Abstract
Inflammatory bone loss in septic and inflammatory conditions is due to increased activity of osteoclasts that requires receptor activator of NF-kappa B-ligand (RANKL). Neutrophils are the predominant infiltrating cells in these conditions. Although disease severity is linked to neutrophils, their role in evolution of bony lesions is not clear. We show that lipopolysaccharide (LPS), a toll-like receptor 4 ligand, up-regulated the expression of membrane RANKL in human blood neutrophils and murine air pouch–derived neutrophils. LPS-activated human and murine neutrophils, cocultured with human monocyte-derived osteoclasts and RAW 264.7 cells, respectively, stimulated bone resorption. Transfection of PLB-985 neutrophil-like cells with RANKL antisense RNA reduced osteoclastogenesis. Synovial fluid neutrophils of patients with exacerbation of rheumatoid arthritis strongly expressed RANKL and activated osteoclastogenesis in coculture systems. Osteoprotegerin, the RANKL decoy receptor, suppressed osteoclast activation by neutrophils from these different sources. Moreover, direct cell-cell contact between neutrophils and osteoclasts was visualized by confocal laser microscopy. Activation of neutrophil membrane-bound RANKL was linked to tyrosine phosphorylation of Src-homology domain–containing cytosolic phosphatase 1 with concomitant down-regulation of cytokine production. The demonstration of these novel functions of neutrophils highlights their potential role in osteoimmunology and in therapeutics of inflammatory bone disease.
Introduction
Inflammation and bone loss are intimately related. This association is seen clinically in septic arthritis, osteomyelitis, rheumatoid arthritis (RA), as well as in periodontitis. Indeed, in most of these conditions, bone loss starts early in the disease process and is a cause of considerable patient morbidity. These destructive bony lesions result from excessive activity of the monocyte-derived bone resorbing cells, osteoclasts (OCs).1 Both osteoclast differentiation and function are regulated by the molecular couple RANK (receptor activator of NF-kappa B) and its ligand, RANKL.2 RANK is a membrane protein expressed by OCs.3 RANKL, belonging to the TNF family, was discovered as a stromal cell–associated protein that required direct cell-to-cell interactions with OCs, and as a soluble protein.2,4,5 Immune cells, such as activated T lymphocytes, also synthesize and release functionally active RANKL.6 Excessive RANKL concentrations are present in local and systemic inflammatory conditions associated with excessive bone resorption. The decoy receptor of RANKL, osteoprotegerin (OPG), negatively regulates OCs. Indeed, the RANKL/RANK/OPG system is the final common pathway through which most osteotropic factors modulate their effects on bone.7
The sites of bony lesions in humans and in animal models show massive infiltration of the prototypic inflammatory cells, neutrophils. Synovial fluids in septic arthritis and in flare-up of RA are almost exclusively neutrophilic. In RA patients, the therapeutic efficacies of the disease-modifying drugs leflunomide and methotrexate are linked to reduced neutrophil activity.8 Neutrophils are also implicated in human periodontitis.9 Similarly, several animal models, such as lipopolysaccharide (LPS)–induced arthritis, the K/BxN mouse RA model, and rabbit Gram-negative periodontitis, are dependent on neutrophils.10-12 Of note, although traditionally considered to be short-lived cells with limited synthetic capacity, activated neutrophils have been shown to synthesize considerable amounts of proteins and lipids that participate in the inflammatory process.13,14
Although neutrophils have been extensively studied as key contributors to the local inflammatory component of active joint diseases, not much is known about the role of neutrophils in the evolution of the bony lesions that characterize these conditions. Given the presence of neutrophils at sites of inflammatory bone loss, we hypothesized that neutrophils might have the potential to directly activate pathologic bone resorption. In a previous work, we showed that human neutrophils have the capacity to express RANKL as an intracellular and as a membrane-bound protein, without any release of the secreted form.15 In the present study, we investigated the potential role of neutrophil RANKL in interactions with osteoclasts and its regulation by proinflammatory conditions.
Methods
Preparation of neutrophils
The institutional review board of the Université Laval approved the study and volunteers signed a consent form in accordance with the Declaration of Helsinki. Human venous blood collected on anticoagulant solution from healthy donors was centrifuged and the platelet rich plasma was removed. Cells were prepared in sterile condition. After dextran sedimentation, neutrophils were purified by centrifugation over Ficoll-Paque (Wisent), and contaminating erythrocytes were eliminated by hypotonic lysis. After washes, neutrophils were resuspended in culture medium constituted of RPMI 1640 + 10% fetal bovine serum (FBS) + 1% penicillin-streptomycin. Cell viability was routinely assessed by propidium iodide (PI) exclusion test (> 99%).
Neutrophils (5 × 106 cells/mL) were incubated in 12-well plates at 37°C, 5% CO2. Cells were incubated with 100 ng/mL LPS (Escherichia coli 0111:B4; Sigma-Aldrich), 1 μg/mL Pam3Cys-Ser-(Lys)4 hydrochloride (Calbiochem), 500 ng/mL flagellin, or 0.1 μg/mL and 1 μg/mL gardiquimod (InvivoGen). The addition of 500 pM GM-CSF (Peprotech) to the incubation medium was used to increase neutrophil viability.16 After 48 hours of incubation, neutrophils were enriched in viable cells by discontinuous Percoll (GE Healthcare) density gradient centrifugation.17 Gradients were equal volumes of 31%, 42%, and 51% of a standard isotonic Percoll solution. Tubes were centrifuged at 610g for 28 minutes at 4°C. Viable cells (annexin V– and PI-negative cells) were collected at the bottom, washed, and counted.
Murine neutrophils were obtained from in vivo air pouch model. Animal experiments were approved by the ethics committee of the Université Laval. Air pouches were raised on the dorsum of 10- to 12-week-old CD-1 mice (Charles River).18 One milliliter of LPS (1 μg/mL) or its diluent (PBS) was injected into the air pouches to induce neutrophil migration. Six hours later, mice were killed by CO2 asphyxiation. Air pouches were washed 3 times with PBS and exudates were centrifuged at 500g for 5 minutes. Cells were counted after acetic blue staining.
Neutrophils of patients with active RA were obtained from sterile synovial fluid (SF) collected on anticoagulant solution and centrifuged at 300g for 10 minutes. Viability (PI exclusion) of pelleted cells was 96.4% plus or minus 1.4% (n = 3). RA-SF leukocytes were 95.6% plus or minus 1.6% neutrophils (n = 3), as assessed by labeling CD66b, a surface protein specific to neutrophils.19 Neutrophils from healthy donors were resuspended in 80% pooled SF/20% culture medium and preincubated at 37°C for 24 hours. Pooled SF was from 3 RA patients, and from 2 patients with osteoarthritis (OA).
Preparation of PLB-985 neutrophil-like cells
PLB-985 cells (Deutsche Sammlung) were grown in RPMI 1640 medium containing 10% FBS in a humidified atmosphere (37°C, 5% CO2). Neutrophil-like cells were obtained with 0.3 mM dibutyryl-cAMP (dbcAMP; Sigma-Aldrich) for 2 days. Transfection was performed using the Nucleofector system (Amaxa Biosystems). After 1 day of differentiation, 2 × 106 PLB-985 cells were transfected with 20 nM RANKL-specific antisense RNA (QIAGEN) or nonsilencing antisense RNA (AllStars negative control antisense RNA; QIAGEN) in nucleofection buffer using the Nucleofector program U-02. The RANKL antisense RNA sequence used was 5′-aguucgagauauagauuga. After nucleofection, cells were resuspended in medium with 0.3 mM dbcAMP. Cell functions were monitored at 48 hours after transfection.
Reverse-transcription–PCR and Western blot detection of RANKL
Total cellular RNA from neutrophils was isolated using Trizol reagent. Subsequently, 1 μg total RNA was reverse-transcribed into cDNA using SuperscriptII (Invitrogen).15 The polymerase chain reaction (PCR) for RANKL was performed as previously described.15 Beta-actin was used as an internal control.
Western blot analyses were performed with neutrophil membranes.20 Membranes (equivalent to 500 000 cells) were lysed in Laemmli sample buffer and immunoblotting was performed using anti–human RANKL IgG primary monoclonal antibody (mAb; MAB6261; R&D Systems). Membrane integrity was verified by reblotting with an anti-FcγRII (cytoplasmic tail of CD32) polyclonal antiserum (CT-10), a gift of Dr P. H. Naccache (CRRI).
Fluorescence staining procedures
Human neutrophils (100 μL of cells at 107/mL) were incubated with 10% pooled human serum for 30 minutes at 4°C to block nonspecific binding sites. Cells were then stained with primary antibody for 30 minutes at 4°C: mouse anti–human surface RANKL (MAB6261), anti–human neutrophil CD66b (eBioscience Inc), and anti–human lymphocyte CD3 (Beckman Coulter) antibodies. Cells were stained with FITC-conjugated F(ab′)2 fragments of goat anti–mouse IgG Ab (Jackson ImmunoResearch Laboratories). Control isotype Ab was a mouse IgG1 Ab. Analysis was performed using a flow cytometer EPICS-XL (Beckman Coulter). Ten thousand cells were counted.
Mouse neutrophil purity was assessed using an anti–mouse neutrophil Ab, clone 7/4 (Cedarlane).21 Neutrophils were incubated for 1 hour in PBS containing 1% BSA, 0.01% azide, 10% normal rat serum (Chemicon), and 10 μg/mL murine Fc block (eBioscience). Cells were washed with PBS containing 1% BSA + 0.01% azide and incubated for 1 hour with 5 μg/mL FITC-conjugated clone 7/4 Ab or isotype IgG2a. Cells were washed and fixed in PBS containing 2% formaldehyde. Experiments were also performed with simultaneous staining of cells with an anti–mouse RANKL PE-conjugated Ab (eBioscience).
Differentiation of human and murine osteoclasts
Human OCs were generated from peripheral blood mononuclear cells (PBMCs). PBMCs obtained by centrifugation over Ficoll-Paque were resuspended (5 × 106 cells/mL) in α MEM (Wisent) supplemented with 10% FBS and depleted of lymphocytes by adherence (5 × 106 cells/mL, 37°C, 90 minutes). Adherent cells that contained OC precursors were incubated with medium alone, or medium supplemented by monocyte-colony stimulating factor (M-CSF, 25 ng/mL), RANKL (40 ng/mL), or OPG (2 μg/mL). After 7 days of incubation, adherent cells were treated with Accutase (eBioscience). These cells, referred to as mature human OCs, were disposed on calcified matrices BioCoat Osteologic discs (BD Biosciences) or OsteoSite (IDS) devitalized dentine discs.
Murine monocyte-macrophage RAW 264.7 cells were plated in 96-well polystyrene plates or on Osteologic discs at 2 × 104 cells/mL in DMEM + 10% FBS, penicillin, streptomycin, with or without 40 ng/mL mRANKL.
Coculture of OCs with PLB-985 cells and neutrophils
Osteoclastogenesis was studied by culture of human OC precursors with fixed PLB-985 neutrophil-like cells transfected with RANKL antisense RNA or nonsilencing antisense RNA. PLB-985 cells (25 × 106) were fixed with 2% sterile paraformaldehyde (PFA) in PBS for 15 minutes, washed, and incubated with human OCs (106/200 μL per well) in RPMI + 10% FBS. Human OC precursors were also coincubated with neutrophils in 96-well plates (250 000-2.5 × 106/200 μL per well), or with purified membranes from activated neutrophils (cellular equivalent of 250 000 to 1 million cells). After 10 days, cells were fixed for TRAP cytochemistry. Similar assays were performed with mature OCs (100 000 cells/well) on Osteologic discs and dentine. After an additional 10 days of culture, discs were evaluated for the presence of resorption pits. Resorption tests were also performed by culturing human OC precursors with conditioned medium from 106 activated neutrophils/well supplemented with 25 ng/mL M-CSF.
Murine neutrophils from air pouches were coincubated with RAW 264.7 cells (100 000-500 000 murine neutrophils/well). Controls were performed with or without exogenous mRANKL (40 ng/mL). Coincubations were continued in 96-well plates for 5 days for TRAP assays and for 10 days on Osteologic discs for resorption.
RA-SF neutrophils and normal neutrophils preincubated in RA-SF or OA-SF were fixed with 2% PFA and coincubated (1 million neutrophils/well) for 10 days with human OC precursors for TRAP and resorption.
Tartrate-resistant acid phosphatase cytochemistry
Tartrate-resistant acid phosphatase (TRAP) was studied following the manufacturer's instructions (Sigma-Aldrich). Cells in culture were washed, fixed with acetone-citrate solution, and stained with 40 mM sodium tartrate. Multinucleated cells exhibiting TRAP activity, as intense cytoplasmic red staining under light microscopy, were considered as differentiated OCs.
Bone resorption assay
After 10 days of culture of mature human OCs (100 000 cells/well) on calcified matrix, discs were washed with 10% sodium hypochlorite solution to remove the cells. Resorption area was evaluated by light microscopy and measured using the image analysis software Imagepro (Media Cybernetics).
Adherence of neutrophils to OCs
Mature human OCs were stained with 2 μM CellTracker Orange CMTMR (Invitrogen) for 30 minutes at 37°C. Human neutrophils, stained with 4 μM PKH-67 green fluorescent cell-linker (Sigma-Aldrich) for 2 minutes at 20°C, were added to confluent OCs (2.5 × 106 cells/mL) and incubated for 1 hour at 37°C followed by 3 vigorous washes. Confocal microscopy analyses were performed with Olympus Fluoview 300 microscope using argon-ion (488 nm) and helium-neon (543 nm) lasers. Forty-three slices of 0.5 μm were scanned (magnification, ×60, plan Apo, NA 1.4) with a Kalman filter 3 to reconstitute z plans. Tests of adherence were also performed with neutrophils (107 cells/mL) prelabeled with 1 μg calcein-AM/mL (Invitrogen) for 30 minutes at 37°C.
Signaling studies
Human neutrophils (20 × 106/mL) were incubated with rhOPG (1 μg/mL) and rhRANK polypeptide (1 μg/mL) at 37°C for 30 minutes. Cells were also preincubated (15 minutes, 37°C) with the Src-kinase inhibitor PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)-pyrazolo[3,4-d] pyrimidine) and its inactive analog PP3 (Calbiochem). Phosphorylation patterns in whole cells were determined as described.22 Membranes were blotted with primary antiphosphotyrosine mAb (UB1 05-321 Clone 4G-10; Upstate Biotechnology Inc) and secondary HRP-labeled goat anti–mouse IgG. Membranes were washed and protein bands were revealed using the enhanced chemiluminescence detection system. Membranes were reblotted with a monoclonal anti-Lyn Ab (sc-7274; Santa Cruz Biotechnology).23 Similar neutrophil incubations were performed for immunoprecipitation studies on cell lysates, as previously described.24 After 24-hour incubation, the production of IL-1β and IL-8 was also evaluated in supernatants by enzyme-linked immunosorbent assay (ELISA; Cayman Chemicals and Biosource International, respectively).
Statistics
Statistical analyses were performed using Instat 3.0 (GraphPad Software Inc). Analyses used the paired or unpaired t test, and the one-way ANOVA followed by Newman-Keuls multiple comparison posttest. Significance was set at P value less than .05.
Results
PLB-985 neutrophil-like cells express RANKL and induce osteoclastogenesis
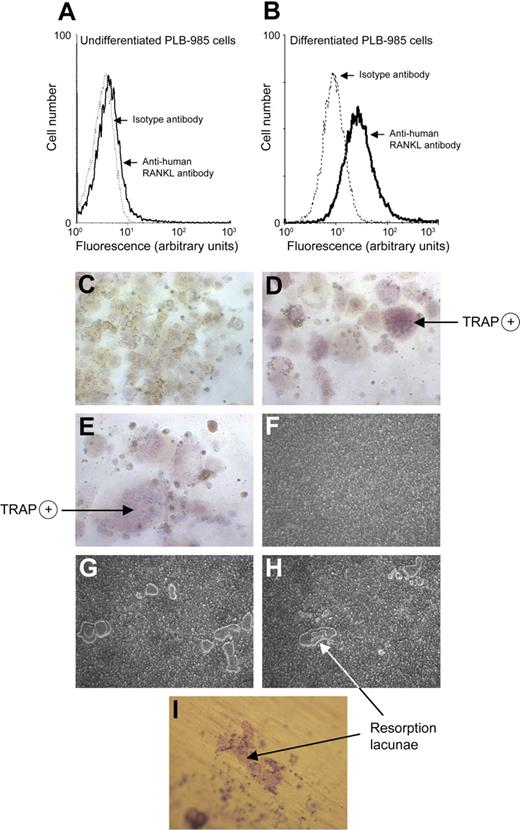
The human myeloid PLB-985 cell line differentiated with dbcAMP allows the study of neutrophil functions.25 RANKL expression was detected at the surface of 7.4% plus or minus 2.3% undifferentiated PLB-985 cells (Figure 1A), whereas 39.7% plus or minus 7.8% dbcAMP-differentiated PLB-985 cells expressed surface RANKL (Figure 1B). Could such differentiated PLB-985 cells induce OC maturation and activity through surface RANKL? Osteoclasts derived from fusion of monocyte-macrophages are characterized by numerous nuclei and acquisition of TRAP activity.26 To determine the functional effect of surface RANKL in PLB-985 cells, we used an in vitro coculture system of human blood monocytes with dbcAMP-differentiated PLB-985 cells fixed with 2% PFA. Monocytes with M-CSF alone were mononucleated and TRAP negative (Figure 1C), but when incubated with rhRANKL they became multinucleated cells with a positive TRAP reaction (Figure 1D). Similarly, coculture of monocytes with dbcAMP-differentiated PLB-985 cells induced the appearance of large multinucleated cells positive for TRAP (Figure 1E).
PLB-985 neutrophil–like cells express RANKL and mediate monocyte maturation into OCs. Flow cytometric analysis of RANKL expression by undifferentiated PLB-985 cells (A) and by PLB-985 cells differentiated for 48 hours with dbcAMP (B). Cells were incubated with an anti–human RANKL mAb followed by a FITC-conjugated anti–mouse F(ab′)2 antibody. Isotype controls are dotted tracings. Results are representative of 5 independent experiments. Monocytes isolated from peripheral blood of healthy human donors were incubated (C) with M-CSF (25 ng/mL) alone, (D) with M-CSF + rhRANKL (40 ng/mL), and (E) with M-CSF + PLB-985 cells (106/well) differentiated for 48 hours with dbcAMP and subsequently fixed with 2% PFA. After 10 days of incubation, cells were stained for TRAP to evaluate TRAP-positive multinucleated cells by light microscopy. (C-E) Magnification, ×200. Mature OCs (100 000/well) on Osteologic discs were incubated (F) with M-CSF (25 ng/mL) alone, (G) with addition of PFA-fixed PLB-985 neutrophil-like cells, and (H) with addition of rhRANKL (40 ng/mL). Cells were removed after 10 days of incubation and bone resorption was evaluated by light microscopy and Imagepro software. (I) Mature OCs deprived of exogenous RANKL were incubated on dentine discs with PFA-fixed PLB-985 neutrophil-like cells. After 10 days, dentine discs were stained with toluidine blue (0.5%). Results were reproduced in 3 independent experiments. (F-I) Magnification, × 100.
PLB-985 neutrophil–like cells express RANKL and mediate monocyte maturation into OCs. Flow cytometric analysis of RANKL expression by undifferentiated PLB-985 cells (A) and by PLB-985 cells differentiated for 48 hours with dbcAMP (B). Cells were incubated with an anti–human RANKL mAb followed by a FITC-conjugated anti–mouse F(ab′)2 antibody. Isotype controls are dotted tracings. Results are representative of 5 independent experiments. Monocytes isolated from peripheral blood of healthy human donors were incubated (C) with M-CSF (25 ng/mL) alone, (D) with M-CSF + rhRANKL (40 ng/mL), and (E) with M-CSF + PLB-985 cells (106/well) differentiated for 48 hours with dbcAMP and subsequently fixed with 2% PFA. After 10 days of incubation, cells were stained for TRAP to evaluate TRAP-positive multinucleated cells by light microscopy. (C-E) Magnification, ×200. Mature OCs (100 000/well) on Osteologic discs were incubated (F) with M-CSF (25 ng/mL) alone, (G) with addition of PFA-fixed PLB-985 neutrophil-like cells, and (H) with addition of rhRANKL (40 ng/mL). Cells were removed after 10 days of incubation and bone resorption was evaluated by light microscopy and Imagepro software. (I) Mature OCs deprived of exogenous RANKL were incubated on dentine discs with PFA-fixed PLB-985 neutrophil-like cells. After 10 days, dentine discs were stained with toluidine blue (0.5%). Results were reproduced in 3 independent experiments. (F-I) Magnification, × 100.
Cocultures were then performed with mature human OCs to verify whether differentiated PLB-985 cells could stimulate bone resorption by RANKL-deprived OCs. Minimal bone resorption activity was observed in wells where mature OCs were incubated without RANKL (Figure 1F). Addition of differentiated PLB-985 cells induced the formation of resorption pits (Figure 1G). Pit appearance resembled that seen with mature OCs incubated with rhRANKL (Figure 1H). Thus, PLB-985 neutrophil-like cells can induce the transformation of OC precursors into mature OCs and stimulate the resorption function of mature OCs. Resorption lacunae with characteristic tracks were also observed when OCs and PLB-985 cells were cocultured on dentine slices (Figure 1I).
Activation of OCs with neutrophil-like PLB-985 cells is reduced by RANKL-directed antisense RNA
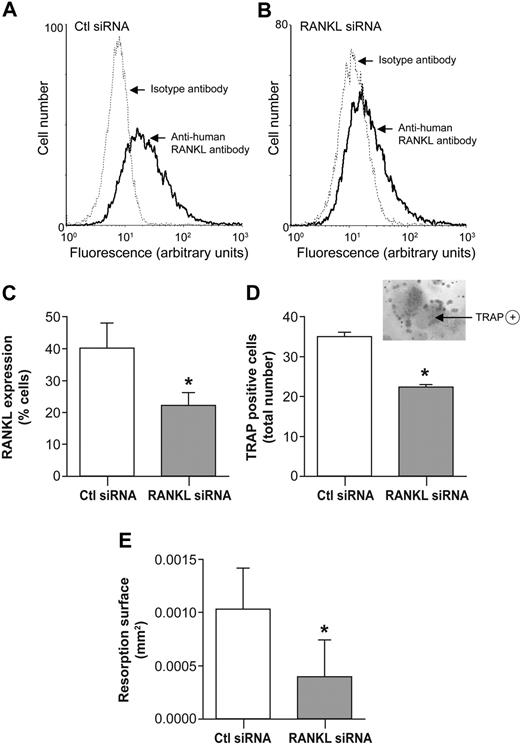
Because neutrophil-like PLB-985 cells can be transfected,27 gene silencing was performed by transfection using RNA interference. Neutrophil-like PLB-985 cells showed a reduction of RANKL-expressing cells in PLB-985 cells transfected with RANKL antisense RNA, compared with cells transfected with nonsilencing antisense RNA (Figure 2A-B). RANKL antisense RNA decreased RANKL-expressing cells by 43.8% plus or minus 1.9% in comparison with control (nonsilencing) antisense RNA (Figure 2C). Cocultures of human OCs with PLB-985 cells transfected with RANKL antisense RNA were associated with a significant reduction of osteoclastogenesis. Knockdown of RANKL in PLB-985 cells inhibited the differentiation of monocytes into OCs, as evaluated by the number of TRAP-positive cells. This number decreased by 35.7% (± 0.1%; Figure 2D). Knockdown of RANKL also reduced the formation of resorption pits by OCs cocultured with transfected PLB-985 cells, by 53.9% (± 11.1%), compared with nonsilencing antisense RNA-transfected control cells (Figure 2E).
RANKL knockdown mediated by antisense RNA in PLB-985 neutrophil-like cells inhibits osteoclastogenesis. Flow cytometric analysis of RANKL expression by dbcAMP-differentiated PLB-985 cells transfected (A) with control nonsilencing antisense RNA (Ctl siRNA), or (B) with RANKL antisense RNA (RANKL siRNA). RANKL expression was examined 48 hours after transfection by incubating cells with an anti–human RANKL mAb followed by an FITC-conjugated anti–mouse F(ab′)2 antibody. Isotype controls are dotted tracings. (C) Histograms represent means (± SEM) of percentage of cells expressing RANKL (n = 3 independent experiments). (D) PLB-985 neutrophil-like cells (106/well) transfected with Ctl antisense RNA or RANKL antisense RNA for 48 hours were fixed with 2% PFA, repeatedly washed, and added to cultures of human peripheral blood monocytes in 96-well plates. After 10 days, TRAP-positive multinucleated cells were counted. Results are expressed as means (± SEM) of the number of multinucleated TRAP-positive cells/well (n = 3 independent duplicated experiments). (Inset) TRAP-positive multinucleated cell obtained after cocultures of human peripheral blood monocytes with transfected dbcAMP-differentiated PLB-985 cells (magnification, ×200). (E) PFA-fixed PLB-985 neutrophil-like cells transfected with Ctl antisense RNA or RANKL antisense RNA, were added to mature OCs on Osteologic discs. After 10 days of cocultures, cells were removed and surfaces of resorption (mm2) were measured by light microscopy using Imagepro software. Results are expressed as means (± SEM) of resorption area (n = 3 independent duplicated experiments). Statistical analysis: paired t test, *P < .05 (Ctl antisense RNA vs RANKL antisense RNA).
RANKL knockdown mediated by antisense RNA in PLB-985 neutrophil-like cells inhibits osteoclastogenesis. Flow cytometric analysis of RANKL expression by dbcAMP-differentiated PLB-985 cells transfected (A) with control nonsilencing antisense RNA (Ctl siRNA), or (B) with RANKL antisense RNA (RANKL siRNA). RANKL expression was examined 48 hours after transfection by incubating cells with an anti–human RANKL mAb followed by an FITC-conjugated anti–mouse F(ab′)2 antibody. Isotype controls are dotted tracings. (C) Histograms represent means (± SEM) of percentage of cells expressing RANKL (n = 3 independent experiments). (D) PLB-985 neutrophil-like cells (106/well) transfected with Ctl antisense RNA or RANKL antisense RNA for 48 hours were fixed with 2% PFA, repeatedly washed, and added to cultures of human peripheral blood monocytes in 96-well plates. After 10 days, TRAP-positive multinucleated cells were counted. Results are expressed as means (± SEM) of the number of multinucleated TRAP-positive cells/well (n = 3 independent duplicated experiments). (Inset) TRAP-positive multinucleated cell obtained after cocultures of human peripheral blood monocytes with transfected dbcAMP-differentiated PLB-985 cells (magnification, ×200). (E) PFA-fixed PLB-985 neutrophil-like cells transfected with Ctl antisense RNA or RANKL antisense RNA, were added to mature OCs on Osteologic discs. After 10 days of cocultures, cells were removed and surfaces of resorption (mm2) were measured by light microscopy using Imagepro software. Results are expressed as means (± SEM) of resorption area (n = 3 independent duplicated experiments). Statistical analysis: paired t test, *P < .05 (Ctl antisense RNA vs RANKL antisense RNA).
RANKL expressed at the surface of human blood neutrophils is modulated by TLR ligands
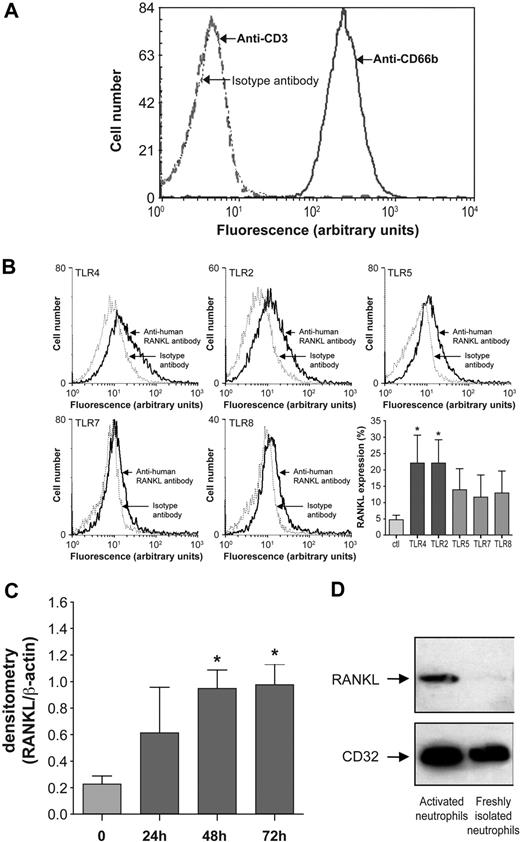
The low RANKL expression by human blood neutrophils and the strong expression of RANKL in neutrophils from RA-SF15 suggest that neutrophils could be up-regulated by local factors. Toll-like receptor (TLR) ligands are effectors present in RA-SF,28,29 and neutrophils respond to TLR agonists.30 To determine whether TLR ligands could modulate RANKL expression by neutrophils, normal human blood neutrophils were incubated with the following TLR agonists: Pam3Cys-Ser-(Lys)4 hydrochloride (TLR2),31 LPS (TLR4),32 flagellin (TLR5),33 and gardiquimod at 0.1 μg/mL and at 1 μg/mL (TLR7 and TLR7/TLR8, respectively).34,35 Because mononuclear leukocytes can express RANKL, the purity of neutrophil preparations was analyzed by cell surface expression of CD66b (a specific neutrophil marker)19 and of CD3 and CD36 (specific for T lymphocytes and monocytes, respectively). After purification, 98% neutrophils were positive for CD66b with less than 2% of cells expressing CD3 (Figure 3A). Expression of CD36 was absent (data not shown). Surface RANKL was expressed by less than 5% of normal human blood neutrophils, as previously reported.15 The number of RANKL-expressing neutrophils was significantly and similarly augmented by TLR4 and TLR2 agonists (4.7- and 4.5-fold, respectively). Interestingly, TLR4 and TLR2 have been shown to have interdependent expression and signaling.36,37 The number of RANKL-expressing neutrophils was also increased by TLR5 (3-fold) and TLR7 agonist (2.5-fold), whereas TLR8 (2.7-fold) was not involved (as revealed by gardiquimod at 1 μg; Figure 3B). Neutrophils incubated with LPS exhibited increased levels of RANKL mRNA after 48 hours (Figure 3C), and their membranes have a robust RANKL protein signal (Figure 3D). Note that LPS-activated neutrophils did not release any soluble RANKL to the supernatant (evaluated by ELISA, detection limit = 15 pg/mL), as reported for RA-SF neutrophils.15
Expression of RANKL by human blood neutrophils. (A) Purity of neutrophils isolated from healthy human subjects. Flow cytometry was performed after incubation of neutrophils with FITC-conjugated mouse anti–human CD66b (blue line), and FITC-conjugated mouse anti–human CD3 (red dotted line). Isotype controls (black dotted line) were performed with equal concentrations of corresponding mouse IgG1. Results shown are representative of neutrophils from 10 healthy subjects. (B) Flow cytometry analysis of RANKL expression by neutrophils incubated with the TLR4 agonist LPS (100 ng/mL), the TLR2 agonist Pam3Cys-Ser-(Lys)4 hydrochloride (1 μg/mL), the TLR5 agonist flagellin (500 ng/mL), and the TLR7 and TLR 8 agonist gardiquimod (at 0.1 μg/mL and 1 μg/mL, respectively) for 48 hours. After incubation, neutrophils were treated with an anti–human RANK-L mAb followed by a FITC-conjugated anti–mouse F(ab′)2 antibody. Isotype controls (dotted line) were performed for each condition. Results are representative of 3 independent experiments. Histograms represent means (± SEM) of the percentage of cells expressing RANKL; control cells (Ctl) are freshly isolated neutrophils (n = 3). Statistical analysis: unpaired t test, *P < .05 (TLR vs Ctl). (C) Expression of RANKL mRNA by human blood neutrophils incubated with 100 ng/mL LPS for 24, 48, and 72 hours. RNA extraction and semiquantitative reverse-transcription–PCR were performed for freshly isolated neutrophils (0) and LPS-stimulated neutrophils; β-actin gene served as an internal control. Histograms represent means (± SEM) of ratios of densitometric values for RANKL/β-actin (n = 3). Student paired t test: *P < .05 (LPS-stimulated vs freshly isolated neutrophils). (D) RANKL is detected in membranes of LPS-activated neutrophils by Western blotting with a primary mouse anti–human RANKL IgG Ab and a secondary HRP-conjugated anti–mouse Ab. Membranes correspond to 500 000 freshly isolated or LPS-activated neutrophils. CD32 serves to verify the integrity of neutrophil membranes.
Expression of RANKL by human blood neutrophils. (A) Purity of neutrophils isolated from healthy human subjects. Flow cytometry was performed after incubation of neutrophils with FITC-conjugated mouse anti–human CD66b (blue line), and FITC-conjugated mouse anti–human CD3 (red dotted line). Isotype controls (black dotted line) were performed with equal concentrations of corresponding mouse IgG1. Results shown are representative of neutrophils from 10 healthy subjects. (B) Flow cytometry analysis of RANKL expression by neutrophils incubated with the TLR4 agonist LPS (100 ng/mL), the TLR2 agonist Pam3Cys-Ser-(Lys)4 hydrochloride (1 μg/mL), the TLR5 agonist flagellin (500 ng/mL), and the TLR7 and TLR 8 agonist gardiquimod (at 0.1 μg/mL and 1 μg/mL, respectively) for 48 hours. After incubation, neutrophils were treated with an anti–human RANK-L mAb followed by a FITC-conjugated anti–mouse F(ab′)2 antibody. Isotype controls (dotted line) were performed for each condition. Results are representative of 3 independent experiments. Histograms represent means (± SEM) of the percentage of cells expressing RANKL; control cells (Ctl) are freshly isolated neutrophils (n = 3). Statistical analysis: unpaired t test, *P < .05 (TLR vs Ctl). (C) Expression of RANKL mRNA by human blood neutrophils incubated with 100 ng/mL LPS for 24, 48, and 72 hours. RNA extraction and semiquantitative reverse-transcription–PCR were performed for freshly isolated neutrophils (0) and LPS-stimulated neutrophils; β-actin gene served as an internal control. Histograms represent means (± SEM) of ratios of densitometric values for RANKL/β-actin (n = 3). Student paired t test: *P < .05 (LPS-stimulated vs freshly isolated neutrophils). (D) RANKL is detected in membranes of LPS-activated neutrophils by Western blotting with a primary mouse anti–human RANKL IgG Ab and a secondary HRP-conjugated anti–mouse Ab. Membranes correspond to 500 000 freshly isolated or LPS-activated neutrophils. CD32 serves to verify the integrity of neutrophil membranes.
From these tests, the TLR4 agonist LPS was chosen to increase RANKL-expressing neutrophils. In addition, the concomitant presence of GM-CSF in medium increased neutrophil viability as evaluated by PI exclusion (12.2% ± 1.1% viable cells in medium alone, 33.8% ± 8.9% viable cells with LPS alone, and 56.5% ± 5.4% viable cells with LPS and GM-CSF, as previously reported).16,38 After 48 hours of incubation followed by removal of cell debris and dead cells, 90% to 95% of Percoll-enriched neutrophils were viable cells (PI exclusion test). These neutrophils were used for subsequent functional assays and are referred to in the text as activated neutrophils.
Activated human neutrophils induce osteoclastogenesis
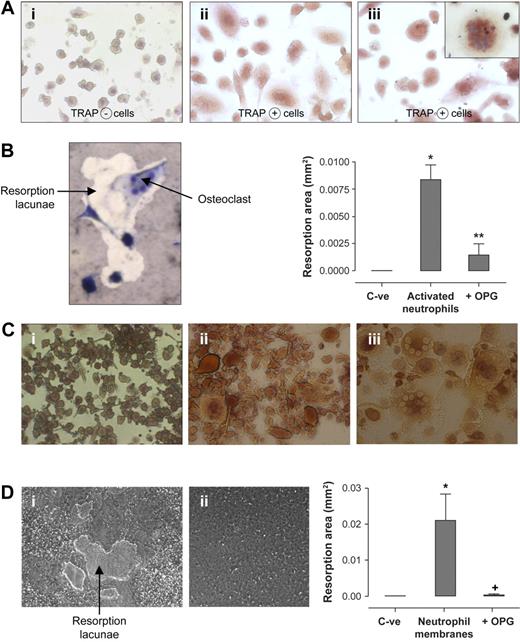
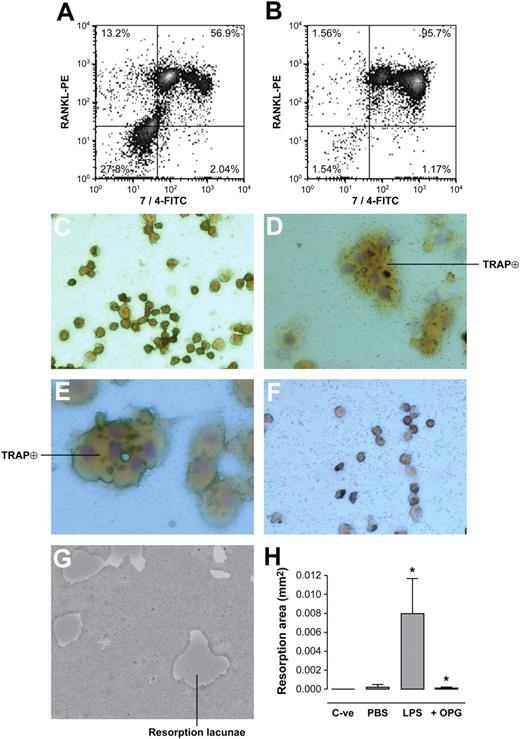
Could activated neutrophils, which are in the vicinity of developing and mature OCs at sites of inflammatory bone resorption, stimulate osteoclastogenesis via surface RANKL? Activated neutrophils in the absence of exogenous RANKL induced the appearance of large TRAP-positive multinucleated cells and bone resorption (Figure 4). These experiments harnessed the capacity of normal human OC precursors from the monocytic fraction of PBMCs to differentiate into mature OCs in the presence of RANKL. After 10 days of differentiation, monocytes cultured with M-CSF alone were TRAP negative (Figure 4Ai); monocytes cultured with activated neutrophils (106 cells/well) were differentiated into large TRAP-positive multinucleated cells (Figure 4Aiii) and resembled cells cultured with the addition of exogenous RANKL (Figure 4Aii). Activated neutrophils added to RANKL-deprived mature OCs also stimulated the formation of pits in the calcified matrix. Resorption lacunae generated by functional OCs cultured with activated neutrophils are presented in Figure 4B. RANKL-deprived mature OCs (C-ve) did not generate resorption pits. When activated neutrophils were preincubated with OPG, the resorptive activity of OCs was significantly diminished (histogram, Figure 4B). This neutralization of OC function demonstrated that surface RANKL in activated neutrophils was responsible for OC activation.
Activated human neutrophils mediate functional osteoclastogenesis from human monocyte precursors. (A) TRAP expression by osteoclast precursors in culture with activated neutrophils: human monocytes were incubated with (i) M-CSF (25 ng/mL), (ii) M-CSF + exogenous rhRANKL (40 ng/mL), and (iii) M-CSF + LPS-activated neutrophils (106/well). After 10 days, TRAP-positive multinucleated cells were observed with light microscope. Results were reproduced in 3 independent experiments. (i-iii) Magnification, × 200. (Inset, iii): Enlarged view of a TRAP-positive multinucleated cell in coincubation with activated neutrophils (magnification, ×400). (B) Formation of resorption pits in neutrophil-osteoclast cocultures: mature OCs deprived of exogenous RANKL were incubated on Osteologic discs for 10 days in the presence of activated neutrophils and were then stained by toluidine blue vital coloration. Osteoclasts (multinucleated giant cells) are visualized in the interior and in the periphery of the resorption lacunae. Histograms represent total resorption area by mature human OCs incubated on Osteologic discs in the absence of exogenous RANKL and activated neutrophils (C-ve), and in the presence of activated neutrophils (106/well) with or without 2 μg/mL recombinant OPG. Results are expressed in square millimeter (mm2) of resorption area (mean ± SEM, n = 3 independent experiments). Statistical analysis: unpaired t test, *P < .002, **P < .003 (activated neutrophils vs C-ve; activated neutrophils vs activated neutrophils + OPG). (C) TRAP expression by osteoclast precursors in culture with neutrophil membranes: monocytes were incubated with (i) M-CSF (25 ng/mL), (ii) M-CSF + exogenous rhRANKL (40 ng/mL), and (iii) M-CSF + membranes of LPS-activated neutrophils added at a cellular equivalent of 500 000 cells/well. After 10 days of cultures, TRAP-positive cells were observed with light microscope (i-iii: magnification, ×200). Results were reproduced in 3 independent experiments. (D) Formation of resorption pits in cultures of osteoclasts with neutrophil membranes: mature OCs without exogenous RANKL were incubated on Osteologic discs with (i) membranes of activated neutrophils added at a cellular equivalent of 500 000 cells/well, and (ii) conditioned medium from activated neutrophils (200 μL/well). Formation of resorption pits was evaluated after an additional 10 days of coculture. Histograms represent total resorption area by mature human OCs incubated on Osteologic discs in the absence of exogenous RANKL and neutrophil membranes (C-ve), and in the presence of membranes from activated neutrophils (cellular equivalent of 500 000 cells/well) with or without 2 μg/mL recombinant OPG. Results are expressed in square millimeter (mm2) of resorption area (mean ± SEM, n = 3 independent experiments). Statistical analysis: Student unpaired t test, *P < .02, +P < .02 (neutrophil membranes vs C-ve; neutrophil membranes vs neutrophil membranes + OPG).
Activated human neutrophils mediate functional osteoclastogenesis from human monocyte precursors. (A) TRAP expression by osteoclast precursors in culture with activated neutrophils: human monocytes were incubated with (i) M-CSF (25 ng/mL), (ii) M-CSF + exogenous rhRANKL (40 ng/mL), and (iii) M-CSF + LPS-activated neutrophils (106/well). After 10 days, TRAP-positive multinucleated cells were observed with light microscope. Results were reproduced in 3 independent experiments. (i-iii) Magnification, × 200. (Inset, iii): Enlarged view of a TRAP-positive multinucleated cell in coincubation with activated neutrophils (magnification, ×400). (B) Formation of resorption pits in neutrophil-osteoclast cocultures: mature OCs deprived of exogenous RANKL were incubated on Osteologic discs for 10 days in the presence of activated neutrophils and were then stained by toluidine blue vital coloration. Osteoclasts (multinucleated giant cells) are visualized in the interior and in the periphery of the resorption lacunae. Histograms represent total resorption area by mature human OCs incubated on Osteologic discs in the absence of exogenous RANKL and activated neutrophils (C-ve), and in the presence of activated neutrophils (106/well) with or without 2 μg/mL recombinant OPG. Results are expressed in square millimeter (mm2) of resorption area (mean ± SEM, n = 3 independent experiments). Statistical analysis: unpaired t test, *P < .002, **P < .003 (activated neutrophils vs C-ve; activated neutrophils vs activated neutrophils + OPG). (C) TRAP expression by osteoclast precursors in culture with neutrophil membranes: monocytes were incubated with (i) M-CSF (25 ng/mL), (ii) M-CSF + exogenous rhRANKL (40 ng/mL), and (iii) M-CSF + membranes of LPS-activated neutrophils added at a cellular equivalent of 500 000 cells/well. After 10 days of cultures, TRAP-positive cells were observed with light microscope (i-iii: magnification, ×200). Results were reproduced in 3 independent experiments. (D) Formation of resorption pits in cultures of osteoclasts with neutrophil membranes: mature OCs without exogenous RANKL were incubated on Osteologic discs with (i) membranes of activated neutrophils added at a cellular equivalent of 500 000 cells/well, and (ii) conditioned medium from activated neutrophils (200 μL/well). Formation of resorption pits was evaluated after an additional 10 days of coculture. Histograms represent total resorption area by mature human OCs incubated on Osteologic discs in the absence of exogenous RANKL and neutrophil membranes (C-ve), and in the presence of membranes from activated neutrophils (cellular equivalent of 500 000 cells/well) with or without 2 μg/mL recombinant OPG. Results are expressed in square millimeter (mm2) of resorption area (mean ± SEM, n = 3 independent experiments). Statistical analysis: Student unpaired t test, *P < .02, +P < .02 (neutrophil membranes vs C-ve; neutrophil membranes vs neutrophil membranes + OPG).
Because supernatants of activated neutrophils contained no soluble RANKL and RANKL was detected at the surface of neutrophils, OCs were then cultured with the membrane fraction from activated neutrophils. Dose-response curves were performed to determine optimal cellular equivalents for induction of osteoclastogenesis (results not shown). Controls were normal human OC precursors cultured with or without exogenous RANKL (Figure 4Ci-ii). Addition of membranes from activated neutrophils at an equivalent of 500 000 cells/well to OC precursors stimulated the appearance of TRAP-positive cells (Figure 4Ciii). These membranes also stimulated mature OCs to resorb calcified matrix without exogenous RANKL (Figure 4Di). This resorptive activity was reversed by concomitant addition of OPG (histogram, Figure 4D). In contrast, conditioned medium from activated neutrophils added to OC did not induce any resorption (Figure 4Dii).
Resorption was also observed when activated neutrophils fixed with 2% PFA were added to OCs (resorption area: 0.041 ± 0.014 mm2, mean ± SEM, n = 3). OC activity induced by fixed neutrophils was also almost completely reversed by addition of rhOPG (resorption area: 0.0032 ± 0.0004 mm2, n = 3). These results confirmed that the membrane-bound RANKL of activated neutrophils was functional and responsible for their osteoclastogenic properties.
Mouse neutrophils transmigrating into LPS-injected air pouch express RANKL
Because LPS injected into an air pouch in mice activated the migration of neutrophils from blood to the air pouch, this model of inflammation was used to determine whether neutrophils activated by LPS in vivo would increase their expression of RANKL. Cells positive for the FITC-conjugated 7/4 mAb specific for mouse neutrophils increased from 56% to 96% in exudates of PBS- and LPS-injected air pouches, respectively (Figure 5A-B). Thus, cellular exudates from LPS-stimulated air pouches were almost entirely composed of neutrophils. Note that the anti–mouse neutrophil 7/4 antibody detected 2 subpopulations of transmigrated neutrophils in both conditions of the experiment. Moreover, LPS-activated neutrophils showed a shift in fluorescence and granularity (Figure 5B), suggesting an important difference between the phenotype of neutrophils that transmigrated in the presence of PBS compared with LPS. More than 95% of neutrophils from LPS-stimulated air pouches were positive for the PE-labeled anti–mouse RANKL mAb (Figure 5B). In addition, a 52% increase in total RANKL expression (percentage of 7/4-positive neutrophils expressing RANKL × fluorescent intensity per cell) was observed in mouse neutrophils migrating into an LPS-stimulated air pouch compared with a vehicle-stimulated air pouch.
Neutrophils from LPS-injected mouse air pouch express RANKL and mediate functional osteoclastogenesis from murine monocyte-macrophage RAW264.7 cells. Cells obtained from (A) PBS-injected and (B) LPS-injected air pouches were labeled with a FITC-conjugated anti–mouse neutrophil antibody (clone 7/4), and PE-conjugated anti-mRANKL antibody simultaneously. Nonspecific sites were blocked by murine Fc block for 1 hour. Graphs represent flow cytometric analysis of pooled neutrophils from 5 mice/group and are representative of 3 such groups. Percentages in each quadrant correspond to numbers of cells present in each quadrant relative to 10 000 cells counted. RAW264.7 mononuclear cells were incubated (C) with medium (DMEM + 10% FBS) alone, (D) with medium + 20 ng/mL recombinant murine RANKL, (E) with medium + murine neutrophils (2.5 × 105/well) from LPS-injected air pouches, and (F) with medium + murine neutrophils (2.5 × 105/well) from LPS-injected air pouches and preincubated with 1 μg/mL recombinant OPG for 1 hour at 37°C. After 5 days of incubation, TRAP-positive multinucleated cells were analyzed by light microscopy (magnification, ×200). Results shown are representative of 3 independent experiments. (G) RAW264.7 cells (2 × 104/well) deposited on Osteologic discs were incubated with murine neutrophils (2.5 × 105/well) from LPS-injected air pouches. Cells were removed after 10 days of culture and resorption lacunae were observed under light microscopy (magnification, ×100). (H) Histograms represent total resorption area by RAW264.7 cells incubated in the absence of exogenous RANKL and neutrophils (C-ve), and in the presence of murine neutrophils (2.5 × 105/well) from PBS- or LPS-injected air pouches with or without 1 μg/mL recombinant OPG. Results are expressed in square millimeter (mm2) of resorption area (mean ± SEM, pool of 5 mice/group representative of 4 such groups). Statistical analysis: unpaired t test, *P < .05 (LPS vs PBS; LPS vs + OPG).
Neutrophils from LPS-injected mouse air pouch express RANKL and mediate functional osteoclastogenesis from murine monocyte-macrophage RAW264.7 cells. Cells obtained from (A) PBS-injected and (B) LPS-injected air pouches were labeled with a FITC-conjugated anti–mouse neutrophil antibody (clone 7/4), and PE-conjugated anti-mRANKL antibody simultaneously. Nonspecific sites were blocked by murine Fc block for 1 hour. Graphs represent flow cytometric analysis of pooled neutrophils from 5 mice/group and are representative of 3 such groups. Percentages in each quadrant correspond to numbers of cells present in each quadrant relative to 10 000 cells counted. RAW264.7 mononuclear cells were incubated (C) with medium (DMEM + 10% FBS) alone, (D) with medium + 20 ng/mL recombinant murine RANKL, (E) with medium + murine neutrophils (2.5 × 105/well) from LPS-injected air pouches, and (F) with medium + murine neutrophils (2.5 × 105/well) from LPS-injected air pouches and preincubated with 1 μg/mL recombinant OPG for 1 hour at 37°C. After 5 days of incubation, TRAP-positive multinucleated cells were analyzed by light microscopy (magnification, ×200). Results shown are representative of 3 independent experiments. (G) RAW264.7 cells (2 × 104/well) deposited on Osteologic discs were incubated with murine neutrophils (2.5 × 105/well) from LPS-injected air pouches. Cells were removed after 10 days of culture and resorption lacunae were observed under light microscopy (magnification, ×100). (H) Histograms represent total resorption area by RAW264.7 cells incubated in the absence of exogenous RANKL and neutrophils (C-ve), and in the presence of murine neutrophils (2.5 × 105/well) from PBS- or LPS-injected air pouches with or without 1 μg/mL recombinant OPG. Results are expressed in square millimeter (mm2) of resorption area (mean ± SEM, pool of 5 mice/group representative of 4 such groups). Statistical analysis: unpaired t test, *P < .05 (LPS vs PBS; LPS vs + OPG).
Neutrophils from LPS-injected air pouch activate the mouse RAW 264.7 cells to become OCs
The mononuclear RAW264.7 cells cultured 5 days without RANKL remained unchanged (Figure 5C). They became large and multinucleated TRAP-positive cells with mRANKL (Figure 5D) or in cocultures with activated neutrophils from LPS-stimulated air pouches (Figure 5E). The transformation of RAW264.7 cells into OC-like cells was abrogated when neutrophils preincubated with OPG were added to RAW264.7 cells (Figure 5F). Murine neutrophils from LPS-injected air pouches significantly stimulated resorptive activity of RAW 264.7 cells, with formation of resorption pits (Figure 5G). Cells transmigrating into vehicle-injected air pouches stimulated negligible bone resorption, with the presence of a few zones of partial bone resorption. Preincubation of activated neutrophils with OPG almost completely abrogated resorptive activity of RAW 264.7 cells (Figure 5H). Thus, neutralization of the osteoclastogenic effects of mouse neutrophils with OPG showed that neutrophils from LPS-injected air pouches express functional surface RANKL.
Adhesion of neutrophils to OCs
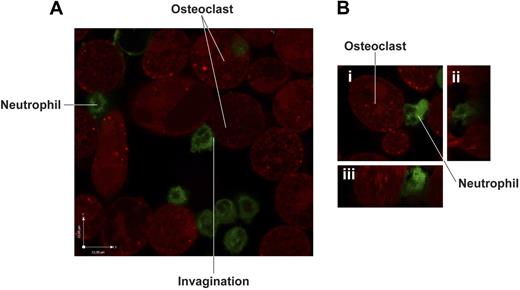
Because RANKL is not implicated in heterotypic adhesion to OCs39 and activated neutrophils release no soluble RANKL, effective cell surface interactions require direct contact between OCs and neutrophils. When examined by live-cell confocal microscopy, activated neutrophils showed a firm adhesion to OCs (Figure 6). Membranes of neutrophils in contact with OCs displayed invaginations and expansions, and the contact zone of the neutrophil membrane took on the contour of its adherent counterpart. This confirms the existence of direct contact between the 2 cell types. Although both freshly isolated neutrophils and activated neutrophils adhered to OCs, adhesion of freshly isolated neutrophils to OCs was, however, significantly reduced compared with adhesion of activated neutrophils to OCs (1.6 ± 0.1 × 106 neutrophils/106 OCs vs 2.0 ± 0.1 × 106 neutrophils/106 OCs, P < .05, n = 3).
Confocal microscopy analysis of the interaction between activated neutrophils and human monocyte-derived OCs. (A) LPS-activated human neutrophils prelabeled with PKH67 green fluorescent linker were incubated for 1 hour at 37°C with mature OCs previously labeled with CellTracker Orange CMTMR. Cells were washed vigorously to eliminate nonadherent neutrophils and visualized by live-confocal microscopy using Olympus Fluoview microscope (magnification, ×1500). (B) Details of cell-cell contact between activated neutrophils and OCs: (i) stacking view; (ii) z plan of y-axis; (iii) z plan of x-axis.
Confocal microscopy analysis of the interaction between activated neutrophils and human monocyte-derived OCs. (A) LPS-activated human neutrophils prelabeled with PKH67 green fluorescent linker were incubated for 1 hour at 37°C with mature OCs previously labeled with CellTracker Orange CMTMR. Cells were washed vigorously to eliminate nonadherent neutrophils and visualized by live-confocal microscopy using Olympus Fluoview microscope (magnification, ×1500). (B) Details of cell-cell contact between activated neutrophils and OCs: (i) stacking view; (ii) z plan of y-axis; (iii) z plan of x-axis.
Activation of the neutrophil membrane-bound receptor RANKL is associated with phosphorylation of the SHP-1 protein tyrosine phosphatase and down-regulation of cytokine production
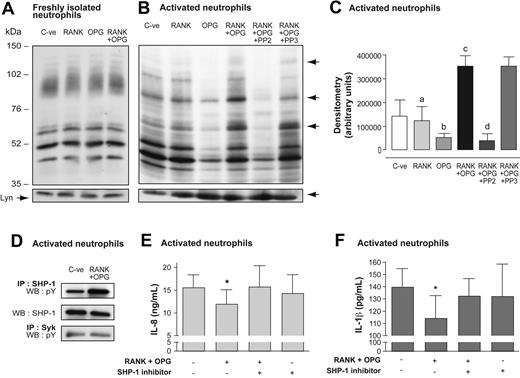
Because protein tyrosine phosphorylation is a hallmark of neutrophil activation, stimulation of surface RANKL could potentially induce protein phosphorylation in neutrophils. Freshly isolated neutrophils and activated neutrophils were optimally incubated with OPG and RANK polypeptide alone or in combination. The tyrosine phosphorylation profile of freshly isolated neutrophils was not modulated in response to RANK, OPG, or RANK + OPG (Figure 7A). Activated neutrophils in the presence of vehicle showed several tyrosine phosphorylated bands that were not influenced by RANK alone (Figure 7B). Addition of OPG to activated neutrophils diminished the phosphorylation of several substrates. The most striking effect was obtained, however, when OPG and RANK were added simultaneously to activated neutrophils. This condition stimulated tyrosine phosphorylation of several substrates, with prominent phosphorylated bands at the apparent 76-, 90-, and 120-kDa regions (Figure 7B). Preincubation of activated neutrophils with PP2 abrogated these bands, whereas the inactive analog PP3 demonstrated no inhibitory activity on phosphorylated bands at the apparent 120- and 76-kDa regions. Histograms of the global densitometry of immunorecognized bands in activated neutrophils showed significant differences between RANK + OPG, RANK or OPG alone, and RANK + OPG + PP2 (Figure 7C).
Signaling through surface RANKL of activated neutrophils: tyrosine phosphorylation of proteins and regulation of cytokine production. (A) Freshly isolated human neutrophils and (B) LPS-activated neutrophils (20 × 106/mL) were incubated for 30 minutes at 37°C with vehicle alone (C-ve), 1 μg/mL rhRANK polypeptide, 1 μg/mL rhOPG, and 1 μg/mL rhRANK peptide + 1 μg/mL rhOPG. Activated neutrophils were also preincubated for 15 minutes with 10 μM PP2 or PP3, and further incubated for 30 minutes at 37°C with rhRANK peptide + rhOPG. Cells were preincubated with 1 mM di-isopropyl-fluorophosphate for 10 minutes. Equal volume of boiling 2 × modified Laemmli sample buffer was added to incubations. Membranes were immunoblotted with a primary antiphosphotyrosine mAb followed by a secondary HRP-labeled goat anti–mouse IgG antibody. Bottom panels indicate sample loading after reblotting the same membrane with an anti-Lyn mAb. Results are representative of 3 independent experiments. (C) Histograms represent global densitometry (expressed in arbitrary units) of immunorecognized bands. Values are means ± SEM (n = 3). Statistical analysis: one-way ANOVA and Newman-Keuls Multiple comparison posttest (RANK vs RANK + OPG, a for P < .05; OPG vs RANK + OPG, b for P < .01; C-ve vs RANK + OPG, c for P < .05, RANK + OPG + PP2 vs RANK + OPG or RANK + OPG + PP3, d for P < .01). (D) Activated neutrophils (20 × 106/mL) were incubated for 30 minutes at 37°C with vehicle alone (C-ve) and rhRANK peptide + rhOPG. Cells were lysed in nondenaturing lysis buffer containing 0.6% CHAPS, centrifuged at 13 000g at 4°C, and the supernatants were immunoprecipitated (IP) for SHP-1 and Syk proteins using anti–SHP-1 (sc-287) or anti-Syk (MAB88906; Chemicon) antibodies. SHP-1 immunoprecipitates were revealed with antiphosphotyrosine mAb (pY) or anti–SHP-1 mAb (ab660; Abcam) by Western blotting (WB). Syk immunoprecipitates were revealed with antiphosphotyrosine mAb. Results are representative of 3 independent experiments. (E-F) LPS-activated neutrophils (5 × 106/mL) were preincubated with vehicle or with 20 μM SHP-1 inhibitor α-bromo-4-hydroxyacetophenone 4-hydroxyphenacyl Br (Calbiochem) for 15 minutes and then incubated for 24 hours with vehicle or with rhRANK peptide + rhOPG. IL-8 and IL-1β were measured in supernatants by ELISA. Results of IL-8 and IL-1β are expressed in nanograms per milliliter (ng/mL) and picograms per milliliter (pg/mL), respectively (mean ± SEM, n = 5 for IL-8 and n = 4 for IL-1β). Statistics: paired t test, *P < .05 (RANK + OPG vs vehicle).
Signaling through surface RANKL of activated neutrophils: tyrosine phosphorylation of proteins and regulation of cytokine production. (A) Freshly isolated human neutrophils and (B) LPS-activated neutrophils (20 × 106/mL) were incubated for 30 minutes at 37°C with vehicle alone (C-ve), 1 μg/mL rhRANK polypeptide, 1 μg/mL rhOPG, and 1 μg/mL rhRANK peptide + 1 μg/mL rhOPG. Activated neutrophils were also preincubated for 15 minutes with 10 μM PP2 or PP3, and further incubated for 30 minutes at 37°C with rhRANK peptide + rhOPG. Cells were preincubated with 1 mM di-isopropyl-fluorophosphate for 10 minutes. Equal volume of boiling 2 × modified Laemmli sample buffer was added to incubations. Membranes were immunoblotted with a primary antiphosphotyrosine mAb followed by a secondary HRP-labeled goat anti–mouse IgG antibody. Bottom panels indicate sample loading after reblotting the same membrane with an anti-Lyn mAb. Results are representative of 3 independent experiments. (C) Histograms represent global densitometry (expressed in arbitrary units) of immunorecognized bands. Values are means ± SEM (n = 3). Statistical analysis: one-way ANOVA and Newman-Keuls Multiple comparison posttest (RANK vs RANK + OPG, a for P < .05; OPG vs RANK + OPG, b for P < .01; C-ve vs RANK + OPG, c for P < .05, RANK + OPG + PP2 vs RANK + OPG or RANK + OPG + PP3, d for P < .01). (D) Activated neutrophils (20 × 106/mL) were incubated for 30 minutes at 37°C with vehicle alone (C-ve) and rhRANK peptide + rhOPG. Cells were lysed in nondenaturing lysis buffer containing 0.6% CHAPS, centrifuged at 13 000g at 4°C, and the supernatants were immunoprecipitated (IP) for SHP-1 and Syk proteins using anti–SHP-1 (sc-287) or anti-Syk (MAB88906; Chemicon) antibodies. SHP-1 immunoprecipitates were revealed with antiphosphotyrosine mAb (pY) or anti–SHP-1 mAb (ab660; Abcam) by Western blotting (WB). Syk immunoprecipitates were revealed with antiphosphotyrosine mAb. Results are representative of 3 independent experiments. (E-F) LPS-activated neutrophils (5 × 106/mL) were preincubated with vehicle or with 20 μM SHP-1 inhibitor α-bromo-4-hydroxyacetophenone 4-hydroxyphenacyl Br (Calbiochem) for 15 minutes and then incubated for 24 hours with vehicle or with rhRANK peptide + rhOPG. IL-8 and IL-1β were measured in supernatants by ELISA. Results of IL-8 and IL-1β are expressed in nanograms per milliliter (ng/mL) and picograms per milliliter (pg/mL), respectively (mean ± SEM, n = 5 for IL-8 and n = 4 for IL-1β). Statistics: paired t test, *P < .05 (RANK + OPG vs vehicle).
Given the phosphorylation profile and possible inhibition of SHP-1 and Syk by PP2, protein tyrosine phosphatase SHP-1 and tyrosine kinase Syk could be the possible downstream phosphorylation candidates.40,41 Both tyrosine phosphorylated SHP-1 and Syk were present in activated neutrophils, as shown after their immunoprecipitation. Interestingly, the incubation of activated neutrophils with RANK + OPG selectively increased the phosphorylation of SHP-1 (Figure 7D). Thus, activated neutrophils present a membrane-bound RANKL receptor that signals into neutrophils through, at least in part, the tyrosine phosphatase SHP-1.
Could RANKL-mediated SHP-1 activation influence neutrophil functions? After 24 hours of incubation, activated neutrophils produced significantly more IL-8 (16 ± 3 ng/mL) and IL-1β (140 ± 15 pg/mL) than did freshly isolated neutrophils (124 ± 40 pg/mL and 0.7 ± 0.3 pg/mL, respectively; n = 3). The production of IL-8 and IL-1β was significantly diminished in the presence of RANK + OPG (Figure 7E-F), whereas neutrophil viability was not affected by RANK + OPG. Moreover, preincubation of activated neutrophils with a SHP-1 inhibitor abrogated this diminution, showing that SHP-1 increased through RANKL down-regulated the production of cytokines by activated neutrophils.
Neutrophils from RA-SF and neutrophils activated by cell-free RA-SF induce osteoclastogenesis through RANKL
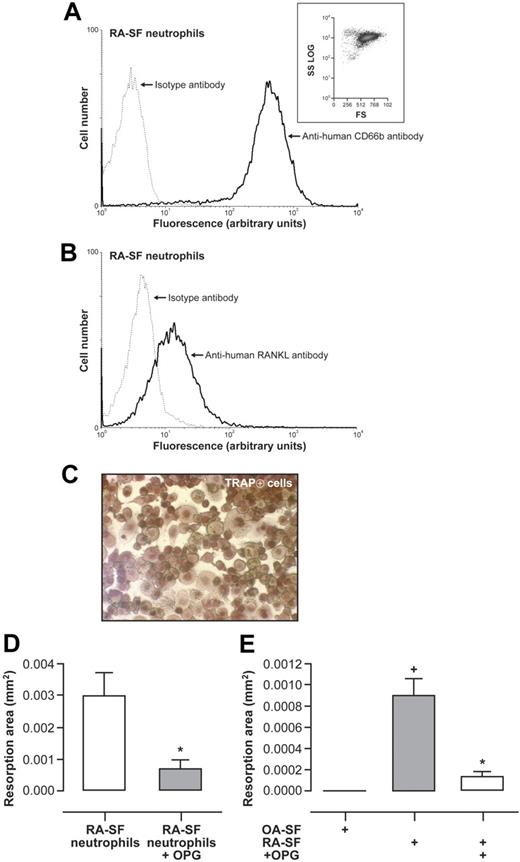
RA-SF neutrophils and normal neutrophils incubated in cell-free RA-SF exhibit increased expression of RANKL.15 RA-SF neutrophils from 3 different patients with flare-up were added to OC precursors to evaluate TRAP-positive cells and their bone resorption capacity after 10 days of culture. RA-SF neutrophils did not incorporate the cell death marker PI (results not shown) and the majority (95.6% ± 1.6%) expressed CD66b (Figure 8A). These neutrophils strongly expressed surface RANKL (Figure 8B), and 55% plus or minus 4% RA-SF neutrophils had surface RANKL detected by flow cytometry. OC precursors cultured with RA-SF neutrophils resulted in the appearance of TRAP-positive cells (Figure 8C). RANKL-deprived mature OCs cultured with RA-SF neutrophils on Osteologic discs were able to resorb the calcified matrix (Figure 8D). This resorption was significantly diminished by the addition of OPG.
RA-SF neutrophils and blood neutrophils preactivated by cell-free RA-SF mediate functional osteoclastogenesis from human monocyte precursors via RANKL. (A) Purity of neutrophils from RA-SF. Flow cytometry was performed after incubation of neutrophils with FITC-conjugated mouse anti–human CD66b (solid line). Isotype controls (dashed line) were performed with equal concentrations of corresponding mouse IgG1. Inset represents forward and side light scatter. Results are representative of neutrophils from SF of 3 RA patients with flare-up. (B) Surface expression of RANKL by neutrophils from RA-SF. Flow cytometry was performed after incubation of neutrophils with an anti–human RANKL mAb followed by a FITC-conjugated anti–mouse F(ab′)2 antibody. Results are representative of neutrophils from SF of 3 RA patients with flare-up. (C) RA-SF neutrophils were fixed with 2% PFA, repeatedly washed, and added (106/well) to cultures of human peripheral blood monocytes in 96-well plates without addition of exogenous RANKL. After 10 days of coculture, TRAP-positive cells were observed with light microscope (magnification, ×200). Results are representative of neutrophils from 3 RA-SF. (D) Mature OCs without exogenous RANKL were incubated on Osteologic discs with fixed RA-SF neutrophils in the presence or absence of 2 μg/mL rhOPG. Resorption pits were evaluated after an additional 10 days of coculture. Histograms represent resorption area expressed in square millimeter (mm2; mean ± SEM). Results are representative of 3 independent experiments performed in duplicates. Statistical analysis: unpaired t test, *P < .02. (E) Freshly isolated neutrophils preincubated in cell-free RA-SF or cell-free OA-SF for 24 hours were fixed with 2% PFA, repeatedly washed, and added (106/well) to mature OCs on Osteologic discs without addition of exogenous RANKL, and with or without pretreatment with 2 μg/mL rhOPG. Cells were removed after 10 days of culture and resorption lacunae were analyzed under light microscope. Histograms represent resorption area expressed in square millimeter (mm2; mean ± SEM). Results shown are representative of 3 independent experiments performed in duplicates. Statistical analysis: unpaired t test, *P < .02 (RA-SF vs RA-SF + OPG); +P < .02 (RA-SF vs OA-SF).
RA-SF neutrophils and blood neutrophils preactivated by cell-free RA-SF mediate functional osteoclastogenesis from human monocyte precursors via RANKL. (A) Purity of neutrophils from RA-SF. Flow cytometry was performed after incubation of neutrophils with FITC-conjugated mouse anti–human CD66b (solid line). Isotype controls (dashed line) were performed with equal concentrations of corresponding mouse IgG1. Inset represents forward and side light scatter. Results are representative of neutrophils from SF of 3 RA patients with flare-up. (B) Surface expression of RANKL by neutrophils from RA-SF. Flow cytometry was performed after incubation of neutrophils with an anti–human RANKL mAb followed by a FITC-conjugated anti–mouse F(ab′)2 antibody. Results are representative of neutrophils from SF of 3 RA patients with flare-up. (C) RA-SF neutrophils were fixed with 2% PFA, repeatedly washed, and added (106/well) to cultures of human peripheral blood monocytes in 96-well plates without addition of exogenous RANKL. After 10 days of coculture, TRAP-positive cells were observed with light microscope (magnification, ×200). Results are representative of neutrophils from 3 RA-SF. (D) Mature OCs without exogenous RANKL were incubated on Osteologic discs with fixed RA-SF neutrophils in the presence or absence of 2 μg/mL rhOPG. Resorption pits were evaluated after an additional 10 days of coculture. Histograms represent resorption area expressed in square millimeter (mm2; mean ± SEM). Results are representative of 3 independent experiments performed in duplicates. Statistical analysis: unpaired t test, *P < .02. (E) Freshly isolated neutrophils preincubated in cell-free RA-SF or cell-free OA-SF for 24 hours were fixed with 2% PFA, repeatedly washed, and added (106/well) to mature OCs on Osteologic discs without addition of exogenous RANKL, and with or without pretreatment with 2 μg/mL rhOPG. Cells were removed after 10 days of culture and resorption lacunae were analyzed under light microscope. Histograms represent resorption area expressed in square millimeter (mm2; mean ± SEM). Results shown are representative of 3 independent experiments performed in duplicates. Statistical analysis: unpaired t test, *P < .02 (RA-SF vs RA-SF + OPG); +P < .02 (RA-SF vs OA-SF).
We next conducted similar experiments with normal blood neutrophils preincubated in cell-free RA-SF or OA-SF. Exposure to RA-SF significantly increased neutrophil cell surface RANKL, whereas exposure to OA-SF did not influence this expression (results not shown). Addition of neutrophils preincubated in RA-SF to RANKL-deprived mature OCs induced the formation of resorption pits, and this effect was inhibited by OPG (Figure 8E). In contrast, neutrophils preincubated in OA-SF did not activate RANKL-deprived mature OCs to resorb. Thus, these results indicate that RANKL spontaneously expressed by RA-SF neutrophils or induced by RA-SF in normal neutrophils was functionally active during osteoclastogenesis.
Discussion
Accelerated bone resorption is presently considered an early event in the pathogenesis of several septic and inflammatory diseases, where RANKL mediates increased osteoclastogenesis and, consequently, abnormal bone erosions.6,10 Neutrophils are the predominant infiltrating cells in this stage and are present in large numbers in areas of osteolysis and adjacent inflammatory sites. Previously, we reported that human neutrophils from inflammatory sites expressed high levels of RANKL.15 Here, we show that human, as well as murine, neutrophils strongly up-regulate their expression of membrane RANKL after LPS stimulation, and thus have the capacity to activate osteoclastic bone resorption through neutrophil-osteoclast interactions. As a corollary, RANKL increased at the surface of activated neutrophils links this cell type to inflammatory bone destruction through the cell population's capacity to directly activate osteoclastogenesis.
The osteoclastogenic effect of neutrophil RANKL, demonstrated with human and murine activated neutrophils (purity > 95%), was reproduced with purified neutrophil membranes and fixed neutrophils, but not with culture supernatants of activated neutrophils in which no secreted RANKL was detected. Human neutrophils have also been shown by others to release no RANKL.42 Thus, RANKL expression in neutrophils differed from that in activated CD3+ lymphocytes, which express both cell surface and soluble RANKL.6,43 Differences in activity have been, however, observed between membrane-bound RANKL and soluble RANKL, the former being more efficient in stimulating OCs.44 The functional activity of neutrophil RANKL, which was substantiated by the inhibitory effects on osteoclastogenesis of RANKL-directed antisense RNA in pure PLB-985 neutrophil-like cells, confirms the importance of the endogenous generation of RANKL in neutrophils.
Parallel studies with human and mouse neutrophils allowed us to consider 2 aspects of the regulation of neutrophil RANKL: whereas the in vitro influence of LPS on isolated neutrophils focused on the functional activation of a stable population of neutrophils (with 23% ± 7% cells that expressed RANKL after activation), in vivo LPS injections stimulated the neoexpression of RANKL in the entire population of neutrophils, leading to the appearance of another RANKL-expressing subset of neutrophils. However, neutrophils from LPS-injected air pouches compared with circulating neutrophils showed differences in granularity and cell surface expression of 7/4 mAb that could suggest mobilization of bone marrow–resident neutrophils with osteoclast-activating potential. LPS injected in rat pleural cavity has been reported to stimulate the pleural accumulation of neutrophils that was related to the mobilization of neutrophils from the bone marrow.45
Our results show that TLR4 and TLR2 ligands, which correspond to Gram-positive and Gram-negative bacterial determinants, are able to stimulate RANKL expression. This might be of particular relevance in infective conditions associated with rapid inflammatory bone loss such as septic arthritis, osteomyelitis, and periodontitis. Interestingly, TLR ligands of microbial origin have been found in joints of patients with RA, and infections could be initiating factors of the disease.29,46 This suggests a link between the major pathogenetic moieties of septic and inflammatory conditions and neutrophil induction of abnormal bone resorption. Furthermore, endogenous molecules such as fibronectin fragments and heat shock proteins, activating mainly TLR4, are present in rheumatoid synovium.47,48 Neutrophils are present in synovial tissue from patients with early and late RA.49 Moreover, the hallmark of exacerbations of active RA is the great number of neutrophils that colonize the joints by migrating from blood to the synovial space through the rheumatoid pannus. Thus, studies with neutrophils from RA-SF and with neutrophils preincubated with different sources of SF might have pathologic implications: (1) infiltrating neutrophils from RA-SF that express membrane RANKL demonstrate the osteoclastogenic potential of this cell; (2) RA-SF can induce RANKL expression in blood neutrophils with subsequent activation of osteoclastogenesis, whereas OA-SF cannot. The latter is, thus, a causal demonstration of the inflammatory character of the RA-SF, which drives the acquisition of a functional trait.
Given the predominance and number of neutrophils at sites of rapid bone loss as well as in flare-ups of chronic diseases, these cells might constitute a nonnegligible source of RANKL in pathologic bone lesions. Indeed, neutrophil expression of RANKL, at mRNA and protein levels, adds this osteotropic molecule to the range of inflammatory cytokines synthesized by the neutrophil.50 Neutrophil-derived mediators enhance its scope in acute and chronic inflammatory reactions, triggering a complex interplay with other cell types such as endothelial cells, platelets, fibroblasts, synoviocytes, lymphocytes, and dendritic cells (DCs). The possible cross-talk between neutrophils and osteoclasts is suggested by the histologic presence of neutrophils in RA synovial tissues.49 Our studies are the first to show that RANKL permits neutrophil interaction with the OCs. Indeed, our results showed the induction of both stages of osteoclastogenesis: enhanced differentiation of circulating precursors as evidenced by appearance of TRAP+ multinucleated cells, as well as OC activity with the formation of resorption pits. The conceptual importance of these results is highlighted in inflammatory bone diseases where neutrophil RANKL acts, not only on resident OCs, but also on the influx of mononuclear precursors of OCs.51 Another point to stress is that synovial inflammatory tissue in RA can break the cortical bone barrier.52 This might be accompanied by profound changes of cellularity of synovial inflammatory tissue, releasing numerous leukocytes and their precursors, thus favoring neutrophil-OC interactions. Osteoclasts lining trabecular bone are also in intimate contact with mature neutrophils and neutrophil precursors in the medullary cavity. Interestingly, we found mouse marrow neutrophils and neutrophil precursors to express RANKL (results not shown), suggesting trabecular bone to be a fertile ground for interaction of the 2 cell types. Studies with osteoblasts and OCs have shown that though the RANK-RANKL interaction is not involved in cell adhesion, effective interactions between RANKL and RANK necessitate cell-to-cell contact through adhesion molecules, notably LFA-1/ICAM-1.39,53 ICAM-1 forms a part of the neutrophil cell surface repertoire and has been shown to be up-regulated in neutrophils by LPS.54 Microscopic demonstration of direct cell-cell contact between neutrophils and OCs also supports the hypothesis that physical contact between cells is closely related to functional exchanges. Neutrophils in contact with DCs that activate DC production of IL-12 offer another example of this functionality.55
Besides activating OCs through neutrophil RANKL, we also show membrane-bound RANKL to signal within the neutrophil itself, activating SHP-1 through tyrosine phosphorylation. The functional association of a membrane-bound ligand to membrane-bound receptors, termed reverse signaling, is seen in many members of the TNF superfamily.56 Production of interferon-γ by activated T cells, mediated by RANKL signaling, demonstrates the biologic significance of this system.57 As a type II integral membrane protein, RANKL would require the association with molecular adaptors to link it to intracellular signaling cascades. We note that protein tyrosine phosphorylation was particularly pronounced in the presence of RANKL receptors, OPG, and RANK. Indeed, RANKL-OPG-RANK have been shown by surface plasmon-resonance studies to constitute a functional heteromolecular complex in RANK-expressing OCs.58 The biologic significance of SHP-1 as a negative regulator in immunity is seen in the severe autoimmunity and neutrophil dysfunctions developed by motheaten mice.59,60 Negative regulation of the neutrophil production of cytokines by RANKL-activated SHP-1 could be of particular importance during inflammation where great numbers of activated neutrophils strongly affect adjacent tissues.
In conclusion, the present study describes the neutrophil as a cell that acquires roles beyond that of a prototypic inflammatory cell, directly capable of activating osteoclasts. It paves the way for future work toward exploring the role of this cell in the growing field of osteoimmunology. Indeed, development of therapeutic strategies targeting neutrophil-RANKL might be particularly effective in arresting the early evolution of bony lesions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mrs Andrée-Anne Marceau for her excellent technical assistance, Dr Maurice Dufour for cytofluorometry analyses, Dr Christophe Pivot-Pajot for useful discussions on antisense RNA transfections, and Dr Paul H. Naccache for his expertise on neutrophil signaling.
These studies were supported by grants from the Canadian Institutes of Health Research (Ottawa, ON).
Authorship
Contribution: A.C. designed and performed experiments, analyzed data, and wrote the paper; M.-A.R. and P.T. contributed mouse experiments; and P.E.P. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrice E. Poubelle, CRRI, CRCHUL, 2705 Boulevard Laurier, Québec, QC Canada G1V 4G2; e-mail: patrice.poubelle@crchul.ulaval.ca.