Abstract
An important hallmark of cancer progression is the ability of tumor cells to evade immune recognition. Understanding the relationship between neoplastic cells and the immune microenvironment should facilitate the design of improved immunotherapies. Here we identify impaired T-cell immunologic synapse formation as an active immunosuppressive mechanism in follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL). We found a significant reduction in formation of the F-actin immune synapse in tumor-infiltrating T cells (P < .01) from lymphoma patients compared with age-matched healthy donor cells. Peripheral blood T cells exhibited this defect only in patients with leukemic-phase disease. Moreover, we demonstrate that this T-cell defect is induced after short-term tumor cell contact. After 24-hour coculture with FL cells, previously healthy T cells showed suppressed recruitment of critical signaling proteins to the synapse. We further demonstrate repair of this defect after treatment of both FL cells and T cells with the immunomodulatory drug lenalidomide. Tissue microarray analysis identified reduced expression of the T-cell synapse signature proteins, including the cytolytic effector molecule Rab27A associated with poor prognosis, in addition to reduced T-cell numbers and activity with disease transformation. Our results highlight the importance of identifying biomarkers and immunotherapeutic treatments for repairing T-cell responses in lymphoma.
Introduction
Follicular lymphoma (FL) is currently incurable, but characterized by an indolent course and variable survival that is significantly worse with disease transformation (t-FL).1 There is therefore a need for prognostic markers that can accurately predict clinical behavior to aid in management decisions based on the prognosis in individual cases. FL outcome is strongly influenced by the immune cell microenvironment,2,3 and immune therapy including monoclonal antibodies,4 stem cell transplantation,5 and therapeutic vaccines6 are used increasingly. T-cell modulation enhances vaccine responses in animal models,7 and rituximab surprisingly enhances immune responses against FL.8
The mechanisms whereby the immune microenvironment impacts FL outcome is poorly understood, but tumor cells alter antitumor immune responses, including recruitment of protumor macrophages and suppression of cytolytic T cells.9,10 Chronic lymphocytic leukemia (CLL) cells actively impair the T-cell actin cytoskeleton, essential for activation and function.11 To understand mechanisms of FL-induced T-cell dysfunction we investigated immunologic synapse function in tumor-infiltrating T cells (TILs) and identified a major tumor-induced defect, reversible with the immunomodulatory drug lenalidomide, that has clinical activity in lymphoma.12 Our results define a novel immunosuppressive mechanism induced by FL and diffuse large B-cell lymphoma (DLBCL) tumor cells and identify functional tumor microenvironment biomarkers that should facilitate development of enhanced immunotherapeutic strategies for lymphoma patients.
Methods
Patient cells and controls
All samples were obtained after written informed consent, in accordance with the Declaration of Helsinki, and approval from the North East London Research Ethics Committee. Peripheral blood (PB) and lymph node (LN) samples were obtained from 15 FL, 3 transformed DLBCL (t-FL), and 3 de novo DLBCL patients undergoing diagnostic biopsies (untreated) and PB from age-matched healthy donors. FL patients were selected to represent the heterogeneity of the disease including clinical grade (grades 1, 2, and 3A) and stage of disease (the patients were stages I to III in those cases with nodal involvement only, and stage IV for those cases with leukemic-phase disease). Of note, clinical factors were not shown to be associated with extent of immune synapse defect. Three additional untreated patients were studied who had leukemic-phase FL, with bone marrow involvement, PB lymphocyte counts higher than 20 × 109 (24-81 × 109), and immunophenotypic confirmation of circulating lymphoma cells. The nonleukemic-phase FL samples had no immunophenotypic evidence of PB involvement. Age-matched healthy donor mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation. Healthy lymphocytes for the coculture assays were obtained from buffy coats prepared by the National Blood Transfusion Service. CD4+, CD8+, and CD3+ T cells were negatively selected using Miltenyi Biotec (magnetic-activated cell sorting [MACS]) cell isolation kits and columns. Normal and malignant B cells were positively selected using MACS CD19 microbeads. The total number of purified T-cell TILs after isolation and purification ranged between 1.5 × 107 and 2.8 × 108 cells, depending on the size of lymph node biopsy material available. PB T cells were isolated from the same patients from whom the LN biopsies were available. For mixed lymphocyte reaction 105 T cells/well were plated with 105 irradiated (96 Gy) allogeneic Epstein-Barr virus–transformed lymphoblastoid cell line as stimulator. Thymidine incorporation was assessed as an index of mitogenic activity for the last 16 hours of a 5-day culture. Cells (104) were cultured with phorbol myristate acetate (PMA) at 1 ng/mL and ionomycin at 100 ng/mL, anti-CD3 and anti-CD28 monoclonal antibodies were added at 1 μg/mL, and thymidine incorporation was assessed during the last 8 hours of the 72-hour culture. The purity of isolated lymphocytes was always more than 95%, determined with flow cytometry.
Antibodies and reagents
RPMI 1640 (GlutaMax), pooled AB human serum, gentamicin, rhodamine phalloidin, CellTracker Blue CMAC (7-amino-4-chloromethylcoumarin), and Alexa Fluor 488–labeled goat anti–mouse and anti–rabbit immunoglobulin G (IgG) antibodies were all purchased from Invitrogen. Anti-integrin αL/leukocyte function-associated antigen 1α (LFA-1; monoclonal antibody [mAb]) was from BD Biosciences. Anti–Filamin-A (mAb) was from Chemicon. Anti-inducible T-cell kinase (Itk) and anti-leukocyte-specific protein tyrosine kinase (Lck) were from Santa Cruz Biotechnology. Antiphosphotyrosine, clone 4G10 (mAb), was purchased from Millipore. Anti-Rab27A, forkhead box P3 (FOXP3; 236A/E7), and anti-Itk (Y401) were from Abcam.
Cell conjugation assays
Cell conjugates were formed as previously described.11 Healthy or malignant B cells (2 × 106) were stained with CellTracker Blue CMAC following the manufacturer's instructions and pulsed with or without 2 μg/mL of a “cocktail” of staphylococcal superantigens (sAgs; SEA and SEB; Sigma-Aldrich) for 30 minutes at 37°C. CD40-ligated B cells were generated by coculture of NIH3T3 fibroblasts transfected with human CD40 ligand with healthy B cells as described previously.13 B cells were centrifuged (200g for 5 minutes) with an equal number of T cells, incubated at 37°C for 10 minutes (CD8+ T cells) or 15 minutes (CD4+ or CD3+ T cells). Phosphotyrosine and Lck were assessed at 5-minute conjugation times. Cells were transferred onto microscope slides using a cell concentrator (Cytofuge 2; Rapid Sample Processing Ltd) and fixed for 15 minutes at room temperature with 3% methanol-free formaldehyde in phosphate-buffered saline (PBS).
Immunofluorescence and confocal microscopy image acquisition
Immunofluorescent labelings were done as previously described using Cytofuge 2 cell concentrator units.11 The specificity of staining was optimized and controlled using appropriate dilutions of isotype-control primary antibodies and subsequent fluorescent secondary antibodies. Background staining with control antibodies was compared with positively stained cells and was not visible using identical acquisition settings. Medial optical section images were captured with a Zeiss 510 confocal laser-scanning microscope and LSM imaging software (Carl Zeiss) using a 63×/1.40 NA oil objective. Detectors were set to detect an optimal signal below the saturation limits. Image sets to be compared were acquired during the same session and using the same acquisition settings.
Quantitative image analysis of synapse signaling protein polarization
Quantification of T-cell conjugation efficiency, F-actin, LFA-1, Lck, Itk, Rab27A, Filamin-A, and phosphotyrosine polarization at the immune synapse was based on our previously described methods,11 including independent scoring by the histopathologists of at least 50 random conjugate images containing a CellTracker Blue–stained healthy or malignant B cell contacting a T cell. Those conjugates showing a distinct polymerized protein band at the T-cell contact site were considered polarized (score = 1). Conjugates lacking protein polymerization (score = 0) or showing weak protein polymerization (score = 0.5) were included in this analysis. All polarization results were verified using ImageJ quantitative algorithm software (multimeasure plug-in; National Institutes of Health) to calculate the relative recruitment index of proteins to the T-cell synapse site as previously described11 (data not shown).
Coculture assays
Coculture assays were carried out as previously described.11 To analyze the impact on T cells of FL cell direct contact, T cells (3 × 106 per milliliter [mL]) and FL cells or healthy allogeneic B cells (1.5 × 106/mL) were cocultured together in full culture medium for 24 hours (24-well culture plate). For cell adhesion blocking coculture experiments, T cells were cocultured with FL cells that had been pretreated with blocking antibody (anti-CD54, intercellular adhesion molecule 1 [ICAM-1], 10 μg/mL) for 1 hour and subsequently washed to remove any unbound antibody. All experiments were performed using isotype-matched IgG as controls. To analyze the impact on T cells of soluble-derived tumor factors only, T cells (lower well) were incubated with FL cells or healthy allogeneic B cells (upper chamber; 2:1 ratio) in 24-well transwell plates (0.4-μm pore) for 48 hours. After coculture, cells were harvested and T cells isolated by MACS negative selection for subsequent flow cytometry purity analysis (> 95%) and functional assays.
Lenalidomide treatment
FL cells and autologous T cells were treated separately with 0.5μM lenalidomide in acidic PBS for 24 hours in full culture medium at 37°C before cell conjugation assays. Lenalidomide was a kind gift from Dr J. Byrd (The Ohio State University).14 Lenalidomide was prepared as previously described.11 Untreated autologous cells were also cultured separately using the acidic PBS alone without drug for 24 hours in full culture medium at 37°C before conjugation assays.
Tissue microarray, IHC, and analysis
Representative extremes of survival tissue microarrays (TMAs) from St Bartholomew's Hospital were used and analyzed by immunohistochemistry (IHC) as previously described (34 samples from patients whose survival was less than 5 years and 25 samples from patients whose survival was more than 15 years from diagnosis).15 Patients who lived less than 5 years from diagnosis, the short-survival group, had a median survival of 2 years and all died as a result of disease. Patients who lived more than 15 years from diagnosis, the long-survival group, had a median survival of 21 years. We also examined TMAs before/after transformation (35 patients) from St Bartholomew's Hospital.16 CD3, Itk, Rab27A, and Filamin-A staining was evaluated for percentage of positive cells and expression (mean intensity) using automated IHC analysis (Ariol automated image analysis system; Applied Imaging Corp) including location relative to the neoplastic follicle: interfollicular, intrafollicular, and total core area (interfollicular + intrafollicular). Expert histopathologist analysis (M.C.) independently validated the IHC staining and automated analysis results.
Statistics
Statistical differences between experimental groups (% polarization) were evaluated by 2-tailed Student t test. A P value less than .05 was considered statistically significant. Tissue microarray protein expression statistical analysis was performed by an expert biostatistician (G.K.) using a mixed effect linear model (difference in average measures between 2 disease groups with survival status as a fixed effect and patient identification as a random effect) and generalized linear model analysis (predictors of disease status; http://lme4.r-forge.r-project.org; http://www.R-project.org).
Results
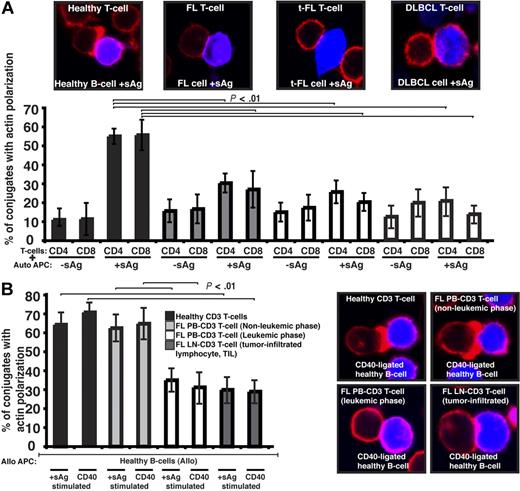
We first sought to characterize the tumor-infiltrating CD4+ or CD8+ lymphocytes (TILs) from lymph node biopsies of patients with germinal center lymphomas. To determine whether there were defects in these TILs in their response to lymphoma cells, we visualized conjugates11 between CD4+ or CD8+ TILs and autologous FL tumor cells (acting as antigen-presenting cells [APCs]). Our first observation was that the percentage efficiency of FL T cells to form conjugates was suppressed compared with healthy donor experiments (data not shown). Assessment of antigen-dependent F-actin immune synapse accumulation demonstrated significant reduction in FL TILs compared with T-cell conjugates using PB from age-matched healthy donors (P < .01; Figure 1A). Similar magnitude defects were also present in TILs from t-FL patient and de novo DLBCL samples (Figure 1A). Of note, whereas TILs had impaired conjugate and immune synapse formation, this was not seen in PB T cells of these patients (data not shown). Because none of these patients had circulating lymphoma cells, we next examined PB T-cell synapse function from patients presenting with leukemic-phase FL. This analysis revealed a significantly reduced ability of these FL PB T cells to form immune synapses with healthy allogeneic APCs including CD40-stimulated B cells (acting as “professional” APCs) compared with PB T cells from nonleukemic-phase patients and age-matched healthy donor cells (Figure 1B). The synapse defect identified in PB T cells that had been exposed to circulating lymphoma cells (leukemic phase) was comparable with that seen with LN TILs interacting with healthy allogeneic APCs (Figure 1B). These data strongly suggested that the presence of lymphoma cells impacts on the immune T-cell population.
Follicular lymphoma cells exhibit defective T-cell synapse formation with autologous antigen-pulsed tumor cells. (A) Healthy age-matched donor T cells (healthy) or tumor-infiltrated T cells from FL, transformed DLBCL (t-FL), or de novo DLBCL (DLBCL) patients, were allowed to conjugate with autologous healthy B, FL, t-FL, or DLBCL cells, respectively, ± sAg as APCs (CMAC dyed, blue). Conjugates were then fixed and stained with rhodamine phalloidin to detect F-actin (red). Conjugates were selected at random for imaging and were scored for F-actin polarization at the immune synapse. Each dataset is the mean ± SD from 5 independent patient experiments (t-FL and DLBCL, n = 3) with at least 50 conjugates analyzed per experiment. The confocal images shown are CD4+ T cells. (B) Healthy age-matched T cells, nonleukemic-phase FL peripheral blood (PB) T cells, leukemic-phase FL PB T cells, and lymph node (LN) tumor-infiltrated FL T cells were allowed to conjugate with stimulated allogeneic healthy APCs (sAg pulsed or CD40 ligated; CMAC dyed, blue). Conjugates were then fixed and stained with rhodamine phalloidin to detect F-actin (red). Conjugates were selected at random for imaging and were scored for F-actin polarization at the synapse. Each dataset is the mean ± SD from 3 independent patient experiments with at least 50 conjugates analyzed per experiment. Original magnification ×63.
Follicular lymphoma cells exhibit defective T-cell synapse formation with autologous antigen-pulsed tumor cells. (A) Healthy age-matched donor T cells (healthy) or tumor-infiltrated T cells from FL, transformed DLBCL (t-FL), or de novo DLBCL (DLBCL) patients, were allowed to conjugate with autologous healthy B, FL, t-FL, or DLBCL cells, respectively, ± sAg as APCs (CMAC dyed, blue). Conjugates were then fixed and stained with rhodamine phalloidin to detect F-actin (red). Conjugates were selected at random for imaging and were scored for F-actin polarization at the immune synapse. Each dataset is the mean ± SD from 5 independent patient experiments (t-FL and DLBCL, n = 3) with at least 50 conjugates analyzed per experiment. The confocal images shown are CD4+ T cells. (B) Healthy age-matched T cells, nonleukemic-phase FL peripheral blood (PB) T cells, leukemic-phase FL PB T cells, and lymph node (LN) tumor-infiltrated FL T cells were allowed to conjugate with stimulated allogeneic healthy APCs (sAg pulsed or CD40 ligated; CMAC dyed, blue). Conjugates were then fixed and stained with rhodamine phalloidin to detect F-actin (red). Conjugates were selected at random for imaging and were scored for F-actin polarization at the synapse. Each dataset is the mean ± SD from 3 independent patient experiments with at least 50 conjugates analyzed per experiment. Original magnification ×63.
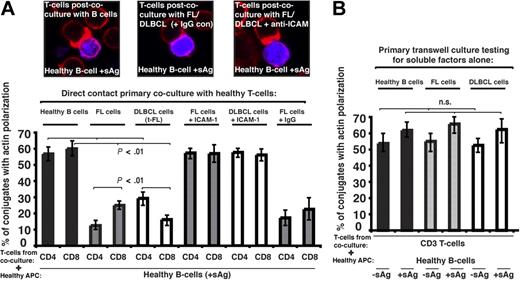
To investigate a direct role of the tumor cell in T-cell dysfunction we used 2-part functional assays. First, healthy CD4+ or CD8+ T cells were cocultured for 24 hours with either allogeneic healthy B cells (n = 9), allogeneic FL, transformed DLBCL (t-FL) cells, or de novo DLBCL (n = 9). Second, these T cells from primary coculture were purified and used in subsequent conjugation assays with third-party healthy allogeneic antigen-pulsed B cells. Primary coculture with healthy B cells had no apparent effect on the ability of these T cells to form immune synapses. However, in all cases studied, coculture with FL or t-FL resulted in significant suppression of T-cell F-actin synapse formation (P < .01; Figure 2A) compared with primary coculture with healthy B cells. Of interest, there was enhanced suppression of CD4+ T-cell synapses after FL coculture assays and CD8+ T cells in the t-FL setting. De novo DLBCL tumor cells also induced a subsequent T-cell defect, with reduction comparable with that seen with FL or t-FL (data not shown). Pretreatment of FL or DLBCL cells with anti–ICAM-1 mAb to block cell adhesion (Figure 2A) or coculture in primary transwell culture assays (Figure 2B) prevented subsequent immune dysfunction, demonstrating requirement of direct lymphoma cell contact for induction of this T-cell defect. Similar T-cell synapse defects were induced by coculture of healthy allogeneic T cells with the lymphoma cell lines tested: CRL, DOHH2, and U937 (data not shown). It has been previously described that culture of healthy T cells with FL cells induces expression of FOXP3, but that this does not occur with coculture with healthy B cells.17 We confirm these findings using our 2-part functional assay, but were not able to demonstrate any involvement of the FOXP3-expressing cells in the observed immune synapse defect. This defect was seen most frequently in cells not expressing FOXP3 and, not surprisingly, we were unable to demonstrate any recruitment of FOXP3 to the synapse site. The question arises as to whether the lymphoma cells induce defects specific to T cells. To test this, we used the 2-part functional assay using FL cells and healthy allogeneic natural killer (NK) cells. Healthy NK cells form effective immune synapses with allogeneic APCs, and this was significantly impaired after initial coculture with FL in 3 independent experiments performed using different FL cells and different NK donors (data not shown). Although these findings are suggestive of the ability of lymphoma cells to interact with other immune cells also, this requires considerable further investigation because of the complexity of NK cell–allogeneic cell interactions.
Follicular lymphoma and DLBCL cells induce defective immune synapse formation in previously healthy T cells via direct contact interaction. (A) Healthy T cells were cocultured in direct contact for 24 hours with either healthy allogeneic B cells or allogeneic FL or transformed DLBCL (t-FL) and subsequently used in conjugation assays with sAg-pulsed third-party allogeneic healthy donor B cells (APCs, blue). Conjugates were selected at random for imaging and were scored for polarization of F-actin (red) at the immune synapse. Note the prevention of the synapse defect when cell adhesion was blocked by pretreatment of FL or DLBCL cells with anti–ICAM-1 monoclonal antibody before primary coculture with healthy T cells but not with isotype control antibody treatment (IgG). Data are the mean ± SD from 9 independent experiments with at least 50 conjugates analyzed per experiment. The confocal images shown are CD8+ T cells. (B) Healthy T cells cocultured for 48 hours with either healthy allogeneic B cells or allogeneic FL or DLBCL cells in transwell culture plates and subsequently used in conjugation assays with ± sAg-pulsed third-party allogeneic healthy donor B cells (APCs). Conjugates were selected at random for imaging and were scored for accumulation of F-actin at the immune synapse. Data are the mean ± SD from 9 independent experiments with 50 conjugates analyzed per experiment.
Follicular lymphoma and DLBCL cells induce defective immune synapse formation in previously healthy T cells via direct contact interaction. (A) Healthy T cells were cocultured in direct contact for 24 hours with either healthy allogeneic B cells or allogeneic FL or transformed DLBCL (t-FL) and subsequently used in conjugation assays with sAg-pulsed third-party allogeneic healthy donor B cells (APCs, blue). Conjugates were selected at random for imaging and were scored for polarization of F-actin (red) at the immune synapse. Note the prevention of the synapse defect when cell adhesion was blocked by pretreatment of FL or DLBCL cells with anti–ICAM-1 monoclonal antibody before primary coculture with healthy T cells but not with isotype control antibody treatment (IgG). Data are the mean ± SD from 9 independent experiments with at least 50 conjugates analyzed per experiment. The confocal images shown are CD8+ T cells. (B) Healthy T cells cocultured for 48 hours with either healthy allogeneic B cells or allogeneic FL or DLBCL cells in transwell culture plates and subsequently used in conjugation assays with ± sAg-pulsed third-party allogeneic healthy donor B cells (APCs). Conjugates were selected at random for imaging and were scored for accumulation of F-actin at the immune synapse. Data are the mean ± SD from 9 independent experiments with 50 conjugates analyzed per experiment.
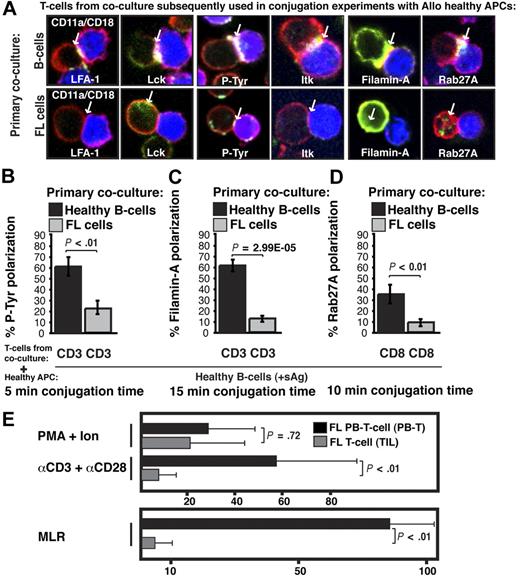
Next we examined the molecular nature of the FL-induced T-cell defect by quantifying recruitment of cytoskeletal signaling proteins to the immune synapse. LFA-1 contributes to T-cell adhesive conjugate interactions with APCs facilitating proximal T-cell receptor–mediated tyrosine kinase recruitment to the synapse site including Lck and Itk, and subsequent formation of the mature synapse containing polarized cytoskeletal signaling molecules such as Filamin-A.18 Rab27A mediates targeted secretion of cytolytic granules by CD8+ T cells toward target APCs via synapselike junctions. Recruitment of cytoskeletal signaling proteins to the immune synapse was assessed in previously healthy CD3+ T cells after primary coculture with FL cells (n = 5) compared with coculture with healthy B cells and showed significantly decreased recruitment of LFA-1, Lck, tyrosine-phosphorylated protein, Itk, Filamin-A, and Rab27A to the T-cell/APC contact site (P < .01, Figure 3A-D and supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Similar T-cell synapse defects were induced by the lymphoma cell lines CRL, DOHH2, and U937 (data not shown). After tumor contact, Filamin-A was strongly expressed by T cells, but did not polarize to the synapse site, demonstrating that it is not only the level of expression of a protein that is important, but also the induction of tumor-induced cytoskeletal recruitment defects. These data demonstrate that FL cells induce major actin cytoskeletal organizational defects in previously healthy T cells. This cellular mechanism of immune suppression likely contributes to the weak autologous and allogeneic T-cell functional responses observed in FL,19,20 and we observed decreased mitogenic responses with anti-CD3/CD28 mAbs and decreased allogeneic MLR responses with TILs compared with matched PB T cells (nonleukemic phase) from patients with FL (Figure 3E).
Follicular lymphoma induces defective actin cytoskeletal signaling in previously healthy T cells, and FL TILs exhibit defective downstream functional responses. (A) Healthy CD3+ T cells were cocultured (primary coculture) as described in Figure 2A with either healthy allogeneic B cells (B, top image panels) or allogeneic FL cells (bottom image panels) and subsequently used in conjugation assays with sAg-pulsed third-party allogeneic healthy donor B cells (APCs, blue). T-cell conjugates formed were analyzed by immunofluorescence and confocal microscopy (F-actin was stained red using rhodamine phalloidin). Images shown are representative of evaluation of 250 conjugates from 5 independent experiments for each protein analyzed (stained green), including CD11a/CD18 (LFA-1), Lck, tyrosine-phosphorylated protein (P-Tyr), Itk, Filamin-A, and Rab27A (CD8+ T cells). White arrows denote protein localization. Colocalization of proteins is shown in yellow. (B-D) Quantitative analysis of protein accumulation (green) at the synapse site is shown for (B) P-Tyr, (C) Filamin-A, (D) Rab27A, and LFA-1, Lck, and Itk in supplemental Figure 1. Data represent means ± SD from 5 independent experiments (50 conjugates analyzed per experiment). (E) Mitogenic activity of PB T cells or TILs from FL patients was assessed by thymidine incorporation using PMA and ionomycin, or anti-CD3 and anti-CD28 mAbs. Results are shown as counts per minute. For mixed lymphocyte (MLR), T cells were stimulated with irradiated allogeneic Epstein-Barr virus–transformed lymphoblastoid cell line as stimulator and results shown as the stimulation index. The results shown are the mean + SD of 8 paired (LN and PB) patient samples studied.
Follicular lymphoma induces defective actin cytoskeletal signaling in previously healthy T cells, and FL TILs exhibit defective downstream functional responses. (A) Healthy CD3+ T cells were cocultured (primary coculture) as described in Figure 2A with either healthy allogeneic B cells (B, top image panels) or allogeneic FL cells (bottom image panels) and subsequently used in conjugation assays with sAg-pulsed third-party allogeneic healthy donor B cells (APCs, blue). T-cell conjugates formed were analyzed by immunofluorescence and confocal microscopy (F-actin was stained red using rhodamine phalloidin). Images shown are representative of evaluation of 250 conjugates from 5 independent experiments for each protein analyzed (stained green), including CD11a/CD18 (LFA-1), Lck, tyrosine-phosphorylated protein (P-Tyr), Itk, Filamin-A, and Rab27A (CD8+ T cells). White arrows denote protein localization. Colocalization of proteins is shown in yellow. (B-D) Quantitative analysis of protein accumulation (green) at the synapse site is shown for (B) P-Tyr, (C) Filamin-A, (D) Rab27A, and LFA-1, Lck, and Itk in supplemental Figure 1. Data represent means ± SD from 5 independent experiments (50 conjugates analyzed per experiment). (E) Mitogenic activity of PB T cells or TILs from FL patients was assessed by thymidine incorporation using PMA and ionomycin, or anti-CD3 and anti-CD28 mAbs. Results are shown as counts per minute. For mixed lymphocyte (MLR), T cells were stimulated with irradiated allogeneic Epstein-Barr virus–transformed lymphoblastoid cell line as stimulator and results shown as the stimulation index. The results shown are the mean + SD of 8 paired (LN and PB) patient samples studied.
Lenalidomide is a currently used immunomodulatory drug thought to act in B-cell malignancies at least in part by enhancing immunologic responses.21 Here we provide direct functional evidence that lenalidomide enhances T-cell immune recognition of FL cells. Ex vivo treatment of both FL and autologous T cells (n = 6) with lenalidomide (0.5 μM for 24 hours) was required to repair formation of the F-actin immune synapse and recruitment of tyrosine-phosphorylated protein irrespective of the presence of exogenous antigen (P < .01; Figure 4A). Moreover, the percentage of FL T cells interacting and forming conjugates with autologous FL cells was significantly enhanced after drug treatment (Figure 4B), again irrespective of the presence of exogenous antigen. Of note, lenalidomide was found to significantly enhance the T-cell synapse response in all of the patient samples tested to date irrespective of clinical grade. We postulate that this immunotherapeutic agent critically restores appropriate T-cell actin cytoskeletal signaling and enhances the APC function of FL cells. This immune repair strategy is in agreement with recent studies where other strategies have been used to repair T-cell defects in FL7 and with our previous findings in CLL.11,22
Lenalidomide repairs FL T-cell immunologic synapse dysfunction with autologous tumor cells. (A) Autologous T-cell conjugates (with or without sAg-pulsed FL cells stained blue) from untreated (UT) or lenalidomide (Len)–treated (0.5 μM for 24 hours) T and FL cells were scored for accumulation (polarization) of F-actin (red) at the immune synapse. As controls, autologous age-matched healthy T and B cells were used. Data are the mean ± SD from 6 independent experiments with at least 50 conjugates analyzed per experiment. Lenalidomide treatment also enhanced polarization of tyrosine phosphorylated protein (P-Tyr; stained green) to the FL T-cell synapse site. Statistical differences between experimental groups were evaluated by 2-tailed Student t test. P < .05 was considered statistically significant. Nonsignificant findings are denoted by ns. (B) Percentage of FL TIL conjugate efficiency with autologous FL cells (± sAg) before (untreated [UT]) and after lenalidomide (Len) was scored by visual counting using a confocal microscope. Each dataset is the mean ± SD from 6 independent experiments with at least 50 random T cells analyzed per experiment.
Lenalidomide repairs FL T-cell immunologic synapse dysfunction with autologous tumor cells. (A) Autologous T-cell conjugates (with or without sAg-pulsed FL cells stained blue) from untreated (UT) or lenalidomide (Len)–treated (0.5 μM for 24 hours) T and FL cells were scored for accumulation (polarization) of F-actin (red) at the immune synapse. As controls, autologous age-matched healthy T and B cells were used. Data are the mean ± SD from 6 independent experiments with at least 50 conjugates analyzed per experiment. Lenalidomide treatment also enhanced polarization of tyrosine phosphorylated protein (P-Tyr; stained green) to the FL T-cell synapse site. Statistical differences between experimental groups were evaluated by 2-tailed Student t test. P < .05 was considered statistically significant. Nonsignificant findings are denoted by ns. (B) Percentage of FL TIL conjugate efficiency with autologous FL cells (± sAg) before (untreated [UT]) and after lenalidomide (Len) was scored by visual counting using a confocal microscope. Each dataset is the mean ± SD from 6 independent experiments with at least 50 random T cells analyzed per experiment.
Genes encoding T-cell immunologic synapse-associated proteins including Itk, Filamin-A, and Rab27A were up-regulated in the favorable prognosis immune-response 1 signature in FL based on gene expression profiling experiments.2 We evaluated expression of these proteins using FL tissue microarrays (TMAs) that include patients who lived less than 5 years (short-survival group) compared with patients with greater than 15-year survival (long-survival group).15 Poor-prognosis FL patients had no difference in location (interfollicular versus intrafollicular) or expression of Itk (supplemental Figure 2) or Filamin-A (supplemental Figure 3). The genes encoding these proteins were found to be important prognostic markers in the immune-response 1 signature of the gene expression profiling study.2 Our present results using the synapse functional assays and TMAs are consistent with the hypothesis that it is not only protein level and site of expression within the LN that is functionally important but also the ability of signaling proteins to polarize to the T-cell synapse. The short-survival patient group was found to have fewer total T cells (P < .05), significantly enhanced mean intensity of expression of total CD3 (P = .001), and significantly reduced Rab27A expression (P = .001) and number of Rab27A-expressing intrafollicular T cells (P = .001; Figure 5A). Interfollicular expression of Rab27A was not significantly different in short- and long-survival groups (supplemental Figure 4). However, higher intrafollicular Rab27A expression (P = .001) and number (P = .001) were predictive of being a long-survivor and had prognostic significance (P = .001) for the whole group (Figure 5A-B).
Altered expression of the T-cell synapse cytolytic effector molecule Rab27A is associated with patient prognosis. (A) Representative high-power (× 40 magnification) images of Rab27A intrafollicular expression in CD3+ T cells on TMA cores from extremes of survival diagnostic FL biopsies (< 5 years [y] [short-survival group] and > 15 years [long-survival group]). Dot plot charts show significantly reduced intrafollicular Rab27A expression (mean intensity, left chart) and percentage of positive cells (right chart) in short-survival group. Statistical analysis was performed using a mixed effect linear model (P values shown). Intrafollicular and interfollicular IHC quantitative analysis for Itk, Filamin-A, and Rab27A is shown in supplemental Figures 2–4. (B) Overall survival by Rab27A expression on tissue microarray of previously untreated FL.
Altered expression of the T-cell synapse cytolytic effector molecule Rab27A is associated with patient prognosis. (A) Representative high-power (× 40 magnification) images of Rab27A intrafollicular expression in CD3+ T cells on TMA cores from extremes of survival diagnostic FL biopsies (< 5 years [y] [short-survival group] and > 15 years [long-survival group]). Dot plot charts show significantly reduced intrafollicular Rab27A expression (mean intensity, left chart) and percentage of positive cells (right chart) in short-survival group. Statistical analysis was performed using a mixed effect linear model (P values shown). Intrafollicular and interfollicular IHC quantitative analysis for Itk, Filamin-A, and Rab27A is shown in supplemental Figures 2–4. (B) Overall survival by Rab27A expression on tissue microarray of previously untreated FL.
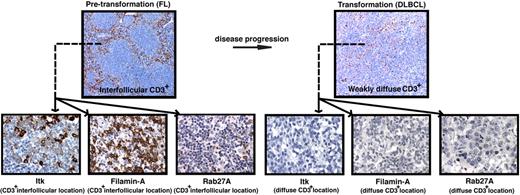
We used disease transformation TMAs16 to investigate whether T-cell signaling molecular expression was a biomarker for aggressive disease and identified significantly reduced total core area expression and percentage positive CD3+ cells expressing Itk, Filamin-A, and Rab27A in transformed (t-FL) patient's samples compared with their respective pretransformation FL samples. Expression levels of these synapse proteins accurately predicted disease status (Figure 6 and supplemental Figures 5–8). These results suggest an active immunosuppressive mechanism impairing T-cell function may be important in lymphoma progression and this is under further investigation in TMAs of FL patients who have and have not undergone subsequent transformation.
Reduced T-cell numbers and expression of T-cell immunologic synapse proteins are associated with disease transformation in FL. (A) Representative low-power (× 10 magnification) images (top panels) of CD3+ expression on TMA cores from pretransformation (FL) biopsies compared with the patient's disease progression/transformation (DLBCL) biopsies. Note the distinct strong interfollicular CD3+ expression on FL biopsy compared with weakly diffuse expression on the patient's transformed biopsy. Representative high-power (×40 magnification) images (bottom panels) of interfollicular (CD3+ location) Itk, Filamin-A, and Rab27A expression on FL biopsies compared with weak diffuse expression of these T-cell synapse signature proteins on the patient's transformed biopsies. Quantitative analysis of expression and percentage of positive cells for these synapse signature proteins comparing total FL biopsy core area (sum of intrafollicular and interfollicular location expression) to total DLBCL biopsy core area is shown in supplemental Figures 5–8.
Reduced T-cell numbers and expression of T-cell immunologic synapse proteins are associated with disease transformation in FL. (A) Representative low-power (× 10 magnification) images (top panels) of CD3+ expression on TMA cores from pretransformation (FL) biopsies compared with the patient's disease progression/transformation (DLBCL) biopsies. Note the distinct strong interfollicular CD3+ expression on FL biopsy compared with weakly diffuse expression on the patient's transformed biopsy. Representative high-power (×40 magnification) images (bottom panels) of interfollicular (CD3+ location) Itk, Filamin-A, and Rab27A expression on FL biopsies compared with weak diffuse expression of these T-cell synapse signature proteins on the patient's transformed biopsies. Quantitative analysis of expression and percentage of positive cells for these synapse signature proteins comparing total FL biopsy core area (sum of intrafollicular and interfollicular location expression) to total DLBCL biopsy core area is shown in supplemental Figures 5–8.
Discussion
Cancer cells induce unique microenvironments that appear conducive to cancer cell growth. Although an inflammatory and immune infiltrate is a feature of most cancers, there is a failure of the host immune response against cancer. The immune microenvironment does play an important role in outcome, and in gene expression profiling studies in FL, the genes expressed by infiltrating T cells and macrophages appear among the most important predictors of survival.2 Here we further characterized the T cells in FL and demonstrate that tumor-infiltrating CD4+ and CD8+ T cells have defects in their ability to mobilize F-actin at the immune synapse. We extend this finding to t-FL and DLBCL. Of note, unlike CLL, the defect was not found in PB T cells but only in the TILs. However, when we examined the PB of patients with a leukemic component of their disease, defects in PB T cells were also found. This implied that it is the interaction with the tumor that is driving the defect. We examined this question by coculturing healthy allogeneic T cells with lymphoma cells or lymphoma cell lines and demonstrate that these defects can be rapidly induced by ex vivo culture. Induction of this defect requires direct cell-cell contact and is not induced in transwell chambers, demonstrating that release of soluble factors alone from the tumor cells does not play a role in mediating the induced T-cell defect studied here, although soluble factors may have other effects on other T-cell functions.
The molecular mechanism(s) whereby lymphoma cells induce these T-cell defects remains unknown and is currently under investigation. Irrespective of the ligand interactions involved, once lymphoma cells make cell contact with T cells they appear to induce rapid changes in actin cytoskeletal organization. This leads to the observed failure of recruitment of cytoskeletal signaling proteins to the immune synapse, including LFA-1, Lck, Itk, Filamin-A, and Rab27A and failure to initiate the early tyrosine phosphorylation of proteins after T-cell receptor activation. Itk, Filamin-A, and Rab27A were chosen for further study as proteins of interest because the genes encoding these proteins were found to be up-regulated in the favorable prognosis immune response 1 signature in FL.2 The TMA has the advantage not only of examining protein rather than gene expression levels, but also of determination of where in the LN the protein is being expressed. In addition to the demonstration that these proteins fail to recruit to the immune synapse in FL, we also demonstrate that the intrafollicular expression of Rab27a protein has important prognostic significance, whereas interfollicular expression had no prognostic significance. The inability to demonstrate prognostic significance of protein expression of Itk and Filamin-A may reflect either mechanisms of posttranslational modifications that affect protein expression or the locality within the LN of the cells expressing these proteins. Moreover, the functional assays provide additional information, because we have observed that it is not only the level of expression of the protein, but also whether it is able to polarize to the immune synapse that is important. We have even observed increased levels of expression of proteins in T cells after tumor contact, but failure of these proteins to traffic to the synapse site. An example of this is shown for Filamin-A.
There has been great excitement regarding the use of immune-based therapies in lymphomas. Previous studies have demonstrated that induction of tumor-specific immune responses after vaccination is associated with improved outcome.23-26 Despite its early promise, the results of phase 3 trials examining the impact of idiotypic vaccination have been disappointing. The failure to translate promising induction of measurable immune response into improved survival may reflect the poor immunogenicity of idiotype as a vaccination approach, or may reflect the fact that in FL the tumor cells have developed effective mechanisms of avoiding immune recognition. The ability of a T cell to respond to antigen is dependent upon both the affinity of binding of antigen to the T-cell receptor and to the additional signals provided by APCs that enable T-cell functional molecules to be recruited to the immune synapse site. The finding here that the T cells in the immune microenvironment are modified by the tumor cells to decrease the effectiveness of these cells to recruit molecules to the immune synapse and respond to antigen may help explain the disappointing clinical results of cancer vaccination strategies. This suggests that in addition to improving cancer vaccines, attention has to be paid to repairing the immune defects induced in cancer-bearing patients. We show here that among the immune defects induced in patients with FL are defects in actin cytoskeletal organization. The immunomodulatory drugs, including lenalidomide, are able to activate RhoA and Rac1 and enhance F-actin formation.27 We now demonstrate that this drug is able to repair the autologous T-cell defects in TILs in FL, as we have previously described in PB T cells in CLL.11 Of note, the drug acts not only on the FL cells but also on the T cells and treatment of both populations is required. There are limited data on the use of lenalidomide in FL,12 but our current findings suggest that strategies to repair immune defects in the tumor-bearing host, such as can be performed here with lenalidomide, should be considered in combination with vaccination in clinical trials of immunotherapy.
In summary, we identified a novel tumor-induced immune dysfunction in FL that can be reversed rapidly after lenalidomide treatment. F-actin immunologic synapse formation and cytoskeletal molecules regulating T-cell effector function may provide important functional biomarkers for assessing whether immunotherapeutic strategies repair and enhance T-cell antitumor responses in lymphoma.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sameena Iqbal and Karin Summers for organizing and preparing the FL patient samples.
This work was supported by Cancer Research UK and by funding from the National Cancer Institute (P01 CA95426; J.G.G.).
National Institutes of Health
Authorship
Contribution: A.G.R. designed the research, performed experiments, and wrote the paper; A.J.C. performed all tissue microarray experiments; G.K. provided expert statistician analysis; R.F. performed experiments; J.M. and F.M. provided patient sample clinical information and analysis of outcome; T.A.L provided patient samples and contributed to study design; A.M.L. and M.C. provided expert histopathologist analysis; and J.G.G. designed the research, supervised the study, and wrote the paper.
Conflict-of-interest disclosure: J.G.G. has received honoraria from Celgene for work on advisory boards. The remaining authors declare no competing financial interests.
Correspondence: John G. Gribben, Institute of Cancer, Barts and The London School of Medicine, Charterhouse Square, London EC1M 6BQ, United Kingdom; e-mail: j.gribben@qmul.ac.uk.

![Figure 4. Lenalidomide repairs FL T-cell immunologic synapse dysfunction with autologous tumor cells. (A) Autologous T-cell conjugates (with or without sAg-pulsed FL cells stained blue) from untreated (UT) or lenalidomide (Len)–treated (0.5 μM for 24 hours) T and FL cells were scored for accumulation (polarization) of F-actin (red) at the immune synapse. As controls, autologous age-matched healthy T and B cells were used. Data are the mean ± SD from 6 independent experiments with at least 50 conjugates analyzed per experiment. Lenalidomide treatment also enhanced polarization of tyrosine phosphorylated protein (P-Tyr; stained green) to the FL T-cell synapse site. Statistical differences between experimental groups were evaluated by 2-tailed Student t test. P < .05 was considered statistically significant. Nonsignificant findings are denoted by ns. (B) Percentage of FL TIL conjugate efficiency with autologous FL cells (± sAg) before (untreated [UT]) and after lenalidomide (Len) was scored by visual counting using a confocal microscope. Each dataset is the mean ± SD from 6 independent experiments with at least 50 random T cells analyzed per experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/21/10.1182_blood-2009-04-217687/4/m_zh89990945010004.jpeg?Expires=1769102454&Signature=tdfkhzVode9saCyNrmzR5TFwBy5k6LJCFwjQXJVtjknNOHuR0X676vJvpblkK2EIhxnYC5lR7cHNh-d1~FNgii6BhsP3XiPXXBEm~ddE9MwRryyok-taUaQ2yo771k0l6dje7r9hLtg0vYZ341OHOH-K0WxKLZSnBqnTYVAuY71JShFEHXtJW4GQ6eKmVwC~WTpOXTpxaRtWkNr-LRxVjDJ6pKbW36eSe3nnQBf02DuQnaQ~71hN4eFwKlPGhJEmnzEVj-UmIGy33hj3awckZPOncQ3MZSVsww2sVVxasRILlPApI3QdFi4xT~4B96cmW6W4g~6xu4O3mv6SzPyRcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Altered expression of the T-cell synapse cytolytic effector molecule Rab27A is associated with patient prognosis. (A) Representative high-power (× 40 magnification) images of Rab27A intrafollicular expression in CD3+ T cells on TMA cores from extremes of survival diagnostic FL biopsies (< 5 years [y] [short-survival group] and > 15 years [long-survival group]). Dot plot charts show significantly reduced intrafollicular Rab27A expression (mean intensity, left chart) and percentage of positive cells (right chart) in short-survival group. Statistical analysis was performed using a mixed effect linear model (P values shown). Intrafollicular and interfollicular IHC quantitative analysis for Itk, Filamin-A, and Rab27A is shown in supplemental Figures 2–4. (B) Overall survival by Rab27A expression on tissue microarray of previously untreated FL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/21/10.1182_blood-2009-04-217687/4/m_zh89990945010005.jpeg?Expires=1769102454&Signature=tX~pvgctFolBgPtAyLCu0~dKKj-RTUgMgwbNdky~63hO2h~vJTOJXo6EEhd-IfW~ttbYTOFIkZK0EROJDUH6X2crWbSyZhSIgH6w3J1gq-f~SJ3GPy2iPHL1miaEfV7drhWCbYgjnH~U1G4jgSpC3d9SENmXet~~316eLGmtB~3bWvCIxUn7wXqC8njd4A0ey0DuZ5EfrVfjhfUibR2KK7lzXYitlD3WjQDiliD541WlMPQGK2dE~4M576oGZCL6yvq3yaSLFoT~H2wcA5-zbwlnZBt2AWWtXosRrni9JTupqVfmmZEKtSCLvxNsAe2wGPMVsI9aMZQvJiDdZYevMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal