Abstract
Intracranial hemorrhage (ICH) is a rare but devastating complication of childhood immune thrombocytopenia purpura (ITP). A survey of ICH from 1987 to 2000 identified cases of ICH in childhood ITP in the United States. Forty patients with ICH and 80 matched ITP control subjects were accrued. The estimated incidence of ICH was 0.19% to 0.78%. Platelet counts were less than 20 × 109/L in 90% and less than 10 × 109/L in 75% of children with ICH. Eighteen (45%) children developed ICH within 7 days of diagnosis of ITP; for 10 of these, ICH was the presenting feature of ITP. Twelve (30%) children had chronic ITP. Head trauma and hematuria were the most prominent features associated with ICH, identified in 33% and 22.5% of the patients with ICH and 1 and none of the controls (both P < .001). Bleeding beyond petechiae and ecchymoses was also linked to ICH. Mortality was 25%; a further 25% had neurologic sequelae. Strategies by which high-risk children could be identified were considered, and the costs of preventive combination treatment were estimated. Children with severe thrombocytopenia plus head trauma and/or hematuria appeared to be at particularly high risk of ICH. Aggressive treatment of these children may be appropriate.
Introduction
Intracranial hemorrhage (ICH) is the most devastating complication of immune thrombocytopenic purpura (ITP) in children,1 and prevention of ICH is the primary goal of ITP treatment. However, the great majority of patients with ITP, even those with very low platelet counts, do not experience severe bleeding,2 and ICH occurs in less than 1 in 100 children with ITP.3-6 Existing case series are small, and reviews of published case reports may suffer from publication bias. The features that predispose patients to develop ICH in addition to severe thrombocytopenia remain poorly defined. Potential risk factors include platelet counts below 10 to 20 × 109/L, nonsteroidal anti-inflammatory drugs (NSAIDs), head trauma, vasculitis associated with systemic lupus erythematosis (SLE), and cerebral arteriovenous malformations (AVMs).1,3,7-9
This study aimed to define the features associated with ICH in ITP. Information was obtained on 40 children with ICH and 80 children with ITP as matched controls by yearly mail survey and follow-up phone calls to pediatric hematologist-oncologists across the United States from January 1987 to December 2000. This study, which constitutes by far the largest primary case series of ICH in children with ITP, had 3 aims: (1) distinguishing children with ITP who develop an ICH from those who do not; (2) determining the outcome of ICH; and (3) exploring the implications for management of children with ITP.
Methods
A nationwide survey requested information on cases of ICH in patients with ITP aged 17 years and younger in the United States over the preceding 10 years (1987-1997) and yearly during a 4-year period from January 1997 to December 2000. ITP was defined according to the American Society for Hematology (ASH) guidelines.10 The questionnaire was sent to members of the American Society of Pediatric Hematology and Oncology and selected members of ASH. Follow-up phone calls were made. Whenever possible, redacted chart copies were obtained.
Each site was asked to provide as controls their next 2 children with ITP who presented with a platelet count less than 30 × 109/L and matched the patient with ICH by duration of ITP (acute or chronic); when unavailable, supplementary controls were provided by Weill Medical College of Cornell University (New York, NY) and St Josephs Hospital (Patterson, NJ). Acute ITP was defined as less than 6 months in duration because the study was conducted before the new terminology.11 Only children with primary ITP were included; cases of ICH in patients with secondary ITP are described in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). ICH was verified by computed tomography (CT) scan in all but one case. Institutional review board (IRB) approval was obtained at Weill Cornell to acquire patient information without specific identifiers and therefore without consent.
Data were analyzed using means, medians, ranges, 2-tailed Fisher Exact tests for 2 × 2 tables, sensitivity (true positives divided by true positives plus false negatives), specificity (true negatives divided by true negatives plus false positives), and positive and negative predictive values using GraphPad Prism software. For the cost utility analyses of treatment strategies, the 10 children who had an ICH as the presenting feature of their ITP were excluded, as these cases were deemed not preventable.
Results
Study patients
A total of 120 children with primary ITP were included, 40 with ICH and 80 as controls with platelet counts less than 30 × 109/L (Table 1). A total of 17 patients with ICH were identified retrospectively in the 10 years before 1997, and 23 during the4 prospective years of the study (1997-2000). The 40 patients with ICH were reported by 36 hematologists.
Comparison of patients with an ICH to the case controls
| . | ICH cases, n = 40 (%) . | Case controls, n = 80 (%) . |
|---|---|---|
| Sex | ||
| Female | 22 (55) | 35 (44) |
| Male | 18 (45) | 45 (56) |
| ITP duration | ||
| Acute ITP less than 6 mo | 28 (70) | 66 (82.5) |
| Chronic ITP 6 mo or more | 12 (30) | 14 (17.5) |
| Platelet counts | ||
| Median, platelets/μL | 5000 | 8000 |
| Range, platelets/μL | 1 to 61 000 | 1 to 28 000 |
| Platelet count less than 20 000/μL | 36 (90) | 68 (85) |
| Platelet count less than 10 000/μL | 30 (75) | 47 (59) |
| Age | ||
| Age range | 3 mo-17 y | 2 mo-17 y |
| Number of patients younger than 10 y old | 23 (58) | 53 (66) |
| Number of patients 10 y or older | 17 (43) | 27 (34) |
| NSAID use* | ||
| Number of patients reporting recent NSAID use | 3 (7.5) | 1 (1.25) |
| Head trauma* | 13 (33) | 1 (1) |
| Bleeding symptoms* | ||
| No bleeding | 3 (7.5) | 4 (5) |
| Any bleeding | 37 (92.5) | 76 (95) |
| Petechiae | 25 (63) | 64 (80) |
| Ecchymosis | 30 (75) | 60 (75) |
| Bleeding other than petechiae and ecchymosis | 25 (63) | 35 (44) |
| Wet purpura | 11 (27.5) | 15 (19) |
| Epistaxis | 15 (37.5) | 19 (24) |
| Gum bleeding | 9 (22.5) | 8 (10) |
| GI bleeding | 7 (17.5) | 5 (6) |
| U bleeding | 9 (22.5) | 0 (0) |
| GYN bleeding | 0 | 3 (4) |
| Head trauma and bleeding symptoms | ||
| Both head trauma and bleeding other than petechiae and ecchymosis | 4 (10) | 0 (0) |
| Head trauma and no bleeding other than petechiae and ecchymosis | 9 (23) | 1 (1) |
| No head trauma but bleeding other than petechiae and ecchymosis | 20 (51) | 35 (44) |
| No head trauma and no bleeding other than petechiae and ecchymosis | 6 (15) | 44 (55) |
| . | ICH cases, n = 40 (%) . | Case controls, n = 80 (%) . |
|---|---|---|
| Sex | ||
| Female | 22 (55) | 35 (44) |
| Male | 18 (45) | 45 (56) |
| ITP duration | ||
| Acute ITP less than 6 mo | 28 (70) | 66 (82.5) |
| Chronic ITP 6 mo or more | 12 (30) | 14 (17.5) |
| Platelet counts | ||
| Median, platelets/μL | 5000 | 8000 |
| Range, platelets/μL | 1 to 61 000 | 1 to 28 000 |
| Platelet count less than 20 000/μL | 36 (90) | 68 (85) |
| Platelet count less than 10 000/μL | 30 (75) | 47 (59) |
| Age | ||
| Age range | 3 mo-17 y | 2 mo-17 y |
| Number of patients younger than 10 y old | 23 (58) | 53 (66) |
| Number of patients 10 y or older | 17 (43) | 27 (34) |
| NSAID use* | ||
| Number of patients reporting recent NSAID use | 3 (7.5) | 1 (1.25) |
| Head trauma* | 13 (33) | 1 (1) |
| Bleeding symptoms* | ||
| No bleeding | 3 (7.5) | 4 (5) |
| Any bleeding | 37 (92.5) | 76 (95) |
| Petechiae | 25 (63) | 64 (80) |
| Ecchymosis | 30 (75) | 60 (75) |
| Bleeding other than petechiae and ecchymosis | 25 (63) | 35 (44) |
| Wet purpura | 11 (27.5) | 15 (19) |
| Epistaxis | 15 (37.5) | 19 (24) |
| Gum bleeding | 9 (22.5) | 8 (10) |
| GI bleeding | 7 (17.5) | 5 (6) |
| U bleeding | 9 (22.5) | 0 (0) |
| GYN bleeding | 0 | 3 (4) |
| Head trauma and bleeding symptoms | ||
| Both head trauma and bleeding other than petechiae and ecchymosis | 4 (10) | 0 (0) |
| Head trauma and no bleeding other than petechiae and ecchymosis | 9 (23) | 1 (1) |
| No head trauma but bleeding other than petechiae and ecchymosis | 20 (51) | 35 (44) |
| No head trauma and no bleeding other than petechiae and ecchymosis | 6 (15) | 44 (55) |
GI indicates gastrointestinal; U, urinary; and GYN, gynecological/vaginal.
Data for bleeding symptoms, NSAID use, and head trauma were considered negative if no specific response was provided on the questionnaire.
At total of 6 patients with ICH and one control were excluded. Of these, 4 patients with ICH had secondary ITP (described in supplemental Table 1). The other 2 patients with ICH were excluded because they were older than 17 years or the ICH occurred before the study period. One control subject had a platelet count greater than 30 × 109/L.
No differences in terms of age, sex, platelet count, duration of ITP, head trauma, bleeding symptoms, treatment, and outcome of ICH were found between the patients with ICH accrued “prospectively” from 1997 to 2000 and those identified “retrospectively” from 1987 to 1997. Therefore, these patients were combined for all analyses.
There were no differences in age, sex, or duration of ITP between the 40 patients with ICH and the 80 case controls (Table 1).
Platelet counts
The median platelet count of the 40 patients with ICH was 5 × 109/L (range, 1-61 × 109/L); for the 80 control subjects, the median platelet count was 8 × 109/L. Thirty-six (90%) of the 40 cases occurred at platelet counts below 20 × 109/L, and 30 (75%) cases below 10 × 109/L (Table 1).
A total of 4 patients had platelet counts of 35 to 61 × 109/L at the time of their ICH. They were all boys; 3 had preceding head trauma and recovered with no neurologic sequelae. The fourth patient presented with wet purpura, gastrointestinal (GI) hemorrhage, and subsequent ICH after an upper respiratory tract infection; this patient died 24 hours after the ICH. A postmortem bone marrow biopsy was compatible with ITP.
Time from diagnosis of ITP to occurrence of ICH
A total of 18 (45%) of 40 children developed an ICH within 7 days of diagnosis of ITP. In 10 (25%) children, the ICH was the first manifestation of their ITP, whereas in 8 (20%) children, an ICH developed in the week after diagnosis. Of the remaining 22 patients, 10 (25%) had an ICH between 1 week and 6 months from diagnosis, and 12 (30%) patients had chronic ITP when their ICH occurred (Table 1).
Risk factors
Head trauma preceded ICH in 13 (33%) of 40 patients compared with only one control (P < .001).
Bleeding beyond skin petechiae and ecchymoses tended to be more frequent among the patients with ICH than the case controls (63% vs 44%, P = .08; Table 1). A total of 9 (22%) patients with ICH had hematuria (5 macroscopic, 3 microscopic, 1 unspecified), whereas no control reported hematuria (P < .001). The incidence of ecchymoses was similar for ICH and controls, but petechiae were reported in fewer patients with ICH (63% vs 80%, P = .05).
NSAIDs were recently used in 3 (7%) of 40 patients with ICH and 1 control (nonsignificant [NS], P = .1). No AVMs were identified.
Outcome
The ICH was lethal in 25% (10 of 40 patients; Table 2). Follow-up data were available in survivors for a median of 11 months (range, 5 days to 6 years). A total of 10 (33%) had neurologic sequelae, including 4 with hemiparesis; 4 with facial weakness and/or cranial nerve palsy; 1 with cognitive deficit; 1 with epilepsy; 1 with speech deficit; and 1 with unavailable details.
Outcome according to clinical variables
| Clinical variable . | Total . | Number of patients (%) . | ||
|---|---|---|---|---|
| Survived with full recovery (%) . | Survived with neurological sequalae (%) . | Died (%) . | ||
| Overall | 40 | 20 (50) | 10 (25) | 10 (25) |
| Sex | ||||
| Male | 18 | 8 (45) | 5 (28) | 5 (28) |
| Female | 22 | 12 (55) | 5 (23) | 5 (23) |
| Platelet count | ||||
| Platelet count less than 10 000 | 31 | 14 (45) | 9 (29) | 8 (26) |
| Platelet count more than 10 000 | 9 | 6 (67) | 1 (11) | 2 (22) |
| Head trauma | ||||
| Yes | 13 | 9 (69) | 3 (15) | 2 (15) |
| No | 27 | 11 (41) | 7 (26) | 8 (30) |
| Bleeding | ||||
| Petechiae and ecchymoses only | 15 | 10 (67) | 4 (27) | 1 (7)* |
| Bleeding other than petechiae and ecchymoses | 25 | 10 (40) | 6 (24) | 9 (36)* |
| NSAID use | ||||
| Yes | 3 | 1 (33) | 2 (67) | 0 |
| No | 37 | 19 (51) | 8 (22) | 10 (27) |
| Time from diagnosis of ITP to ICH | ||||
| Presented ITP with ICH | 10 | 7 (70) | 1 (10) | 2 (20) |
| ICH in first week following diagnosis | 8 | 1 (13) | 3 (38) | 4 (50)† |
| ICH later than 1st week following dignosis | 22 | 12 (55) | 6 (27) | 4 (18) |
| Clinical variable . | Total . | Number of patients (%) . | ||
|---|---|---|---|---|
| Survived with full recovery (%) . | Survived with neurological sequalae (%) . | Died (%) . | ||
| Overall | 40 | 20 (50) | 10 (25) | 10 (25) |
| Sex | ||||
| Male | 18 | 8 (45) | 5 (28) | 5 (28) |
| Female | 22 | 12 (55) | 5 (23) | 5 (23) |
| Platelet count | ||||
| Platelet count less than 10 000 | 31 | 14 (45) | 9 (29) | 8 (26) |
| Platelet count more than 10 000 | 9 | 6 (67) | 1 (11) | 2 (22) |
| Head trauma | ||||
| Yes | 13 | 9 (69) | 3 (15) | 2 (15) |
| No | 27 | 11 (41) | 7 (26) | 8 (30) |
| Bleeding | ||||
| Petechiae and ecchymoses only | 15 | 10 (67) | 4 (27) | 1 (7)* |
| Bleeding other than petechiae and ecchymoses | 25 | 10 (40) | 6 (24) | 9 (36)* |
| NSAID use | ||||
| Yes | 3 | 1 (33) | 2 (67) | 0 |
| No | 37 | 19 (51) | 8 (22) | 10 (27) |
| Time from diagnosis of ITP to ICH | ||||
| Presented ITP with ICH | 10 | 7 (70) | 1 (10) | 2 (20) |
| ICH in first week following diagnosis | 8 | 1 (13) | 3 (38) | 4 (50)† |
| ICH later than 1st week following dignosis | 22 | 12 (55) | 6 (27) | 4 (18) |
P = .009 for number of patients surviving versus died between two bleeding categories.
P = .02 for number of patients surviving versus died among the 8 children who had ICH in first week versus all other patients.
Interrelationship of clinical variables
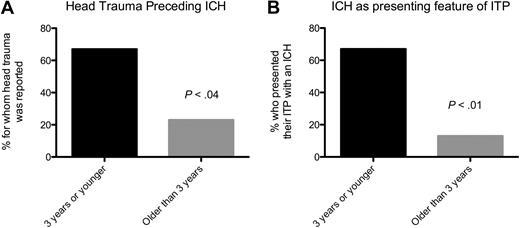
The 10 children whose ITP presented with ICH were more likely to have had head trauma than the other patients with ICH (60% vs 23%, P = .05). Young infants (3 years of age or younger) were more likely to have head trauma (P < .04; Figure 1A) and more likely to have an ICH as the presenting feature of their ITP (P < .001; Figure 1B). Compared with the 8 children who had an ICH in the first week after diagnosis, the 10 whose ITP presented with an ICH had a significantly lower incidence of bleeding beyond petechiae and ecchymoses (P = .02).
Younger patients were significantly more likely to have head trauma preceding their ICH and to have an ICH as the presenting feature of their ITP. (A) Head trauma preceding ICH. (B) ICH as presenting feature of ITP.
Younger patients were significantly more likely to have head trauma preceding their ICH and to have an ICH as the presenting feature of their ITP. (A) Head trauma preceding ICH. (B) ICH as presenting feature of ITP.
The 8 patients with an ICH in the first week after diagnosis of their ITP had significantly poorer outcomes than the other 32 patients. Only 1 made a full recovery: 38% had neurologic sequelae and 50% died (P = .02). In contrast, 8 (80%) of the 10 patients who presented their ITP with an ICH survived without sequelae.
Mortality was higher in the patients who had bleeding beyond petechiae and ecchymoses (36% vs 7%, P < .01; Table 2), whereas it tended to be lower in patients with head trauma.
Treatments at any time before the ICH
A total of 28 (70%) of the 40 patients with ICH had received treatment for their thrombocytopenia: 16 (57%) of 28 of patients with acute ITP and 11 (92%) of 12 with chronic ITP. The most common treatments were intravenous immunoglobulin (IVIG; 65%), steroids (50%), anti-D (25%), immunosuppressive chemotherapy (15%), platelet transfusions (15%), and splenectomy (15%). Unfortunately, information on platelet response to therapies and timing of administration before ICH were not consistently provided.
Treatments after ICH
All 40 children received at least 1 treatment after the ICH: 78% received IVIG, 75% received platelet transfusions, 73% received corticosteroids, and 43% underwent splenectomy; 35% had a craniotomy. There was no difference in outcome between emergency splenectomy after ICH and medical management. In contrast, of the 14 who had a craniotomy, only 2 (14%) made a full recovery (P < .001).
Combinations of risk factors and likelihood of ICH
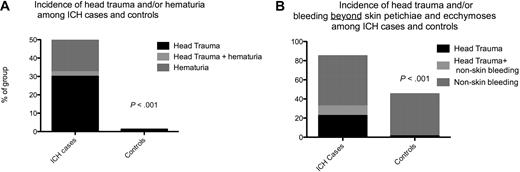
Combining the risk factors head trauma and/or hematuria (Figure 2A) would have identified 21 (53%) of the 40 patients with ICH. However, only 6 patients with ICH (15%) had neither head trauma nor bleeding beyond skin petechiae and ecchymoses, compared with 44 (55%) of the case controls (P ≤ .001; Figure 2B). Table 3 illustrates different algorithms that may be used to select high-risk patients.
Combination of the risk factors of head trauma and bleeding to identify patients with ICH. (A) Incidence of head trauma and/or hematuria among patients with ICH and control subjects. (B) Incidence of head trauma and/or bleeding beyond skin petechiae and ecchymoses among patients with ICH and control subjects.
Combination of the risk factors of head trauma and bleeding to identify patients with ICH. (A) Incidence of head trauma and/or hematuria among patients with ICH and control subjects. (B) Incidence of head trauma and/or bleeding beyond skin petechiae and ecchymoses among patients with ICH and control subjects.
Combinations of risk factors for ICH to explore their use in diagnostic and management algorithms
| Combination of high-risk factors for ICH used to identify patients for treatment . | Sensitivity, % . | Specificity, % . | Estimated or potential use of combination therapy . | Positive predictive value, % . | Negative predictive value, % . | Number (%) of ITP patients with ICH identified for treatment* . | Number (%) of patients who will test positive but not have an ICH† . | Number of patients without ICH treated per ICH case treated . | Approximate cost of preventing one ICH‡ . |
|---|---|---|---|---|---|---|---|---|---|
| Any bleeding other than skin petechiae and ecchymoses and/or head trauma and/or a platelet count < 10 000/mL | 100 | 23 | Possibly useful | 33 | 100 | 9 (100) | 2944 (77) | 327 | $1.7 million |
| Any bleeding other than skin petechiae and ecchymoses and/or head trauma | 83 | 55 | Probably useful | 41 | 90 | 7.5 (83) | 1727 (45) | 230 | $1.2 million |
| Hematuria and/or head trauma | 50 | 99 | Use in all cases | 94 | 84 | 4.5 (50) | 38 (1) | 8.5 | $45 300 |
| Combination of high-risk factors for ICH used to identify patients for treatment . | Sensitivity, % . | Specificity, % . | Estimated or potential use of combination therapy . | Positive predictive value, % . | Negative predictive value, % . | Number (%) of ITP patients with ICH identified for treatment* . | Number (%) of patients who will test positive but not have an ICH† . | Number of patients without ICH treated per ICH case treated . | Approximate cost of preventing one ICH‡ . |
|---|---|---|---|---|---|---|---|---|---|
| Any bleeding other than skin petechiae and ecchymoses and/or head trauma and/or a platelet count < 10 000/mL | 100 | 23 | Possibly useful | 33 | 100 | 9 (100) | 2944 (77) | 327 | $1.7 million |
| Any bleeding other than skin petechiae and ecchymoses and/or head trauma | 83 | 55 | Probably useful | 41 | 90 | 7.5 (83) | 1727 (45) | 230 | $1.2 million |
| Hematuria and/or head trauma | 50 | 99 | Use in all cases | 94 | 84 | 4.5 (50) | 38 (1) | 8.5 | $45 300 |
The 10 children who presented their ITP with an ICH are excluded from these analyses since their ICH could not be prevented.
These calculations assume an incidence of ICH of 3850 cases/year and that this study captured 50% of true cases of ICH, suggesting an incidence of ICH among children with ITP of 0.3%, that is, 12 cases of ICH per year. As 25% of cases of ICH were the first presenting feature of ITP, we considered 75%, that is, 9 cases of ICH, to be preventable. The number of ITP patients identified for treatment by each combination of risk factors = (9 × specificity).
The number of patients who will test positive by not have an ICH was calculated as (100 − specificity) × (number of children with ICH who do not have ICH, that is, 3850 − 12 = 3838).
This calculation assumes that each child identified as high risk for ICH receives 1 dose of IVIG at 1g/kg and I course of intravenous anti-D at 75 mcg/kg. For average 20-kg child, total cost is $5313.
Discussion
This study accrued 40 cases of ICH in children with primary ITP in the United States from 1987 to 2001; these children were compared with 80 matched controls. A total of 17 “retrospective” patients with ICH that had occurred in the previous 10 years were obtained in 1997, and 23 “prospective” patients with ICH were obtained through yearly surveys from 1997 to 2000.
Are these data consistent with previous estimates of the incidence of ICH in children with ITP?
There are 70 million children in the United States,12 and it is estimated that the annual incidence of ITP is 5.5 children per 100 000.5 Therefore, approximately 3850 new cases of ITP are diagnosed annually. A total of 6 cases of ICH were reported per year on average during the 4-year “prospective” phase of the study. Accordingly, the yearly incidence of ICH in ITP could range from 7.5 (if 80% of all US patients with ICH had been captured in this survey) to 30 (20% capture). Assuming that there are 3850 cases of ITP per year, the incidence of ICH in children with ITP in the United States would be 0.19% to 0.78%. This is consistent with previous estimates.1,3,5,7,13,14
What distinguishes children with ITP who develop an ICH from those children with ITP who do not?
Severe thrombocytopenia, head trauma, and hematuria were strongly and significantly associated with ICH.
Severe thrombocytopenia appears to be permissive but not sufficient for ICH. The overwhelming majority of children with ITP who are severely thrombocytopenic do not experience an ICH. This is illustrated by the case controls, half of whom had platelet counts of 10 × 109/L or less. A total of 75% of cases of ICH occurred when platelet counts were less than 10 × 109/L and 90% occurred when platelet counts were less than 20 × 109/L. Head trauma was more frequent in patients with ICH, particularly among children aged 3 years or less, in cases where the ICH lead to the diagnosis of ITP and in cases that occurred at platelet counts greater than 20 × 109/L.
Bleeding symptoms beyond petechiae and bruises tended to occur more frequently in ICH cases than controls. Hematuria was reported in 9 patients with ICH (microscopic in 3 patients) but not in any controls (P < .001); the number of urinalyses in the control group is unknown.11,15 GI bleeding, epistaxis, gum bleeding, and wet purpura were also more frequent in the patients with ICH, but not significantly so (Table 1). Detailed information describing bleeding events (eg, duration of epistaxis) was often unavailable.
NSAID use and AVMs were not identified as risk factors for ICH. A total of 4 cases of vasculitis in patients with SLE were excluded (these cases are described in supplemental Table 1).
What is the outcome of patients with ITP-associated ICH?
The mortality rate was 25%, lower than reviews of published cases of ICH that may have been biased toward severe cases.3,16 A further 10 had neurologic sequelae. Therefore, although the incidence of ICH is low, 50% of children died or had neurologic disability. This is particularly striking given that ITP (without ICH) is typically a self-limited disease with no sequelae. The children who had an ICH in the first week after diagnosis of ITP seemed to do particularly poorly, possibly because children with severe hemorrhage may respond less well to treatment.1,17
What are the implications for management of children with ITP?
Serious bleeding in children with ITP is quite infrequent both at diagnosis and later in the course.2,18 Considerable debate regarding management therefore remains, as attempts to prevent ICH must be balanced against treatment-related toxicities and cost implications in the many patients with very low counts but little bleeding.2,19-24 In addition, ITP is typically a short-lived problem that may resolve in weeks to months. Conservative (ie, noninterventional) management therefore appears appropriate for most patients.
On the other hand, this survey suggests that it may be possible to identify high-risk cases prospectively,1,2 except when the ICH is the presenting feature of the ITP. In cases of severe thrombocytopenia, head trauma and bleeding beyond petechiae and ecchymoses (especially hematuria) appear to predispose to ICH.1,3,15 Other types of bleeding, such as wet purpura, GI and vaginal bleeding, and the use of NSAIDs may also be important but were not as clearly related to ICH in this study.
For patients with a high likelihood of ICH, could it be prevented by timely therapy? We believe, as do others, that single-agent therapy may often be ineffective in preventing ICH. We were unable to ascertain whether specific treatments may have prevented ICH in some cases in this series, had they been administered earlier or repeatedly. Not only were details of response to therapies generally not available, but also response to therapy after ICH has occurred may not necessarily be indicative of response to therapy before or during ICH.
An option to consider, given the potential insufficiency of single-agent treatment, is multiagent combination therapy using IVIG, high-dose intravenous methylprednisolone with intravenous anti-D and/or intravenous vincristine.25 In the Boruchov study, 10 children and 25 adults who were completely unresponsive to either steroids or IVIG given alone (median platelet count after initial treatment, 8 × 109/L) had a 71% response rate to combination therapy, achieving peak platelet counts of 30 × 109/L or higher. The cases reported by Boruchov, with severe thrombocytopenia and refractoriness to initial therapy, are comparable with the cases reported here in which ICH actually occurred.
Additional treatment needs to be considered early in patients with ITP at high risk for ICH who are not responding to standard therapy, especially in the first week after diagnosis of ITP. In dire cases, platelet transfusions (bolus followed by continuous infusion) or even recombinant human factor VIIa could be added. Thrombopoietic agents might also have a role in combination therapy, although they generally require at least 5 to 8 days to increase the platelet count.26
The estimated treatment-related cost of preventing an ICH varies, depending upon the approach used to identify high-risk patients and the efficacy of combination therapy. Table 3 describes a preliminary consideration of selected strategies exploring prospective approaches to identifying children for treatment. The strategies ranged from 100% sensitivity (with only 23% specificity) to 99% specificity (with 50% sensitivity). Although an oversimplification, the treatment cost per ICH prevented among the 3850 children with ITP per year (assuming an average weight of 20 kg) ranged from $45 300 (50% sensitivity, 100% efficacy of treatment, and 99% specificity) to $3 400 000 (100% sensitivity, 50% efficacy, and 23% specificity; Table 3).
Other management considerations
The proportion of patients with ICH that occurred more than 6 months from diagnosis (29%) is substantial. Management of these patients is different, since the time horizon is potentially unlimited because spontaneous remission is no longer as likely. Treatment (eg, danazol and azathioprin, rituximab, splenectomy, or thrombopoietic agents)25-31 may need to be continued indefinitely for those whose persistent severe thrombocytopenia does not improve and who have ongoing risk factors for ICH, even if the platelet increment in response to treatment is small.
No specific medical treatment of ICH appeared to be associated with an improved neurologic outcome. Emergency splenectomy was not associated with a better outcome and craniotomy was associated with a poorer outcome, suggesting that it may have been reserved for more severe cases.
Limitations
As with any survey study, details were limited especially in certain areas. For example, information on the efficacy of treatment was quite limited. Also, not all centers responded to the survey, although mail-outs were sent yearly for 3 years and every listed center was called at least twice. Reporting biases are possible. An illustration of this is that petechiae were reported less frequently among the patients with ICH than the controls, which seems counterintuitive. However, the higher mortality among the patients with ICH who had more bleeding manifestations supports the accuracy of documentation of bleeding symptoms in the patients with ICH. It is possible that the severity of the ICH influenced the likelihood of responding to the survey. Furthermore, a disproportionate number of the controls had to be taken from the Weill Cornell Center because accompanying control subjects were not always provided by the physicians reporting the patients with ICH. These and the other controls received treatment of their ITP as determined by their hematologists. In this survey study, investigation of the underlying pathophysiology of the ICH in patients with ICH was not possible. Finally, only a very preliminary analysis of strategies to identify and manage patients at high risk of ICH was performed, and no formal cost-benefit analysis was conducted.
Conclusion
There have been 2 schools of thought regarding management of markedly thrombocytopenic children with ITP. One is that given the rarity of ICH, therapy should almost always be avoided because in the majority of cases it is unnecessary, as the ITP will spontaneously resolve and treatment may be toxic and ineffective. The other approach considers that although ICH is rare, it may be devastating, treatments are reasonably benign when used appropriately, and there are quality-of-life benefits to increasing the platelet count in addition to avoiding ICH. Both approaches, however, treat patients as if they all have equal risks of severe hemorrhage, and neither provides a suitable approach to children with persistent, chronic, severe ITP. As is well known, it is impossible to do a prospective, randomized study of treatment to prevent ICH in children with ITP.6 This study argues that individual patients with ITP could be stratified according to their risk of impending ICH. Treatment or nontreatment could be decided accordingly. Given the possibility that single agents are not always effective, in high-risk patients, combination therapy may need to be considered.25
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank George Buchannan, Paul Imbach, and Kim Ritchey for their helpful comments on this manuscript.
B.P. was supported by a Fulbright Scholarship in Cancer Research and a Kay Kendall Leukemia Fund Traveling Fellowship. This work was also partly supported by Dana Hammond Stubgen, the Children's Blood and Cancer Foundation, and National Institutes of Health grants U01 HL072186 and UL1RR024996 (J.B.B.).
National Institutes of Health
Authorship
Contribution: B.P. analyzed the data and wrote the manuscript with J.B.B. and L.K.P.; A.P. performed research and contributed to writing the paper; L.K.P. analyzed data and contributed to writing the manuscript; J.M. and M.S. performed research; and J.B.B. designed the study, performed research, and wrote the manuscript together with the coauthors.
Conflict-of-interest disclosure: J.B.B. receives clinical research support from the following companies: Amgen, Cangene, GlaxoSmithKline, Eisai, Rigel, Ligand, Gensyme, and Sysmex. J.B.B. has also participated for Baxter and Sysmex in their speaker's bureau program. His family owns less than $100 000 in stock in Amgen and GlaxoSmithKline in an IRA and a trust, respectively. J.B.B. has served on advisory boards for Amgen, GlaxoSmithKline, Talecris, and Ligand. B.P. has served on advisory boards for GlaxoSmithKline and has received research support from Sysmex. The remaining authors declare no competing financial interests.
Correspondence: James B. Bussel, Department of Pediatrics, Weill Medical College, 525 East 68th St, P-695, New York, NY 10021; e-mail: jbussel@med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal