Abstract
Abstract 2820
Poster Board II-796
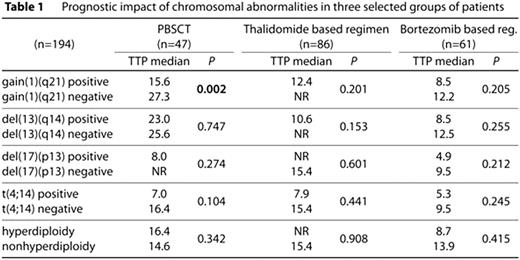
The presence of chromosomal abnormalities detected by FISH in plasma cells is considered to be an important prognostic factor for patients with multiple myeloma. In this study we analysed the impact of gain(1)(q21), del(13)(q14), del(17)(p13), t(4;14) and (non)hyperdiploidy on prognosis in MM patients. Taking together 194 MM patients (median age 66 years) were successfully examined by FISH for presence of above named chromosomal abnormalities in plasma cells. A total of 47 patients (median follow-up 43.2 months; 91% first line treatment, 9% second line treatment) were treated by peripheral blood stem cells transplantation (PBSCT). A total of 86 patients (median follow-up 38.4 months; 38% first line treatment, 43% second line treatment, 19% >2 previous treatment lines) were treated by thalidomide based regimen (86% together with glucocorticoids and alkylating agens). A total of 61 patients (median follow-up 46.5 months; 16% first line treatment, 36% second line treatment, 33% third line treatment, 15% >3 previous treatment lines) were treated by bortezomib based regimen (53% with glucocorticoids and alkylating agens, 20% with glucocorticoids + anthracycline). Gain(1)(q21) was found in 52% (101/194), del(13)(q14) in 55% (69/125), del(17)(p13) in 12% (13/112), t(4;14) in 20% (18/90) and 44% (56/126) of cases were hyperdiploid. In any of the three treatment based groups of patients we haven't found any significant difference in TTP, OS and treatment response (ORR) between any studied positive/negative chromosomal abnormality except gain(1)(q21). See Table 1 for results overview (TTP only). In patients treated by PBSCT (regardless of their pre-treatment) significant difference in TTP and OS for positive/negative patients was observed (15.6 vs. 27.3 months, p=0.002 for TTP; 47.2 vs. NR months, p=0.001 for OS). Furthermore, we entirely focused on newly diagnosed patients divided them into three subgroups: (1) patients treated by PBSCT in first line (n=42), (2) patients treated by thalidomide based regimen in first line (n=33) and (3) patients treated by bortezomib based regimen in first line (n=10). We confirmed unfavourable impact of gain(1)(q21) in patients treated with PBSCT whereas TTP and OS in gain(1)(q21) positive/negative patients was 15.6 vs. 27.3 months, p=0.003 (TTP); 47.2 vs. 66.9 months, p=0.002 (OS). This difference in TTP/OS among gain(1)(q21) positive/negative patients was observed neither in thalidomide based subgroup [NR vs. 11.7 months, p=0.956 (TTP); NR vs. 34.4 months, p=0.683 (OS)] nor in bortezomib based subgroup [(8.5 vs. 6.5 months, p=0.549 (TTP); NR vs. 6.8, p=0.254 (OS)]. See Table 2 for results overview (TTP only). In this study we revealed gain(1)(q21) almost in one half of MM cases, and we defined the newly diagnosed MM patients carrying gain(1)(q21) as a unique group of patients with poor prognosis. Nevertheless, treatment based on bortezomib / thalidomide combination probably suppresses unfavourable prognostic impact of all studied chromosomal abnormalities including gain(1)(q21). This study was supported by grant LC06027 of Masaryk University, Brno, Czech Republic, and by grants MSM0021622415 and MSM0021622434 of Ministry of Education, Czech Republic, and by IGA grants NR/9317-3 and NS/10207-3/2009 of Ministry of Medicine, Czech Republic.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal