Abstract
Dendritic cell (DC) development is efficiently supported by Flt3-ligand or GM-CSF in vitro, and lymphoid-organ DC maintenance in vivo is critically dependent on Flt3-ligand. However, the relevance of GM-CSF for lymphoid-tissue DC maintenance and the importance of both cytokines for nonlymphoid organ DC homeostasis are not defined. Here, we show that, although Gm-csfr and Flt3 are both expressed in DC progenitors, Gm-csfr is expressed predominantly in monocytes, classical DCs (cDCs), and skin DCs, whereas Flt3 is expressed in both cDCs and plasmacytoid DCs (pDCs). In accordance with the respective cytokine receptor expression, DC progenitor and pDC numbers are primarily affected by Flt3-ligand deficiency, whereas both splenic and lymph node cDCs and dermal DCs are reduced in the absence of either GM-CSF or Flt3-ligand. Combined lack of GM-CSF and Flt3-ligand in newly generated double-deficient mice leads to further significant reductions of DC progenitors and dermal DCs. In line with the decrease of respective DC subsets, T-cell and antigen-specific IgG responses decline progressively, from wild-type to GM-CSF– to Flt3-ligand– to double-deficient mice, upon subcutaneous antigen delivery. These data thus show the concerted action of GM-CSF and Flt3-ligand on DC homeostasis in vivo.
Introduction
Steady-state maintenance of tolerance and induction of the adaptive immune response during infection and inflammation both require the specialized functions of dendritic cells (DCs). Although the role of DCs in immune regulation is crucial, they represent a very small fraction of short-lived cells of the hematopoietic system distributed throughout the body with particularly high concentrations at environmental interfaces and in lymphoid organs. DCs can be divided into multiple subsets based on location, function, and surface markers. Here, we divide them into plasmacytoid DCs (pDC) that are uniquely equipped to produce type I interferons during infection, lymphoid-tissue resident DCs (also called classical DCs; cDCs), and nonlymphoid tissue, migratory DCs such as epidermal DCs (Langerhans cells; LCs) and dermal DCs that are, in contrast to pDCs, more efficient in extracellular antigen uptake, presentation, and activation of lymphocytes.1-3
DCs can efficiently be differentiated in vitro by stimulating monocytes or hematopoietic progenitors with granulocyte macrophage colony-stimulating factor (GM-CSF).4,5 At the same time, GM-CSF inhibits in vitro pDC development through activation of STAT5 signaling.6-8 Surprisingly, mice lacking GM-CSF or its receptor had only small decreases in lymphoid-organ DCs with a maximum reduction of 3-fold in lymph node cDCs and only a modest reduction of LCs, whereas GM-CSF transgenic mice showed similar opposite effects.9,10 Thus, at least in the presence of compensatory cytokines, GM-CSF seemed to add little to steady-state DC maintenance, and it was suggested that GM-CSF mostly contributes to inflammatory DC generation, potentially from monocytes, in vivo. Indeed, it was recently shown that adoptively transferred monocytes only generate DCs in nonlymphoid tissue and spleen in an inflammatory environment (eg Naik et al11 and Varol et al12 ), and bone marrow–derived DCs cultured in GM-CSF represent tumor necrosis factor-α– and inducible nitric oxide synthase–producing inflammatory DCs observed in vivo.13
In contrast, Flt3-ligand (FL) supports the in vitro differentiation of progenitor cells, but not monocytes, into both cDCs and pDCs7,14 and genetic deletion of FL or treatment of mice with Flt3 (fms-related tyrosine kinase 3; Flk2) inhibitors leads to a 10-fold reduction of lymphoid-organ pDCs and cDCs,15,16 whereas LCs are little or not affected.9 In addition, FL injection or overexpression of FL results in the expansion of both pDCs and DCs in all lymphoid and nonlymphoid organs.17-20 In line with this, it has been shown that DC development is confined to hematopoietic precursors in the bone marrow expressing Flt3,18,21 that Flt3 signaling can also instruct Flt3-negative precursors to differentiate into both pDCs and cDCs,22 and that FL is involved in all lymphoid-organ DC development and expansion from early progenitors in the bone marrow to immediate DC progenitors in lymphoid tissues.18,23 Moreover, early progenitors such as “macrophage and DC progenitors” (MDPs)24 and “MDPΔ”23 that give rise to monocytes, macrophages, and DCs, and probably further downstream25,26 “common DC progenitors” (CDPs)27 and “pro-DCs,”28 that give rise solely to pDCs and DCs, have recently been identified. All express c-kit (CD117, the receptor for stem cell factor) and Flt3 (CD135), but no mature lineage marker.
To further elucidate the roles of GM-CSF and FL on DC homeostasis, we here systematically compared GM-CSF and Flt3 receptor expression on ex vivo–isolated DC progenitors, monocytes, and lymphoid and nonlymphoid tissue DC populations, and tested the effect of in vivo GM-CSF, FL, and combined GM-CSF and FL deficiency on lymphoid and nonlymphoid organ DCs at steady-state and on immune responses upon vaccination.
Methods
Mice
GM-CSF−/− mice were obtained from Dr J.A. Whitsett (Hospital Medical Center, Cincinnati, OH),29 FL−/− mice were obtained from Dr J.J. Peschon (Immunex Corporation, Seattle, WA).15 GM-CSF−/− FL−/− double-knockout (DKO) mice were generated by crossbreeding GM-CSF−/− and FL−/− mice. All knockout mice were on the C57BL/6 background. C57BL/6 mice were used as wild-type (WT) controls. Sex- and age-matched, 6- to 12-week-old mice were used in the studies. All mice were bred and maintained at the Institute for Research in Biomedicine animal facility. Mice were treated in accordance with guidelines of the Swiss Federal Veterinary Office, and experiments were approved by the Dipartimento della Sanità e Socialità.
Antibodies
All antibodies were purchased from eBiosciences, unless otherwise stated. The following monoclonal antibodies conjugated to different fluorochromes or biotin were used: CD3ϵ (145-2C11), c-kit (ACK2), M-CSFR (AFS98), Flt3 (A2F10), IL7Rα (A7R34), MHCII (M5/114.15.2), CD11c (N418), B220 (RA3-6B2), CD45RA (14.8; Becton Dickinson), CD40 (3/23; Becton Dickinson), Gr-1 (RB6-8C5), CD11b (M1/70), NK1.1 (PK136), CD19 (MB19-1 and ID3; Becton Dickinson), CD45 (30-F11; Becton Dickinson), CD45.1 (A20), CD45.2 (104), CD4 (RM4-5), CD8α (53-6.7), and Ter119 (Ter119). Biotinylated antibodies were visualized with streptavidin–fluorescein isothiocyanate (FITC), streptavidin-APC or streptavidin-APC-Cy7.
Cell preparation and flow cytometry
Quantitative reverse transcription–polymerase chain reaction analysis
Cells were sorted and resuspended in TRIzol LS reagent (Invitrogen). RNA was extracted, followed by DNase I treatment with the use of the DNA-free kit (Applied Biosystems). Equal amounts of RNA were used for cDNA synthesis and real-time polymerase chain reaction (PCR), which was performed and analyzed as previously described.27 Taqman probes for 18S (HS99999901_s1), mouse Gm-csfr (Csf2ra; Mm00438331_g1), Flt3 (Mm00438996_m1), and M-csfr (Mm00432689_m1) were purchased from Applied Biosystems. Results were normalized to 18S.
Immunofluorescence
Epidermal sheets were separated from the dermis with the use of 0.5 M ammonium thiocyanate (Sigma-Aldrich) at 37°C for 30 minutes, stained, and prepared on slides. Images were taken on a Nikon Eclipse E800 microscope with a CCD Qimaging camera with the use of OpenLab software. All images were acquired with the use of a Nikon Plan Apo 20×/0.75 numeric aperture (NA) objective lens.
In vivo T-cell proliferation assay
Naive OT-II T cells (CD4+CD8−CD25−CD44loCD62Lhi) were sorted from spleens and lymph nodes (LNs) of OT-II/RAG1−/− transgenic mice. Cells were labeled with 2.5 μM CFSE (carboxyfluorescein diacetate succinimidyl ester; Invitrogen), and 6 × 104 cells were injected intravenously into mice. Sixteen hours later, mice were immunized with 2 μg whole OVA protein (Sigma-Aldrich) and 4 μg monophosphoryl lipid A (InvivoGen) in the flank. Draining LNs were analyzed by flow cytometry after 3 days.
Antibody response
Mice were immunized in the footpads as described in the T-cell proliferation assay. A booster immunization was given 3 weeks later. Serum was collected and analyzed by enzyme-linked immunoabsorbent assay (ELISA). Plates were coated with 10 μg/mL OVA and blocked with 2% BSA in PBS, and serial dilutions of sera from immunized mice were added. OVA-specific antibodies were detected with alkaline phosphatase–conjugated goat anti–mouse antibodies (Southern Biotech) and pNPP substrate (Sigma-Aldrich). Plates were read at 405 nm with a microplate reader (Molecular Devices). Pooled sera from hyperimmunized mice were used as a standard to calculate relative units.
Statistics
Data were analyzed by Prism 4 (GraphPad Software) with the use of the nonparametric unpaired Mann-Whitney U test. Graphs show the mean plus or minus SEM. P was considered significant at values less than .05.
Results
Cell-specific expression of Flt3, Gm-csfr, and M-csfr
We tested the relative mRNA expression of Flt3 and Gm-csfr between ex vivo–isolated progenitor cells, subpopulations of monocytes, and lymphoid and nonlymphoid organ DCs with the use of quantitative real-time PCR. Cells were sorted from bone marrow, spleen, lymph nodes, epidermis, and dermis.
In the earliest bone marrow progenitor population analyzed, c-kithi cells, which contain hematopoietic stem cells, as well as multiple early lineage-restricted progenitors, Flt3 and Gm-csfr expression were relatively low, whereas expression increased slightly on MDPΔs and even more so on CDPs (Figure 1A-B; progenitor population and sorting gate defined in supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).23,27 A comparative back-gating analysis of MDPΔ and CDP showed that approximately two-thirds of CDPs are contained within the MDPΔ gate, whereas the MDPΔ gate includes, with respect to c-kit and Flt3 expression, a heterogeneous population of cells (supplemental Figure 1).
Flt3, Gm-csfr, and M-csfr mRNA expression in progenitor and myeloid cells. Real-time PCR analysis of mRNA expression of Flt3 (A), Gm-csfr (B), and M-csfr (C) in cell populations sorted from WT mice based on the following markers: c-kithi (lin−c-kithi); MDPΔ (lin−M-CSFRhi); CDP (lin−c-kitintFlt3+IL7Rα−); spleen (Sp) and LN cDC (CD19−MHCII+CD11c+) and pDC (CD19−CD45RA+CD11c+); LN CD40hi DC (CD19−CD40hiCD11cint); Langerhans cells (LC from epidermis) and dermal DC (MHCII+CD45+); blood (Bl), spleen, and bone marrow (BM) monocytes (mo) (M-CSFR+CD11b+ and Gr-1+ or Gr-1−). Data shown are averages of 3 independent experiments.
Flt3, Gm-csfr, and M-csfr mRNA expression in progenitor and myeloid cells. Real-time PCR analysis of mRNA expression of Flt3 (A), Gm-csfr (B), and M-csfr (C) in cell populations sorted from WT mice based on the following markers: c-kithi (lin−c-kithi); MDPΔ (lin−M-CSFRhi); CDP (lin−c-kitintFlt3+IL7Rα−); spleen (Sp) and LN cDC (CD19−MHCII+CD11c+) and pDC (CD19−CD45RA+CD11c+); LN CD40hi DC (CD19−CD40hiCD11cint); Langerhans cells (LC from epidermis) and dermal DC (MHCII+CD45+); blood (Bl), spleen, and bone marrow (BM) monocytes (mo) (M-CSFR+CD11b+ and Gr-1+ or Gr-1−). Data shown are averages of 3 independent experiments.
In both the spleen and lymph node, cDCs and pDCs expressed Flt3 at high levels, and similar levels were also observed in CD40hiCD11cint cells that include both LCs and dermal DCs that have trafficked to the lymph node from the skin.31 Conversely, LCs and dermal-derived DCs, which were sorted directly from the skin, had much lower levels of Flt3 expression compared with CD40hiCD11cint cells. Flt3 levels were also very low or absent in either Gr1+ or Gr1− monocytes isolated from the blood, spleen, and bone marrow (BM; Figure 1A).32
As expected, given their responsiveness to GM-CSF in culture, all sorted monocytes expressed high levels of Gm-csfr. Furthermore, skin-derived DCs and cDCs had similarly high levels of Gm-csfr gene expression. As in the case with Flt3, Gm-csfr expression levels reversed in the migratory skin DCs isolated from skin-draining LNs compared with DCs isolated directly from the skin, suggesting that upon maturation and migration, skin-derived DCs down-regulate Gm-csfr expression and at the same time up-regulate Flt3 expression. In contrast to cDCs, pDCs expressed relatively low levels of Gm-csfr (Figure 1B).
In addition to Flt3 and GM-CSF signaling in DC development, recent studies have suggested a role for macrophage colony-stimulating factor receptor (M-CSFR, CSF1-R) ligands in DC development. As shown by M-CSFR reporter mice, M-csfr mRNA is expressed by most lymphoid organ DCs,33 and DC progenitors, such as MDPΔs and CDPs, are sorted based on their M-CSF receptor expression.23,27 Furthermore, M-CSFR–deficient mice have slightly reduced pDC and cDC numbers,33 M-CSFR is required for in vivo LC development,30 and M-CSFR was also shown to support pDC and cDC development in vitro and in vivo.27,34
We therefore analyzed the mRNA expression levels of M-csfr in all respective populations. Although both monocyte subsets and progenitors were sorted on the basis of high surface M-CSFR expression, the mRNA levels in all monocyte subsets tested were much higher than in MDPΔs or CDPs. Similar high levels of M-csfr mRNA were detected in LCs and dermal DCs compared with monocytes. The LC data are in agreement with the finding that LCs develop from a monocyte precursor and require M-CSFR for development.30 Only cDCs in the spleen and LN expressed detectable M-csfr, whereas M-csfr expression was very low or absent in pDCs and in CD40hiCD11cint cells (Figure 1C).
Generation of GM-CSF and FL DKO mice
To test the effect of the combined absence of GM-CSF and FL on DC homeostasis in vivo, we generated GM-CSF and FL DKO mice by cross-breeding the 2 single-knockout mice. The lack of gene sequences for Flt3l and Gm-csf and the absence of respective serum cytokines were confirmed. Interestingly, no significant compensatory increase of FL, GM-CSF, or M-CSF was detected in serum from any of the cytokine-deficient animals (supplemental Figure 2).
Absence of GM-CSF and FL reduces DC progenitors in the BM
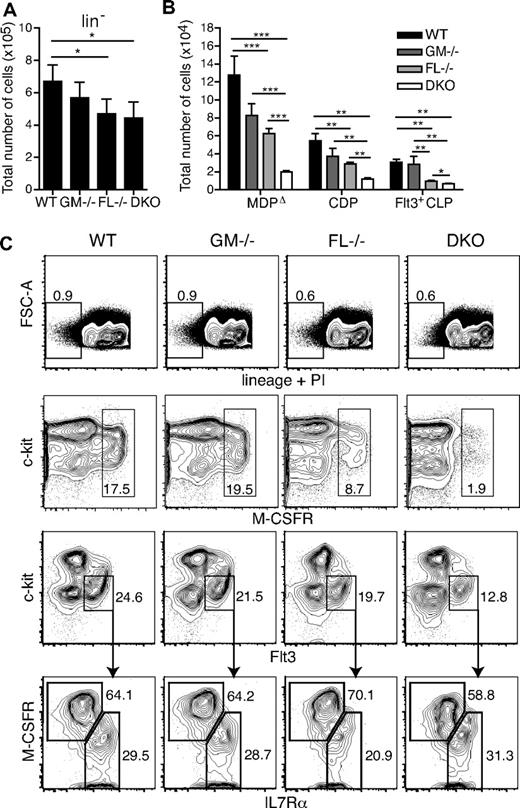
To determine the effects of GM-CSF and FL on DC progenitors, BM cells from GM-CSF−/−, FL−/−, and DKO mice were analyzed. Cells were first gated on those lacking the following lineage markers: B220, CD11b, CD19, CD3ϵ, CD4, CD8α, Gr-1, Ter119, and NK1.1 (Figure 2A,C first row). There was a significant reduction of approximately one-third in the absolute numbers of lineage-negative cells in FL−/− and DKO mice compared with WT mice. When cells were further subdivided into MDPΔ (M-CSFRhi) and CDP (c-kitintFlt3+M-CSFR+IL7Rα−) populations (supplemental Figure 1), FL−/− mice had 2-fold fewer MDPΔs and CDPs compared with WT mice at 6 to 9 weeks of age, a finding that was significant for both absolute and relative reductions (Figure 2B-C; Table 1; supplemental Tables 1-2). This finding parallels the decreased myeloid colony-forming unit (CFU) activity measured from the BM of FL−/− mice.15
Dendritic cell progenitors are significantly reduced in the absence of GM-CSF and FL. Quantification of total cell numbers of lineage-negative (lin−) cells (A) and for each progenitor population (B) (n = 5-7 mice/group). *P < .05; **P < .01; ***P < .001. (C) Representative fluorescence-activated cell sorting (FACS) plots of stained BM cells from WT, GM-CSF−/−, FL−/−, and DKO mice. Gating of lin− cells (dead cells excluded by PI staining; first row). Second and third rows were first gated on lin− cells. MDPΔ gate: M-CSFRhi (second row). CDP and Flt3+ CLP gates were as follows: CDP, c-kitintFlt3+M-CSFR+IL7Rα−; Flt3+ CLP, c-kitintFlt3+M-CSFRlo/−IL7Rα+ (third and fourth rows). Numbers in or beside boxes indicate percentage of cells shown.
Dendritic cell progenitors are significantly reduced in the absence of GM-CSF and FL. Quantification of total cell numbers of lineage-negative (lin−) cells (A) and for each progenitor population (B) (n = 5-7 mice/group). *P < .05; **P < .01; ***P < .001. (C) Representative fluorescence-activated cell sorting (FACS) plots of stained BM cells from WT, GM-CSF−/−, FL−/−, and DKO mice. Gating of lin− cells (dead cells excluded by PI staining; first row). Second and third rows were first gated on lin− cells. MDPΔ gate: M-CSFRhi (second row). CDP and Flt3+ CLP gates were as follows: CDP, c-kitintFlt3+M-CSFR+IL7Rα−; Flt3+ CLP, c-kitintFlt3+M-CSFRlo/−IL7Rα+ (third and fourth rows). Numbers in or beside boxes indicate percentage of cells shown.
Relative and absolute fold reductions of progenitor cells and DCs in knockout mice compared with WT mice
| Cell population . | GM-CSF−/− . | FL−/− . | DKO . |
|---|---|---|---|
| MDPΔ | 1.0/1.5 | 1.3*/2.3* | 2.9*/7.1* |
| CDP | 1.0/1.5 | 1.3*/1.9* | 2.4*/4.6* |
| Spleen cDC | 1.7*/1.8 | 5.7*/7.5* | 4.9*/7.0* |
| Spleen pDC | 1.2/0.8 | 6.2*/5.6* | 6.7*/9.9* |
| LN cDC | 3.4*/4.0* | 28*/8.3* | 7.5*/41.6* |
| LN pDC | 1.4/1.8 | 10.9*/7.5* | 19.4*/79* |
| LN CD40hiCD11cint DC | 1.8/2.3 | 1.5/2.3 | 3.3*/8.3* |
| CD45+MHCII+ dermal DC | 1.4*/ND | 2.2*/ND | 4.8*/ND |
| LC | ND/2.0* | ND/1.3* | ND/1.5* |
| Cell population . | GM-CSF−/− . | FL−/− . | DKO . |
|---|---|---|---|
| MDPΔ | 1.0/1.5 | 1.3*/2.3* | 2.9*/7.1* |
| CDP | 1.0/1.5 | 1.3*/1.9* | 2.4*/4.6* |
| Spleen cDC | 1.7*/1.8 | 5.7*/7.5* | 4.9*/7.0* |
| Spleen pDC | 1.2/0.8 | 6.2*/5.6* | 6.7*/9.9* |
| LN cDC | 3.4*/4.0* | 28*/8.3* | 7.5*/41.6* |
| LN pDC | 1.4/1.8 | 10.9*/7.5* | 19.4*/79* |
| LN CD40hiCD11cint DC | 1.8/2.3 | 1.5/2.3 | 3.3*/8.3* |
| CD45+MHCII+ dermal DC | 1.4*/ND | 2.2*/ND | 4.8*/ND |
| LC | ND/2.0* | ND/1.3* | ND/1.5* |
Values are the mean relative fold reduction/absolute fold reduction. DKO indicates double knockout; and ND, not determined.
Significant reduction compared with WT (P < .05).
Although in GM-CSF−/− mice the total numbers of both MDPΔs and CDPs seemed slightly reduced, this reduction was not statistically significant with the number of animals analyzed (Figure 2B-C). In DKO mice, however, a synergistic effect through the absence of GM-CSF and FL was observed, with 7.1-fold and 4.6-fold lower numbers of MDPΔs and CDPs, respectively, compared with WT mice. These results were significant for both absolute and relative reductions (Figure 2B-C; Table 1; supplemental Tables 1-2). Therefore, macrophage and DC-specific progenitors express Flt3 and Gm-csfr mRNA (Figure 1), and both cytokines are critical for their differentiation and/or maintenance in steady-state.
Although the Flt3+ fraction of the common lymphoid progenitor (CLP; c-kitintFlt3+M-CSFRlo/−IL7Rα+) was unchanged in GM-CSF–deficient mice, FL−/− mice, as previously shown for mice deficient in Flt3, had significantly lower numbers of CLPs, and a further significant reduction of absolute CLP numbers was observed in DKO mice (Figure 2B-C).15,35 However, relative CLP frequencies were nearly identical between FL−/− and DKO mice (0.028% ± 0.003% and 0.027% ± 0.003% of total nucleated cells, respectively; data not shown).
Effect of GM-CSF and FL on DC subsets in steady state
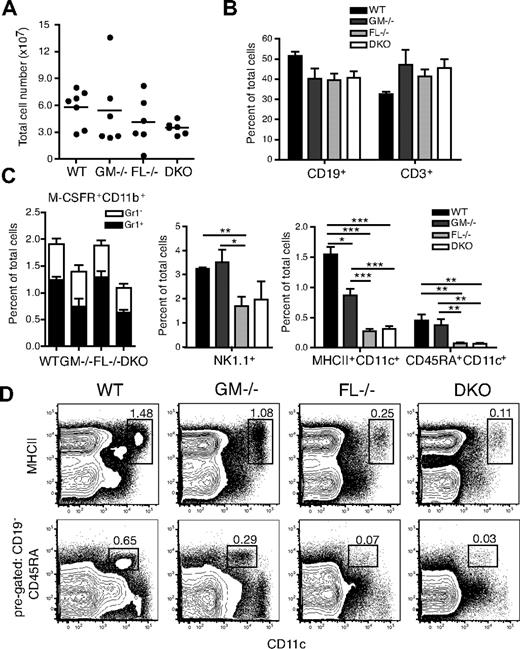
Spleens of WT, GM-CSF−/−, FL−/−, and DKO mice were analyzed in steady state for leukocyte subsets. FL−/− and DKO mice had on average a slightly lower spleen cellularity compared with WT or GM-CSF−/− mice (Figure 3A). Mice lacking GM-CSF, FL, or both cytokines had no significant reduction in the percentages of B cells (CD19+) or T cells (CD3+; Figure 3B). Reductions in both Gr1+ and Gr1− subsets of monocytes (M-CSFR+CD11b+) were equally present in GM-CSF−/− and DKO mice, suggesting, as expected, little involvement of FL in steady-state monocyte homeostasis (Figure 3C; supplemental Figure 3). As described, the percentage of natural killer (NK) cells (CD3−NK1.1+) was significantly reduced in FL−/− compared with WT or GM-CSF−/− mice,15 and DKO mice exhibited a similar percentage of NK1.1+ cells as FL−/− mice (Figure 3C).
Analysis of cell subsets in the spleen of DKO mice in steady state. (A) Total cellularity of spleens from WT, GM-CSF−/−, FL−/−, and DKO mice. Horizontal lines indicate mean values of the results. (B) Percentage of CD19+ and CD3+ cells in the spleen. (C) Percentage of myeloid cell subsets: Monocytes were first gated as M-CSFR+CD11b+ and separated into Gr1+ or Gr1− cells; NK cells were gated as CD3−NK1.1+; DCs were divided into cDCs (MHCII+CD11c+) and pDCs (CD19−CD45RA+CD11c+) with representative FACS plots shown (D). CD45RA versus CD11c plots were first gated on CD19− cells. Numbers in FACS plots are percentages of total nucleated cells. *P < .05; **P < .01; ***P < .001.
Analysis of cell subsets in the spleen of DKO mice in steady state. (A) Total cellularity of spleens from WT, GM-CSF−/−, FL−/−, and DKO mice. Horizontal lines indicate mean values of the results. (B) Percentage of CD19+ and CD3+ cells in the spleen. (C) Percentage of myeloid cell subsets: Monocytes were first gated as M-CSFR+CD11b+ and separated into Gr1+ or Gr1− cells; NK cells were gated as CD3−NK1.1+; DCs were divided into cDCs (MHCII+CD11c+) and pDCs (CD19−CD45RA+CD11c+) with representative FACS plots shown (D). CD45RA versus CD11c plots were first gated on CD19− cells. Numbers in FACS plots are percentages of total nucleated cells. *P < .05; **P < .01; ***P < .001.
Analysis of spleen cDCs (MHCII+CD11c+) showed a significant 1.7-fold reduction in GM-CSF−/− mice compared with WT mice. FL deficiency had an even greater effect, with an average 5.7-fold relative decrease (7.5-fold reduction in absolute cDC numbers). Interestingly, mice lacking both GM-CSF and FL had no further reduction of cDCs compared with FL-only deficient mice (Figure 3C-D; Table 1; supplemental Tables 1-2). In addition, CD8α− and CD8α+ cDCs were equally reduced in both FL−/− and DKO mice (data not shown). GM-CSF−/− and WT mice had similar frequencies of pDCs (CD19−CD45RA+CD11c+), whereas FL−/− and DKO mice had approximately 6-fold reductions (Figure 3C-D; Table 1; supplemental Tables 1-2). In lymph nodes, cDCs were reduced by 3-fold in GM-CSF−/− and 28-fold in FL−/− mice, respectively (supplemental Figure 4; Table 1; supplemental Tables 1-2). However, there was no significant difference in the percentage of cDCs in DKO mice compared with either GM-CSF−/− or FL−/− mice, although DKO mice had significantly lower numbers of cDCs compared with GM-CSF−/− mice because of a total reduction in lymph node cellularity. FL−/− and DKO mice had similar reductions in the frequency of pDCs compared with WT or GM-CSF−/− mice; however, there was a significant reduction in absolute pDC numbers in DKO mice compared with FL−/− mice (supplemental Figure 4; Table 1; supplemental Tables 1-2). As in the spleen, FL−/− and DKO mice had similar frequencies of cDCs and pDCs in the bone marrow and liver (supplemental Figure 4).
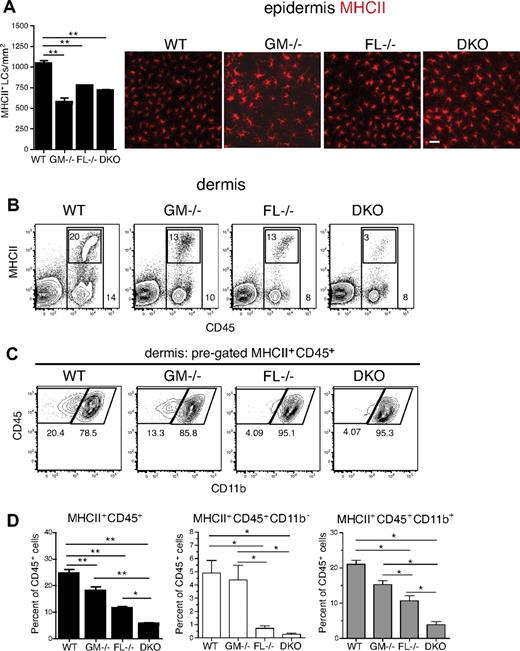
Skin DCs are representative environmental interface tissue DCs that constitutively take up antigen and then migrate to draining lymph nodes where they represent a small fraction of DCs.36 During inflammation the migratory process is largely enhanced, and tissue-derived LN DCs increase in numbers. Analysis of epidermal sheets stained for MHCII showed an overall slight decrease of LCs in all knockout mice examined compared with WT mice with significant reductions of 2-fold, 1.3-fold, and 1.5-fold in GM-CSF−/−, FL−/−, and DKO mice, respectively (Figure 4A; Table 1; supplemental Table 2). Relative numbers of dermal CD45+MHCII+ cells compared with total dermal CD45+ cells were reduced in GM-CSF−/− and FL−/− mice compared with WT mice by 1.3-fold and 2.2-fold, respectively (Figure 4B-D; Table 1; supplemental Table 1). CD45+MHCII+ cells from DKO mice were even further reduced compared with FL or GM-CSF single cytokine knockout mice, with a 4.8-fold reduction compared with WT mice (Figure 4B,D; Table 1; supplemental Table 1). In the mouse, 3 distinct subsets of CD45+MHCII+ dermal-derived DCs have been characterized, based on a combination of surface protein expression. Langerhans cells (langerin+CD11b+) emigrating from the epidermis are found in the dermis, as well as the major dermis-resident DC population (langerin−CD11b−) and a recently identified minor dermal DC subset, the langerin+ dermal DCs (langerin+CD11b−).37-39 Further subdivision of the dermal DC subsets into CD45+MHCII+ CD11b+ or CD11b− cells showed that CD11b+ dermal DCs were increasingly reduced from GM-CSF−/− to FL−/− to DKO mice compared with WT mice, with DKO mice having significant reductions compared with GM-CSF−/− and FL−/− mice (Figure 4C-D). In contrast, CD45+MHCII+CD11b− cells were predominately affected by the absence of FL, with comparable reduced frequencies in FL−/− and DKO mice (Figure 4C-D). Similar results were obtained when gating on CD45+MHCII+CD11b−langerin+ cells (data not shown).
Dermal DCs are significantly reduced in the absence of GM-CSF and FL in steady state. DCs were analyzed from WT, GM-CSF−/−, FL−/−, and DKO mice. (A) Immunofluorescence microscopy of MHCII-stained (red) epidermal sheets. MHCII+ cells were counted on images taken from multiple fields per mouse (n = 4/group). Epidermal sheets were stained with PE-conjugated anti-MHCII antibody and mounted on slides with the use of Eukitt mounting medium. Images were taken at room temperature on a Nikon Eclipse E800 microscope with a CCD Qimaging camera using a Nikon Plan Apo 20×/0.75 NA objective lens and acquired with the use of OpenLab software. Scale bar represents 10 μm. (B-C) Flow cytometry of ex vivo–isolated dermal-derived cells. Representative FACS plots from WT, GM-CSF−/−, FL−/−, and DKO mice are shown. (B) Percentage of all CD45+ cells shown by outer gate. Inner gate represents percentage of MHCII+ cells within CD45+ gate. (C) Dermal DCs were pregated on MHCII+CD45+, followed by gating on CD11b+ and CD11b− populations. (D) Percentages of total dermal-derived MHCII+CD45+ cells, as well as CD11b− and CD11b+ subsets are shown. Results are given as the percentage of CD45+ cells (n = 4-6 mice/group). *P < .05; **P < .01.
Dermal DCs are significantly reduced in the absence of GM-CSF and FL in steady state. DCs were analyzed from WT, GM-CSF−/−, FL−/−, and DKO mice. (A) Immunofluorescence microscopy of MHCII-stained (red) epidermal sheets. MHCII+ cells were counted on images taken from multiple fields per mouse (n = 4/group). Epidermal sheets were stained with PE-conjugated anti-MHCII antibody and mounted on slides with the use of Eukitt mounting medium. Images were taken at room temperature on a Nikon Eclipse E800 microscope with a CCD Qimaging camera using a Nikon Plan Apo 20×/0.75 NA objective lens and acquired with the use of OpenLab software. Scale bar represents 10 μm. (B-C) Flow cytometry of ex vivo–isolated dermal-derived cells. Representative FACS plots from WT, GM-CSF−/−, FL−/−, and DKO mice are shown. (B) Percentage of all CD45+ cells shown by outer gate. Inner gate represents percentage of MHCII+ cells within CD45+ gate. (C) Dermal DCs were pregated on MHCII+CD45+, followed by gating on CD11b+ and CD11b− populations. (D) Percentages of total dermal-derived MHCII+CD45+ cells, as well as CD11b− and CD11b+ subsets are shown. Results are given as the percentage of CD45+ cells (n = 4-6 mice/group). *P < .05; **P < .01.
Cells within the CD45+MHCII+ gate in the dermis include mostly DCs but also some MHCIIlo dermal macrophages. Nevertheless, the specific reduction of dermal-derived DCs in mice lacking GM-CSF and FL was also evident when evaluating the CD40hiCD11cint constitutively migrating skin DCs in the draining LN, which include both migrated LCs and dermal DCs (Figure 5A-B).31 Importantly, only CD40hiCD11cint skin-derived DCs were significantly 2- and 3.5-fold lower in relative and absolute numbers, respectively, in DKO mice compared with FL−/− mice, whereas there were no substantial differences in the LN-resident CD40+CD11chi or CD40−CD11cint DC subsets (Figure 5B; Table 1; supplemental Tables 1-2). Therefore, absence of either GM-CSF or FL caused significant reductions in the frequencies of dermal DCs and, unlike cDCs of the lymphoid organs, combined loss of GM-CSF and FL further greatly diminished dermal DC numbers. Thus, both GM-CSF and FL are major cytokines involved in regulating dermal DC populations in the skin.
Skin-derived DCs in the draining lymph nodes are significantly reduced in GM-CSF– and FL-deficient mice in steady state. (A) Flow cytometry and analysis of DC subsets in skin-draining LNs (pooled inguinal, axillary, and cervical LNs). Horizontal lines indicate mean values of the results. LN DC subsets gated as CD40hiCD11cint, CD40+CD11chi, and CD40−CD11cint. Total LN cell numbers (A) and percentage of each LN DC subset (B) are shown in graphs. *P < .05; **P < .01.
Skin-derived DCs in the draining lymph nodes are significantly reduced in GM-CSF– and FL-deficient mice in steady state. (A) Flow cytometry and analysis of DC subsets in skin-draining LNs (pooled inguinal, axillary, and cervical LNs). Horizontal lines indicate mean values of the results. LN DC subsets gated as CD40hiCD11cint, CD40+CD11chi, and CD40−CD11cint. Total LN cell numbers (A) and percentage of each LN DC subset (B) are shown in graphs. *P < .05; **P < .01.
Reduced immune responses after subcutaneous immunization in the absence of GM-CSF and FL
Given that the strongest reduction in DCs in the absence of GM-CSF and FL was observed in steady-state dermal DCs at primary sites and in LNs, we examined the role of these cytokines in immune responses initiated at these sites. After contact sensitization with a FITC-containing solution of acetone and dibutylphthalate, the total number of FITC+ CD40hiCD11cint cells in skin-draining LNs was consistently lower in DKO mice, compared with WT, GM-CSF−/−, and FL−/− mice (supplemental Figure 5). DKO mice had significantly reduced total lymph node cellularity in steady state; however, by day 3 after FITC treatment, the LN cellularity of DKO mice was similar to FL−/− mice (supplemental Figure 5B). Still, the number of FITC+ cells that migrated from the site of inflammation to the draining LN was much lower in the DKO mice (supplemental Figure 5C). We speculate that the reduction of skin-derived cells observed in the LN after contact sensitization is most likely due to the reduction of dermal DCs in the skin. However, we cannot rule out an additional defect in maturation and migration of skin DCs, although CCR7 surface expression on CD40hiCD11cint cells from DKO mice was similarly up-regulated compared with WT mice (data not shown).
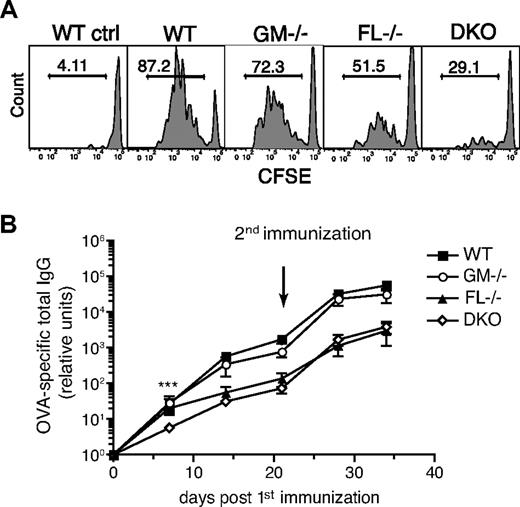
We next tested whether the reduction of dermal DCs in the draining LN in the absence of GM-CSF or FL or both would affect the activation and proliferation of CD4+ T cells after immunization with whole protein antigen. To measure the proliferation of antigen-specific T cells, mice were adoptively transferred with small numbers (6 × 104) of CFSE-labeled naive OVA-specific CD4+ OT-II T cells that homed at similar absolute numbers to skin-draining LNs of WT and knockout mice (data not shown). Mice were subsequently immunized subcutaneously with low amounts of OVA and monophosphoryl lipid A (MPL) as adjuvant. The proliferation of OVA-specific OT-II T cells was assessed by flow cytometry. In GM-CSF−/− mice, OT-II T cells proliferated as well as in WT mice. FL−/− mice had a 1.4-fold reduction in the percentage of proliferating T cells compared with WT mice, whereas DKO mice had on average a 2-fold decrease in proliferating OT-II cells (Figure 6A). Similar results have also been shown by the lack of OT-I CD8+ T-cell proliferation after immunization of CD11c+ cell–depleted mice and by the dependence of proliferation and priming of CD4+ DO11.10 T cells on the number of DCs migrating to the draining LN.40,41 The decrease in T-cell activation in FL−/− and DKO mice was also most likely not due to a defect in maturation of the DCs, because they expressed MHCII and costimulatory molecules (CD40, CD80, and CD86) at levels similar to activated DCs from WT mice (data not shown). Thus, the reduction in OT-II cell proliferation after subcutaneous immunization can probably be attributed to the decrease in the number of antigen-capturing and migrating dermal DCs.
Reduced T-cell and antibody responses after subcutaneous immunization in the absence of GM-CSF and FL. (A) T-cell proliferation assay. Naive OT-II CD4+CD45.1+ T cells were sorted and labeled with CSFE before transfer into CD45.2+ WT, GM-CSF−/−, FL−/−, and DKO mice (6 × 104 cells/recipient). The next day, mice were immunized subcutaneously in the right flank with 2 μg OVA protein and 4 μg MPL. Draining LNs (right axillary and inguinal) and nondraining LNs (shown as representative WT control) were analyzed 3 days later. The proliferation of the transferred OT-II T cells was analyzed by gating on CD45.1+CD3+CD4+ cells. Histograms show the percentage of OT-II T cells having diluted the CFSE label. Results are representative of 2 independent experiments with a total of 3 to 7 mice/group. (B) Production of OVA-specific IgG antibodies. WT, GM-CSF−/−, FL−/−, and DKO mice were immunized in the footpads with 2 μg whole OVA protein and 4 μg MPL, and serum was collected at the indicated time points. A second immunization was given at day 21. OVA-specific IgG antibodies were measured by ELISA (n = 7-8 mice/group). ***P < .001.
Reduced T-cell and antibody responses after subcutaneous immunization in the absence of GM-CSF and FL. (A) T-cell proliferation assay. Naive OT-II CD4+CD45.1+ T cells were sorted and labeled with CSFE before transfer into CD45.2+ WT, GM-CSF−/−, FL−/−, and DKO mice (6 × 104 cells/recipient). The next day, mice were immunized subcutaneously in the right flank with 2 μg OVA protein and 4 μg MPL. Draining LNs (right axillary and inguinal) and nondraining LNs (shown as representative WT control) were analyzed 3 days later. The proliferation of the transferred OT-II T cells was analyzed by gating on CD45.1+CD3+CD4+ cells. Histograms show the percentage of OT-II T cells having diluted the CFSE label. Results are representative of 2 independent experiments with a total of 3 to 7 mice/group. (B) Production of OVA-specific IgG antibodies. WT, GM-CSF−/−, FL−/−, and DKO mice were immunized in the footpads with 2 μg whole OVA protein and 4 μg MPL, and serum was collected at the indicated time points. A second immunization was given at day 21. OVA-specific IgG antibodies were measured by ELISA (n = 7-8 mice/group). ***P < .001.
To assess whether a reduced immune response in GM-CSF and FL-deficient mice would also be observed in the absence of TCR-transgenic cells in a potentially more physiologic setting, T cell–dependent antibody responses were measured after subcutaneous immunization. During steady-state conditions no significant differences were seen in the basal immunoglobulin levels of IgM, IgA, and total IgG between WT, GM-CSF−/−, FL−/−, and DKO mice (supplemental Figure 6). Mice were immunized subcutaneously with OVA and MPL, and OVA-specific total IgG antibody levels were measured by ELISA. At early time points (less than 20 days after immunization), antibody levels were reduced in DKO mice and were significantly lower at day 7 compared with WT, GM-CSF−/−, and FL−/− mice. After a booster immunization, both FL−/− and DKO mice had one-log lower antibody responses compared with WT or GM-CSF−/− mice (Figure 6B). Taken together, although GM-CSF, in combination with FL, plays an important role in the homeostasis of dermal DCs, as well as in the early events in the induction of adaptive immune responses, FL alone seems to be the more critical factor, compared with GM-CSF, contributing to the production of serum antibody levels after subcutaneous immunization.
Discussion
GM-CSF and FL are 2 key cytokines for DC differentiation from progenitors. To date, the effects of GM-CSF and FL on DC development have been primarily studied in lymphoid organ cDC subsets in vivo or in vitro generated DCs. In this study we provide, for the first time, a comprehensive analysis of in vivo DC Gm-csfr and Flt3 receptor expression and the roles of GM-CSF and FL on the development of DCs from restricted progenitors in the BM to the various DC subsets throughout the body by studying GM-CSF−/−, FL−/−, and newly generated GM-CSF−/−FL−/− double-deficient mice.
Although GM-CSF deficiency alone did not lead to a significant reduction of early DC progenitors such as MDPΔs and CDPs in the BM, FL deficiency, and even more so combined GM-CSF and FL deficiency led to a massive reduction of these populations. These results show that committed DC progenitors require GM-CSF and FL during development and/or maintenance in the BM, which is consistent with Gm-csfr and Flt3 expression in both MDPΔs and CDPs. This is in line with previous data showing CDP and MDPΔ expansion upon in vivo stimulation with supraphysiologic levels of FL,23,27 but it contrasts to previous findings in which no significant difference was seen in the numbers of MDPΔs at 9 weeks of age in mice lacking the cognate receptor, Flt3.23 We have obtained similar results in Flt3−/− mice as in the study by Waskow et al23 (data not shown). These differential effects on MDPΔ numbers between Flt3 receptor– and ligand-deficient mice will need further evaluation.
For steady-state cDCs in lymphoid organs, the current results confirm previous findings in GM-CSF and FL single cytokine-deficient mice, with GM-CSF deficiency leading to minor, and FL deficiency leading to major reductions of cDCs.10,15 Because GM-CSF was recently shown to inhibit FL-driven pDC development by STAT5-mediated IRF8 suppression, and increases in pDC frequencies are seen from STAT5-deficient progenitor cells,6-8 it was critical to test if pDC numbers might be elevated in GM-CSF−/− mice. GM-CSF deficiency, however, had no effect on the number of pDCs in all the tissues analyzed. Thus, it is important to note that inhibitory signals from GM-CSF are not a primary mechanism in regulating pDC numbers in vivo, at least not under steady-state conditions.
Although FL is necessary to regulate steady-state numbers of lymphoid organ DCs, a small pool of DCs is still present in FL−/− mice. To determine whether these small numbers of DCs were maintained by a GM-CSF–driven pathway, we intercrossed GM-CSF−/− and FL−/− mice to generate double cytokine-deficient animals. Although double deficiency led to an additional reduction in BM DC progenitors as discussed earlier, it did not lead to further DC reduction compared with FL single deficiency in BM, spleen, liver, and LNs, thus showing compensation of the progenitor deficiency in double-deficient animals on the mature steady-state lymphoid organ DC level. However, analysis of nonlymphoid tissue DCs, ie, dermal DCs, showed significant reductions in both GM-CSF−/− and FL−/− mice with an additional substantial reduction in the combined knockout mice. Furthermore, whereas CD11b+ dermal DCs were progressively reduced from GM-CSF−/− to FL−/− to DKO mice, CD11b− dermal DCs were primarily dependent on FL. These results suggest that these distinct dermal DC populations have differential cytokine requirements. Further studies will be required to determine the roles of GM-CSF and FL in the development, homeostasis, and subsequent function of these dermal DC subsets.
The deficiency of dermal DCs was confirmed on detailed analysis of skin-derived DCs in LNs, suggesting consecutively reduced steady-state migration from skin.3,36 These reduced steady-state DC numbers and migration might translate into impaired immune responses. Indeed, although not formally proven to be directly DC related, we observed increasingly reduced proliferative T-cell and specific antibody responses from single- to double-deficient mice upon subcutaneous immunization. We thus identified a synergistic role for both GM-CSF and FL during the differentiation or maintenance or both of DC progenitors and nonlymphoid organ dermal DCs during steady-state conditions.
Interestingly, the ex vivo analysis of Gm-csfr and Flt3 expression on different lymphoid and nonlymphoid organ DCs closely correlated with the observed reductions of DCs in respective single and double cytokine knockout mice. This indicates that receptor expression not only is relevant for further differentiation or activation of these cells but also plays a role in their respective steady-state generation and maintenance. Because similar results for Flt3 mRNA expression have also been shown for human DCs and myelomonocytic cells, we speculate that the differential and combined roles of these cytokines also hold true in humans.42
FL and GM-CSF are produced by stromal cells and activated T cells.43,44 Although FL is constitutively expressed and measurable in serum, GM-CSF only becomes detectable in systemic inflammation and then might drive a robust GM-CSF–induced pathway of DC differentiation involving monocytes.3,11,12,43 It has, therefore, been suggested that the DC developmental pathways mediated by FL or GM-CSF are isolated events with FL contributing to DC development in steady state, whereas GM-CSF only plays a role in the differentiation of DCs from monocytes under inflammatory conditions. However, our data show that small amounts of local GM-CSF expression must be involved in steady-state DC homeostasis, acting on both DC progenitors in BM and on DCs in nonlymphoid tissues. Furthermore, although FL and GM-CSF are major cytokines for DC development in steady state, cytokines such as IL-4, TNF-α, LTβ, M-CSF, and TGF-β1 will probably have more subtle effects or are only active in inflammatory conditions.3 Thus, future analysis of these cytokines in defined tissues and the visualization of respective receptor-expressing cells should help to further define DC differentiation pathways during steady state and inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank David Jarrossay for assistance with cell sorting and Enrica Mira Catò and Andrea D'Ercole for animal care.
This work was supported by the Swiss National Science Foundation (310000-116637), the European Commission FP6 Network of Excellence initiative (LSHB-CT-2004-512074 DC-THERA), and the Sixth Research Framework Program of the European Union, Project MUGEN (MUGEN LSHG-CT-2005-005203).
Authorship
Contribution: D.K. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; M.A.S., N.O., A.O.-O., and D.B. performed experiments and collected data; and M.G.M. directed the study and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus G. Manz, Institute for Research in Biomedicine (IRB), Via Vincenzo Vela 6, CH-6500 Bellinzona, Switzerland; e-mail: manz@irb.unisi.ch.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal