This prospective longitudinal study examined the quality of life (QOL) after hematopoietic cell transplantation (HCT) and identified risk factors of poor QOL in 312 adult autologous and allogeneic HCT patients. Physical, psychological, social, and spiritual well-being was assessed before HCT, 6 months, and 1, 2, and 3 years after HCT. For all HCT patients, physical QOL was stable from before to after HCT (P > .05); psychologic (P < .001), social (P < .001), and spiritual (P = .03) QOL improved at 6 months. Study noncompleters (because of illness or death) had worse QOL. Allogeneic patients reported worse physical and psychologic well-being (P < .05). Older patients reported worse physical but better social well-being regardless of HCT type (P < .05). Two or more domains were affected by race/ethnicity, household income, and education in autologous patients, and by body mass index (BMI), decline in BMI, primary diagnosis, and chronic graft-versus-host disease (GVHD) in allogeneic patients (P < .05). At 3 years, 74% of HCT patients were employed full or part time. Older autologous patients with lower pre-HCT income were less likely to work (P < .05); allogeneic patients with chronic GVHD were less likely to work (P = .002). Multidisciplinary efforts to identify and support vulnerable subgroups after HCT need to be developed.
Introduction
During the last 30 years, both the number of hematopoietic cell transplantations (HCTs) performed annually and the number of diseases for which HCT is therapeutically indicated have increased.1 Improved transplantation strategies have contributed to survival increments of 10% per decade.2 Patients who survive 2 years after HCT now have long-term survival rates exceeding 70%.3,4 However, cure or control of the underlying malignancy is often not accompanied by full restoration of health or quality of life (QOL). Long-term complications include physical and psychologic morbidity attributed to toxicity from therapeutic exposures, infections, and graft-versus-host disease (GVHD).3,,,,,,,,,,,–15
The growing number of survivors creates a need to identify factors that affect the survivors' long-term QOL. Although cross-sectional studies have examined QOL,16,,,–20 longitudinal studies are essential for elucidating the time course of change. Previous longitudinal studies of QOL21,,,,–26 are limited by small sample size, lack of racial/ethnic diversity, and exclusive focus on allogeneic recipients. Thus, a large prospective longitudinal study, with a broader representation of clinical and demographic characteristics, is needed to define recovery after HCT that is generalizable across all HCT populations.
The objective of our 3-year longitudinal study was to evaluate long-term changes in QOL and return to work as an indicator of return to normalcy in patients who underwent HCT. We identified host- and treatment-related factors that impacted long-term functional recovery to identify high-risk subpopulations for future targeted interventions. We also examined the impact of survival and study participation on the long-term trends in QOL.
Methods
Study participants
The study was approved by the Institutional Review Board at City of Hope and informed consent was obtained in accordance with the Declaration of Helsinki. Between 2001 and 2005, English-literate patients older than 18 years scheduled to undergo HCT were approached for study participation. The City of Hope Quality of Life (COH-QOL-HCT) questionnaire was completed by 316 patients before transplantation. A post-HCT version was mailed at 6 months, and 1, 2, and 3 years after HCT. Follow-up phone calls were made 2 weeks after nonresponse and up to 4 months for the 6-month assessment and up to 10 months for the subsequent assessments, after which patients were considered as refusals for that time point only.
Instrument
The COH-QOL-HCT questionnaire27 contains subscales that assess 4 QOL domains (physical, psychological, social, and spiritual). The physical domain includes 17 subscales related to physical problems such as changes in skin, vision, hearing, appetite, nausea, fatigue, and physical strength. The psychological domain includes 22 subscales on anxiety, depression, fear of recurrence, and satisfaction with life. The social domain comprises 12 subscales on relationships, family, intimacy, work, and social reintegration. The 7 items of spiritual domain encompass global spirituality, religiosity, and life appreciation. Subscale scores range from 0 (worst) to 10 (best). The mean subscale scores within each domain comprise the domain score—computed if less than 25% were missing data; the missing observations were imputed using the patient's domain mean. The COH-QOL-HCT instrument was developed specifically for QOL of HCT patients and has been tested,27,28 with test-retest reliability of r = .71 and internal consistency of r = .85. The instrument has been used in several studies29,–31 and demonstrates discriminant validity between those with and without specific late effects after HCT.31
Demographic and clinical characteristics
The COH-QOL-HCT contains a demographic section focusing on age, sex, race/ethnicity, marital status, height, weight, and, for the post-HCT time points, presence of chronic GVHD. Self-report accounted for 58% of the race/ethnicity data, with the remainder obtained from the COH Cancer Registry (39%) and COH HCT database (1%). Self-report accounted for 77% of the education and 57% of the income data. Missing data were supplemented with the US Census 2000 data. A patient's address at the time of HCT was matched with the geographic unit in the US Census data. Sex-, age-, and race-specific median education level and race-specific income were computed and used as proxy for patients with missing data. Medical records were used to abstract clinical data, including primary diagnosis, conditioning regimens, stem cell source, risk of relapse at HCT, and disease relapse after HCT.
Return to work
The COH-QOL-HCT questionnaire asked whether participants were able to return to work after HCT. The possible responses include “No,” “Yes (part time),” “Yes (full time),” and “Not applicable.” The reason for not being able to return to work was asked as an open-ended question.
Statistical methods
Longitudinal trends of the QOL domain means were estimated using the generalized estimating equation (GEE) method.32 We modeled the QOL domain scores as a linear function between the pre-HCT and 6-month time point, and as either linear or quadratic function of time between the 6-month and 3-year time points. At the post-HCT time points, interactions of covariates with time were included to allow for variation in temporal trends according to covariate values.
Time-independent covariates included sex, age at referent HCT; race/ethnicity (Hispanic; African American; Asian; non-Hispanic white; other); annual household income before HCT (< $20 000; $20 000-$49 999; $50 000-$74 999; $75 000-$100 000; > $100 000; the midpoint in each category [$125 000 for the highest category] was also used), highest education attained at HCT (less than high school; high school diploma/general educational development; some college/Associate of Arts; Bachelor of Science/Bachelor of Arts; postgraduate/professional degree); primary diagnosis (acute lymphoblastic leukemia [ALL]; acute myeloid leukemia [AML]; Hodgkin lymphoma [HL]; non-Hodgkin lymphoma [NHL]; myeloproliferative disorders [MPDs]; multiple myeloma [MM]; and other diagnoses [amyloidosis, chronic lymphocytic leukemia [CLL], chronic myelogenous leukemia [CML], severe aplastic anemia [SAA], other]), conditioning agent used (yes/no for each agent); number of HCTs received (1; > 1); stem cell source (autologous; allogeneic); and risk of relapse at HCT (standard; high). Patients who underwent transplantation in first or second complete remission after AML, ALL, HL or NHL, or first chronic phase of CML, or patients with SAA were considered at standard risk for relapse at HCT; the remainder, at high risk.
Time-dependent covariates included marital status (married/partnered; single; divorced/widowed/separated); body mass index (BMI: kg/m2); change in BMI relative to pre-HCT level; self-reported chronic GVHD (yes/no); and relapse of primary disease (yes/no). Covariate values concurrent with the QOL scores were used. The effects of pre-HCT marital status, BMI, and relapse status were also examined as time-independent covariates. To understand the clinical relevance of the significant modifying covariates, effect size (ES) was computed at each time point as the absolute difference in estimated QOL domain scores between the index group and the referent group divided by the common standard deviation. Average effect size over the 4 follow-up time points was reported.
Factors influencing patients' employment status (part time or full time) at 6 months, and 1, 2, and 3 years after HCT were identified using logistic regression. Sex, age at referent HCT, race/ethnicity, annual household income, education level at HCT, primary diagnosis, risk of relapse at HCT, stem cell source, and conditioning regimen were considered as time-independent covariates. Time-dependent covariates included marital status, BMI, BMI change since before HCT, disease relapse status, and chronic GVHD; values concurrent to assessment times were used. The effects of pre-HCT marital status, BMI, and relapse of primary disease were also examined.
Allogeneic and autologous transplant recipients were analyzed together initially to test for QOL differences by stem cell source. Separate analyses by stem cell source were then conducted to identify transplant-type–specific variables associated with QOL temporal trends. Categoric variables, such as primary diagnosis, were collapsed in the course of analysis by combining categories with similar (statistically nonsignificant) QOL scores.
Comparison of pre-HCT characteristics between participants and nonparticipants was conducted using the t test for continuous and the χ2 test for categoric variables. Multivariate logistic and linear regressions were used to identify independently significant variables. Overall survival with follow-up censored on November 30, 2008, was compared between participants and nonparticipants, using the log-rank test.
To assess the robustness of the estimated longitudinal QOL trends to missing data resulting from death (31%) or study dropout (20%), we supplemented the analysis with pattern mixture model (PMM).33,34 Nonignorable missing data become a concern35 because inference based on GEE could potentially become invalid.33 PMM extends the usual GEE analysis by incorporating additional variables identifying dropout/missing data patterns. We defined 3 patterns/strata based on survival and study completion36 : (1) deceased/noncompleter—patients who died before study completion; (2) alive/noncompleter—patients who were alive at 3 years but dropped out before the 3-year assessment; and (3) alive/completer—patients who were alive at 3 years and provided the 3-year assessment data. Interactions between the missing data strata and time were included; significant interactions indicate nonignorability of missing data and that longitudinal trends should be reported separately for each stratum.
PROCs GEE, LOGIT, and PHREG of SAS 9.1 were used for data analyses (SAS Institute). Two-sided Wald test was used with P value less than .05 considered statistically significant.
Results
Patient characteristics
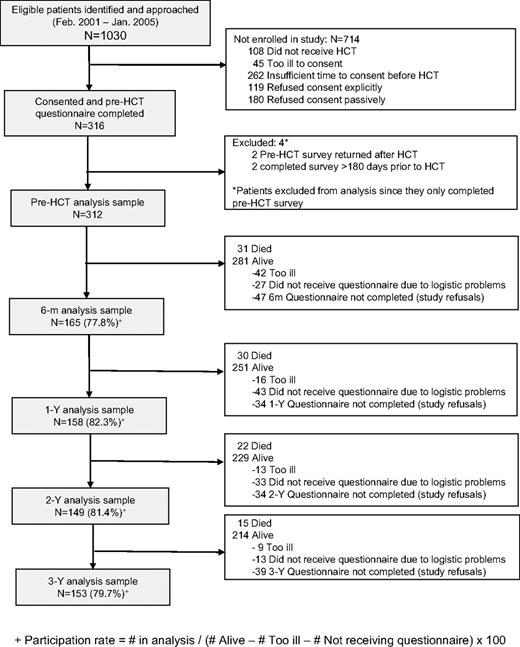
A total of 1030 patients were scheduled to undergo HCT during the study period. Of these, 108 did not proceed to HCT; consent could not be obtained from 45 because of illness and from 262 because of insufficient time before HCT; 299 were offered an opportunity to participate, but did not consent; and 316 consented to participate (participation rate: 51%). Of these, 4 patients who completed only the pre-HCT questionnaire were excluded from the analysis because of inappropriate timing of questionnaire completion. Characteristics of the study participants at each time point are shown in Table 1. Comparison of the 312 study participants with nonparticipants showed that participants were more likely to have at least a college education (P = .047). However, after adjustment for sex, income, stem cell source, and primary diagnosis, survival rates did not differ between participants and nonparticipants (P = .67).
Clinical and demographic characteristics of study cohort before HCT, at 6 months, 1 year, 2 years, and 3 years after HCT
| . | Pre-HCT (n = 312) . | 6 mo (n = 165) . | 1 y (n = 158) . | 2 y (n = 149) . | 3 y (n = 153) . |
|---|---|---|---|---|---|
| Median age at referent HCT,*s y (range) | 48 (18-78) | 50 (19-78) | 48 (19-78) | 49 (19-78) | 48 (18-78) |
| Sex, n (%) | |||||
| Male | 173 (55) | 84 (51) | 76 (48) | 75 (50) | 80 (52) |
| Female | 139 (45) | 81 (49) | 82 (52) | 74 (50) | 73 (48) |
| Race/ ethnicity, n (%) | |||||
| White (non-Hispanic) | 202 (65) | 105 (64) | 104 (66) | 96 (64) | 101 (66) |
| Hispanic/Latino | 57 (18) | 28 (17) | 29 (18) | 27 (18) | 28 (18) |
| African American | 22 (7) | 15 (9) | 11 (7) | 9 (6) | 8 (5) |
| Asian | 26 (8) | 12 (7) | 11 (7) | 12 (8) | 11 (7) |
| Other | 5 (2) | 5 (3) | 3 (2) | 5 (3) | 5 (3) |
| Marital status at pre-HCT, n (%) | |||||
| Married/partnered | 203 (65) | 115 (70) | 107 (68) | 104 (70) | 105 (69) |
| Single | 64 (20) | 27 (16) | 32 (20) | 28 (19) | 28 (18) |
| Divorced/widowed/separated | 45 (14) | 23 (14) | 19 (12) | 17 (11) | 20 (13) |
| Education, highest attained at time of referent HCT, n (%) | |||||
| Did not complete high school (HS) | 18 (6) | 8 (5) | 10 (6) | 6 (4) | 6 (4) |
| HS graduate | 53 (17) | 27 (16) | 24 (15) | 21 (14) | 18 (12) |
| Some college | 105 (34) | 51 (31) | 43 (27) | 44 (30) | 43 (28) |
| Bachelor's degree | 71 (23) | 38 (23) | 40 (25) | 39 (26) | 44 (29) |
| Postgraduate degree | 64 (21) | 41 (25) | 41 (26) | 39 (26) | 42 (27) |
| Unknown | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Annual household income at time of referent HCT, n (%) | |||||
| Less than $20 000 | 24 (8) | 12 (7) | 15 (9) | 10 (7) | 13 (8) |
| $20 000-$49 000 | 78 (25) | 38 (23) | 30 (19) | 29 (19) | 31 (20) |
| $50 000-$74 000 | 98 (31) | 47 (28) | 49 (31) | 43 (29) | 39 (25) |
| $75 000-$100 000 | 47 (15) | 26 (16) | 25 (16) | 22 (15) | 26 (17) |
| More than $100 000 | 63 (20) | 41 (25) | 38 (24) | 44 (30) | 44 (29) |
| Unknown | 2 (.6) | 1 (.6) | 1 (.6) | 1 (.7) | 0 (0) |
| Body mass index in kg/m2 at pre HCT, median (range) | 26.2 (17.0-50.2) | 26.3 (18.5-50.2) | 26.5 (19.1-47.2) | 25.9 (17.0-50.2) | 26.3 (18.1-50.2) |
| Change in body mass index from pre-HCT level, median (range) | NA | −1.3 (−14.9-12.0) | −0.9 (−15.3-10.5) | −0.6 (−17.5-13.5) | 0 (−10.4-15.3) |
| Primary diagnosis, n (%) | |||||
| Non-Hodgkin lymphoma | 86 (28) | 51 (31) | 45 (28) | 46 (31) | 50 (33) |
| Acute myelogenous leukemia | 66 (21) | 31 (19) | 31 (20) | 31 (21) | 33 (22) |
| Multiple myeloma | 60 (19) | 40 (24) | 35 (22) | 28 (19) | 28 (18) |
| Hodgkin lymphoma | 25 (8) | 12 (7) | 13 (8) | 13 (9) | 12 (8) |
| Acute lymphocytic leukemia | 23 (7) | 8 (5) | 8 (5) | 6 (4) | 6 (4) |
| Myeloproliferative disorder | 18 (6) | 9 (5) | 9 (6) | 10 (7) | 9 (6) |
| Other | 34§ (11) | 14 (8) | 17 (11) | 15 (10) | 15 (10) |
| High risk of relapse at referent HCT, n (%) | 162 (52) | 80 (48) | 80 (51) | 75 (50) | 77 (50) |
| Disease in remission, n (%) | 107 (36) | 139 (84) | 135 (85) | 129 (87) | 130 (85) |
| Stem cell source,†n (%) | |||||
| Autologous only | 170 (54) | 103 (62) | 97 (61) | 92 (62) | 93 (61) |
| Allogeneic | 142 (46) | 62 (38) | 61 (39) | 57 (38) | 60 (39) |
| Chronic GVHD,‡ n (%), among allogeneic HCT patients | NA | 22 (35) | 30 (49) | 33 (58) | 33 (55) |
| Number of HCT, n (%) | |||||
| 1 | 253 (81) | 140 (85) | 132 (84) | 125 (84) | 131 (86) |
| 2 | 57 (18) | 25 (15) | 26 (16) | 24 (16) | 21 (14) |
| 3 | 2 (.6) | 0 (0) | 0 (0) | 0 (0) | 1 (.6) |
| Conditioning regimen, n (%) | |||||
| Total body irradiation | 134 (43) | 62 (38) | 56 (35) | 57 (38) | 59 (39) |
| Chemotherapy based | 311 (99) | 164 (99) | 157 (99) | 148 (99) | 152 (99) |
| . | Pre-HCT (n = 312) . | 6 mo (n = 165) . | 1 y (n = 158) . | 2 y (n = 149) . | 3 y (n = 153) . |
|---|---|---|---|---|---|
| Median age at referent HCT,*s y (range) | 48 (18-78) | 50 (19-78) | 48 (19-78) | 49 (19-78) | 48 (18-78) |
| Sex, n (%) | |||||
| Male | 173 (55) | 84 (51) | 76 (48) | 75 (50) | 80 (52) |
| Female | 139 (45) | 81 (49) | 82 (52) | 74 (50) | 73 (48) |
| Race/ ethnicity, n (%) | |||||
| White (non-Hispanic) | 202 (65) | 105 (64) | 104 (66) | 96 (64) | 101 (66) |
| Hispanic/Latino | 57 (18) | 28 (17) | 29 (18) | 27 (18) | 28 (18) |
| African American | 22 (7) | 15 (9) | 11 (7) | 9 (6) | 8 (5) |
| Asian | 26 (8) | 12 (7) | 11 (7) | 12 (8) | 11 (7) |
| Other | 5 (2) | 5 (3) | 3 (2) | 5 (3) | 5 (3) |
| Marital status at pre-HCT, n (%) | |||||
| Married/partnered | 203 (65) | 115 (70) | 107 (68) | 104 (70) | 105 (69) |
| Single | 64 (20) | 27 (16) | 32 (20) | 28 (19) | 28 (18) |
| Divorced/widowed/separated | 45 (14) | 23 (14) | 19 (12) | 17 (11) | 20 (13) |
| Education, highest attained at time of referent HCT, n (%) | |||||
| Did not complete high school (HS) | 18 (6) | 8 (5) | 10 (6) | 6 (4) | 6 (4) |
| HS graduate | 53 (17) | 27 (16) | 24 (15) | 21 (14) | 18 (12) |
| Some college | 105 (34) | 51 (31) | 43 (27) | 44 (30) | 43 (28) |
| Bachelor's degree | 71 (23) | 38 (23) | 40 (25) | 39 (26) | 44 (29) |
| Postgraduate degree | 64 (21) | 41 (25) | 41 (26) | 39 (26) | 42 (27) |
| Unknown | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Annual household income at time of referent HCT, n (%) | |||||
| Less than $20 000 | 24 (8) | 12 (7) | 15 (9) | 10 (7) | 13 (8) |
| $20 000-$49 000 | 78 (25) | 38 (23) | 30 (19) | 29 (19) | 31 (20) |
| $50 000-$74 000 | 98 (31) | 47 (28) | 49 (31) | 43 (29) | 39 (25) |
| $75 000-$100 000 | 47 (15) | 26 (16) | 25 (16) | 22 (15) | 26 (17) |
| More than $100 000 | 63 (20) | 41 (25) | 38 (24) | 44 (30) | 44 (29) |
| Unknown | 2 (.6) | 1 (.6) | 1 (.6) | 1 (.7) | 0 (0) |
| Body mass index in kg/m2 at pre HCT, median (range) | 26.2 (17.0-50.2) | 26.3 (18.5-50.2) | 26.5 (19.1-47.2) | 25.9 (17.0-50.2) | 26.3 (18.1-50.2) |
| Change in body mass index from pre-HCT level, median (range) | NA | −1.3 (−14.9-12.0) | −0.9 (−15.3-10.5) | −0.6 (−17.5-13.5) | 0 (−10.4-15.3) |
| Primary diagnosis, n (%) | |||||
| Non-Hodgkin lymphoma | 86 (28) | 51 (31) | 45 (28) | 46 (31) | 50 (33) |
| Acute myelogenous leukemia | 66 (21) | 31 (19) | 31 (20) | 31 (21) | 33 (22) |
| Multiple myeloma | 60 (19) | 40 (24) | 35 (22) | 28 (19) | 28 (18) |
| Hodgkin lymphoma | 25 (8) | 12 (7) | 13 (8) | 13 (9) | 12 (8) |
| Acute lymphocytic leukemia | 23 (7) | 8 (5) | 8 (5) | 6 (4) | 6 (4) |
| Myeloproliferative disorder | 18 (6) | 9 (5) | 9 (6) | 10 (7) | 9 (6) |
| Other | 34§ (11) | 14 (8) | 17 (11) | 15 (10) | 15 (10) |
| High risk of relapse at referent HCT, n (%) | 162 (52) | 80 (48) | 80 (51) | 75 (50) | 77 (50) |
| Disease in remission, n (%) | 107 (36) | 139 (84) | 135 (85) | 129 (87) | 130 (85) |
| Stem cell source,†n (%) | |||||
| Autologous only | 170 (54) | 103 (62) | 97 (61) | 92 (62) | 93 (61) |
| Allogeneic | 142 (46) | 62 (38) | 61 (39) | 57 (38) | 60 (39) |
| Chronic GVHD,‡ n (%), among allogeneic HCT patients | NA | 22 (35) | 30 (49) | 33 (58) | 33 (55) |
| Number of HCT, n (%) | |||||
| 1 | 253 (81) | 140 (85) | 132 (84) | 125 (84) | 131 (86) |
| 2 | 57 (18) | 25 (15) | 26 (16) | 24 (16) | 21 (14) |
| 3 | 2 (.6) | 0 (0) | 0 (0) | 0 (0) | 1 (.6) |
| Conditioning regimen, n (%) | |||||
| Total body irradiation | 134 (43) | 62 (38) | 56 (35) | 57 (38) | 59 (39) |
| Chemotherapy based | 311 (99) | 164 (99) | 157 (99) | 148 (99) | 152 (99) |
NA indicates not applicable.
Referent HCT refers to the specific transplantation (not necessarily the first) that led to a patient's study enrollment.
Patients who received at least one allogeneic HCT were categorized under allogeneic HCT.
Self-reported.
Chronic myelogenous leukemia (n = 18); severe aplastic anemia (n = 6); chronic lymphocytic leukemia (n = 3); other-amyloidosis (n = 2); 1 each of amyloidosis, hypereosinophillic syndrome, other-leukemia, POEMS syndrome, renal cancer.
The median age at study enrollment was 48 years; 55% of the study participants were male; 65% were white; 65% were married or partnered; 44% had at least a college degree; median annual household income was $50 000 to $74 000; and median BMI was 26.4. NHL, AML, and MM accounted for 69% of all primary diagnoses; 52% were at high risk of relapse; 81% had undergone only one HCT; 54% underwent autologous HCT; 43% were exposed to total body irradiation (TBI).
The number of study participants at 6 months, and 1, 2, and 3 years was 165, 158, 149, and 153, corresponding to participation rates of 77.8%, 82.4%, 81.4%, and 79.7%, respectively, after accounting for death or illness precluding participation at the respective time points (Figure 1). Distribution of age, sex, race/ethnicity, marital status, BMI, risk of relapse, number of HCTs, and TBI-based regimens remained relatively stable over time (< 10% change between before HCT and at 3 years). A comparison of the characteristics of study participants and nonparticipants at each time point revealed that participants at 6 months were more likely to be older and to have disease in remission, and were less likely to be exposed to TBI; participants at 1 year were also less likely to be exposed to TBI and were more likely to be female; participants at 2 years were more likely to have higher income; and participants at 3 years were older and better educated than nonparticipants. Thus, no systematic bias was apparent across the entire study period.
Longitudinal trends
The estimated QOL trends are shown in Figure 2 for all participants. There was no significant change in physical well-being between the pre-HCT and post-HCT time points (P = .10). Psychological, social, and spiritual well-being improved from before HCT to the 6-month time point (P < .001, P < .001, P = .03, respectively) and then remained stable up to the 3-year time point.
GEE estimates of the longitudinal trends of QOL domains for autologous and allogeneic HCT patients combined (solid lines), and for autologous (dotted lines) and allogeneic (dashed lines) patients separately. ○ indicates observed means for autologous HCT patients; and ●, observed means for allogeneic HCT patients.
GEE estimates of the longitudinal trends of QOL domains for autologous and allogeneic HCT patients combined (solid lines), and for autologous (dotted lines) and allogeneic (dashed lines) patients separately. ○ indicates observed means for autologous HCT patients; and ●, observed means for allogeneic HCT patients.
After adjustment for significant covariates (age, primary diagnosis, BMI, pre-HCT income and education), post-HCT physical and psychological well-being were found to be significantly lower among those who underwent allogeneic HCT compared with autologous HCT (difference in physical well-being: 5.3%; ES = 0.24, P = .033; difference in psychological well-being: 5.6%; ES = 0.23, P = .036). Social and spiritual well-being did not differ by stem cell source (P = .25 and P = .99, respectively). Longitudinal trends were examined separately by stem cell sources; the estimated QOL trends are shown in Figure 2.
Autologous HCT.
Compared with the scores reported at the pre-HCT time point, physical well-being improved by 3.4% (ES = 0.16) at 1 year (P = .05) and 5.2% (ES = 0.25) at 2 years (P = .01) after HCT, with a nonsignificant (P = .36) 1.9% (ES = 0.09) increase at 3 years. Both psychological and social well-being improved significantly at 6 months and remained elevated through 3 years (P < .01); psychological well-being improved by 13% (ES = 0.43) and social QOL, by 9% (ES = 0.31) to 15% (ES = 0.52) relative to pre-HCT levels. An improvement in spiritual QOL occurred at 6 months (3.9%; ES = 0.14, P = .02) and remained stable thereafter.
Allogeneic HCT.
Physical well-being declined significantly (3.9% at 6 months [ES = 0.19]; 7.5% at 3 years [ES = 0.38]; P < .01). A significant improvement in psychological well-being (4.7%; ES = 0.18, P = .04) and social well-being (5.5%; ES = 0.20, P = .05) occurred at 6 months; levels at subsequent time points were not statistically different from pre-HCT levels. Except for a 3.0% nonsignificant rise at 6 months (ES = 0.12, P = .08), spiritual well-being remained unchanged at other post-HCT time points.
Factors modifying longitudinal trends of QOL domains by transplant type
Significant modifiers of the longitudinal trends of QOL are shown in Tables 2 and 3 by stem cell source, along with the percentage change in QOL scores of the index group relative to the referent group and the corresponding ES. Pre-HCT values of marital status, BMI, and relapse status were not significantly associated with post-HCT QOL for either transplant type. Therefore, reference to these covariates below corresponds to their inclusion as time-varying covariates, using values concurrent to QOL scores. Unless the effects also existed before HCT, only the post-HCT effects are described.
Modifying factors of the longitudinal trends of QOL domains for autologous transplantation (HCT) patients
| Modifying factor* . | Percent change† (effect size)‡ in QOL domain score by modifying factor . | |||||||
|---|---|---|---|---|---|---|---|---|
| QOL domain . | ||||||||
| Physical . | Psychological . | Social . | Spiritual . | |||||
| Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | |
| Age at referent HCT, per year increase | — | −0.21%** (0.01) | 0.63%†† (0.02) | — | 0.79%†† (0.03) | 0.47%‡‡ (0.02) | — | — |
| Race/ethnicity | ||||||||
| African American, Hispanic (vs white, Asian, other) | — | −4.0%** (0.18) | — | — | — | — | — | — |
| Asian, other (vs white, Hispanics, African American) | — | — | — | — | — | — | — | 15.4%‡‡ (0.70) |
| Married§ (vs single, divorced/widowed/separated) | — | — | — | — | — | — | 9.9%** (0.34) | — |
| Annual household income less than $75 000 (vs $75 000 or more) | — | −10.1%‡‡ (0.45) | — | — | — | −8.4%** (0.29) | — | — |
| Education | ||||||||
| Less than or more than high school (vs high school only) | — | — | −22.4%†† (0.69) | −11.2%** (0.40) | −14.0%** (0.49) | −11.8%** (0.41) | — | — |
| Bachelor degree or higher (vs less than Bachelor degree) | — | — | — | — | — | — | −12.1%‡‡ (0.47) | −11.9%‡‡ (0.58) |
| BMI§ in kg/m2, per unit increase | — | — | — | — | — | −0.16%‡‡ (0.01) | — | — |
| Change in BMI from pre-HCT level,§,‖ per unit decrease | NA | — | NA | −0.73%‡‡ (0.03) | NA | — | NA | — |
| Primary diagnosis | ||||||||
| Group¶,# 2,4,5,7 (vs group 3) | −20.8%†† (−1.27) | — | — | — | — | — | — | — |
| Groups 2,3 (vs groups 4,5,7) | — | — | — | −9.5%** (0.42) | — | — | — | — |
| High risk of relapse at HCT (vs low risk) | — | — | −8.1%** (0.28) | — | — | — | — | — |
| One transplant (vs more than 1 transplant) | — | — | −16.4%‡‡ (0.52) | — | — | — | — | 8.3%** (0.39) |
| Modifying factor* . | Percent change† (effect size)‡ in QOL domain score by modifying factor . | |||||||
|---|---|---|---|---|---|---|---|---|
| QOL domain . | ||||||||
| Physical . | Psychological . | Social . | Spiritual . | |||||
| Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | |
| Age at referent HCT, per year increase | — | −0.21%** (0.01) | 0.63%†† (0.02) | — | 0.79%†† (0.03) | 0.47%‡‡ (0.02) | — | — |
| Race/ethnicity | ||||||||
| African American, Hispanic (vs white, Asian, other) | — | −4.0%** (0.18) | — | — | — | — | — | — |
| Asian, other (vs white, Hispanics, African American) | — | — | — | — | — | — | — | 15.4%‡‡ (0.70) |
| Married§ (vs single, divorced/widowed/separated) | — | — | — | — | — | — | 9.9%** (0.34) | — |
| Annual household income less than $75 000 (vs $75 000 or more) | — | −10.1%‡‡ (0.45) | — | — | — | −8.4%** (0.29) | — | — |
| Education | ||||||||
| Less than or more than high school (vs high school only) | — | — | −22.4%†† (0.69) | −11.2%** (0.40) | −14.0%** (0.49) | −11.8%** (0.41) | — | — |
| Bachelor degree or higher (vs less than Bachelor degree) | — | — | — | — | — | — | −12.1%‡‡ (0.47) | −11.9%‡‡ (0.58) |
| BMI§ in kg/m2, per unit increase | — | — | — | — | — | −0.16%‡‡ (0.01) | — | — |
| Change in BMI from pre-HCT level,§,‖ per unit decrease | NA | — | NA | −0.73%‡‡ (0.03) | NA | — | NA | — |
| Primary diagnosis | ||||||||
| Group¶,# 2,4,5,7 (vs group 3) | −20.8%†† (−1.27) | — | — | — | — | — | — | — |
| Groups 2,3 (vs groups 4,5,7) | — | — | — | −9.5%** (0.42) | — | — | — | — |
| High risk of relapse at HCT (vs low risk) | — | — | −8.1%** (0.28) | — | — | — | — | — |
| One transplant (vs more than 1 transplant) | — | — | −16.4%‡‡ (0.52) | — | — | — | — | 8.3%** (0.39) |
— indicates statistically non-significant factors (P > .05); therefore percent change and ES are not shown. NA indicates not applicable.
Referent groups are shown in parentheses. Other factors examined that were nonsignificant in the multivariate GEE regression include: sex, disease relapse status, pre-HCT exposure to total body irradiation and chemotherapy-based conditioning agents.
Percent change is calculated as [(expected level of the index group − expected level of the referent group)/(expected level of the referent group)] × 100. For age and BMI, expected levels corresponding to their means at pre-HCT were used as referent levels. For post-HCT, the average of the 4 time points is reported. Positive percent change indicates more favorable QOL in the index group.
Effect size at a particular time point is computed as the absolute value of (expected level of the index group − expected level of the referent group)/observed common SD at that time point. At post-HCT, the average effect size of the 4 time points is reported.
Time-varying: values concurrent to QOL scores were used.
BMI at follow-up minus BMI at pre-HCT.
Group 1: ALL; Group 2: AML, APL; Group 3: Amyloidosis, CLL, Hypereosinophilic syndrome, other-Amyl, POEMS, renal cancer, SAA, CML; Group 4: HD'; Group 5: LBL, NHL, Other-lymph; Group 6: MDS, MPD, myelofibrosis, other-MPP; Group 7: MM.
Disease groups 1 and 6 did not occur under autologous HCT.
Value of P < .05.
Value of P < .001.
Value of P < .01.
Modifying factors of the longitudinal trends of QOL domains in allogeneic transplantation (HCT) patients
| Modifying factor* . | Percent change† (effect size)‡ in QOL domain score by modifying factor . | |||||||
|---|---|---|---|---|---|---|---|---|
| QOL domain . | ||||||||
| Physical . | Psychological . | Social . | Spiritual . | |||||
| Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | |
| Age at referent HCT, per year increase | — | −0.69%** (0.03) | — | — | 0.64%†† (0.02) | 0.75%†† (0.02) | — | — |
| Race/ethnicity | ||||||||
| Asian, other (vs white, Hispanic, African American) | — | — | — | — | −14.5%‡‡ (0.53) | −15.1%‡‡ (0.50) | — | — |
| White (vs Hispanic, African American, Asian, other) | — | — | — | — | — | — | −10.0%‡‡ (0.40) | — |
| Annual household income | ||||||||
| $100 000 or less (vs more than $100 000) | — | — | 9.5%†† (0.37) | — | — | — | — | — |
| $75 000 or less (vs more than $75 000) | — | — | — | — | −13.7%‡‡ (0.54) | −12.9%†† (0.45) | — | — |
| BMI§ in kg/m2, per unit increase | 1.0%†† (0.05) | 1.1%** (0.05) | — | 0.43‡‡ (0.02) | — | — | — | — |
| Change in BMI from pre-HCT level,§,‖ per unit decrease | NA | — | NA | −0.71%†† (0.03) | NA | −0.83% (0.03) | NA | — |
| Primary diagnosis | ||||||||
| Groups¶ 1,4,6 (vs groups 2,3,5,7) | −10.0%** (0.52) | — | — | — | — | — | −9.8%‡‡ (0.42) | — |
| Groups 1,4 (vs groups 2,3,5,6,7) | — | −17.0%** (0.75) | — | — | — | — | — | — |
| Groups 3,5,6,7 (vs groups 1,2,4) | — | — | 12.0%‡‡ (0.40) | — | — | — | — | — |
| Groups 6,7 (vs groups 1,2,3,4,5) | — | — | — | 10.6%†† (0.39) | — | — | — | — |
| Groups 2,4,6 (vs groups 1,3,5,7) | — | — | — | — | −11.7%‡‡ (0.44) | — | — | — |
| Disease in remission§ (vs in relapse) | 8.3%‡‡ (0.44) | — | — | — | — | — | — | — |
| Chronic GVHD§,# (vs no chronic GVHD) | NA | −11.2%** (0.51) | NA | −8.0%‡‡ (0.32) | NA | −10.6%‡‡ (0.37) | NA | −7.8%‡‡ (0.34) |
| Busulfan (vs no busulfan) | — | −17.6%** (0.79) | — | — | — | — | — | — |
| Modifying factor* . | Percent change† (effect size)‡ in QOL domain score by modifying factor . | |||||||
|---|---|---|---|---|---|---|---|---|
| QOL domain . | ||||||||
| Physical . | Psychological . | Social . | Spiritual . | |||||
| Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | Pre-HCT . | Post-HCT . | |
| Age at referent HCT, per year increase | — | −0.69%** (0.03) | — | — | 0.64%†† (0.02) | 0.75%†† (0.02) | — | — |
| Race/ethnicity | ||||||||
| Asian, other (vs white, Hispanic, African American) | — | — | — | — | −14.5%‡‡ (0.53) | −15.1%‡‡ (0.50) | — | — |
| White (vs Hispanic, African American, Asian, other) | — | — | — | — | — | — | −10.0%‡‡ (0.40) | — |
| Annual household income | ||||||||
| $100 000 or less (vs more than $100 000) | — | — | 9.5%†† (0.37) | — | — | — | — | — |
| $75 000 or less (vs more than $75 000) | — | — | — | — | −13.7%‡‡ (0.54) | −12.9%†† (0.45) | — | — |
| BMI§ in kg/m2, per unit increase | 1.0%†† (0.05) | 1.1%** (0.05) | — | 0.43‡‡ (0.02) | — | — | — | — |
| Change in BMI from pre-HCT level,§,‖ per unit decrease | NA | — | NA | −0.71%†† (0.03) | NA | −0.83% (0.03) | NA | — |
| Primary diagnosis | ||||||||
| Groups¶ 1,4,6 (vs groups 2,3,5,7) | −10.0%** (0.52) | — | — | — | — | — | −9.8%‡‡ (0.42) | — |
| Groups 1,4 (vs groups 2,3,5,6,7) | — | −17.0%** (0.75) | — | — | — | — | — | — |
| Groups 3,5,6,7 (vs groups 1,2,4) | — | — | 12.0%‡‡ (0.40) | — | — | — | — | — |
| Groups 6,7 (vs groups 1,2,3,4,5) | — | — | — | 10.6%†† (0.39) | — | — | — | — |
| Groups 2,4,6 (vs groups 1,3,5,7) | — | — | — | — | −11.7%‡‡ (0.44) | — | — | — |
| Disease in remission§ (vs in relapse) | 8.3%‡‡ (0.44) | — | — | — | — | — | — | — |
| Chronic GVHD§,# (vs no chronic GVHD) | NA | −11.2%** (0.51) | NA | −8.0%‡‡ (0.32) | NA | −10.6%‡‡ (0.37) | NA | −7.8%‡‡ (0.34) |
| Busulfan (vs no busulfan) | — | −17.6%** (0.79) | — | — | — | — | — | — |
— indicates statistically nonsignificant factors (P > .05); therefore percent change and ES are not shown. NA indicates not applicable.
Referent groups are shown in parentheses. Other factors examined that were nonsignificant in the multivariate GEE regression include: sex, marital status, education, disease relapse status, risk of relapse at HCT, number of transplants, pre-HCT exposure to total body irradiation.
Percent change is calculated as [(expected level of the index group — expected level of the referent group)/(expected level of the referent group)] × 100. For age and BMI, expected levels corresponding to their means at pre-HCT were used as referent levels. For post-HCT, the average of the 4 time points is reported. Positive percent change indicates more favorable QOL in the index group.
Effect size at a particular time point is computed as the absolute value of (expected level of the index group − expected level of the referent group)/observed common SD at that time point. At post-HCT, the average effect size of the 4 time points is reported.
Time-varying: values concurrent to QOL scores were used.
BMI at follow-up minus BMI at pre-HCT.
Group 1: ALL; Group 2: AML, APL; Group 3: Amyloidosis, CLL, Hypereosinophilic syndrome, other-Amyl, POEMS, renal cancer, SAA, CML; Group 4: HD'; Group 5: LBL, NHL, Other-lymph; Group 6: MDS, MPD, Myelofibrosis, other-MPP; Group 7: MM.
Self-reported.
Value of P < .001.
Value of P < .05.
Value of P < .01.
Autologous HCT.
Physical well-being was worse among African Americans and Hispanics; among younger patients; and for patients with pre-HCT annual income less than $75 000. Psychological well-being was worse among patients diagnosed with AML; and for patients who had a large decline in BMI after HCT regardless of pre-HCT BMI status. In addition, at both pre- and post-HCT time points, psychological well-being was worse for those with less than a high-school education, as well as those with at least some college education. At all time points, social well-being was worse among younger patients, those with less than high school education, as well as those with at least some college education. Social well-being was also worse for patients with pre-HCT annual income less than $75 000; and for patients with higher BMI. Spiritual well-being at all times was lower for patients with college degree or higher. However, spiritual well-being was higher among Asians, and among those who had undergone only one HCT. Lower education level was a single factor significantly associated with lower QOL scores for 3 of the 4 QOL domains, with ES ranging from 0.40 to 0.69.
High risk of relapse of the primary disease before HCT was marginally associated with better spiritual QOL after HCT (5.1%; ES = 0.23, P = .062). Relapse of primary disease was also suggestively associated with poorer psychological QOL (−6.1%; ES = 0.27, P = .061) and social QOL (−5.9%; ES = 0.21, P = .08).
Allogeneic HCT.
Physical well-being was better before and after HCT among patients with higher BMI. However, physical well-being declined with increasing age, and was also lower among patients diagnosed with ALL or HL, among patients who received busulfan for conditioning, and among patients reporting chronic GVHD. Psychological well-being was better after HCT for patients with higher BMI, and among patients diagnosed with MM or myeloproliferative disorders. On the other hand, patients who had a large decline in BMI after HCT regardless of pre-HCT BMI status and patients with chronic GVHD had worse psychological well-being. At all time points, social well-being was worse for younger patients; Asians; and patients with pre-HCT annual household income less than $75 000. Patients reporting chronic GVHD and patients who experienced a large BMI decline after HCT regardless of pre-HCT BMI status also had worse social well-being. Chronic GVHD was the only factor significantly associated with concurrently worse spiritual well-being after HCT. Only chronic GVHD was associated significantly with lower QOL scores for all QOL domains; ES ranged from 0.30 to 0.50. Relapse of primary disease was not associated with any QOL domains.
Of note, no significant differences in physical, psychological, and social QOL were evident between patients not reporting chronic GVHD who underwent allogeneic HCT and those who underwent autologous HCT (P = .84, P = .48, and P = .75, respectively). However, spiritual QOL in patients who underwent allogeneic HCT without chronic GVHD was significantly better than among patients who underwent autologous HCT (P = .037).
Pattern mixture model for longitudinal trends
The deceased/noncompleter stratum comprised 31% (n = 98) of the cohort; 20% (n = 61) were alive/noncompleters; and 49% (n = 153) were alive/completers. The 3 groups (alive/completers, deceased/noncompleters, and alive/noncompleters) did not differ significantly in terms of sex, age, race/ethnicity, marital status, BMI, primary diagnosis, risk of relapse, number of HCTs, and exposure to TBI. Noncompleters tended to have lower level of education (P < .001) and pre-HCT annual household income (P = .001), as well as an overrepresentation of allogeneic transplant recipients (P = .03). In addition, there was an overrepresentation of NHL patients (33%) among the alive/completers compared with deceased/noncompleters (19%), and an overrepresentation of ALL patients (16%) among the deceased/noncompleters compared with the other 2 groups (5%).
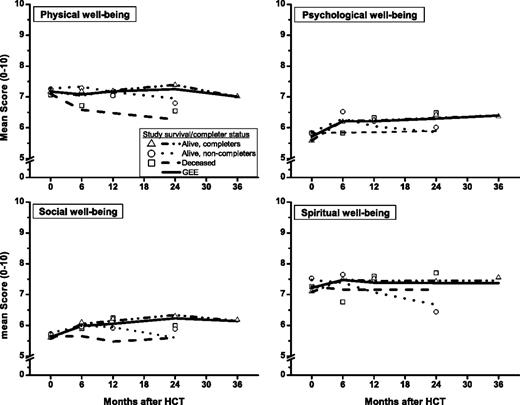
Pre-HCT observations were the only available data for 69% (n = 66) of the deceased/noncompleters and for 38% (n = 23) of the alive/noncompleters. Based on PMM, significant differences in QOL were observed by completion status, for physical well-being (P = .02) and spiritual well-being (P = .05); differences were not statistically significant for psychological well-being (P = .11) and social well-being (P = .07). The predicted parsimonious models of the longitudinal trends in QOL for the 3 groups are shown in Figure 3 along with the overall longitudinal trends for the entire cohort. The overall GEE models generally indicate favorable recovery. However, stratified by survival/completion status, longitudinal QOL scores of the noncompleters were lower than those of the completers at follow-up. Specifically, physical well-being was lower for deceased/noncompleters than for alive/noncompleters (P = .07), which in turn was lower than that for alive/completers (P = .05). Compared with alive/completers, psychological well-being was lower for alive/noncompleters (P = .04). Social well-being was lower for deceased/noncompleters relative to alive/completers (P = .03). Finally, spiritual well-being was lower for alive/noncompleters than for alive/completers (P = .04). These group differences were not significant before HCT.
Pattern mixture model estimates of GEE longitudinal trends. Graphs show GEE longitudinal trends of QOL domains (broken lines) and observed means (symbols) by survival/completer status and GEE longitudinal trends for all patients combined (solid lines).
Pattern mixture model estimates of GEE longitudinal trends. Graphs show GEE longitudinal trends of QOL domains (broken lines) and observed means (symbols) by survival/completer status and GEE longitudinal trends for all patients combined (solid lines).
Of note, QOL trends estimated without regard to survival/dropout status are valid for approximately 50% of the study participants who survived at least 3 years and completed the 3-year assessment (alive/completers), as evidenced by the complete overlap of the overall GEE models with the GEE models for alive/completers (Figure 3). QOL trends after HCT for the remaining 50% of patients were generally worse, likely because of poor health, as evidenced by lower overall survival (67%) for alive/noncompleters compared with alive/completers (85%) (P = .003, after accounting for age and HCT type) from the end of the study participation to November 30, 2008.
Return to work
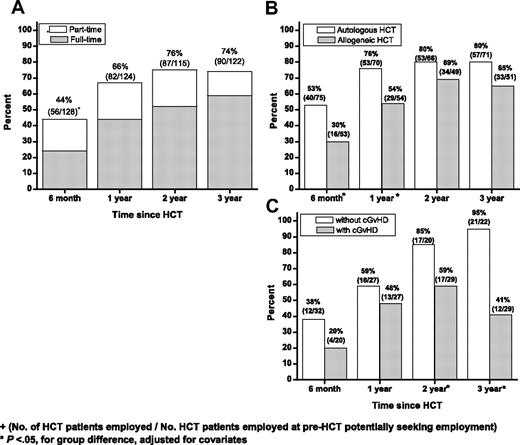
Excluding patients for whom the question regarding return to work was not applicable (eg, retired, student, or never worked), the proportion employed part time or full time increased with time, from 44% (56/128) at 6 months, to 66% (82/124) at 1 year, 76% (87/115) at 2 years, and 74% (90/122) at 3 years (Figure 4A). The proportion of patients working full time increased, from 24% at 6 months to 59% at 3 years. The proportion of patients working part time remained stable at 15% to 23%. Health-related causes comprised 90% of the reasons for not returning to work. Accounting for covariate differences, the proportion of patients returning to work was significantly lower for allogeneic than for autologous HCT patients at 6 months (P = .001) and at 1 year (P = .007), but not at 2 or 3 years (Figure 4B). Among allogeneic patients, the proportion returning to work was significantly lower at 2 and 3 years for those with chronic GVHD compared with those without (P = .01, P = .002, for respective time points). At 3 years, 41% of patients with chronic GVHD were working compared with 95% without chronic GVHD (P = .002; Figure 4C).
Proportion of HCT patients returning to work. Graphs show proportions of HCT patients returning to full- or part-time work by time since HCT according to (A) part-time or full-time status; (B) stem cell source; and (C) chronic GVHD status in allogeneic HCT recipients.
Proportion of HCT patients returning to work. Graphs show proportions of HCT patients returning to full- or part-time work by time since HCT according to (A) part-time or full-time status; (B) stem cell source; and (C) chronic GVHD status in allogeneic HCT recipients.
Covariates significantly associated with returning to work are shown in Table 4 by stem cell source and time from HCT. At 6 months after HCT, patients who underwent autologous HCT in the fourth decade of life were less likely to be working (odds ratio [OR] = 0.25, P = .001), as were patients whose disease was in relapse at 6 months (OR = 0.09, P < .001) or at 1 year (OR = 0.19, P = .01). At 2 and 3 years after HCT, the odds of returning to work were lower for autologous patients with lower pre-HCT annual household income (OR = 0.98 per $10 000 decrease, P ≤ .06). Finally, patients who underwent autologous HCT in the fifth decade of life were significantly less likely to return to work by 3 years (OR = 0.32, P = .045) compared with patients who were either younger or older at HCT.
Factors associated with return to work, by HCT type and time after HCT
| Factor* . | Time since HCT . | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 mo . | 1 y . | 2 y . | 3 y . | |||||
| Odds ratio . | P . | Odds ratio . | P . | Odds ratio . | P . | Odds ratio . | P . | |
| Autologous HCT | ||||||||
| Age at referent HCT | — | — | ||||||
| 40-49 y (vs younger than 40 y, 50 y or older) | 0.25 | < .001 | — | — | — | — | — | |
| 50-59 y (vs younger than 50 y, 60 y or older) | — | — | — | — | 0.32 | .045 | ||
| Annual household income, per $10 000 decrease | — | — | — | — | 0.98 | .06 | 0.98 | .012 |
| Relapsed disease (vs in remission) | 0.09 | < .001 | 0.19 | .010 | — | — | — | |
| Allogeneic HCT | ||||||||
| Age at referent HCT | ||||||||
| 50 y or older (vs younger than 50 y) | — | — | — | — | 0.19 | .002 | — | — |
| Other than single for marital status (vs single) | — | — | 0.04 | .002 | — | — | — | — |
| Annual household income, per $10 000 decrease | — | — | 0.98 | .011 | — | — | — | — |
| BMI less than 30 (vs 30 or more) | 0.12 | .018 | 0.08 | .002 | — | — | — | — |
| Change in BMI from pre-HCT level, per unit decrease | 0.58 | .021 | — | — | — | — | — | — |
| Chronic GVHD | — | — | — | — | 0.24 | .010 | 0.03 | .002 |
| Factor* . | Time since HCT . | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 mo . | 1 y . | 2 y . | 3 y . | |||||
| Odds ratio . | P . | Odds ratio . | P . | Odds ratio . | P . | Odds ratio . | P . | |
| Autologous HCT | ||||||||
| Age at referent HCT | — | — | ||||||
| 40-49 y (vs younger than 40 y, 50 y or older) | 0.25 | < .001 | — | — | — | — | — | |
| 50-59 y (vs younger than 50 y, 60 y or older) | — | — | — | — | 0.32 | .045 | ||
| Annual household income, per $10 000 decrease | — | — | — | — | 0.98 | .06 | 0.98 | .012 |
| Relapsed disease (vs in remission) | 0.09 | < .001 | 0.19 | .010 | — | — | — | |
| Allogeneic HCT | ||||||||
| Age at referent HCT | ||||||||
| 50 y or older (vs younger than 50 y) | — | — | — | — | 0.19 | .002 | — | — |
| Other than single for marital status (vs single) | — | — | 0.04 | .002 | — | — | — | — |
| Annual household income, per $10 000 decrease | — | — | 0.98 | .011 | — | — | — | — |
| BMI less than 30 (vs 30 or more) | 0.12 | .018 | 0.08 | .002 | — | — | — | — |
| Change in BMI from pre-HCT level, per unit decrease | 0.58 | .021 | — | — | — | — | — | — |
| Chronic GVHD | — | — | — | — | 0.24 | .010 | 0.03 | .002 |
— indicates statistically nonsignificant factors (P > .05), therefore odds ratios are not reported.
Referent groups are shown in parentheses. Other factors examined that were not significant include sex, marital status (at pre-HCT and by time point), race/ethnicity, income, and education level at pre-HCT, risk of relapse at HCT, and disease relapse status (at pre-HCT and by time point).
Patients who underwent allogeneic HCT were less likely to be working at 6 months and 1 year if their BMI was lower than 30 (OR = 0.2, P = .031 at 6 months; OR = 0.08, P = .002 at 1 year), as were those who underwent allogeneic HCT who had a larger decline in BMI at 6 months relative to before HCT (OR = 0.6 per unit BMI decrease, P = .02). Also less likely to be working at 1 year were allogeneic patients whose marital status was other than single (OR = 0.04, P = .002) and who had lower pre-HCT annual household income (OR = 0.98 per $10 000 decrease, P = .01). At 2 years after HCT, patients who were 50 years or older at the time of allogeneic HCT were less likely to be working (OR = 0.19, P = .002). Finally, those with chronic GVHD were less likely to report a return to work at the 2-year (OR = 0.24, P = .010) or the 3-year (OR = .03, P = .002) time point.
Discussion
This study demonstrates that if post-HCT attrition is not taken into consideration, the health-related QOL appears stable or improves in the 3 years after transplantation. These findings are consistent with other longitudinal studies.21,22,23,,–26 However, the relatively high mortality and morbidity associated with HCT raises concerns about the possibility of bias35 and the validity of the general trends obtained from conventionally used GEE methods that assume missing data are ignorable or are missing at random.33 Missing data arising from patient dropout because of death or deteriorating health are not random occurrences. These patients may display declining QOL scores before dropping out. Our analysis applying PMM showed that the overall GEE trends are appropriate for half of the HCT study patients who lived at least to the end of the study and completed the last assessment. QOL of dying patients and those in poorer health who dropped out of the study were generally worse, as might be expected. Altmaier et al26 conducted joint analysis of mixed-effects model with time to disease progression/death in a 3-year longitudinal study, and showed a high correlation between change in QOL trajectory and time to disease progression. They concluded that QOL in patients experiencing disease progression who drop out of the study or die from their disease would have worse QOL than survivors if their outcomes could be observed. Lee et al23 in their 2-year longitudinal study also showed that not returning surveys was significantly associated with subsequent relapse or death. Broers et al21 in their 3-year longitudinal study described selective dropout of their study patients, which could have biased the temporal changes observed in QOL. Pidala et al35 concluded that patients with better QOL are more likely to have complete data—an observation that is confirmed conclusively by our study. Hence, in addition to observable covariables, unobservable factors associated with study noncompletion also affect QOL.
Results of our multivariate analyses identified vulnerable subgroups of patients with lower QOL scores for whom closer surveillance and interventions may be beneficial. First, allogeneic and autologous HCT recipients do not experience the same level of health-related QOL in the 3 years after transplantation. Adjusted for covariate differences, mean physical and psychological well-being of allogeneic HCT recipients is statistically significantly lower than that of patients who undergo autologous HCT. The average physical and psychological well-being scores after allogeneic HCT were approximately 5% lower than after autologous HCT. The effect size was approximately 0.25, which is small,37 being less than the 0.3 to 0.5 often considered clinically meaningful.38,–40 Whereas physical, psychological, and social well-being of autologous patients improved at around 6 months to 1 year after HCT and generally stayed elevated to 3 years (average increase of 3%, 13%, and 11%, for physical, psychological, and social well-being, respectively), physical well-being of patients who underwent allogeneic HCT declined (6% on average), and the improvement in psychological and social well-being at 6 months was more modest (average increase of 3% for both domains). The observed difference in QOL by stem cell source is in agreement with some of the larger cross-sectional studies,19,21,41,–43 but contrary to another44 and to 2 longitudinal studies.22,23 The longitudinal studies may have been underpowered because of small sample size at follow-up.
In both allogeneic and autologous transplant recipients, older age was associated with worse physical well-being, consistent with some cross-sectional studies19,45,46 ; however, social well-being was worse in younger patients. Lower pre-HCT annual household income was associated with lower social well-being in both autologous and allogeneic HCT patients. As seen in a cross-sectional study,42 lower education was associated with poorer psychological, social, and spiritual well-being among those who underwent autologous HCT, but not among those who underwent allogeneic HCT.
Although most studies of BMI and QOL show a negative correlation,47,48 this relationship was seen only for social well-being among patients who underwent autologous HCT in our study. In fact, allogeneic patients with higher BMI had better physical and psychological well-being after transplantation; and larger weight loss after HCT was associated with poorer psychological and social well-being. Autologous transplant recipients who lost a larger amount of weight after HCT had poorer psychological well-being. Allogeneic transplant recipients experienced a greater weight loss after HCT (mean change in BMT: −1.4) than autologous transplant recipients (−0.31).
High risk of relapse before HCT was not predictive of post-HCT QOL in allogeneic recipients. After autologous HCT, as anticipated, relapse of primary disease was associated with poorer concurrent psychological and social well-being, whereas receipt of multiple transplants was associated with poorer spiritual QOL. Finally, exposure to busulfan for conditioning in allogeneic transplant recipients was associated with significantly lower physical well-being after HCT. Busulfan is associated with chronic pulmonary disease,49 and could, in this patient population, have contributed to the poorer physical well-being.
As shown by several others,9,46,50,51 the presence of chronic GVHD in patients who underwent allogeneic HCT significantly reduced their QOL. The decline ranged from 7% for psychological well-being to 11% for physical well-being. The large majority of the effect sizes were within the 0.3 to 0.5 range, considered to be clinically relevant.38,–40 Another significant observation in the current study was that no differences in physical, psychological, and social well-being were evident between patients who underwent autologous and allogeneic HCT not reporting chronic GVHD—demonstrating that the differences in QOL between allogeneic and autologous transplant recipients were driven largely by chronic GVHD. Although the proposed new response criteria by the National Institute of Health Consensus Development Project on Criteria for Clinical Trials in Chronic GVHD endorses incorporation of patient self-report as part of response assessment in outcomes research,52 and self-reported chronic GVHD has been shown to correlate well with medical records,53 patient-reported chronic GVHD may still be considered problematic. Therefore, we examined the concordance rate between patient-reported chronic GVHD and clinician report (from medical records), which was 81% for all time points, and increased with time to 94% at 3 years. These findings indicate the reliability of self-reported chronic GVHD and the validity of our results. Misclassification, moreover, tends to reduce rather than enhance the effect size; therefore, our estimate of the chronic GVHD effect is likely an underestimate.
The ability to hold a job is an important indicator of rehabilitation to normal life. The proportion of our study participants working at 6 months (44%), 1 year (66%), and 2 years (76%) was comparable with that reported in 2 longitudinal studies,23,24 but higher than that reported by Syrjala et al,22 possibly because of differences in study composition and nature of data collected. The current study demonstrated that allogeneic HCT patients were less likely to be working compared with autologous HCT patients up to 1 year after transplantation, with the differences disappearing after that, and that overwhelmingly the major reason for not returning to work was related to poor health.
For both transplant types, undergoing HCT in the fourth decade of life or later and lower pre-HCT annual household income were associated with a lower likelihood of returning to work. Not only did allogeneic HCT patients with higher BMI have better QOL, they also were more likely to return to work sooner after HCT than patients with lower BMI. Moreover, patients who experienced greater weight loss had a lower likelihood of working at 6 months after transplantation. Finally, relapse of primary disease was significantly associated with a lower likelihood of return to work for autologous HCT recipients in the first year after HCT, whereas chronic GVHD hampered allogeneic transplant recipients returning to work after the first year.
HCT patients who participated in the current study tended to be better educated than those who did not participate. Because higher education was associated with higher QOL scores among the autologous transplant recipients, the results from this study may be slightly optimistic, although the degree of optimism cannot be quantified.
This limitation notwithstanding, this large longitudinal study demonstrates quite clearly, that among HCT survivors, the QOL is generally good over the 3-year period after transplantation. Patients who underwent allogeneic HCT report poorer QOL compared with those who underwent autologous HCT—a difference that is largely explained by chronic GVHD. Return to work occurs sooner among patients who underwent autologous HCT, especially among those who remain in complete continuous remission. Likelihood of return to work among patients who underwent allogeneic HCT is associated with chronic GVHD. The vulnerable subgroups identified in this study could benefit from more intense surveillance and multidisciplinary approach to follow-up and intervention, including aggressive management of chronic GVHD, as well as exercise for stress reduction,54 and psychosocial interventions55,56 tailored to the specific needs of the patients, which were shown as promising for maintaining or improving QOL after hematopoietic cell transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the National Cancer Institute P01 CA 30206 (S.J.F.) and Lymphoma/Leukemia Society Scholar Award for Clinical Research 2191-02 (S.B.)
National Institutes of Health
Authorship
Contribution: F.L.W. performed the statistical analysis and wrote the paper; L.F., A.B., M. Gonzales, C.H., and M.S. performed data collection; K.T. assisted in data collection and statistical analysis; M. Grant contributed to critical review of the paper; S.J.F. contributed to study design and critical review of the paper; and S.B. contributed to study conception, design, and analysis, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: F. Lennie Wong, Department of Population Sciences, City of Hope National Medical Center, 1500 E Duarte Rd, DPS-173, Duarte, CA 91010-3000; e-mail: lwong@coh.org.
References
Author notes
Presented in abstract form at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, December 8-10, 2007.57