Abstract
The nuclear protein FOG-1 binds transcription factor GATA-1 to facilitate erythroid and megakaryocytic maturation. However, little is known about the function of FOG-1 during myeloid and lymphoid development or how FOG-1 expression is regulated in any tissue. We used in situ hybridization, gain- and loss-of-function studies in zebrafish to address these problems. Zebrafish FOG-1 is expressed in early hematopoietic cells, as well as heart, viscera, and paraspinal neurons, suggesting that it has multifaceted functions in organogenesis. We found that FOG-1 is dispensable for endoderm specification but is required for endoderm patterning affecting the expression of late-stage T-cell markers, independent of GATA-1. The suppression of FOG-1, in the presence of normal GATA-1 levels, induces severe anemia and thrombocytopenia and expands myeloid-progenitor cells, indicating that FOG-1 is required during erythroid/myeloid commitment. To functionally interrogate whether GATA-1 regulates FOG-1 in vivo, we used bioinformatics combined with transgenic assays. Thus, we identified 2 cis-regulatory elements that control the tissue-specific gene expression of FOG-1. One of these enhancers contains functional GATA-binding sites, indicating the potential for a regulatory loop in which GATA factors control the expression of their partner protein FOG-1.
Introduction
Transcription factor GATA-1 is the founding member of a small family of nuclear proteins that bind the DNA consensus sequences [(T/A)GATA(A/G)]1 in a variety of tissues. GATA-1 was initially discovered as a nuclear protein that binds numerous GATA consensus motifs distributed throughout enhancers and promoters of erythroid-specific genes. Moreover, GATA-1 is a key regulator of erythropoiesis, as demonstrated by genetic studies in zebrafish and mice.2-5 GATA-1 null mice showed complete ablation of primitive and definitive erythropoiesis resulting from arrested maturation4,5 and apoptosis of erythroid cells.2 In addition, numerous gain- and loss-of-function experiments show that GATA-1 is important for the development of megakaryocytes,6,7 eosinophils, and mast cells.8-11 In addition to driving the maturation of several hematopoietic lineages, GATA-1 also inhibits the formation of alternate lineages by interacting with the myeloid transcription factor PU.1.11 Specifically, studies in zebrafish and mice demonstrate that GATA-1 and PU.1 proteins antagonize each other during hematopoietic lineage specification.12-15
GATA-1 contains 2 zinc finger domains, which are highly conserved throughout vertebrate evolution. The C-terminal zinc finger is required for DNA binding, whereas the N-terminal finger stabilizes DNA binding and facilitates physical interaction with numerous proteins. Interactions between the GATA-1 N-finger and the multitype zinc finger protein, FOG-1 (Friend-of-GATA-1, zfpm1), appear to be particularly important.16,17 GATA-1/FOG-1 complexes can function as activators for several erythroid and megakaryocytic genes and as repressors for others.18 In both mice and humans, GATA-1 missense mutations that disrupt FOG-1 interaction cause severe anemia and thrombocytopenia, partially recapitulating loss of GATA-1 phenotypes.17 In contrast to GATA-1–deficient mice, FOG-1 knockout animals show complete ablation of the megakaryocytic lineage,19 probably because GATA-2/FOG-1 complexes can partially compensate for loss of GATA-1.7,18 Together these experiments demonstrate that FOG-1 acts as a critical cofactor for GATA-1 and GATA-2 in erythroid and megakaryocytic lineages.
Interestingly, FOG-1 antagonizes GATA-1 activities in other lineages. Thus, GATA-1–dependent eosinophil maturation is inhibited by FOG-1.8 A similar example has been reported for mast cell differentiation in which FOG-1 down-regulation is a prerequisite for mast cell development.9,10 Ectopic expression of FOG-1 in committed mast cell progenitors reprograms them along erythroid/megakaryocytic lineages by repression of GATA-2, an essential GATA factor in mast and eosinophilic cells.9,10 Together, these experiments suggest that erythroid and myeloid specification is achieved by activation and/or repression of FOG-1, which acts largely by modulating GATA factor activity through direct physical interaction.
FOG-1 is also expressed in nonhematopoietic tissues where it exerts GATA-1–independent functions, probably through interaction with other GATA family members. For example, in mice FOG-1 represses GATA-3 activity in naive T-helper cells during their development into T-helper 2 cells.20 FOG-1 also plays a role in heart development, most probably through interactions with GATA factors 4, 5, or 6.18,21-23 In zebrafish, the injection of an antisense morpholino directed against the homolog to murine FOG-1 resulted in embryos with a large pericardial effusion and a looping-deficient heart tube. This looping defect could be rescued by coinjection of cRNA encoding zebrafish or murine FOG-1.24
Together, prior studies indicate that FOG-1 positively and negatively regulates the formation of hematopoietic and nonhematopoietic tissues through interactions with GATA proteins. However, the full extent of these interactions, the associated mechanisms, and their functional implications are unknown. Moreover, little is known about the regulation of FOG-1 expression, which must be exquisitely controlled to positively and negatively regulate GATA factors in different tissues. Using zebrafish genetics and molecular experiments, we show that zebrafish FOG-1 acts independently of GATA-1 and is required for T-cell development in the thymic organ. Our findings also reveal that loss of FOG-1 function significantly expands myeloid precursors at the expense of erythrocytes, similar to what occurs with loss of GATA-1 activity. Thus, FOG-1 is an essential component of GATA-1 activity during erythroid/myeloid cell fate determination. Finally, we used bioinformatics coupled with zebrafish transgenic methods to identify 2 conserved cis-regulatory motifs (CRM) in the FOG-1 locus, FE1 and FE2, which control its expression during hematopoiesis. The FE2 enhancer contains functional GATA-1 motifs, consistent with FOG-1 being a downstream target of GATA factors.
Methods
The nucleotide sequences reported in this paper have been submitted to GenBank with the accession numbers: AY515850 (FOG-1).
Zebrafish maintenance
Wild-type (AB*, Tü) and germline transgenic zebrafish (Danio rerio) were kept and bred according to standard methods.25 Maintenance of zebrafish transgenic lines is detailed in the supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article). All studies described received full approval from the institutional animal care and use committee at Brigham & Women's Hospital.
WISH
A detailed description of the whole mount in situ hybridization (WISH) procedure and molecular probes is provided in the supplemental data.
Knockdown of zebrafish FOG-1 with antisense MO
To knock down the function of zebrafish FOG-1, we used morpholino (MO) oligomers.26 Custom-synthesized MOs were obtained from Gene Tools, LLC. Approximately 0.05 to 0.08 pmol of MO was injected into embryos at the 1- to 2-cell stage. MOs targeting FOG-1 and control are listed in supplemental Table 1. The vlad tepes (vlt) genotyping was performed as previously described.3 Rescues assays were performed by coinjecting approximately 0.08 pmol of FOG-1MO with 100 pg of FOG-1 zebrafish cRNA.
Fluorescence-activated flow cytometry
MO against FOG-1 (∼ 0.08 pmol) was injected into fertilized eggs at the 1- to 2-cell stage from the transgenic zebrafish Tg(GATA-1:eGFP).27 Approximately 100 to 200 embryos were collected from MO-injected and control MO-injected clutches. Disaggregated cells at 24 hpf were sequentially passed through 70-mm and 40-mm cell strainers, washed once in 0.9× phosphate-buffered saline buffer, and then pelleted by gentle centrifugation. The cells were resuspended in a final buffer containing 0.9× phosphate-buffered saline, 2% fetal bovine serum, and propidium iodide. Cells were sorted in a BD Biosciences FACSVantage SE machine, collected by cytospin centrifugation, and stained with Wright-Giemsa dye as described.14
Generation of destination pTol2-FOG-1:eGFP enhancer-reporter clones
We used the Gateway-compatible vectors (Invitrogen) to analyze gene function in transgenic zebrafish.28 Descriptions of the molecular and transgenic techniques are detailed in the supplemental data.
Expression of enhancer transgenes in zebrafish embryos
The pCS2-TP plasmid, encoding the Tol2 transposase, was linearized, and 5′-capped Tol2 cRNA synthesized by in vitro transcription using SP6 RNA polymerase (Ambion). Embryos were coinjected at the 1-cell stage with 25 pg of Tol2 transposase cRNA and 50 pg of Tol2-flanked destination enhanced green fluorescence protein (eGFP) reporter plasmid.28 The expression of the transgenic reporter eGFP was visualized at 24 hpf under a fluorescence microscope (Leica MZFLIII).
ChIP assay and real-time PCR
Mouse erythroleukemia (MEL) cells were initially grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 2% penicillin/streptomycin, and 1% glutamine. MEL cells were induced to undergo erythroid maturation by the addition of dimethyl sulfoxide to final 1.7% (vol/vol) concentration for 24 hours before performing the chromatin immunoprecipitation (ChIP) assay. G1ER cells were grown as described29 for ChIP analysis. ChIP assay was performed as previously described30 with modifications. Protein-A DynaBeads (100.02D; Invitrogen) were preincubated with rabbit anti–rat IgG (H + L; Jackson ImmunoResearch) for 1 hour before incubation with the GATA-1 antibody (sc-265; Santa Cruz Biotechnology) for 3 hours. Chromatin from 4 × 106 MEL cells was added and immunoprecipitated. The immunoprecipitated DNA was purified and analyzed by quantitative real-time polymerase chain reaction (PCR) using the QuantiTect SYBR Green PCR Kit (QIAGEN). A fragment 2 kb upstream of the GATA-1 HS1 site, which lacks the GATA-binding site, was used as an internal control, and fold of enrichment was calculated by the 2ΔCt method. The primers used for PCR amplifications are listed in supplemental Table 2.
Statistical analysis
Pooled data were calculated as the mean plus or minus SD with the number of independent experiments indicated. Pairwise comparisons were performed by the Student t test and multiple comparisons by analysis of variance using the SAS 9.1.3 software (SAS Institute Inc; www.sas.com).
Results
Zebrafish FOG-1 is expressed during early hematopoiesis
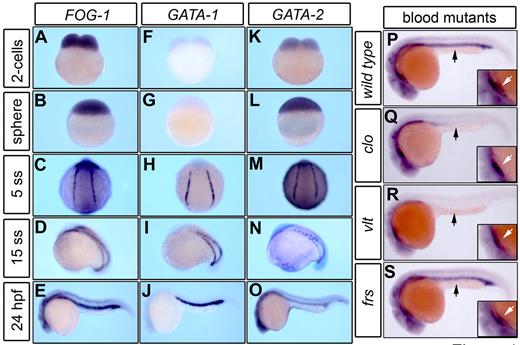
In zebrafish, the cells destined for hematopoiesis express GATA transcription factors in the lateral plate mesoderm (LPM). Subsequently, the LPM gives rise to the intermediate cell mass (ICM), the functional equivalent of the yolk sac blood islands in mammals.31 To assess the role of FOG-1 during zebrafish hematopoiesis, we used WISH to compare the expression patterns of FOG-1, GATA-1, and GATA-2 at early stages of embryogenesis. Zebrafish FOG-1 mRNA is strongly expressed as a maternal transcript during the 2-cell stage (Figure 1A) in a similar expression pattern to GATA-2 (Figure 1K). At the sphere stage, FOG-1 transcripts are observed along with zygotic GATA-2 mRNA (Figure 1B,L). At the 5-somite stage (5ss), the expression of GATA-1 is initiated in the LPM in a common region compared with FOG-1 and GATA-2 (Figure 1C,H,M). At the 15-somite stage (15ss), FOG-1 is coexpressed with those GATA-factors in the ICM (Figure 1D,I,N). At 24 hours postfertilization (hpf), FOG-1 and GATA-1 are strongly expressed in the ICM (Figure 1E,J), whereas GATA-2 levels are reduced in the same region (Figure 1O). We also detected expression of FOG-1 mRNA during organogenesis in the digestive tract. FOG-1 transcripts can be detected in the liver, hepatic duct, intestine, and pancreatic primordia (supplemental Figure 1A), similar to endodermal markers of the viscera, such as pdx1 (supplemental Figure 1B) and GATA-6 (supplemental Figure 1C). At 5 days postfertilization (dpf), FOG-1 is coexpressed with GATA-6 and pdx1, which delineate the liver, stomach, and intestine (supplemental Figure 1D-F). In the heart, FOG-1 transcripts are most intensely visible at the constriction of the atrial-ventricular boundary as well as nkx2.5 and GATA-6 (supplemental Figure 1G-I), consistent with previous observations.24
FOG-1 expression pattern during early hematopoiesis. The expression pattern of FOG-1 and GATA factors was visualized by WISH during zebrafish embryonic development (A-O). The maternal expression of FOG-1 (A-B) and GATA-2 (K-L) transcripts is initially noted at the 2-cell and sphere stages. The zygotic expression pattern of FOG-1 in the blood island is coincident with the expression of GATA-1 and GATA-2 in the LPM (C,H,M) and the ICM (D-E,I-J,N-O). FOG-1 is highly expressed in the ICM and neural tissues of a wild-type embryo at 24 hpf (P). Mutants involved in defective formation of hematopoietic (cloche, clo; Q) or erythroid progenitors (vlad tepes, vlt; R) are deficient in the expression of FOG-1 in the ICM (black arrow), whereas expression in the heart is preserved (white arrow, inset magnification). The expression of FOG-1 in the ICM is normal in a mutant with a defect in late erythroid maturation (frascati, frs; S).
FOG-1 expression pattern during early hematopoiesis. The expression pattern of FOG-1 and GATA factors was visualized by WISH during zebrafish embryonic development (A-O). The maternal expression of FOG-1 (A-B) and GATA-2 (K-L) transcripts is initially noted at the 2-cell and sphere stages. The zygotic expression pattern of FOG-1 in the blood island is coincident with the expression of GATA-1 and GATA-2 in the LPM (C,H,M) and the ICM (D-E,I-J,N-O). FOG-1 is highly expressed in the ICM and neural tissues of a wild-type embryo at 24 hpf (P). Mutants involved in defective formation of hematopoietic (cloche, clo; Q) or erythroid progenitors (vlad tepes, vlt; R) are deficient in the expression of FOG-1 in the ICM (black arrow), whereas expression in the heart is preserved (white arrow, inset magnification). The expression of FOG-1 in the ICM is normal in a mutant with a defect in late erythroid maturation (frascati, frs; S).
The expression pattern of FOG-1 in zebrafish blood mutants suggests a role in hematopoiesis
Using zebrafish blood mutants defective at different stages of hematopoietic differentiation, we examined the genetic interactions and function of FOG-1. The zebrafish blood mutants involved in the early stages of hematopoiesis, such as cloche (clo, encoding a gene that specifies the formation of hematopoietic and vascular progenitors32 ) and vlad tepes (vlt, encoding a deficient form of GATA-13 ), were evaluated for expression of FOG-1 in the ICM. In these mutants, the expression of FOG-1 is retained in neural structures of the head and in the heart but not in the ICM (Figure 1P-R). In contrast, the blood mutation frascati (frs, encoding a defect in the slc25a37 mitochondrial iron transporter33 ), which affects erythroid maturation at a later stage than clo or vlt, displays normal levels of FOG-1 expression in the ICM (Figure 1S). Thus, the deficiency of erythropoietic progenitors in the ICM causes a drastic reduction in mRNA expression of FOG-1 during zebrafish hematopoiesis (Figure 1Q-R).
FOG-1 is necessary but not sufficient for erythroid/thrombocyte formation in zebrafish
To test the role of FOG-1 in zebrafish early hematopoiesis, we depleted embryos of FOG-1 protein by injecting morpholinos26 (hereafter referred to as FOG-1 MO). The FOG-1 MO was designed to target the splice-donor site of exon 4 to selectively interfere with FOG-1 mRNA processing. Using reverse transcription (RT)–PCR, we found that the injection of FOG-1 MO resulted in the production of an aberrantly spliced mRNA (supplemental Figure 2A; supplemental Table 1). Sequence analysis of the mis-spliced cDNA revealed that the variant results from exclusion of exon 5 (data no shown). As a result, a frame shift is generated that would lead to subsequent nonsense-mediated mRNA decay. Consistent with this prediction, we observed that the FOG-1 morphant had drastically reduced the steady-state FOG-1 mRNA compared with the control embryo (supplemental Figure 2B).
Embryos injected with FOG-1 MO show normal early hematopoiesis, as indicated by GATA-1 and scl expression in the erythroid progenitors of the LPM and ICM (supplemental Figure 3). In contrast, at 20 hpf, the expression of band3 was significantly reduced in FOG-1 morphants (supplemental Figure 3J). Interestingly, the overexpression of FOG-1 mRNA in wild-type embryos did not increase the number of band3+-erythroid cells (data not shown). This suggests that FOG-1 is not essential for specification of early erythroid progenitors but is required for maintenance or maturation of erythroid cells.
To quantify our data, the embryos were placed in 3 different categories (normal, reduced, and absent), depending on the level of hemoglobinization or expression for band3 and cd41 in erythrocytes and thrombocytes, respectively (Figure 2). The FOG-1 MO-injected embryos have a strong reduction of band3 expression in the ICM relative to wild-type embryos (Figure 2A-B,K). The loss of erythroid cells was verified by staining with o-dianisidine for hemoglobinized cells, which revealed either drastic reduction or complete absence of mature erythrocytes in FOG-1 morphants (Figure 2D-E,J). The specificity of the morphant phenotype was validated by injections with control MOs (supplemental Table 1), showing no anemia (data not shown). Furthermore, hemoglobinized cells (Figure 2J), band3 expression (Figure 2K), and cardiac morphant phenotype24 were fully restored by coinjection of zebrafish FOG-1 cRNA in FOG-1 morphants (Figure 2C,F; supplemental Figure 4).
FOG-1 is necessary for erythroid/thrombocytic development in zebrafish. Uninjected control embryos (A,D,G), FOG-1 MO-injected embryos (B,E,H), and FOG-1 MO coinjected with FOG-1 cRNA (C,F,I). Lateral views of 24-hpf embryos processed by WISH to reveal band3 expression (A-C). Lateral (D-Di,E-Ei,F-Fi) and ventral (Dii,Eii,Fii) views of 48 hpf embryos processed by o-dianisidine staining (D-F) to reveal hemoglobinized cells. Lateral view at the trunk level of 4-dpf transgenic zebrafish [Tg(cd41:eGFP)] embryos to reveal eGFP+ thrombocytes (G-I). FOG-1 morphant embryos display reduction or complete absence of band3 expression in the ICM (A-B black brackets, inset magnification). The morphant embryos show severe anemia when stained with o-dianisidine (D-E black arrows). Injection of FOG-1 MO in the transgenic zebrafish [Tg(cd41:eGFP)] produces complete absence of eGFP+ thrombocytes at 4 dpf (G-H white brackets). The anemic phenotype is fully rescued by coinjection of FOG-1 cRNA in the morphant embryos (C,F). In contrast, the thrombocytic phenotype is only partially rescued (I, white arrow). The bars represent quantification of the normalized phenotypes (mean ± SD) with the numbers of embryos analyzed in each category indicated above the bar (J-L). White, black, and gray bars represent embryos with normal, reduced, and absence of erythroid/thrombocytic markers, respectively. Data were derived from 3 independent experiments. The statistical significance, marked by lines for each paired condition, was analyzed using analysis of variance; *P < .001; **P < .02.
FOG-1 is necessary for erythroid/thrombocytic development in zebrafish. Uninjected control embryos (A,D,G), FOG-1 MO-injected embryos (B,E,H), and FOG-1 MO coinjected with FOG-1 cRNA (C,F,I). Lateral views of 24-hpf embryos processed by WISH to reveal band3 expression (A-C). Lateral (D-Di,E-Ei,F-Fi) and ventral (Dii,Eii,Fii) views of 48 hpf embryos processed by o-dianisidine staining (D-F) to reveal hemoglobinized cells. Lateral view at the trunk level of 4-dpf transgenic zebrafish [Tg(cd41:eGFP)] embryos to reveal eGFP+ thrombocytes (G-I). FOG-1 morphant embryos display reduction or complete absence of band3 expression in the ICM (A-B black brackets, inset magnification). The morphant embryos show severe anemia when stained with o-dianisidine (D-E black arrows). Injection of FOG-1 MO in the transgenic zebrafish [Tg(cd41:eGFP)] produces complete absence of eGFP+ thrombocytes at 4 dpf (G-H white brackets). The anemic phenotype is fully rescued by coinjection of FOG-1 cRNA in the morphant embryos (C,F). In contrast, the thrombocytic phenotype is only partially rescued (I, white arrow). The bars represent quantification of the normalized phenotypes (mean ± SD) with the numbers of embryos analyzed in each category indicated above the bar (J-L). White, black, and gray bars represent embryos with normal, reduced, and absence of erythroid/thrombocytic markers, respectively. Data were derived from 3 independent experiments. The statistical significance, marked by lines for each paired condition, was analyzed using analysis of variance; *P < .001; **P < .02.
In zebrafish, thrombocytes are the hemostatic cellular equivalent of mammalian platelets.34 To determine the effects of FOG-1 loss of function on thrombopoiesis at 4 dpf, we used a transgenic zebrafish line where the eGFP expression is under the control of the thrombocyte-specific cd41 promoter.35 During zebrafish embryogenesis, the cd41 promoter directs the expression of eGFP reporter in hematopoietic stem cell in the dorsal aorta at 30 hpf. Previous studies demonstrate that these cd41+ hematopoietic stem cells exists only as transitory precursors and disappear by 2 dpf.36 The Tg(cd41:eGFP) embryos injected with FOG-1 MO failed to generate eGFP+ mature thrombocytes by 4 dpf (Figure 2G-H,L). The effects were specific, as shown in the partial rescue of FOG-1 MO phenotype in embryos injected with FOG-1 cRNA (Figure 2H-I,L). The cd41 partial rescue could be explained by reduction of the exogenous cRNA activity after 4 dpf when the cd41 eGFP expression is first detected in zebrafish embryos.35 Altogether, these results demonstrate that zebrafish FOG-1 is essential for erythroid and thrombocytic maturation.
FOG-1 but not GATA-1 is required for lymphoid-specific gene expression in zebrafish thymic organs
In vertebrates, the thymic anlage forms from the pharyngeal endoderm.37,38 The genes for lck and rag-1 are expressed in maturing T lymphocytes of the bilateral thymi in zebrafish.37-40 The ikaros gene is expressed in all cells of the lymphoid lineage, including multipotential hematopoietic progenitors in the caudal hematopoietic tissue (CHT), a transient localization for definitive hematopoiesis in zebrafish.31 The effect of FOG-1 deficiency on lymphoid and thymic development was evaluated by WISH. The control and morphant embryos were placed in 3 different categories (normal, reduced, and absent), depending on the level of expression for lck, rag1, and ikaros in the thymi by WISH assay. Control embryos showed normal expression of lymphoid markers (Figure 3A,E,I arrow), whereas expression of those same markers in the FOG-1 morphant embryos was generally either reduced or absent (Figure 3B-D,F-H,J-L). In contrast, the morphant embryos showed a less severe effect on ikaros expression in the ventral CHT (Figure 3M-N insets). This result suggests that FOG-1 participates in T-lymphocyte maturation, but not during early lymphoid progenitor specification in the CHT. Previous studies in mouse have shown the endodermal requirement of pax9 expression during thymus development.41 We did not detect significant differences in the level of pax9 expression between control and FOG-1 morphant embryos (Figure 3O-Q). However, the patterning of the endodermal pouches was abnormal in FOG-1 morphants. This suggests that the initial phase of the endoderm specification does not require FOG-1 but that FOG-1 alone or in conjunction with a partner is required for proper endodermal patterning (Figure 3O-Q). We injected MOs into transgenic embryos that express eGFP regulated by the T-lymphocyte–specific lck promoter [Tg(lck:eGFP)].38 The transgenic embryos injected with FOG-1 MO were sorted by the absence of eGFP+-T lymphocytes in the thymi, which indirectly reflects a defect in thymic development. To test whether endoderm-derived structures are normally developed in FOG-1 morphants, we used Alcian blue staining of cartilage. The morphant embryos (eGFP−) show gross hypoplasia of the pharyngeal arches (Figure 3S) compared with eGFP+ wild-type siblings (Figure 3R). This defect was complemented by coinjection with FOG-1 cRNA (supplemental Figure 4C). In summary, these data suggest that the deficiency of FOG-1 disturbs the formation of the later stages of endoderm-derived pharyngeal structures, such as pharyngeal arches and thymic anlage.
Loss of zebrafish FOG-1 results in defective lymphopoiesis in the thymic organs. Lateral views of 4-dpf control (wild-type [wt], A,E,I,M,O) and FOG-1 morphant embryos (B-C,F-G,J-K,N,P) labeled for the following markers: lck (A-C), rag-1 (E-G), ikaros (I-K,M-N), and pax9 (O-P). In control embryos, the thymic cells express normal levels of lck (A), rag-1 (E), and ikaros (I). In contrast, the morphant embryos show strong reduction or absence of lck (B-C), rag-1 (F-G), and ikaros (J-K) expression in the thymi. The expression of ikaros is maintained in the CHT, indicating normal formation of lymphoid progenitors (M,N, brackets and insets). In the lateral views of 4 dpf embryos, the expression of pax9 is normal in the FOG-1 MO-injected embryos, indicating that the initial phase of pharyngeal endoderm formation is not affected (O-Q). In addition, the embryos were processed with Alcian blue staining to delineate the morphologic architecture of the pharyngeal arch cartilages in control (wt; R) and morphant (S) embryos. The morphant embryos display dysplastic development of the pharyngeal arches, indicating a disruption of late endodermal derivatives: pharyngeal arch cartilage and thymic anlage (S). The bars represent quantification of the normalized phenotypes (mean ± SD) with the numbers of embryos analyzed in each category indicated above the bars. White, black, and gray bars indicate embryos with normal, reduced, and absent expression for lymphoid markers, respectively (D,H,L,Q). Results were derived from 3 independent experiments. The statistical significance, marked by lines for each paired conditions, was analyzed using analysis of variance; *P < .05.
Loss of zebrafish FOG-1 results in defective lymphopoiesis in the thymic organs. Lateral views of 4-dpf control (wild-type [wt], A,E,I,M,O) and FOG-1 morphant embryos (B-C,F-G,J-K,N,P) labeled for the following markers: lck (A-C), rag-1 (E-G), ikaros (I-K,M-N), and pax9 (O-P). In control embryos, the thymic cells express normal levels of lck (A), rag-1 (E), and ikaros (I). In contrast, the morphant embryos show strong reduction or absence of lck (B-C), rag-1 (F-G), and ikaros (J-K) expression in the thymi. The expression of ikaros is maintained in the CHT, indicating normal formation of lymphoid progenitors (M,N, brackets and insets). In the lateral views of 4 dpf embryos, the expression of pax9 is normal in the FOG-1 MO-injected embryos, indicating that the initial phase of pharyngeal endoderm formation is not affected (O-Q). In addition, the embryos were processed with Alcian blue staining to delineate the morphologic architecture of the pharyngeal arch cartilages in control (wt; R) and morphant (S) embryos. The morphant embryos display dysplastic development of the pharyngeal arches, indicating a disruption of late endodermal derivatives: pharyngeal arch cartilage and thymic anlage (S). The bars represent quantification of the normalized phenotypes (mean ± SD) with the numbers of embryos analyzed in each category indicated above the bars. White, black, and gray bars indicate embryos with normal, reduced, and absent expression for lymphoid markers, respectively (D,H,L,Q). Results were derived from 3 independent experiments. The statistical significance, marked by lines for each paired conditions, was analyzed using analysis of variance; *P < .05.
Given the close interrelationship between GATA-1 and FOG-1 during erythroid and megakaryocytic development, we evaluated whether FOG-1 acts in the context of GATA-1 interactions to specify T-cell progenitors during thymus formation. At 5 dpf, Tg(lck:eGFP) embryos injected with FOG-1 MO were sorted by phenotype (Figure 4A-C) and eGFP expression in the thymi (Figure 4G-L). Furthermore, o-dianisidine staining was used to assess erythropoiesis in the morphants (Figure 4D-F white arrows). Transgenic embryos injected with either GATA-1 MO or FOG-1 MO are profoundly anemic (Figure 4D-F). Knockdown of FOG-1 reduced eGFP expression in the thymi compared with the controls (Figure 4). To exclude a nonspecific MO effect, we coinjected the anti-p53 MO simultaneously with FOG-1 MO. The results demonstrate that the phenotypic alterations in zebrafish FOG-1 morphant embryos are specific and not caused by generalized apoptosis mediated by p5342 (supplemental Figure 5). No differences in eGFP+-T lymphocytes were observed between control embryos and morphants injected with GATA-1 MO, indicating that GATA-1 is dispensable for FOG-1 activity during zebrafish thymic development (Figure 4H-K).
FOG-1 acts independently of GATA-1 during thymic development. Lateral views of uninjected/Tg(lck:eGFP)/control embryos (A,D,G,J) compared with embryos injected with GATA-1MO (B,E,H,K) or FOG-1MO (C,F,I,L). The pericardial effusion is noted in the FOG-1 morphant (C black arrow). Knocking down of either GATA-1 or FOG-1 shows drastic anemia when stained with o-dianisidine for hemoglobinized cells (D-F). The transgenic zebrafish [Tg(lck:eGFP)] injected with FOG-1 MO shows complete absence of eGFP+-T lymphocytes in the thymi (comparing G,J with I,L). In contrast, GATA-1 morphant shows normal levels of eGFP expression in the thymi (H,K).
FOG-1 acts independently of GATA-1 during thymic development. Lateral views of uninjected/Tg(lck:eGFP)/control embryos (A,D,G,J) compared with embryos injected with GATA-1MO (B,E,H,K) or FOG-1MO (C,F,I,L). The pericardial effusion is noted in the FOG-1 morphant (C black arrow). Knocking down of either GATA-1 or FOG-1 shows drastic anemia when stained with o-dianisidine for hemoglobinized cells (D-F). The transgenic zebrafish [Tg(lck:eGFP)] injected with FOG-1 MO shows complete absence of eGFP+-T lymphocytes in the thymi (comparing G,J with I,L). In contrast, GATA-1 morphant shows normal levels of eGFP expression in the thymi (H,K).
Loss of FOG-1 drives hematopoietic progenitor cells along the myeloid lineage
Zebrafish deficient in GATA-1 (vlt) shows expansion of myeloid cells. These data were interpreted to suggest that GATA-1 inhibits myelopoiesis by antagonizing PU.1 activity in hematopoietic precursors in the ICM.14,15 Because FOG-1 is a critical cotranscription factor of GATA-1 in formation of the erythroid/megakaryocytic cell lineage, it may also play an essential role during myeloid cell fate determination.
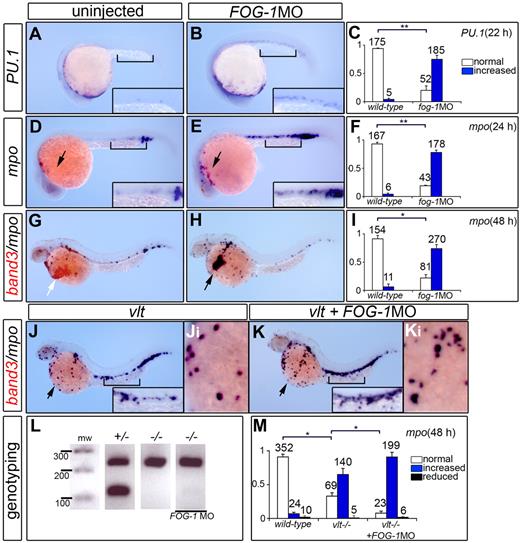
To investigate the role of FOG-1 in zebrafish myelopoiesis, embryos injected with FOG-1 MO were processed by in situ hybridization to reveal the expression of PU.1 in myeloid progenitor cells and mpo in myelomonocytes. Quantification of the FOG-1 morphants indicated a significant increase in the number of myeloid cells expressing PU.1 (Figure 5A-C) and mpo (Figure 5D-F). Double in situ hybridization of FOG-1 morphants showed a reduction in the number of erythroid band3+ cells (Figure 5G-H) with concomitant increase of granulocyte-specific mpo+ cells (Figure 5I), suggesting that loss of FOG-1 redirects erythroid progenitor cells into myeloid precursors. We also tested whether the mpo expansion in GATA-1 null animals (vlt)14,15 could be further increased by knockdown of FOG-1 function in vlt embryos. Injection of FOG-1 MO in vlt mutants produced even greater numbers of mpo-expressing cells in the common cardinal vein compared with vlt uninjected animals (Figure 5J-M). These results suggest that FOG-1 could act independently of GATA-1 in myeloid cell fate commitment.
FOG-1 is required for myelopoiesis in developing zebrafish. Uninjected control embryos (A,D,G), FOG-1 MO-injected embryos (B,E,H), vlt mutants (J), and vlt mutants injected with FOG-1 MO (K). Lateral views of embryos fixed at 22 and 24 hpf were processed by single-labeled WISH to reveal the expression of the myeloid markers, PU.1 (A-B) and mpo (D-E), respectively. Lateral views of embryos fixed at 48 hpf were processed by double-labeled WISH to reveal the expression of the myeloid-specific marker mpo (purple) and the erythroid-specific marker band3 (red; G-H,J-K). Black arrow indicates myeloid cells; white arrow, erythroid cells. Higher magnification views of the ICM (square bracket, D-E) and the ducts of Cuvier on the yolk (Ji-Ki). Loss of FOG-1 function results in an increased expression of myeloid-specific markers, PU.1/mpo (A-F) and decreased expression of the erythroid-specific marker, band3 (G-H). Mutation in the GATA-1 gene causes an increase in the number of mpo+ cells in the vlt mutants compared with wild-type embryos. Injection of FOG-1MO in vlt further expands mpo+ cells compared with vlt control embryos (compare J with K). Genotyping of injected embryos (FOG-1 MO) with increased myeloid markers was performed to verify their vlt (−/−) genotype (L). Bars represent quantification of the normalized phenotypes (mean ± SD) with the numbers of embryos analyzed in each category indicated above the bars (C,F,I,M). White, blue, and black bars represent embryos with normal, increased, and reduced expression for myeloid markers, respectively. Results were derived from 3 independent experiments. The statistical significance, marked by lines for each paired conditions, was analyzed using analysis of variance: *P < .001; **P < .05.
FOG-1 is required for myelopoiesis in developing zebrafish. Uninjected control embryos (A,D,G), FOG-1 MO-injected embryos (B,E,H), vlt mutants (J), and vlt mutants injected with FOG-1 MO (K). Lateral views of embryos fixed at 22 and 24 hpf were processed by single-labeled WISH to reveal the expression of the myeloid markers, PU.1 (A-B) and mpo (D-E), respectively. Lateral views of embryos fixed at 48 hpf were processed by double-labeled WISH to reveal the expression of the myeloid-specific marker mpo (purple) and the erythroid-specific marker band3 (red; G-H,J-K). Black arrow indicates myeloid cells; white arrow, erythroid cells. Higher magnification views of the ICM (square bracket, D-E) and the ducts of Cuvier on the yolk (Ji-Ki). Loss of FOG-1 function results in an increased expression of myeloid-specific markers, PU.1/mpo (A-F) and decreased expression of the erythroid-specific marker, band3 (G-H). Mutation in the GATA-1 gene causes an increase in the number of mpo+ cells in the vlt mutants compared with wild-type embryos. Injection of FOG-1MO in vlt further expands mpo+ cells compared with vlt control embryos (compare J with K). Genotyping of injected embryos (FOG-1 MO) with increased myeloid markers was performed to verify their vlt (−/−) genotype (L). Bars represent quantification of the normalized phenotypes (mean ± SD) with the numbers of embryos analyzed in each category indicated above the bars (C,F,I,M). White, blue, and black bars represent embryos with normal, increased, and reduced expression for myeloid markers, respectively. Results were derived from 3 independent experiments. The statistical significance, marked by lines for each paired conditions, was analyzed using analysis of variance: *P < .001; **P < .05.
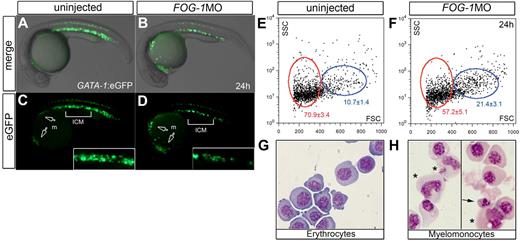
To rigorously quantify the extent of the erythroid/myeloid switch in FOG-1 null embryos, we analyzed hematopoietic cells by flow cytometry43 using the Tg(GATA-1:eGFP) transgenic line, which expresses the eGFP reporter in erythroid and myeloid progenitors of the rostral ICM.14,15 The transgenic zebrafish injected with FOG-1 MO showed expansion of the anterior eGFP+-myeloid cells at the expense of the posterior eGFP+-erythroid cells (Figure 6A-D). Fluorescence-activated sorting of eGFP+ cells in uninjected control embryos showed that approximately 71% were erythrocytes located in a low forward scatter fraction (Figure 6E,G). Moreover, approximately 11% were myelomonocytic cells located in the high forward scatter population. Examination of eGFP+ cells in FOG-1 knockdown animals showed a significant reduction of erythrocytes together with an increase in myelomonocytes compared with controls (Figure 6F,H). The increase in the number of myelomonocytic cells provides further evidence that loss of FOG-1 preferentially shifts the hematopoietic progenitors to a myeloid fate in zebrafish. In summary, our findings demonstrate that loss of FOG-1 function in the presence of normal GATA-1 levels suppresses erythroid commitment and results in a switch to the myeloid cell fate commitment (Figures 5–6).
Loss of FOG-1 expands myelopoiesis at the expense of erythropoiesis. Lateral views at 24 hpf of Tg(GATA-1:eGFP) embryos uninjected (A,C) or injected with FOG-1MO (B,D). White arrows indicate eGFP expression in the myeloid precursor cells (m); square bracket, ICM expression in insets (C-D). Gated erythroid (red circle) and myeloid cells (blue circle) are shown using forward (FSC) and side scatter (SSC) flow cytometry (E-F). The eGFP+ cells were purified by fluorescence-activated flow cytometry (G-H). Histologic analysis of sorted eGFP+ cells from FOG-1 morphants shows myelomonocytic (H asterisks) and dyserythropoietic morphology (H arrows); in comparison, the sorted cells from control embryos are predominantly erythroblasts (G). A representative from 3 independent experiments is shown. Populations of cells within the gate are enumerated as mean percentages of total cells ± SD, showing statistically significant differences (*P < .05; E-F).
Loss of FOG-1 expands myelopoiesis at the expense of erythropoiesis. Lateral views at 24 hpf of Tg(GATA-1:eGFP) embryos uninjected (A,C) or injected with FOG-1MO (B,D). White arrows indicate eGFP expression in the myeloid precursor cells (m); square bracket, ICM expression in insets (C-D). Gated erythroid (red circle) and myeloid cells (blue circle) are shown using forward (FSC) and side scatter (SSC) flow cytometry (E-F). The eGFP+ cells were purified by fluorescence-activated flow cytometry (G-H). Histologic analysis of sorted eGFP+ cells from FOG-1 morphants shows myelomonocytic (H asterisks) and dyserythropoietic morphology (H arrows); in comparison, the sorted cells from control embryos are predominantly erythroblasts (G). A representative from 3 independent experiments is shown. Populations of cells within the gate are enumerated as mean percentages of total cells ± SD, showing statistically significant differences (*P < .05; E-F).
Identification of transcriptional elements for FOG-1 expression in zebrafish
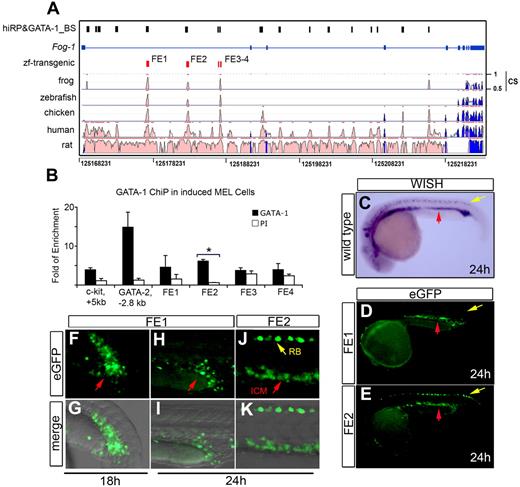
To gain further insight into the regulation of the FOG-1 genes during vertebrate development, we assessed their conservation and divergence through extensive sequence comparison over coding and noncoding domains for predicted transcriptional enhancer elements.44 Using an in silico bioinformatics strategy, we identified FOG-1 CRM necessary for expression in the erythroid compartment. This analysis combines 2 parameters: (1) a positive regulatory potential (RP), which detects similarity to patterns in alignments distinctive for regulatory regions; and (2) conservation of binding site motifs for the essential erythroid transcription factor GATA-1.45,46 Using this algorithm, 17 high-RP and GATA-1–binding sites (hiRP and GATA-1_BS) in the FOG-1 locus with shared homology in zebrafish, mouse, and human were identified (Figure 7A). Rather than testing each of the 17 hiRP and GATA-1_BS individually, extreme evolutionary sequence conservation was used as a filter to select for candidate regions with a high likelihood of enhancer activity.44
Conserved cis-enhancer fragment regulates the expression of FOG-1 in zebrafish blood and heart. Cis-regulatory modules in the mouse FOG-1 locus on chromosome 8 are compared with other vertebrates (A). The high regulatory potential (hi-RP) and GATA-binding sites (GATA-1_BS) are represented as black boxes (hiRP&GATA-1_BS), the exons as blue boxes, the FOG-1 enhancer candidates (FE1-FE4) as red boxes, and the conservation score as “CS.” These 4 FE fragments were interrogated for in vivo GATA-1–binding activity using ChIP assay and real-time PCR (B). The relative occupancy levels of GATA-1 are indicated by the fold enrichment at each of the sites shown, normalized to levels at the negative control region (2 kb 5′ to the GATA-1 gene enhancer HS1). The bar graphs represent quantification (mean ± SD) for GATA-1 binding (3 independent experiments) using nuclear extracts from differentiated MEL cells. The mouse GATA-2 (GATA-2, −2.8 kb) and the c-kit (c-kit, + 5 kb) promoters were used as positive controls for GATA-1 occupancy.30 *The only significant GATA-1 occupancy in the FOG-1 locus at the FE2 site (P < .001). PI indicates the preimmune sera control. WISH shows endogenous expression of FOG-1 mRNA in the intraembryonic blood island (ICM, red arrow) and Rohon-Beard neurons (yellow arrow) (C). The FE1 from mouse (D,F-G) and zebrafish (H-I) robustly drives the expression of eGFP in the ICM (red arrow), but only FE2 from either zebrafish or mouse is expressed in Rohon-Beard paraspinal neurons (E,J-K and yellow arrows in E,J). The developmental stages are properly indicated.
Conserved cis-enhancer fragment regulates the expression of FOG-1 in zebrafish blood and heart. Cis-regulatory modules in the mouse FOG-1 locus on chromosome 8 are compared with other vertebrates (A). The high regulatory potential (hi-RP) and GATA-binding sites (GATA-1_BS) are represented as black boxes (hiRP&GATA-1_BS), the exons as blue boxes, the FOG-1 enhancer candidates (FE1-FE4) as red boxes, and the conservation score as “CS.” These 4 FE fragments were interrogated for in vivo GATA-1–binding activity using ChIP assay and real-time PCR (B). The relative occupancy levels of GATA-1 are indicated by the fold enrichment at each of the sites shown, normalized to levels at the negative control region (2 kb 5′ to the GATA-1 gene enhancer HS1). The bar graphs represent quantification (mean ± SD) for GATA-1 binding (3 independent experiments) using nuclear extracts from differentiated MEL cells. The mouse GATA-2 (GATA-2, −2.8 kb) and the c-kit (c-kit, + 5 kb) promoters were used as positive controls for GATA-1 occupancy.30 *The only significant GATA-1 occupancy in the FOG-1 locus at the FE2 site (P < .001). PI indicates the preimmune sera control. WISH shows endogenous expression of FOG-1 mRNA in the intraembryonic blood island (ICM, red arrow) and Rohon-Beard neurons (yellow arrow) (C). The FE1 from mouse (D,F-G) and zebrafish (H-I) robustly drives the expression of eGFP in the ICM (red arrow), but only FE2 from either zebrafish or mouse is expressed in Rohon-Beard paraspinal neurons (E,J-K and yellow arrows in E,J). The developmental stages are properly indicated.
Several evolutionarily conserved regions (ECRs) were found within the FOG-1 locus. These modules were plotted with the ECR browser server (http://ecrbrowser.dcode.org/) and compared with the relative position of mouse FOG-1 on chromosome 8 (Figure 7A). In intron 1 of the FOG-1 gene, we identified 4 candidate regulatory elements showing high nucleotide identity among vertebrate species. In Figure 7A, the exons are represented by the blue boxes with transcription directionally oriented left to right. The “hiRP” score is the log-likelihood measurement estimates of the probability that the sequence is involved in regulating expression. Matches to weighted matrices for potential GATA-1–binding sites were identified in the mouse sequences (track labeled “hiRP&GATA-1_BS,” indicated by black boxes). FE1 contains one of the largest ultra-conserved elements: approximately 250 base pairs with almost complete identity (∼ 90% nucleotide identity) among zebrafish, Xenopus, chicken, mouse, and human. For FE2, only chicken, mouse, and human showed higher conservation, whereas the zebrafish sequence is only partially conserved (∼ 50% nucleotide identity).
The FOG-1 enhancer FE2 is a GATA-1 target
As shown in Figure 7A, the 4 putative GATA-1–binding sites FE1-FE4 have hiRP and GATA-1_BS, which led to the prediction of functional occupancy of these 2 sites by GATA-1 in regulating FOG-1 expression during erythropoiesis. ChIP assays using chromatin from Friend MEL cells were performed to test for in vivo occupancy by GATA-1 at these 4 putative sites (FE1-FE4). As shown in Figure 7B, FE2 is an in vivo binding site for GATA-1. We also tested whether FE1 or FE2 is a target of GATA-2. Considering that MEL cells express very low levels of GATA-2, we performed the ChIP assays using chromatin from uninduced and induced G1ER cells.29 The results showed that none of the enhancers (FE1-FE2) was bound by GATA-2 (data not shown).
The FE-1 and FE-2 enhancers drive expression of a reporter in zebrafish hematopoietic and cardiac tissues
The FE1 to FE4 regions of the FOG-1 locus were individually cloned using the Gateway system into a Tol2 transposon-based vector. The final constructs contain the individual putative enhancers with a minimal TATA-box promoter driving an eGFP reporter28 (supplemental Figure 7). Injection of either the mouse (Figure 7F-G) or zebrafish FE1 (Figure 7H-I) gives rise to transgenic zebrafish expressing eGFP in the ICM, mimicking the endogenous expression of FOG-1 mRNA (Figure 7C). In zebrafish, FOG-1 is also expressed in the developing heart from 24 through 48 hpf.24 Analogous to the endogenous FOG-1 mRNA, FE1 directs expression of eGFP in the heart during early cardiac development and later during heart morphogenesis (supplemental Figure 6). None of the transgenic embryos carrying FE1 directed reporter activity to Rohon-Beard neurons (Figure 7F-I). In contrast, transgenic embryos carrying FE2 showed eGFP expression in both the ICM and Rohon-Beard (Figure 7J-K) in a manner comparable with FOG-1 mRNA expression (Figure 7C,E). Like FE1, FE2 directs expression of the eGFP reporter in the developing heart (supplemental Figure 6), suggesting that both enhancers play a role during early heart formation. No consistent or significant expression of the eGFP reporter was seen in any of the zebrafish transgenic embryos similarly injected with FE3 or FE4 (supplemental Table 3).
Discussion
We demonstrate that FOG-1 is expressed in early erythroid progenitor cells in a pattern similar to that of GATA-1 and GATA-2 during zebrafish hematopoiesis. Our finding in FOG-1 knocked down embryos indicates that GATA-1 expression is independent of FOG-1 activity (supplemental Figure 3). Moreover, in the absence of GATA-1, FOG-1 expression is drastically reduced, suggesting that FOG-1 functions genetically downstream of GATA-1 (Figure 1R). Our data demonstrate that FOG-1 activity is necessary for erythroid cell maturation (Figure 2) but is not sufficient to expand the number of erythroid cells in the embryonic ICM (data not shown). These findings are generally consistent with the mouse model, in which the absence of either FOG-1 or GATA-1 perturbs erythroid/megakaryocytic cell formation.6,7,19
Relying on compelling evidence from the mouse model, FOG-1 is recognized as a cotranscriptional factor for GATA-1 during erythrocyte as well as megakaryocyte development.6,7,19 However, the role of FOG-1 in differentiation of the other blood cell lineages remains unclear. In this study, we evaluated the effect of zebrafish FOG-1 knockdown during myeloid and lymphocyte development. Our in vivo analysis in zebrafish shows that FOG-1 activity is required for the expression of lymphoid markers in the thymi; however, the expression of ikaros in hematopoietic/lymphoid progenitors in the CHT region remains unaffected (Figure 3). This finding suggests that FOG-1 is specifically required for expression of T-lymphoid genes in the thymi but not during formation of hematopoietic/lymphoid ikaros+ progenitors in the CHT.31 Based on pax9a expression in the pharyngeal region of FOG-1 morphant embryos, we conclude that early endodermal specification is normal whereas patterning is abnormal (Figure 3O-Q). In zebrafish and mouse, there is evidence showing that FOG-1 requires GATA-4/-5/-6 during proper cardiac and endoderm development.21-24 Although FOG/GATA interactions have been detected in zebrafish and mouse embryos, there has hitherto been no evidence for the role of FOG-1 during thymus development. Our results indicate that in zebrafish FOG-1 is required for proper patterning of endoderm, leading to disruption of normal arch architecture and defects in thymic and T-cell development. We also demonstrate that these phenotypic alterations are not caused by apoptosis mediated by p53 (supplemental Figure 5). Future transplantation experiments using FOG-1–deficient cells into a wild-type background would definitively help resolve the question of whether FOG-1 acts in a cell-autonomous or non–cell-autonomous manner for thymic anlage formation and residence of mature T lymphocytes.
From overexpression studies, it is clear that GATA-1 and PU.1 are able to specify erythroid and myeloid cell fate.12-15 It is well documented that both GATA-1 and PU.1 cross-antagonize each other's activity.11 However, the precise mechanism of how GATA-1 and PU.1 initiate the transcriptional network that determines the specification of erythroid/myeloid cells remains unknown. In the case of GATA-1/FOG-1 interactions, the situation is especially complicated by the establishment of different activating and repressive transcriptional complexes depending on the cellular context.11,18 Given the cooperative interaction between FOG-1 and GATA-1,18 we found that the inhibition of FOG-1 function with MOs resulted in expansion of myeloblasts at the expense of erythroblasts (Figures 5–6). On the other hand, knockdown of FOG-1 in zebrafish GATA-1 mutants (vlt) increases the number of myeloid precursor cells, suggesting that FOG-1 itself could regulate myeloid specification (Figure 5J-M). Supportive of our observations, a recent ChiP analysis to functionally interrogate predicted GATA-binding motifs in the PU.1 locus demonstrated that FOG-1 occupies the PU.1 gene in the same regions bound by GATA factors.47,48 Both GATA-1 and GATA-2 recruit FOG-1 to the PU.1 transcriptional regulatory elements. Interestingly, Chou et al also found that FOG-1 and its associated NuRD component47 bound to an upstream regulatory enhancer called URE49 at −14 kb, where they did not detect GATA factors. Thus, FOG-1 probably regulates PU.1 by GATA-dependent and -independent mechanisms.47 Collectively, our data extend well-established observations indicating that the ability of GATA-1 to inhibit myelopoiesis requires FOG-1.
To determinate whether GATA-1 regulates FOG-1 in vivo, we used bioinformatics to predict whether functionally important GATA-binding motifs are present in the FOG-1 gene.44-46 Using this model together with comparative genomic tools from the ECR browser (http://ecrbrowser.dcode.org/), we identified 4 predicted GATA-1-binding CRMs within the FOG-1 locus. The 4 CRMs (FE1-FE4) were conserved in evolution and contain canonical GATA-1 DNA-binding sites T/A(GATA)A/G.1 Two of these regions, FE1 and FE2, individually drive the proper temporal and spatial expression of an eGFP reporter in hematopoietic organs in transgenic zebrafish (Figure 7). Using ChIP assays, we demonstrate that only FE2 is bound by GATA-1 (Figure 7B). We propose that GATA-1 binds to the FE2 enhancers to activate FOG-1 expression during hematopoiesis. As previously discussed, the mutual antagonism between GATA-1 and PU.1 during lineage specification12-15 may be enhanced by the participation of FOG-1. In this context, the mutual activation of GATA-1 and FOG-1 expression would establish a sustained autoregulatory circuit and promote the lineage specification from myeloid to erythroid differentiation. We found that GATA-1 binds to FE2 but not FE1 (Figure 7B). This observation indicates that other transcription factors, besides GATA-1, could be involved in the activation of FE1 during hematopoiesis. Such considerations are pertinent in the context of GATA-2/FOG-1 interactions, which may be either positive or negative depending on the presence or use of additional partner protein.11,18 Two recent studies proposed that GATA-2 acts to repress FOG-1 expression during mast cell development.9,10 However, we did not find significant binding of GATA-2 on FE1-2 in G1E cells (data not shown). Thus, it is possible that GATA-2 regulates other enhancer elements within the FOG-1 promoter in mast cells. This study integrates results from 3 types of analyses and shows that the data from bioinformatics or ChIP binding do not reveal specific biologic functions. Our analysis of the in vivo transcriptional activity of the FE candidates using zebrafish transgenesis serves as a robust functional assay.
Although FOG-1 has been shown to participate in erythroid and megakaryocytic maturation in mice, its role in myeloid and T-lymphoid development in vivo remains completely unknown. Our study provides the first evidence that FOG-1 regulates cell fate determination between erythroid and myeloid development, as well as T-lymphoid maturation during thymic development in the zebrafish. We advance well-established observations indicating that GATA-1 requires FOG-1 to regulate myelopoiesis. Our strategy, combining bioinformatics algorithms with Tol2-Gateway cloning and transgenesis in zebrafish, greatly facilitates the rapid, functional analysis of conserved vertebrate regulatory elements that regulate tissue-specific expression. Here, we identified and characterized mouse and zebrafish FOG-1 transcriptionally active enhancers during hematopoiesis. We showed that the enhancers contain critical GATA motifs, consistent with FOG-1 being a direct target of GATA factors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the Paw laboratory and Mitchell Weiss for critical review of the manuscript; Xiao Ming Shang and Matthew Keefe for technical assistance; Christian Lawrence, Jason Best, and the crew of the zebrafish facility for the animal husbandry; Shuo Lin for providing the Tg(GATA-1:eGFP) transgenic zebrafish line; and Nathan Lawson for the pCS2-Tol2 cDNA.
This work was supported in part by grants from the National Institutes of Health (B.H.P., A.B.C., N.S.T., D.T., J.P.K.), William Randolph Hearst Foundation (B.H.P.), March of Dimes Foundation (B.H.P.), American Society of Hematology (A.B.C., G.C.S.), American Heart Association (J.D.C.), and Swiss National Science Foundation (G.E.A.).
National Institutes of Health
Authorship
Contribution: J.D.A., G.E.A., J.J.C., J.D.C., D.M., N.B.L., E.S., G.C.S., W.H., N.S.T., A.J.D., B.A.B., Y.Z., S.A.W., D.T., M.Y., T.B.M., G.K., K.H., J.P.K., D.I.S., and B.H.P. designed experiments, performed research, and analyzed data; J.D.A, G.E.A., and B.H.P. wrote the manuscript; H.F.L. and R.I.H. provided reagents; and J.P.K., D.T., N.S.T., A.J.D., A.B.C., and B.H.P. designed experiments and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Current affiliations are as follows: Johnson & Johnson Pharmaceutical, Zürich (G.E.A); Baystate Medical Center, Tufts University (J.J.C); Columbia University, College of Physicians & Surgeons (N.B.L.); Pall Corporation (E.S.); University of Virginia (G.C.S); Huntsman Cancer Institute, University of Utah (W.H., N.S.T.); Massachusetts General Hospital (A.J.D.); Harvard Medical School (S.A.W.); University of California–San Diego (D.T.); University of Rhode Island (G.K.); and Merck Research Labs (K.H).
Correspondence: Barry H. Paw, Brigham & Women's Hospital, Hematology Division, 1 Blackfan Cir, Karp Bldg 06.213, Boston, MA 02115; e-mail: bpaw@rics.bwh.harvard.edu.
References
Author notes
*J.D.A., G.E.A., and J.J.C. contributed equally to this study.

![Figure 2. FOG-1 is necessary for erythroid/thrombocytic development in zebrafish. Uninjected control embryos (A,D,G), FOG-1 MO-injected embryos (B,E,H), and FOG-1 MO coinjected with FOG-1 cRNA (C,F,I). Lateral views of 24-hpf embryos processed by WISH to reveal band3 expression (A-C). Lateral (D-Di,E-Ei,F-Fi) and ventral (Dii,Eii,Fii) views of 48 hpf embryos processed by o-dianisidine staining (D-F) to reveal hemoglobinized cells. Lateral view at the trunk level of 4-dpf transgenic zebrafish [Tg(cd41:eGFP)] embryos to reveal eGFP+ thrombocytes (G-I). FOG-1 morphant embryos display reduction or complete absence of band3 expression in the ICM (A-B black brackets, inset magnification). The morphant embryos show severe anemia when stained with o-dianisidine (D-E black arrows). Injection of FOG-1 MO in the transgenic zebrafish [Tg(cd41:eGFP)] produces complete absence of eGFP+ thrombocytes at 4 dpf (G-H white brackets). The anemic phenotype is fully rescued by coinjection of FOG-1 cRNA in the morphant embryos (C,F). In contrast, the thrombocytic phenotype is only partially rescued (I, white arrow). The bars represent quantification of the normalized phenotypes (mean ± SD) with the numbers of embryos analyzed in each category indicated above the bar (J-L). White, black, and gray bars represent embryos with normal, reduced, and absence of erythroid/thrombocytic markers, respectively. Data were derived from 3 independent experiments. The statistical significance, marked by lines for each paired condition, was analyzed using analysis of variance; *P < .001; **P < .02.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/21/10.1182_blood-2008-12-189910/4/m_zh89990944120002.jpeg?Expires=1770056503&Signature=aFwvtmWlSGRWldPWWE3R7OOObtJyGHdcsgJ99-9TayzMsaVrV3T96oXBcRddk0mzkoPNY9yzdhVDvdGTe1ppXgalEwbAyFo9feIjk9kDunD-Py5TvGcatwWnM1GwfbkNM9fNF76c2aXiOAKM9CDdzTFg38cl2LuShR49JxNcZ7-pigQvyiLUTpujGlsAFEqHeXk9fmRylEhEbM-SIbf3eBLh7UCWf00scFdbe4I~Tbi4QDdWqfxcyjV0kFK~gyCrvhc0iPnzofTiXuxb9Mk9dfwicfJlgwN56JGqY-Zd4uH8pKFHNBLl3nog~7ppPnQQPDoic7PgUN40vrwX9K~49Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Loss of zebrafish FOG-1 results in defective lymphopoiesis in the thymic organs. Lateral views of 4-dpf control (wild-type [wt], A,E,I,M,O) and FOG-1 morphant embryos (B-C,F-G,J-K,N,P) labeled for the following markers: lck (A-C), rag-1 (E-G), ikaros (I-K,M-N), and pax9 (O-P). In control embryos, the thymic cells express normal levels of lck (A), rag-1 (E), and ikaros (I). In contrast, the morphant embryos show strong reduction or absence of lck (B-C), rag-1 (F-G), and ikaros (J-K) expression in the thymi. The expression of ikaros is maintained in the CHT, indicating normal formation of lymphoid progenitors (M,N, brackets and insets). In the lateral views of 4 dpf embryos, the expression of pax9 is normal in the FOG-1 MO-injected embryos, indicating that the initial phase of pharyngeal endoderm formation is not affected (O-Q). In addition, the embryos were processed with Alcian blue staining to delineate the morphologic architecture of the pharyngeal arch cartilages in control (wt; R) and morphant (S) embryos. The morphant embryos display dysplastic development of the pharyngeal arches, indicating a disruption of late endodermal derivatives: pharyngeal arch cartilage and thymic anlage (S). The bars represent quantification of the normalized phenotypes (mean ± SD) with the numbers of embryos analyzed in each category indicated above the bars. White, black, and gray bars indicate embryos with normal, reduced, and absent expression for lymphoid markers, respectively (D,H,L,Q). Results were derived from 3 independent experiments. The statistical significance, marked by lines for each paired conditions, was analyzed using analysis of variance; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/21/10.1182_blood-2008-12-189910/4/m_zh89990944120003.jpeg?Expires=1770056503&Signature=oF3BlG40bmS8GBYncWgCQ-C0QeVwqmLor7D8mbAdkKdY7ESxd1xlhebPfIY8qmVasObcD-GiQXmxorsLRtWwY-m49xl7R~j4ILnSm77Q5A5ANcbFs5L5iCXF68dGAhYVmTW7U-QNsdniRI2ESfHoa9ywCrNMouHI0idrLB2bq2smqJrwBShl6ExsHHbP8mnmCWvXqqYYEGW97lo4qOgPxahkxjOpU-I3~e-6V2kfvrWppnp~-i~D~pthO5dNIGKCKdifdahNo-8Vo5b8xM9K7-RPuIQx0Jbuz7DH5b6QOIT6l20BrUMcoYMq98mx73uL6FlGH9-ddmF-q6iwU~O9ww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. FOG-1 acts independently of GATA-1 during thymic development. Lateral views of uninjected/Tg(lck:eGFP)/control embryos (A,D,G,J) compared with embryos injected with GATA-1MO (B,E,H,K) or FOG-1MO (C,F,I,L). The pericardial effusion is noted in the FOG-1 morphant (C black arrow). Knocking down of either GATA-1 or FOG-1 shows drastic anemia when stained with o-dianisidine for hemoglobinized cells (D-F). The transgenic zebrafish [Tg(lck:eGFP)] injected with FOG-1 MO shows complete absence of eGFP+-T lymphocytes in the thymi (comparing G,J with I,L). In contrast, GATA-1 morphant shows normal levels of eGFP expression in the thymi (H,K).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/21/10.1182_blood-2008-12-189910/4/m_zh89990944120004.jpeg?Expires=1770056503&Signature=Yyr7SUmPb~gNj-5FiKh0VvLbatGDfBDfqncHRMDGCq0FtW6PrquEQ5TAq3Fj0r4d60~E5~pszRRfG~d2YpRO5Ho3zwOH7dO~XlSuJcC43NCbhVtWm5wKWKHBhjNlGcFOYJ77CgrnYrSrbmvygP4QS0yh5i5bXqaMClNlaMu45WRa~C0VdfhLDrSG43RFB6ns8nz5BSF8-QLCjTmixGohY22eGGX8m1FSymYC9ij7VjaMQRuZf0qCI~Iqi-pIx7Vi5HjH~IHzzdaIsCfYRJ~SVUlYj7-DVfOdAz7-ObFrfJRRGGZvkO~DLfg5mC4DatHBMcgkF7eAOvc268spoIAxIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)