level ranges best summarizes when SF may be useful as a clinical tool to monitor iron, and when it is of limited value (Table 2).
This study also examined LIC performed in a clinical setting as a measure of iron overload. Complications from liver biopsy requiring hospitalization were observed in 2%. One patient developed a liver abscess, similar to a previous report.34 No patients died from the procedure.35 In a smaller group of patients, the linear regression equation between TIL and LIC assayed by ICPMS, which was the predominant method used, was similar to that observed in patients with thalassemia major after bone marrow transplantation.10 In that study, TIL in milligrams per kilogram measured by phlebotomy was equal to 10.6 times LIC in milligrams per gram dry weight, assayed by atomic absorption spectroscopy. In the present study, the correlation coefficient between LIC and TIL was more modest. LIC specimen weight10 or transportation medium (paraffin block vs other)36 was not evaluated, as it is not usually reported in commercially assayed LICs, and may have affected the variability of results. Recommendations for routine use of LIC by biopsy are tempered by small but finite risk of severe complications,34,35 lack of cross-validation of assays,18,37-39 and generally unremarkable liver histology.9 Safety and reproducibility of LIC by biopsy could be improved if prophylactic antibiotics were administered in stable patients35 at the time of ultrasound-guided biopsy, and if samples weighing the equivalent of 1 g dry weight10 were sent in the same transport media36 (eg, all embedded in paraffin block) only to laboratories that perform routinely LICs by validated analytical chemistry methods.18,39 Adequacy of sample size could be determined if total dry weight was reported with LIC result.
In light of these observations, an approach to monitor iron overload can be proposed, using a combination of methods. Before the start of chelation, determination of iron load from transfusion history, either by TIL or just by counting the number of plain transfusions, appears to be the simplest and most accurate measure of iron load to determine when to start chelation therapy (eg, TIL 75 mg/kg ≈ 90-130 mL/kg of blood ≈ 9-13 transfusions at 10 mL/kg). SF levels less than 1500 ng/mL (eg, before the response curve flattens) indicate acceptable iron load in most patients. However, approximately 15% to 25% of patients may be “high”11 or “low” SF responders. These can be detected by comparing SF with TIL before the start of chelation. “Low responders,” once detected, may require a lower SF threshold, as seemingly adequate ferritin levels in such patients may give a false sense of security despite significant iron overload. Observing SF trends with frequent measures (eg, at each transfusion) overcomes much of the variability seen over time in individual patients, and can also help avoid overchelation, another potential source of toxicity. Serial ALT measures can also be informative, as levels change in response to iron overload.31,40
In contrast, more than three-fourths of patients with SF levels of 2250 ng/mL or more, 88% of those with 3000 ng/mL or more, and all measures of 4000 ng/mL or more were associated with LICs of 10 mg/g or more dry weight. Clearly, patients with repeated high SF measures require intensified iron removal therapy, as such levels indicate significant and potentially toxic iron overload in most.
Thus, maintaining a lower SF threshold (eg, 750 to < 1250 ng/mL) in all patients may result in adequate iron load control, as 83% of patients with such SF levels have an acceptable iron load as assessed by TIL. However, safety and effectiveness of such strategies would need to be evaluated prospectively long term. Pitfalls of such a strategy may include possible fluctuations of ferritin trends unrelated to iron load (eg, from chronic inflammation or other reasons), the possibility of missing “low responders” (eg, TIL not available), and SF level changes that differ between chelation agents (different iron compartment mobilization).
However, there is no way of knowing with precision if a person with SF levels in the 1500 or more to less than 3000 ng/mL range, who was on transfusion and chelation therapy for a period of time, has an acceptable iron load or has developed significant iron overload. For these reasons, optimal iron load assessment should also include periodic (yearly) tissue iron determination, especially in patients with intermediately elevated SF levels (1500 to < 3000 ng/mL).
Noninvasive methods of tissue iron assessment include the investigational superconducting interference device susceptometer7,41 and MRI.42-45 Clinically meaningful correlations between MRI R2,42,43 R2*,43,45 and biopsy-derived LICs were reported in patients with transfusion iron overload and are currently the most accessible noninvasive methods. Cardiac MRI should be considered in patients with a prolonged history of transfusions, as heart iron overload seems to follow that of the liver.44 Elucidating how tissue iron levels are measured by MRI46 may help improve the method further and allow its precise calibration and standardization, without the need of tissue biopsies.
In patients with thalassemia major,3 SF levels exceeding 2500 ng/mL were associated with decreased survival from iron overload-related heart complications. Although in patients with SCD, the relationship between iron measures and outcome needs to be further defined, in a recent study, transfused patients with SF of 2000 ng/mL or more or LIC of 10 mg/g or more dry weight had similar risk of death as those with thalassemia major with equivalent iron load and transfusion history.47 In the current study, most patients became iron overloaded during the course of the trials, as most developed SF levels more than 2500 ng/mL after approximately 30 transfusions, reflecting difficulties in administering desferrioxamine. Studies of populations at risk, including surveys using TIL and tissue iron assessments, will help ascertain novel iron removal methods1 and help validate simpler, cheaper, but equally effective iron-monitoring strategies that may be more generally applicable.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Duane Bonds, National Institutes of Health Project Officer for STOP/STOP2, for her support; Dianne Gallagher, New England Research Institutes, for retrieving initial LIC data; Dr Paul Harmatz and Dr Ellen Butensky, Children's Hospital & Research Center Oakland; Jessica Peterson and Elizabeth Rackoff, Medical University of South Carolina; Manuela Merelles-Pulcini, Hospital for Sick Children, Toronto; Elizabeth Dackiw, Johns Hopkins University; Dr Neil Grossman, University of Maryland Medical Center; Dr Joseph Wiley, Sinai Hospital of Baltimore; Kristin Stegenga, Children's Mercy Hospital, Kansas City; Dr Kathleen Loomes, Children's Hospital of Philadelphia; Dr Karen Viviera, Dr Porshia Bradford, Howard Hughes/Emory Summer Undergraduate Program and Dr Carlos Abramowsky, Emory University, for assistance with LIC data collection; Mimi Lou, Medical College of Georgia, for assistance with data management; Keisha Harville, Morehouse School of Medicine, for administrative support; Dr Gregory Strayhorn and Dr Harry Strothers, Morehouse School of Medicine and Dr Jennifer Buskey, Novartis Corporation, for general assistance; and Dr Mitch Klein and Dr Kevin Sullivan, Emory Rollins School of Public Health, for statistical advice.
This work was supported in part by the National Heart, Lung, and Blood Institute (1K23HL0425101A1, T.V.A.; U01HL052193, U01HL052016, R.J.A.) and by Novartis Corporation (CICL670AUS06, T.V.A.).
Authorship
Contribution: T.V.A., M.R.A., C.P., E.V., and R.J.A. designed the study; T.V.A., N. Olivieri, M.K.-A., E.V., J.F.C., O.A.A., J.C.B., M.T.L., R.V.I., A.K., K.M.M., V.M., N. Odo, B.G., J.L.K., G.M.W., and T.C. collected data; T.V.A. and N. Odo prepared and analyzed data; and T.V.A., M.R.A., C.P., N. Olivieri, E.V., J.F.C., O.A.A., M.T.L., N. Odo, J.L.K., G.M.W., T.C., W.W., and R.J.A. performed analysis review and prepared the manuscript.
Conflict-of-interest disclosure: C.P. is an employee of Novartis Corporation. The remaining authors declare no competing financial interests.
Correspondence: Thomas V. Adamkiewicz, NCMHD Southeastern Exploratory Sickle Cell Center of Excellence, MSM Hemoglobinopathy/Genomics Training Program, Department of Family Medicine, Morehouse School of Medicine, 1513 E Cleveland #100, 3rd Fl, Ste 300-A, East Point, GA 30344; e-mail: tadamkiewicz@msm.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal