Abstract
von Willebrand factor (VWF) is an essential mediator of platelet adhesion to the vessel wall, but little is known about its role in megakaryocytopoiesis. VWF and its platelet receptor, glycoprotein Ibα (GPIbα), are both expressed during megakaryocyte (MK) maturation. This study was designed to evaluate whether the enhanced VWF-GPIbα interactions typical of patients with von Willebrand disease type 2B (VWD2B) modify platelet production. Platelets from 9 patients with VWD2B with 7 different gain-of-function mutations were examined by electron microscopy (EM) and immunofluorescence labeling. For the patients with VWD2B, EM characteristically showed variable numbers of structurally abnormal giant platelets, sometimes in agglutinates. Cultures of MKs from controls performed with or without purified VWF confirmed a positive influence of VWF on platelet production with specific inhibition by an antibody blocking VWF binding to GPIbα. VWD2B MK cultures examined by EM showed a disorganized demarcation membrane system and abnormal granule distribution. They produced platelets with structural abnormalities typical of VWD2B. Confocal examination of MK revealed limited extension of pseudopods with few large proplatelets. These results confirm that megakaryocytopoiesis is modified by the enhanced VWF-GPIbα interactions. These data obtained for controls and patients with VWD2B suggest a novel regulatory role of VWF-GPIbα interactions in platelet production.
Introduction
von Willebrand factor (VWF) is a large multimeric adhesive glycoprotein that circulates in plasma and is also found in platelets, megakaryocytes (MKs), endothelial cells, and the subendothelial matrix.1,2 VWF is synthesized both in endothelial cells and MKs, where under normal conditions it is stored in secretory organelles as ultralarge multimers (ULVWF) that are processed on secretion by a disintegrin and metalloproteinase with thrombospondin type I motif-13 (ADAMTS13).3 VWF is a well-known mediator of platelet adhesion to the vessel wall and of platelet-platelet interactions under high shear-stress conditions.1 VWF has also been identified as a sensitive and distinct marker for early MKs4 and, more recently, exposure of human MKs to VWF at high shear rates was reported to accelerate platelet production.5
Reduced or dysfunctional levels of VWF cause inherited von Willebrand disease (VWD), currently classified within 6 different types.6 VWD type 2B (VWD2B) results from a gain-of-function of VWF that has an increased affinity for platelet glycoprotein (GP) Ibα.7,8 The mutations responsible for the gain of function are located in exon 28 of the VWF gene coding for the A1 domain. Thrombocytopenia is often present in VWD2B, and the presence of giant platelets has been reported in isolated cases.9-12 Circulating platelet agglutinates have been found in rare families.12-14 Studies using a nanobody recognizing soluble VWF in its gain-of-function state confirmed that soluble VWF in its GPIbα-binding conformation was present in greater amounts in most patients with VWD2B with thrombocytopenia.13
We recently reported that impaired megakaryocytopoiesis results from an abnormal interaction between GPIbα with newly synthesized VWF in the MKs of a family with VWD2B due to a R1308P mutation.14 Severe thrombocytopenia and enlarged platelets were accompanied by the presence of platelet agglutinates in freshly drawn blood. Our observation that the impaired interaction between GPIbα and VWF R1308P was seen in maturing MKs suggested a direct effect of VWF on MK maturation and platelet production. Previously, thrombocytopenia in VWD2B had been considered to result from premature platelet elimination after the abnormal VWF had bound to the platelet surface. But, our work suggested that the abnormal VWF structure in VWD2B was directly influencing megakaryocytopoiesis. More recently, the very rare Montreal platelet syndrome, an autosomal-dominant form of inherited thrombocytopenia associated with lifelong mucocutaneous bleeding, giant platelets, and spontaneous platelet agglutination, has been recognized to be associated with the V1316M VWF mutation typical of VWD2B.15
Given this complex background, we tested here the hypothesis of a role for VWF in human megakaryocytopoiesis. Studies were performed to evaluate the role on platelet morphology and production of the enhanced VWF-GPIb interaction using the human model of patients with VWD2B who are characterized by a gain of function of VWF. Platelets were first examined by electron microscopy; then in in vitro studies, we assessed the role of native VWF and mutated VWF on platelet production by MKs derived from peripheral blood precursors. Not only were platelet ultrastructural abnormalities found to be widespread, suggestive of a generalized abnormal megakaryocytopoiesis in patients with VWD2B; but cultured CD34+ or CD45+ MKs from patients with VWD2B have an altered morphology and produced abnormal and fewer platelets than those observed from control donors, suggesting that the presence of abnormal VWF during MK maturation influenced the formation of platelets. Significantly, the newly produced platelets in vitro have the same abnormal morphologic abnormalities as found in peripheral blood. These data suggest a novel and relevant regulatory role of VWF-GPIb interactions in megakaryocytopoiesis.
Methods
Patients and blood collection
A total of 9 patients with VWD were enrolled in the study after informed consent was obtained in accordance with the Declaration of Helsinski. Diagnosis of VWD was based on the criteria of the International Society of Thrombosis and Haemostasis Scientific and Standardization Committee (ISTH-SSC) VWF Subcommittee.6 The methods used to obtain clinical and laboratory information were as previously described.8 The characteristics of the patients, bleeding score, levels of VWF activities, values of ristocetin-induced platelet aggregation (RIPA), and distribution of VWF multimers are reported in Table 1. All patients were symptomatic with a bleeding score greater than 5. Included were 9 patients with VWD2B from 8 families with the following mutations (Table 1): P1266L (n = 1), R1306W (n = 1), R1308C (n = 1), I1309V (n = 1), V1316M (n = 2), R1341Q (n = 2), and R1341W (n = 1). The platelet count was decreased at the time of examination for 5 of 9 patients with VWD2B and normal for the remainder (Table 1).
Clinical and laboratory parameters of the patients with VWD2B enrolled in the study listed according to mutations of the VWF A1 domain
| Patient no. . | Mutation . | Bleeding score . | Platelet count, × 109/L . | RIPA, mg/mL . | VWF:Ag, U/dL . | VWF:RCo, U/dL . | FVIII:C, U/dL . | VWF:RCo/Ag ratio . | HMW/ intermediate multimers . |
|---|---|---|---|---|---|---|---|---|---|
| P1 | P1266L | 6 | 251 | 0.4 | 59 | 45 | 57 | 0.76 | Present/normal |
| P2 | R1306W | 12 | 175 | 0.6 | 45 | 28 | 51 | 0.62 | Absent/partial loss |
| P3 | R1308C | 9 | 121 | 0.5 | 54 | 21 | 45 | 0.39 | Absent/complete loss |
| P4 | I1309V | 9 | 96 | 0.3 | 160 | 125 | 140 | 0.78 | Absent/normal |
| P5 | V1316M | 13 | 274 | 0.6 | 33 | 12 | 6 | 0.21 | Absent/complete loss |
| P6 | V1316M | 17 | 60 | 0.25 | 70 | 25 | 52 | 0.4 | Absent/complete loss |
| P7 | R1341Q | 9 | 98 | 0.25 | 95 | 70 | 69 | 0.7 | Absent/partial loss |
| P8 | R1341Q | 10 | 120 | 0.5 | 79 | 48 | 47 | 0.6 | Absent/partial loss |
| P9 | R1341W | 9 | 195 | 0.4 | 72 | 42 | 73 | 0.58 | Absent/partial loss |
| Patient no. . | Mutation . | Bleeding score . | Platelet count, × 109/L . | RIPA, mg/mL . | VWF:Ag, U/dL . | VWF:RCo, U/dL . | FVIII:C, U/dL . | VWF:RCo/Ag ratio . | HMW/ intermediate multimers . |
|---|---|---|---|---|---|---|---|---|---|
| P1 | P1266L | 6 | 251 | 0.4 | 59 | 45 | 57 | 0.76 | Present/normal |
| P2 | R1306W | 12 | 175 | 0.6 | 45 | 28 | 51 | 0.62 | Absent/partial loss |
| P3 | R1308C | 9 | 121 | 0.5 | 54 | 21 | 45 | 0.39 | Absent/complete loss |
| P4 | I1309V | 9 | 96 | 0.3 | 160 | 125 | 140 | 0.78 | Absent/normal |
| P5 | V1316M | 13 | 274 | 0.6 | 33 | 12 | 6 | 0.21 | Absent/complete loss |
| P6 | V1316M | 17 | 60 | 0.25 | 70 | 25 | 52 | 0.4 | Absent/complete loss |
| P7 | R1341Q | 9 | 98 | 0.25 | 95 | 70 | 69 | 0.7 | Absent/partial loss |
| P8 | R1341Q | 10 | 120 | 0.5 | 79 | 48 | 47 | 0.6 | Absent/partial loss |
| P9 | R1341W | 9 | 195 | 0.4 | 72 | 42 | 73 | 0.58 | Absent/partial loss |
VWF:Ag indicates VWF antigen; VWF:RCo: VWF as ristocetin cofactor activity; FVIII:C, procoagulant activity of FVIII; VWF:RCo/Ag, calculated ratio with antigen; and HMW/intermediate, high-molecular-weight vs intermediate multimers.
All patients with VWD2B gave an enhanced RIPA in platelet-rich plasma (PRP). The mutation P1266L corresponds to VWD2B “Malmo or New York,” which is associated with increased RIPA, but with normal multimeric distribution in plasma and a normal platelet count.8 Whereas all patients enrolled in the study could be tested for platelet morphology, only 3 patients with VWD2B (1 patient with the R1306W mutation [P2] and 2 patients with the V1316M mutation [P5 and P6] found also in the Montreal platelet syndrome) were available for in vitro studies performed in MK cultures. Since a relative large amount of venous blood was required for these in vitro studies on platelet production by MKs, only male patients with normal levels of hemoglobin were studied after obtaining ethical board approval from all participating institutions and informed consent. The in vitro studies on CD34+/MK were performed at the Department of Anatomy in Parma using blood obtained from healthy donors (HDs) and patients and were done blind (ie, without knowing in advance samples from VWD2B donors), whereas in Bordeaux, cultures were performed from CD45+ cells from HDs or patients.
Proteins
Native VWF was purified from human cryoprecipitate obtained from the Division of Hematology and Transfusion Medicine at the L. Sacco Hospital, as previously described.16 It was characterized by VWF activities and multimer composition typical of normal plasma: no fibrinogen or fibronectin were present. Recombinant mutant R1306W-VWF (VWF2B) was obtained using stable cell lines as described.17 Fibrinogen (FG) was purified from human plasma as reported.18 Human fibronectin (FN) was obtained from Sigma-Aldrich.
Antibodies
A monoclonal antibody (MoAb) directed against αIIbβ3 (AP-2 from Dr T. Kunicki, The Scripps Research Institute) as reported previously was used.19 LJIb1 (anti-GPIbα inhibitor antibody) and LJIb10 (anti-GPIbα antibody control) were prepared from hybridomas made with splenocytes harvested from the same mouse (a kind gift of Dr Z. M. Ruggeri, The Scripps Research Institute). Both are IgG1k and have been characterized in detail. They each recognize a distinct epitope located in the 45-kDa amino-terminal domain of GPIbα. Purifid IgG was stored at −70°C. The antibody LJIb10 has only a minimal inhibitory effect on VWF binding to platelets, but obliterates completely the high affinity α-thrombin binding site, whereas LJ-Ib1 inhibits both binding sites.20,21 Tirofiban (αIIbβ3 inhibitor) was obtained from Merck Sharp Dohme-Chibret and was used according to previously reported recommendations.22
Electron microscopy: standard procedure
Venous blood was taken in ACD-A (1 vol/7 vol) and centrifuged for 10 minutes at 80g, and the PRP up to the buffy coat was carefully aspirated. After incubation for 20 minutes at 37°C, platelets were fixed in 1.25% glutaraldehyde (Fluka Chemie) and diluted in 0.1M phosphate buffer (pH 7.2) for 1 hour at room temperature. For MK cultures, at day 14, cells were fixed under the same conditions after their concentration by centrifugation at 120g for 10 minutes. Samples were processed for EM as previously described.14 Sections were observed with a Jeol JEM-1010 transmission electron microscope (Jeol) at 80 kV. In morphometric studies, a minimum of 100 sections of platelets obtained using standard EM procedures were analyzed for each subject. Platelet diameters and platelet surface area were measured using the software ImageJ (National Institutes of Health). Platelets from 4 HDs have been examined under the same conditions.
Immunofluorescence studies
Smears on glass slides were prepared using the same preparations of PRP as used for EM for each patient. They were fixed with cold acetone, washed in phosphate-buffered saline (PBS)–albumin 0.5% and then incubated with a predetermined optimal dilution of AP-2 directed against αIIbβ3. Highly cross-adsorbed polyclonal antibody to mouse IgG conjugated to Alexa-Fluor 488 (green; Interchim) was then used to reveal bound mouse IgG. After further washings, a drop of a solution of 4,6-diamidino-2-phenylindole (DAPI) was used to visualize DNA. The slides were examined with a Nikon Eclipse 80i fluorescent microscope (Nikon France SAS) equipped with a fluorescence illumination system EXFO Xcite and a Nikon DXM1200F camera. The objectives were Nikon Plan Fluor 100×/1.30 (oil) and Nikon Plan Fluor 40×/0.75. Images were analyzed using Nikon NIS Elements D2.20 imaging software. To study the formation of proplatelets from MK in culture, cells were replated on a poly-L-lysine–coated coverslip in a 4-well plate. The MK-extending proplatelets were studied after 24 hours with a Leica SP2 confocal microscope equipped with Leica confocal software using a 40×/1.25 or a 63×/1.40 objective (Leica), after labeling with AP-2 directed against αIIbβ3 and a species-specific goat anti–mouse antibody conjugated with FITC (DakoCytomation) and by DAPI to visualize DNA as described in the next paragraph.
CD34+ or CD45+ purification and MK differentiation
Primary CD34+ cells were isolated from peripheral blood of HDs or 3 patients with VWD2B by immunomagnetic positive selection using the CD34+ (Parma; 150-200 mL of blood) or CD45+ (Bordeaux; 40 mL of blood) cell isolation Kit (Miltenyi Biotec) in the magnetic field of a Vario-MACS apparatus (Miltenyi Biotec), according to the manufacturer's protocols.23 The purity of CD34+ cell preparations was immediately verified by a R-phycoerythrin (RPE)–conjugated anti-CD34 MoAb (Beckman Coulter) and flow cytometry. Only samples exceeding 95% purity were used for subsequent experiments. Purified human CD34+ cells were cultured up to 7 days (or 14 days in some experiments), at an optimal cell density of 1 × 106 cells/mL, in serum-free X-vivo medium (BioWhittaker) supplemented every 3 days with 3 ng/mL of recombinant human interleukin-3 (IL-3), 40 ng/mL recombinant human stem cell factor (SCF), and 100 ng/mL recombinant human thrombopoietin (TPO), in the presence or absence (controls) of 10 μg/mL of purified VWF. To test whether the effects of VWF were dependent on its concentration, we cultured CD34+ cells from HDs with increasing doses of native purified VWF (2.5, 5, 10, 20, and 40 μg/mL). In some experiments, we also used FG (200 μg/mL) or FN (200 μg/mL) instead of VWF. To test the specificity of exogenous VWF in its interactions with GPIbα, in some experiments purified CD34+ cells were cultured in the presence of 80 μg/mL LJIb1 or 50 μg/mL LJIb10 (both anti-GPIbα MoAbs), or 20 μg/mL tirofiban (αIIbβ3 inhibitor). In other types of experiments (in Bordeaux), the cultures were performed under the same conditions but from CD45+ isolated cells. In case of morphologic studies, the MK cultures were performed without addition of exogenous VWF.
Flow cytometric analysis
To quantify the platelet production in culture, fixed volumes of the culture supernatants were collected, incubated with anti-CD41–RPE and calcein AM (Sigma; to exclude fragments) and added to a fixed volume of calibration beads (DAKO) at a known concentration, as described previously.23,24 Both the platelet and bead populations were simultaneously identified in flow cytometry on the forward scatter (FS) versus logarithmic side scatter (SS) dot plot. The absolute platelet count was performed on the CD41+/Calcein am+ platelet population. Analysis of the samples was performed by an Epics XL flow cytometer and the Expo ADC software (both Beckman Coulter). The instrument was calibrated daily with a set of standardized beads (Dako).23,24
Statistics
To evaluate differences among various groups of samples, a Bonferroni-type analysis was applied to the data obtained in different experiments: P values less than .05 were considered significant. Error bars represent the standard error of the mean (SEM) or the standard deviation (SD), as indicated in the figure legends.
Results
Ultrastructure of platelets in VWD2B
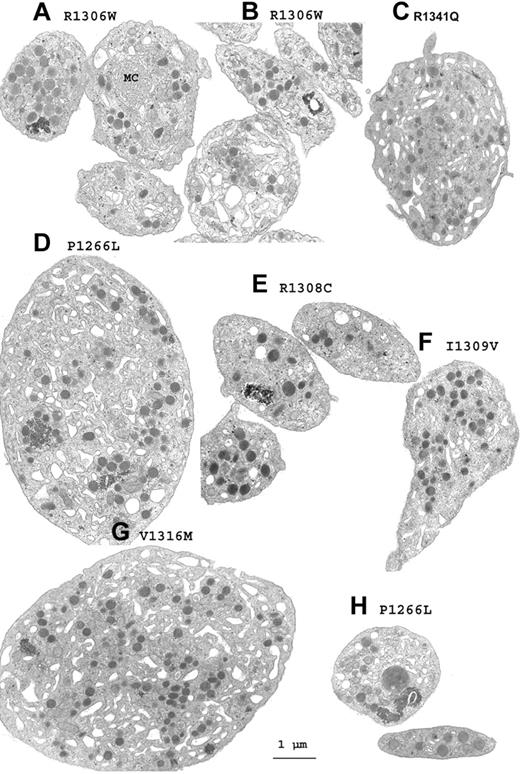
For each patient, platelets with an abnormal morphology were found within the normal population. Figure 1 shows a gallery of electron micrographs of platelets chosen to illustrate the morphologic changes that were detected in VWD2B. Illustrations are given for each mutation. Globally, the characteristics included platelet agglutinates without signs of activation and sometimes with what appear to be fused surface membranes (Figure 1A-B,E), whereas enlarged but not totally spherical platelets with an altered distribution of membranes and granules were abundant (Figure 1C-D,G). Even when not giant, platelet size and shape was often heterogeneous and occasional enlarged α-granules were seen (Figure 1H). These observations led us to perform a morphometric analysis.
A gallery of electron micrographs of platelets from patients with VWD2B. (A-B,E) Platelet agglutinates. (A) Membrane complexes (MCs) in an enlarged platelet. (D) Giant platelet from P1 with the P1266L mutation where high-molecular-weight (HMW) multimers are present despite a positive RIPA. (H) α-granule of increased size for P1. (G) Giant platelet from P6 with the V1316M mutation. (C,F) Enlarged platelets of abnormal form are shown for P7 (C) and for P4 (F). Many of the illustrated platelets contain increased amounts of surface-connected canalicular systems, and the granules are not homogeneously distributed. Scale bar equals 1 μm for all the electron micrographs.
A gallery of electron micrographs of platelets from patients with VWD2B. (A-B,E) Platelet agglutinates. (A) Membrane complexes (MCs) in an enlarged platelet. (D) Giant platelet from P1 with the P1266L mutation where high-molecular-weight (HMW) multimers are present despite a positive RIPA. (H) α-granule of increased size for P1. (G) Giant platelet from P6 with the V1316M mutation. (C,F) Enlarged platelets of abnormal form are shown for P7 (C) and for P4 (F). Many of the illustrated platelets contain increased amounts of surface-connected canalicular systems, and the granules are not homogeneously distributed. Scale bar equals 1 μm for all the electron micrographs.
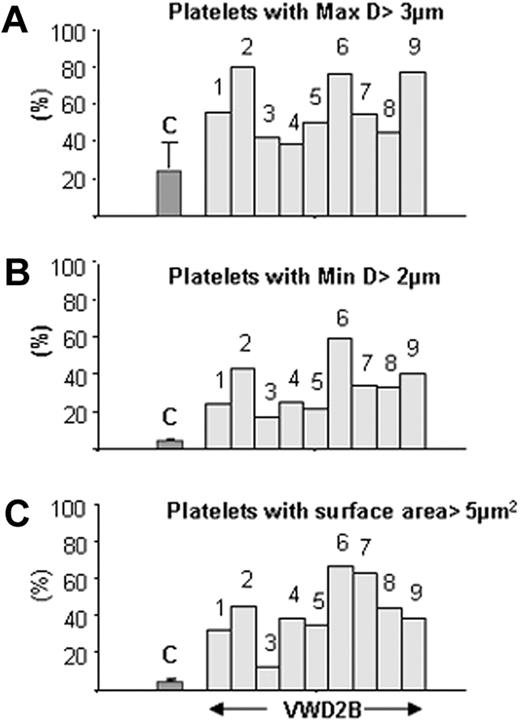
Morphometric studies of platelet size in VWD2B
Results summarized in Table 2 show that mean maximal platelet diameter was increased for most patients with VWD2B; nonetheless, results were variable and remained at the upper limit of normal values for P3, P4, and P8. This heterogeneity extended to platelet surface area, where mean values were always greater than that seen for normal platelets and sometimes by several-fold. We show in Figure 2 the proportion of platelets with increased size for each patient. Results confirm that for each patient with VWD2B, platelet maximal diameter and minimal diameter increased almost proportionally, with the largest platelets found for P2 (R1306W), P6 (V1316M), and P9 (R1341W). Notably, quite marked size differences were seen between P5 and P6, both of whom possessed the V1316M mutation.
Morphometric studies of the platelets
| Patient no. . | Mutations . | Maximum diameter, μm . | Minimum diameter, μm . | Area, μm2 . |
|---|---|---|---|---|
| Control (n = 4) | — | 2.7 ± 0.5 | 1.1 ± 0.5 | 2.4 ± 1.3 |
| P1 | P1266L | 3.7 ± 1.7 | 1.6 ± 1.0 | 5.5 ± 5.7 |
| P2 | R1306W | 3.5 ± 0.7 | 1.8 ± 0.7 | 4.7 ± 2.4 |
| P3 | R1308C | 2.9 ± 0.6 | 1.4 ± 0.6 | 3.2 ± 2 |
| P4 | I1309V | 2.9 ± 1.0 | 1.5 ± 0.6 | 6.8 ± 6.8 |
| P5 | V1316M | 3.1 ± 1.1 | 1.5 ± 0.6 | 6.0 ± 6.1 |
| P6 | V1316M | 3.7 ± 1.2 | 2.4 ± 1.0 | 10.4 ± 11.2 |
| P7 | R1341Q | 3.2 ± 0.6 | 1.8 ± 0.7 | 9.9 ± 8.7 |
| P8 | R1341Q | 3.1 ± 0.7 | 1.8 ± 0.7 | 7.6 ± 7.7 |
| P9 | R1341W | 3.6 ± 1.0 | 1.9 ± 0.9 | 5.6 ± 5.4 |
| Patient no. . | Mutations . | Maximum diameter, μm . | Minimum diameter, μm . | Area, μm2 . |
|---|---|---|---|---|
| Control (n = 4) | — | 2.7 ± 0.5 | 1.1 ± 0.5 | 2.4 ± 1.3 |
| P1 | P1266L | 3.7 ± 1.7 | 1.6 ± 1.0 | 5.5 ± 5.7 |
| P2 | R1306W | 3.5 ± 0.7 | 1.8 ± 0.7 | 4.7 ± 2.4 |
| P3 | R1308C | 2.9 ± 0.6 | 1.4 ± 0.6 | 3.2 ± 2 |
| P4 | I1309V | 2.9 ± 1.0 | 1.5 ± 0.6 | 6.8 ± 6.8 |
| P5 | V1316M | 3.1 ± 1.1 | 1.5 ± 0.6 | 6.0 ± 6.1 |
| P6 | V1316M | 3.7 ± 1.2 | 2.4 ± 1.0 | 10.4 ± 11.2 |
| P7 | R1341Q | 3.2 ± 0.6 | 1.8 ± 0.7 | 9.9 ± 8.7 |
| P8 | R1341Q | 3.1 ± 0.7 | 1.8 ± 0.7 | 7.6 ± 7.7 |
| P9 | R1341W | 3.6 ± 1.0 | 1.9 ± 0.9 | 5.6 ± 5.4 |
Maximum diameter, minimum diameter, and area are shown as median ± SD.
Quantitative morphometric evaluation of platelet size parameters in patients with VWD. The results are given as percentage of platelets with maximal diameter greater than 3 μm (A), with minimal diameter greater than 2 μm (B), and with surface area greater than 5 μm2 compared with controls (C) (n = 4, median ± SD).
Quantitative morphometric evaluation of platelet size parameters in patients with VWD. The results are given as percentage of platelets with maximal diameter greater than 3 μm (A), with minimal diameter greater than 2 μm (B), and with surface area greater than 5 μm2 compared with controls (C) (n = 4, median ± SD).
Further evidence for an abnormal megakaryocytopoiesis using immunofluorescence labeling
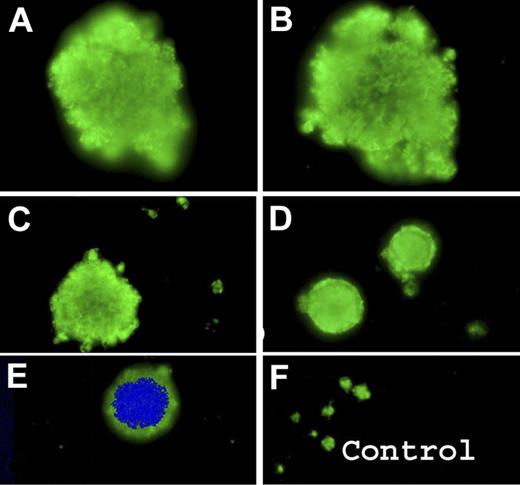
Figure 3 illustrates the results for platelets from the patients with VWD2B (P2) with the R1306W mutation and was typical of the results seen for all the patients. Selected images for αIIbβ3 are shown in Figure 3A-E. Compared with normal platelets (Figure 3F), there was considerable size heterogeneity with the presence of giant platelets. Occasional nucleated cells were identified as MKs by their labeling for αIIbβ3.
Detection of circulating MKs or their fragments by immunofluorescence labeling of blood smears from VWD2B using a MoAb specific for the αIIbβ3 complex. (A-E) Results for P2 with the R1306W VWD2B mutation show enlarged platelets confirming size diversity with occasional MK staining both with DAPI and for αIIbβ3. Control platelets are shown for comparison (F).
Detection of circulating MKs or their fragments by immunofluorescence labeling of blood smears from VWD2B using a MoAb specific for the αIIbβ3 complex. (A-E) Results for P2 with the R1306W VWD2B mutation show enlarged platelets confirming size diversity with occasional MK staining both with DAPI and for αIIbβ3. Control platelets are shown for comparison (F).
Effects on platelet production of exogenous VWF and other ligands or inhibitors
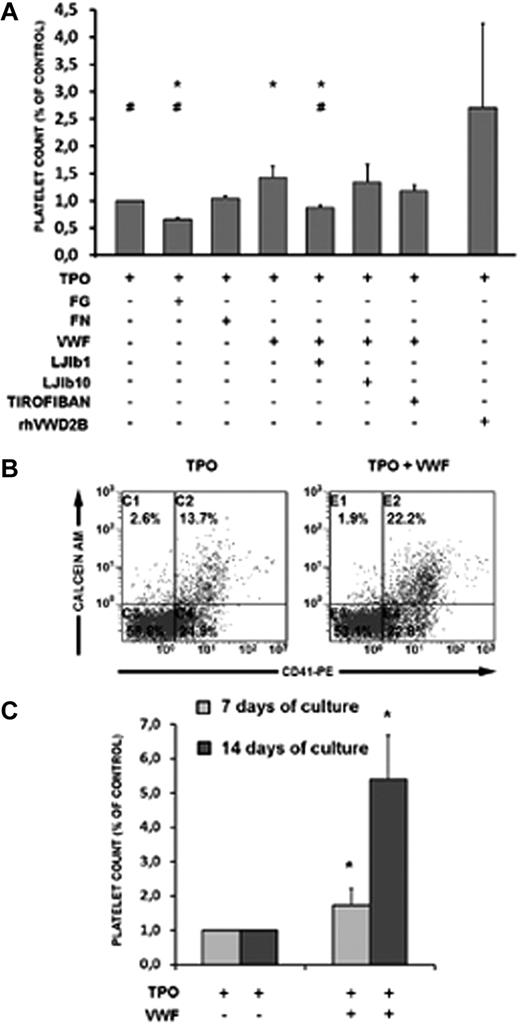
We first studied the effects of exogenous VWF on platelet production after in vitro MK differentiation. Primary CD34+ cells from HDs cultured for 7 days in the presence of TPO and VWF released a higher number of platelets compared with TPO alone (Figure 4 A-B). In contrast, αIIbβ3 ligands, such as FG and FN, did not boost platelet production. VWF effects on platelet production were indeed related to GPIbα because these effects were selectively blocked by its functional inhibition by the antibody LJIb1, but not by LJIb10, reactive with the thrombin-binding site. Conversely, tirofiban, which inhibits αIIbβ3, was not able to block the VWF-induced enhancement of platelet production in TPO-treated CD34+ cell cultures. Of note, the addition of exogenous R1306W-recombinant VWF to CD34+ cells from HDs strongly stimulated platelet production (Figure 4A).
Platelet production from HD CD34+ cell cultures. Relative number of platelets at day 7 of treatment with TPO or TPO and VWF (A). The effects of fibrinogen (FG) and fibronectin (FN) and different inhibitors on platelet production in the presence or absence of VWF is shown. The number of platelets was calculated by flow cytometry on a CD41+/calcein am+-labeled population; a representative dot plot is shown (B) analyzed in combination with a known number of calibration beads. (C) Time-course kinetics of platelet production after 7 and 14 days of treatment with TPO (control) or TPO and VWF. Data from 4 independent experiments are shown. The statistical analyses according to Bonferroni test were grouped as follows: In Group 1, samples from A to G were all calculated against A. In this group, only sample D showed statistical significance (means ± SD; *P < .05 vs TPO treatment); VWF promotes platelet production. In Group 2, samples from A to G were all calculated against D. In this group, samples A, B, and E were significantly different; GPIba inhibition selectively counteracts VWF stimulation (means ± SD; #P < .05 vs TPO and VWF treatment). In Group 3, samples A, D, and H were all calculated against A and D. In this group, the sample H showed statistical significance; recombinant mutated VWF strongly promotes platelet production from normal CD34+ cells (means ± SD; *P < .05 vs TPO treatment; #P < .05 versus TPO and VWF treatment).
Platelet production from HD CD34+ cell cultures. Relative number of platelets at day 7 of treatment with TPO or TPO and VWF (A). The effects of fibrinogen (FG) and fibronectin (FN) and different inhibitors on platelet production in the presence or absence of VWF is shown. The number of platelets was calculated by flow cytometry on a CD41+/calcein am+-labeled population; a representative dot plot is shown (B) analyzed in combination with a known number of calibration beads. (C) Time-course kinetics of platelet production after 7 and 14 days of treatment with TPO (control) or TPO and VWF. Data from 4 independent experiments are shown. The statistical analyses according to Bonferroni test were grouped as follows: In Group 1, samples from A to G were all calculated against A. In this group, only sample D showed statistical significance (means ± SD; *P < .05 vs TPO treatment); VWF promotes platelet production. In Group 2, samples from A to G were all calculated against D. In this group, samples A, B, and E were significantly different; GPIba inhibition selectively counteracts VWF stimulation (means ± SD; #P < .05 vs TPO and VWF treatment). In Group 3, samples A, D, and H were all calculated against A and D. In this group, the sample H showed statistical significance; recombinant mutated VWF strongly promotes platelet production from normal CD34+ cells (means ± SD; *P < .05 vs TPO treatment; #P < .05 versus TPO and VWF treatment).
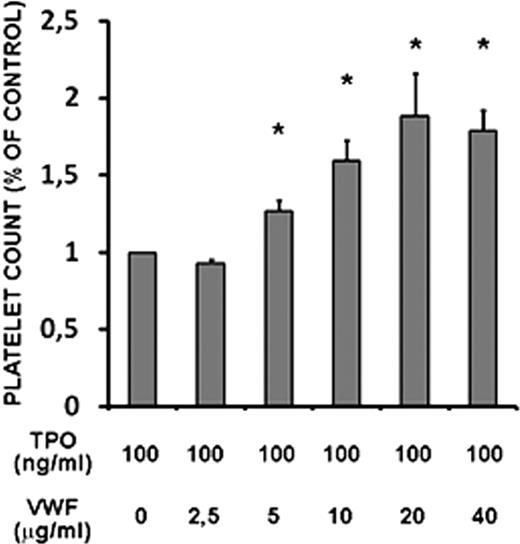
The presence of VWF in the TPO-treated CD34+ cell cultures not only boosted the production of platelets in vitro at 7 days, but continued to promote terminal MK differentiation even after 14 days of culture (Figure 4C). Moreover, the effects of VWF on platelet production were dose-dependent, as shown in Figure 5.
Platelet production from healthy donor (HD) plus CD34+ cell cultures in the presence of increasing concentrations of native VWF. Relative number of platelets at day 7 of treatment with TPO or TPO and VWF at the indicated doses. The number of platelets was calculated by flow cytometry on a CD41+/calcein am+-labeled population analyzed in combination with a known number of calibration beads. Data are from 2 independent experiments. All the samples were analyzed with Bonferroni test against TPO treatment (means ± SD; *P < .05 vs TPO treatment).
Platelet production from healthy donor (HD) plus CD34+ cell cultures in the presence of increasing concentrations of native VWF. Relative number of platelets at day 7 of treatment with TPO or TPO and VWF at the indicated doses. The number of platelets was calculated by flow cytometry on a CD41+/calcein am+-labeled population analyzed in combination with a known number of calibration beads. Data are from 2 independent experiments. All the samples were analyzed with Bonferroni test against TPO treatment (means ± SD; *P < .05 vs TPO treatment).
Platelet production by normal versus VWD2B MKs (endogenous VWF)
Quantitative aspects.
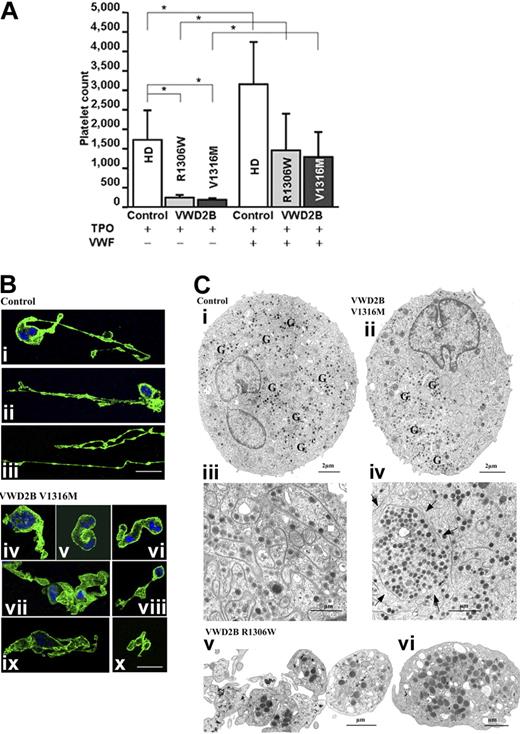
We finally tested the effects of purified native VWF on CD34+ cells isolated from the peripheral blood of 3 patients with VWD2B and 3 HDs (Figure 6A). In the absence of exogenous VWF, platelet production from CD34+ cells from patients with VWD2B cultured for 7 days with 100 ng/mL of TPO was reduced compared with CD34+ cells from the HDs. However, the addition of native exogenous VWF with the TPO was able to increase platelet production levels from CD34+ VWD2B cells, even if the global level of platelet production remained lower than that of VWF-treated CD34 from HDs (Figure 6A).
Platelet production and megakaryocytopoiesis in culture. (A) Platelet production from cultured CD34+ MKs obtained from 3 different HDs (empty histograms) used as controls and from 3 patients with VWD2B: 1 patient with mutation R1306W (light gray histograms) and 2 patients with mutation V1316M (dark gray histograms). Relative number of platelets at day 7 of treatment with TPO (left) or TPO and VWF (right). The number of platelets was calculated by flow cytometry on a CD41+/calcein am+-labeled population analyzed in combination with a known number of calibration beads. Data from 2 independent experiments (means ± SD; *P < .05 HD versus each mutant VWF as well as HD and each mutant VWF exposed to VWF versus TPO treatment only). The statistical analyses according to Bonferroni test were grouped as follows. In Group 1, all samples were calculated against TPO-treated HDs. In this group, the TPO-treated VWD2B samples show statistical significance; endogenous VWD2B inhibits platelet production. In Group 2, samples from VWD2B were calculated against TPO-treated VWD2B. In this group, samples treated with normal exogenous VWF are significantly different compared with TPO treatment; normal exogenous VWF normalizes platelet production from VWD2B CD34+ cells. (B) Morphologic studies of the formation of proplatelets as seen by confocal microscopy of MK cultures labeled with an anti-αIIbβ3 (green) antibody and by DAPI (blue). In panels Bi and Bii, the progressive disruption of MK cytoplasm and formation of proplatelets can be seen; MKs appeared with long pseudopods decorated with small blisters corresponding to proplatelets. In panel Biii, the very long pseudopods have a bead-like appearance. For the VWD2B MKs, instead of thin pseudopods, the cytoplasm forms thick protusions (Biv-ix) with interwoven membranes (vii, ix). In panel Bx, 3 distinct but adjacent proplatelets are to be seen. (C) Examination of culture MKs from controls and patients by EM. On the left side (Ci,Ciii), we illustrate the morphology of control MKs, and in panels Cii and Civ, the morphology of VWD2B V1316M MKs. (Ci) Homogeneous presence of granules and DMS. (Cii) All the granules (G) are concentrated in one part of the MK cytoplasm; most of the DMS is also seen in this same area, suggesting a defect in the process of partitioning the components. (Ciii) Shown at a higher magnification is the cytoplasm of another control mature MK, where small platelet territories are well delimited by abundant and well-organized DMS. (Civ) Shown at a high magnification is part of a mature VWD2B MK where an abnormal large platelet territory (arrows) surrounded by DMS can be seen. Globally abnormal distribution of DMS suggests a defect in the organization of membranes during maturation. (Cv-vi) Newly produced platelets from the R1306W MK. (Cv) the platelets are heterogeneous in size, not separated/or agglutinated, and the distribution of granules is heterogeneous. (Cvi) Illustration of a giant platelet as found in the circulation.
Platelet production and megakaryocytopoiesis in culture. (A) Platelet production from cultured CD34+ MKs obtained from 3 different HDs (empty histograms) used as controls and from 3 patients with VWD2B: 1 patient with mutation R1306W (light gray histograms) and 2 patients with mutation V1316M (dark gray histograms). Relative number of platelets at day 7 of treatment with TPO (left) or TPO and VWF (right). The number of platelets was calculated by flow cytometry on a CD41+/calcein am+-labeled population analyzed in combination with a known number of calibration beads. Data from 2 independent experiments (means ± SD; *P < .05 HD versus each mutant VWF as well as HD and each mutant VWF exposed to VWF versus TPO treatment only). The statistical analyses according to Bonferroni test were grouped as follows. In Group 1, all samples were calculated against TPO-treated HDs. In this group, the TPO-treated VWD2B samples show statistical significance; endogenous VWD2B inhibits platelet production. In Group 2, samples from VWD2B were calculated against TPO-treated VWD2B. In this group, samples treated with normal exogenous VWF are significantly different compared with TPO treatment; normal exogenous VWF normalizes platelet production from VWD2B CD34+ cells. (B) Morphologic studies of the formation of proplatelets as seen by confocal microscopy of MK cultures labeled with an anti-αIIbβ3 (green) antibody and by DAPI (blue). In panels Bi and Bii, the progressive disruption of MK cytoplasm and formation of proplatelets can be seen; MKs appeared with long pseudopods decorated with small blisters corresponding to proplatelets. In panel Biii, the very long pseudopods have a bead-like appearance. For the VWD2B MKs, instead of thin pseudopods, the cytoplasm forms thick protusions (Biv-ix) with interwoven membranes (vii, ix). In panel Bx, 3 distinct but adjacent proplatelets are to be seen. (C) Examination of culture MKs from controls and patients by EM. On the left side (Ci,Ciii), we illustrate the morphology of control MKs, and in panels Cii and Civ, the morphology of VWD2B V1316M MKs. (Ci) Homogeneous presence of granules and DMS. (Cii) All the granules (G) are concentrated in one part of the MK cytoplasm; most of the DMS is also seen in this same area, suggesting a defect in the process of partitioning the components. (Ciii) Shown at a higher magnification is the cytoplasm of another control mature MK, where small platelet territories are well delimited by abundant and well-organized DMS. (Civ) Shown at a high magnification is part of a mature VWD2B MK where an abnormal large platelet territory (arrows) surrounded by DMS can be seen. Globally abnormal distribution of DMS suggests a defect in the organization of membranes during maturation. (Cv-vi) Newly produced platelets from the R1306W MK. (Cv) the platelets are heterogeneous in size, not separated/or agglutinated, and the distribution of granules is heterogeneous. (Cvi) Illustration of a giant platelet as found in the circulation.
Morphologic aspects.
We used confocal microscopy to examine MKs in culture at day 14 at a late stage of maturation (Figure 6B). First illustrated are control MKs typically showing very thin and long extensions with small swellings (Figure 6Bi-ii); beads extending for a long distance can be also observed in Figure 6Biii. Conversely, in MK cultures from the V1316M patients, the extensions are shorter and the swellings larger (Figure 6Bi-vi), with proplatelets remaining distinct but associated (Figure 6Bvii). MK cultures were also performed until day 14 and examined by EM (Figure 6C). For the controls, EM showed a normal MK morphology and granules were uniformly distributed, whereas the demarcation membrane system (DMS) uniformly invaded the cytoplasm (Figure 6Ci). For VWD2B V1316M cultures, it can be seen that granules are distributed heterogeneously within most of the MKs, whereas the DMS is poorly developed (Figure 6Cii). At higher magnification, in mature MKs, platelet territories are small for controls and delimitated by a well-developed membrane system (Figure 6Ciii), whereas for the VWD2B V1316M, a defective DMS delimits large platelet territories containing varying numbers of granules, whereas other parts of the cytoplasm contains little DMS (Figure 6Civ). Similar observations were made for MK from the R1306W patient (results not shown). In Figure 6Cv and vi, platelets produced in vitro from the VWD2B R1306W are shown; note the heterogeneity in size, in granule distribution, and again the presence of giant platelets as illustrated in Figure 6Cvi.
Discussion
VWF plays a major role in hemostasis, and molecular abnormalities or deficiency of this protein cause inherited VWD.1,2 By associating with collagen in the vascular matrix, VWF permits the anchoring of platelets at the site of vascular lesions under shear and then to the arrest of bleeding.25,26 In VWD2B, conformational changes in the mutated VWF are responsible for an up-regulated binding to GPIbα.27 A consequence of this gain-of-function is the selective binding of the larger multimers to the platelets and the loss of VWF multimers from the circulation. The concentration of circulating VWF multimers in a GPIbα-binding conformation is particularly elevated in patients with VWD2B and thrombocytopenia.8 Such a result suggests that accelerated platelet loss from the circulation is linked to the presence of platelet-reactive VWF multimers.8 Our current working hypotheses is that an altered platelet production in addition to the loss of the larger VWF multimers may intervene to cause a low platelet count in VWD2B and, more generally, that the VWF-GPIb interactions is crucial for normal megakaryocytopoiesis. Earlier reports showing (1) impaired megakaryocytopoiesis in a family with the R1308P mutation of VWF, (2) altered megakaryocytopoiesis for patients with Bernard-Soulier syndrome (BSS) and heterozygotes for the Bolzano mutation of GPIbα, and (3) that platelet production was accelerated by high shear forces in presence of VWF constitute additional reasons to suggest a role for the interaction between GPIbα and VWF in megakaryocytopoiesis.5,14,28,29
Now, we report that in a large series of patients with VWD2B with different mutations, variable populations of platelets have increased size with ultrastructural characteristics of giant platelet syndromes. Giant granules were occasionally found; independent of the nature of the mutation, these granules were confirmed to contain VWF by immunogold labeling (data not shown). Giant platelets were regularly present in higher numbers than in HDs, a finding seen whether or not the patient was thrombocytopenic. Giant platelets have also been reported in the Montreal platelet syndrome first considered to be a platelet disease,30 but now shown to be VWD2B-associated with the presence of platelet agglutinates.15 Giant granules are rare but well described in the Paris-Trousseau syndrome31 given by mutations of the Fli gene, but the mechanism responsible for their formation is unknown. Our results implied that enhanced binding of endogenous VWF to GPIbα observed in patients with VWD2B can interfere with the fine regulation of megakaryocytopoiesis.
The first confirmation of this came from cultures of CD34+ cells obtained from HDs when platelet production was shown to be increased by the addition of VWF with more platelets liberated at day 7 and day 14 of culture than in the presence of TPO alone. The potentiating effect of VWF was specifically mediated by the VWF-binding domain of GPIbα. In fact, the use of the antibody against GPIbα (LJIb1) could block such a VWF-dependent accelerated platelet production, and this was specific, since another MoAb against the thrombin-binding site of GPIbα (LJIb10) did not interfere with this positive effect. The lack of inhibition with tirofiban further showed that VWF was not mediating this effect through αIIbβ3. In our sets of experiments, we could confirm that the VWF-dependent positive effect on platelet production does require an intact and functional GPIbα. Moreover, other ligands of αIIbβ3 (FN and FG) were not able to boost MK differentiation. Interestingly, antibodies against GPIbα, which selectively block VWF effects on MK cells, have been previously shown to interfere with MK maturation and platelet production in idiopathic thrombocytopenic purpura (ITP).32
Further evidence came when we found that not only native VWF, but also recombinant R1306W-VWF mutant protein was able to boost in vitro MK differentiation from normal CD34+ cells, and even more efficiently than its native form when platelet production was studied after 7 and 14 days of maturation. This observation suggested that interaction of exogenous VWF to membrane GPIbα was able to promote MK differentiation, and that an increased VWF/GPIb interaction can accelerate the last step of platelet release. The fact that (1) the same morphologic characteristics were present in these platelets as well as platelets derived from the peripheral blood, and (2) that abnormalities were present during megakaryocytopoiesis implied a role for newly synthesized VWF and GPIbα. Performing the opposite experiments using VWD2B-MK, we could also show that the exposure of MK cultures from patients with VWD2B to normal purified VWF was able to improve platelet production, suggesting the same VWF-dependent positive effect on platelet production independently of the source of the MKs (HDs or patients with VWD2B). In our hands, VWF accelerated platelet production when added to the media and this effect was dose-dependent and independent of high shear rate. Recent evidence from studies performed in cultures point to a role for interactions between mature MKs and stromal adhesive proteins in proplatelet formation.25,26 Interestingly, GPIbα is a signaling molecule,33 offering the possibility that the GPIb-VWF axis may play a regulatory role in MK maturation. GPIbα serves as an anchor between extracellular matrix proteins and the actin network; significantly, macrothrombocytopenia was improved in a BSS mouse model in which the GPIbα cytoplasmic domain was retained.34
In parallel to the work performed in patients with BSS (see Nurden and George28 ) or GPIbα-deficient mice,35 or with the patients with Bolzano heterozygote mutation of GPIbα characterized by an abnormal GPIbα,29 we could demonstrate that the CD34+/MKs obtained from patients with VWD2B characterized by 2 relatively common mutations (R1306W and V1316M) showed significantly abnormal platelet formation. Although GPIbα is expressed later than αIIbβ3,36 it is present in maturing MKs and may play a role in the interaction in the development of internal MK membranes and formation of pseudopods. Using confocal microscopy, it was possible to visualize the extending pseudopods, and we confirmed with cultures from CD34+ or CD45+ cells that major differences were present for the patients. For controls, the proplatelets were mostly long and thin and decorated by protrusions or beads corresponding to the small future platelets; for VWD2B, the pseudopods remained thick, were fewer in number, and the protrusions larger. EM of the cultured MKs confirmed the presence of morphologic abnormalities in the cytoplasm with an abnormal distribution of the DMS present in decreased amounts at the later stages of maturation. In VWD2B, the interaction between newly synthesized VWF and GPIbα begins at an early stage of differentiation and has the potential to also occur intracellularly.14 Confirming our original work, platelet agglutinates have recently been shown in a marrow aspirate taken from a new unrelated patient with VWD with the R1308P mutation.37 Circulating platelet agglutinates were also a feature of VWD2B Tampa,12 and they are also present in the Montreal platelet syndrome recently ascribed to a V1316M mutation as discussed in the “Introduction.”15
BSS and VWD2B are 2 syndromes with giant platelets and thrombocytopenia. The platelet morphology differs, however: BSS has round giant platelets deficient in internal membranes.33 In VWD2B, the platelets, although large, often did not appear to be spheroid. It is possible that the binding of the gain-of-function VWF in the circulation while promoting platelet elimination may also exert a negative influence during megakaryocytopoiesis, modifying the organization of intracellular DMS and reducing the extension of pseudopods so important for platelet formation. Platelet formation is a complex and well-orchestrated phenomenon.38 In VWD2B, the fine balance of platelet production is disrupted.
In conclusion, our data show that megakaryocytopoiesis requires a normal VWF-GPIb interaction. Taken together, our results suggest a cytokine-like function of VWF as a physiologic promoter of MK differentiation. However, the pathophysiology of altered megakaryocytopoiesis in VWD2B may also be due to an altered intracellular interaction between the abnormal VWF2B and GPIbα, whereas extracellular VWF2B maintains its promoting effects on proplatelet formation. It will be studied in detail in the near future whether such a difference might be due to the activation of specific signaling pathways downstream of membrane GPIbα,
Preliminary data were presented in part at the 50th Annual Meeting of the American Society of Hematology in San Francisco, CA, December 6, 2008.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Rosanna Garavaglia for her help with the patients and Jean-Max Pasquet for his technical help. We are grateful to Drs Z. M. Ruggeri and T. Kunicki for the gift of their monoclonal antibodies.
This study was supported by grants from the Italian Ministry of Health to A.B.F., from the Bayer Hemophilia Awards Program to L.B., and by the Diagnostic Différentiel des Purpuras Thrombopéniques Idiopatiques Chroniques et des Thrombopénies Congénitales (DIATROC “Hospital Project of Clinical Research” to P.N. Support from the French network “Gis-Maladies Rares,” from the French Health Ministry to the Centre de Référence des Pathologies Plaquettaires (CRPP), and from Inserm (ANR-08-GENO-028-03) is acknowledged by A.N. and P.N.
Authorship
Contribution: A.B.F. and P.N. were responsible for study initiation and coordination; P.N., G.G., A.N., J.E., I.Y.-M., E.C., C.C., S.L.M., M.P., L.B., L.D.M., M.V., and A.B.F. were involved in study design, data collection, and performing laboratory analyses; A.B.F, A.N., and P.N. wrote the manuscript; G.G., L.B., M.P., L.D.M., and M.V. were responsible for revisions of draft manuscripts; and all authors were responsible for approval of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Augusto B. Federici, Department of Internal Medicine University of Milan, Via Pace 9, 20122 Milan, Italy; e-mail: augusto.federici@unimi.it.
References
Author notes
P.N. and G.G. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal