Abstract
Replacement therapy with exogenous factor VIII (FVIII) to treat hemorrhages induces anti-FVIII inhibitory immunoglobulin G in up to 30% of patients with hemophilia A. Chronic inflammation associated with recurrent bleedings is a proposed risk factor for FVIII inhibitor development. Heme oxygenase-1 (HO-1) is a stress-inducible enzyme with potent anti-inflammatory activity. Here, we demonstrate that induction of HO-1 before FVIII administration drastically reduces the onset of the anti-FVIII humoral immune response. The protective effect was specific for HO-1 because it was reproduced on administration of the end products of HO-1 activity, carbon monoxide, and bilirubin, and prevented by the pharmacologic inhibition of HO-1 using tin mesoporphyrin IX. HO-1 induction was associated with decreased major histocompatibility complex class II expression by splenic antigen-presenting cells and reduced T-cell proliferation. Triggering the endogenous anti-inflammatory machinery before FVIII administration may represent a novel therapeutic option for preventing the development of FVIII inhibitors in hemophilia A patients.
Introduction
Patients with severe hemophilia A lack functional endogenous factor VIII (FVIII). In up to 30% of the patients, administration of exogenous FVIII to treat hemorrhages results in the development of anti-FVIII immunoglobulin G (IgG) that inhibit the therapeutically administered FVIII.1 The reasons for the elevated immunogenicity of therapeutic FVIII, compared with other protein therapeutics, are not clear. One of the invoked hypotheses states that repeated bleeding in the joints of the patients creates a chronic inflammatory environment favoring the local recruitment and activation of antigen-presenting cells (APCs) and of immune effectors. Accordingly, polymorphisms in the promoters of genes encoding proinflammatory (tumor necrosis factor-α) and anti-inflammatory (interleukin-10) cytokines have been shown to be associated with a risk for inhibitor development.2 Hence, reducing inflammation or triggering natural anti-inflammatory mechanisms at the time of therapeutic FVIII administration should help in preventing the onset of FVIII inhibitors.
Heme-oxygenase-1 (HO-1) is a potent antioxidant and anti-inflammatory enzyme. The expression of HO-1 is induced by its substrate heme, as well as by a variety of physical, chemical, or biologic stimuli that share the ability to induce cellular stress.3,4 HO-1 catabolizes heme into carbon monoxide (CO), biliverdin, and ferrous ions. Biliverdin is further transformed into bilirubin by biliverdin reductase, whereas ferrous ions induce heavy chain ferritin synthesis and iron efflux from cells. CO and biliverdin have potent anti-inflammatory, antiproliferative, antiapoptotic, and antioxidant activities and exert their effects on many cell types, including cells of the immune system.3,5 Congenital defect in HO-1 expression in mice and human is associated with systemic inflammation.3,4 Conversely, overexpression of HO-1 suppresses graft rejection.6 HO-1 and CO were shown to protect animals from experimental autoimmune encephalomyelitis, sepsis, pulmonary infection, and cardiovascular pathologies.7-10 Here, we investigated whether triggering the anti-inflammatory properties of HO-1 prevents the anti-FVIII immune response in FVIII-deficient mice.
Methods
Seven- to 10-week-old male FVIII-deficient mice were administered intravenously with 1 IU recombinant FVIII (Kogenate FS; BayerPharma) once a week for 3 weeks. Animals were handled with approval from the regional ethical authorities (Comité régional d'éthique p3/2005/002). Blood was collected one week after the third FVIII administration. Anti-FVIII IgG in serum was detected by enzyme-linked immunosorbent assay (ELISA) using the mouse monoclonal anti-FVIII IgG mAb6 (a gift from Prof J. M. Saint-Remy, KUL, Belgium) as a standard, and in a functional assay for FVIII inhibitors, as described in our previous works.11 Mice were treated intravenously with 0.2μM-filtered ferriprotoporphyrin IX (hemin, 50-750μM, considering 5 mL of total extracellular body fluid/mouse), or intraperitoneally with tin (IV) mesoporphyrin IX (SnMP, 10 mg/kg; Frontier Scientific), bilirubin (750μM; Fluka), or CORM-3 (10 mg/kg). Induction of HO-1 expression in livers of hemin-treated mice was investigated by Western blot. The expression of activation markers by splenocytes of hemin-treated mice and the levels of T regulatory cells (Treg) in spleen were analyzed by flow cytometry. Experimental details are available in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results and discussion
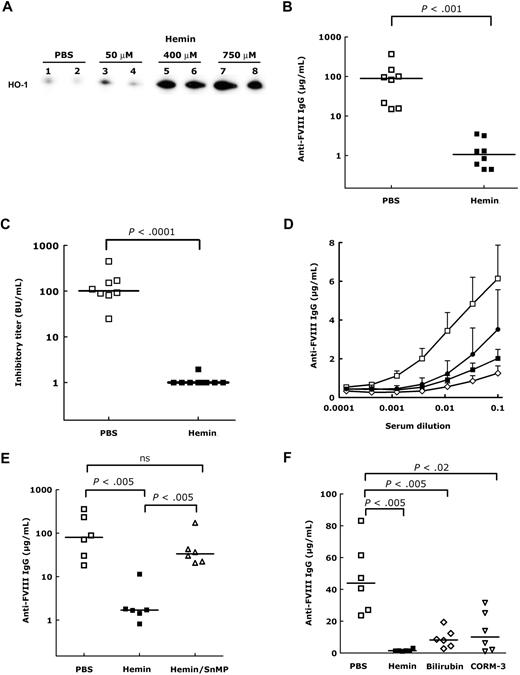
The intravenous administration of hemin (an oxidized form of heme) to FVIII-deficient mice induced a dose-dependent expression of HO-1 both 24 and 48 hours after hemin injection, as shown by the detection of increased levels of HO-1 protein (Figure 1A), mRNA, and activity in liver extracts (data not shown). Administration of therapeutic human FVIII to FVIII-deficient mice induces the production of inhibitory anti-FVIII IgG.12 Induction of HO-1 expression before FVIII administration resulted in a drastic reduction in the levels of inhibitory anti-FVIII IgG, compared with phosphate-buffered saline (PBS)-treated mice (P < .001, Figure 1B-C). Administration of quantities as low as 50μM hemin was sufficient to reduce the anti-FVIII humoral response (Figure 1D). Coadministration to mice of hemin and SnMP, a specific inhibitor of HO-1 activity, along with FVIII, reverted the protective effect of hemin injected alone (Figure 1E), thus confirming the involvement of HO-1 in suppression of the anti-FVIII response. Pretreatment of FVIII-deficient mice with SnMP in the absence of hemin did not interfere with anti-FVIII IgG development (not shown), thus demonstrating that SnMP does not counteract the immunosuppressive effect of HO-1. The physiologic effects of HO-1 may be substituted by administration of the end products of the enzymatic pathway.4,13-15 Indeed, treatment of mice with CORM-3, a CO-releasing compound,16 or with bilirubin before FVIII administration resulted in a significant reduction of the anti-FVIII IgG response (Figure 1F, P < .02 and P < .005, respectively), although the effect was not as drastic as that seen in the case of hemin injection or as that expected on coinjection of CORM-3 and bilirubin.
Induction of HO-1 in FVIII-deficient mice protects from the development of FVIII inhibitors. (A) Induction of HO-1 in the liver of hemin-treated mice. FVIII-deficient mice were treated by intravenous administration of 150 μL of PBS or hemin (50, 400, and 750μM). After 24 hours, livers (200 mg) were recovered and snap-frozen. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions and transferred to polyvinylidene fluoride membrane. HO-1 was detected using an anti–HO-1 rat monoclonal IgG (R&D Systems), a horseradish peroxidase–conjugated rabbit anti–rat IgG, and the enhanced chemiluminescence kit. As a control of protein loading, membranes were stained with Protogold after chemiluminescence detection (not shown). Representative of 4 mice per group. Increased HO-1 expression was also detected 48 hours after hemin treatment (not shown). (B-C) Levels of anti-FVIII IgG in hemin-treated mice. Mice were treated with PBS (□) or hemin (750μM, ■) 3 times at weekly intervals. Twenty-four hours after each treatment, mice received intravenously 1 IU FVIII (200 μL). Blood was collected by retro-orbital puncture 1 week after the third FVIII administration. Anti-FVIII IgG were quantified by ELISA using a mouse monoclonal anti-FVIII IgG (mAb6) as a standard. Results are expressed as micrograms per milliliter of anti-FVIII IgG mAb6-equivalent (B). The anti-FVIII inhibitory titer was measured in serum using the Bethesda assay and is expressed in Bethesda units (BU) per milliliter (C). (D) Dose-response of hemin treatment. FVIII-deficient mice (6 mice/group) were treated 3 times at weekly intervals with PBS (□) or with 50 (●), 400 (◇), and 750 (■) μM hemin, 24 hours before administration of 1 IU FVIII. Anti-FVIII IgG were detected in the serum collected 1 week after the third FVIII administration by ELISA. (E) Induction of anti-FVIII IgG by FVIII on neutralization of HO-1. Mice received FVIII (1 IU) 24 hours after treatment with heme (750μM, ■) or PBS (□), 3 times at weekly intervals. Some mice (▵) were treated intraperitoneally with 10 mg/kg SnMP on each day of FVIII administration and 24 hours later. Levels of anti-FVIII IgG were quantified by ELISA. (F) Protective effect of hemin-degradation products by HO-1. Mice received FVIII (1 IU) 24 hours after intravenous injection of heme (750μM, ■), or after intraperitoneal injection of bilirubin (750μM, ◇), or CORM-3 (10 mg/kg, ▵), 3 times at weekly intervals. Bilirubin and CORM-3 were also injected at the time of FVIII administration. Anti-FVIII IgG titers were determined. Statistical differences depicted in all panels were assessed using the nonparametric Mann-Whitney test.
Induction of HO-1 in FVIII-deficient mice protects from the development of FVIII inhibitors. (A) Induction of HO-1 in the liver of hemin-treated mice. FVIII-deficient mice were treated by intravenous administration of 150 μL of PBS or hemin (50, 400, and 750μM). After 24 hours, livers (200 mg) were recovered and snap-frozen. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions and transferred to polyvinylidene fluoride membrane. HO-1 was detected using an anti–HO-1 rat monoclonal IgG (R&D Systems), a horseradish peroxidase–conjugated rabbit anti–rat IgG, and the enhanced chemiluminescence kit. As a control of protein loading, membranes were stained with Protogold after chemiluminescence detection (not shown). Representative of 4 mice per group. Increased HO-1 expression was also detected 48 hours after hemin treatment (not shown). (B-C) Levels of anti-FVIII IgG in hemin-treated mice. Mice were treated with PBS (□) or hemin (750μM, ■) 3 times at weekly intervals. Twenty-four hours after each treatment, mice received intravenously 1 IU FVIII (200 μL). Blood was collected by retro-orbital puncture 1 week after the third FVIII administration. Anti-FVIII IgG were quantified by ELISA using a mouse monoclonal anti-FVIII IgG (mAb6) as a standard. Results are expressed as micrograms per milliliter of anti-FVIII IgG mAb6-equivalent (B). The anti-FVIII inhibitory titer was measured in serum using the Bethesda assay and is expressed in Bethesda units (BU) per milliliter (C). (D) Dose-response of hemin treatment. FVIII-deficient mice (6 mice/group) were treated 3 times at weekly intervals with PBS (□) or with 50 (●), 400 (◇), and 750 (■) μM hemin, 24 hours before administration of 1 IU FVIII. Anti-FVIII IgG were detected in the serum collected 1 week after the third FVIII administration by ELISA. (E) Induction of anti-FVIII IgG by FVIII on neutralization of HO-1. Mice received FVIII (1 IU) 24 hours after treatment with heme (750μM, ■) or PBS (□), 3 times at weekly intervals. Some mice (▵) were treated intraperitoneally with 10 mg/kg SnMP on each day of FVIII administration and 24 hours later. Levels of anti-FVIII IgG were quantified by ELISA. (F) Protective effect of hemin-degradation products by HO-1. Mice received FVIII (1 IU) 24 hours after intravenous injection of heme (750μM, ■), or after intraperitoneal injection of bilirubin (750μM, ◇), or CORM-3 (10 mg/kg, ▵), 3 times at weekly intervals. Bilirubin and CORM-3 were also injected at the time of FVIII administration. Anti-FVIII IgG titers were determined. Statistical differences depicted in all panels were assessed using the nonparametric Mann-Whitney test.
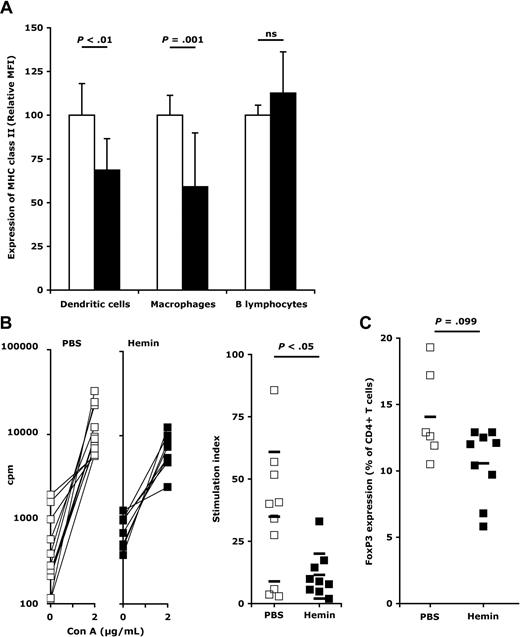
After each administration of hemin, we observed a significant decrease of major histocompatibility complex (MHC) class II expression on splenic macrophages (Figure 2A; supplemental Figures 1-2), a relevant observation in view of the recent description of an accumulation of exogenous FVIII at the level of splenic macrophages of FVIII-deficient mice.11,17 This was also observed in the case of splenic dendritic cells after the second and third administration of hemin. An increase in the HO-1 activity had no impact on the expression of CD80, CD86, and CD40 on any of the APCs, as reported previously in other disease models,7 and on the cytokine secretion profile, after each administration of hemin (not shown). Induction of HO-1 or exposure to CO also has antiproliferative effects on T lymphocytes.18 Accordingly, splenic T cells from hemin-treated FVIII-deficient mice demonstrated a decreased proliferation on challenge with convanavalin A (ConA; Figure 2B) and with FVIII (supplemental Figure 3). However, induction of HO-1 expression in FVIII-deficient mice was not associated with significant changes in CD4+CD25+Foxp3+ Tregs (Figure 2C), in agreement with published data.7,19 In hemophilia A patients, it is unclear as yet whether failure to develop FVIII inhibitors is associated with the generation of Tregs.20 Our data suggest that tolerance toward exogenous FVIII mediated by HO-1 involves, at least in part, a decreased antigen presentation and a reduced T-cell proliferative capacity.
Induction of HO-1 results in reduced T-cell proliferation and expression of MHC class II by APCs. (A) Expression of MHC class II by splenic APCs. Mice were treated twice with PBS (□, 11 mice) or hemin (750μM, ■, 7 mice) at a 7-day interval. Spleens were recovered 72 hours after the second treatment. The expression of MHC class II was measured using an LSRII flow cytometer (BD Biosciences) on dendritic cells (identified as CD11c+ and F4/80− cells), macrophages (F4/80+ and CD11c− cells), and B lymphocytes (B220+ and F4/80− and CD11c− cells). Geometric mean fluorescence intensities (MFI) were measured for each mouse on each individual cell population with FACSDiva software Version 5.0.1 (BD Biosciences) and are expressed for each mouse relative to the mean MFI calculated for all PBS-treated mice. Data represent mean ± SD. Statistical significance in the differences were assessed using the Mann-Whitney test. The expression of MHC class II by APCs after the first and third series of treatments is shown in supplemental Figure 2. (B) Proliferation of splenic T cells. Spleens from PBS- (□) and hemin-treated mice (■) were recovered 72 hours after the second treatment. Splenocytes (1.25 × 106 cells/mL) were incubated for 72 hours alone or with 2 μg/mL ConA. Cell proliferation was measured by incorporation of tritiated thymidine (0.5 μCi/well) for an additional 16 hours, and is expressed as counts per minute (cpm; left panels). Stimulation indexes were calculated for the splenocytes of each mouse by dividing the cpm obtained in the presence of ConA by that obtained in medium alone (right panel). Horizontal bars represent mean ± SD. Significance of differences was statistically assessed using the Mann-Whitney test. (C) Levels of regulatory T cells on HO-1 induction in FVIII-deficient mice. Levels of T cells positive for CD4, CD25, and FoxP3 expression were analyzed by flow cytometry in spleens from mice treated twice with PBS (□) or hemin (■) at a 7-day interval, 72 hours after the second treatment. Horizontal bars represent the arithmetic means. All data are from 2 independent experiments.
Induction of HO-1 results in reduced T-cell proliferation and expression of MHC class II by APCs. (A) Expression of MHC class II by splenic APCs. Mice were treated twice with PBS (□, 11 mice) or hemin (750μM, ■, 7 mice) at a 7-day interval. Spleens were recovered 72 hours after the second treatment. The expression of MHC class II was measured using an LSRII flow cytometer (BD Biosciences) on dendritic cells (identified as CD11c+ and F4/80− cells), macrophages (F4/80+ and CD11c− cells), and B lymphocytes (B220+ and F4/80− and CD11c− cells). Geometric mean fluorescence intensities (MFI) were measured for each mouse on each individual cell population with FACSDiva software Version 5.0.1 (BD Biosciences) and are expressed for each mouse relative to the mean MFI calculated for all PBS-treated mice. Data represent mean ± SD. Statistical significance in the differences were assessed using the Mann-Whitney test. The expression of MHC class II by APCs after the first and third series of treatments is shown in supplemental Figure 2. (B) Proliferation of splenic T cells. Spleens from PBS- (□) and hemin-treated mice (■) were recovered 72 hours after the second treatment. Splenocytes (1.25 × 106 cells/mL) were incubated for 72 hours alone or with 2 μg/mL ConA. Cell proliferation was measured by incorporation of tritiated thymidine (0.5 μCi/well) for an additional 16 hours, and is expressed as counts per minute (cpm; left panels). Stimulation indexes were calculated for the splenocytes of each mouse by dividing the cpm obtained in the presence of ConA by that obtained in medium alone (right panel). Horizontal bars represent mean ± SD. Significance of differences was statistically assessed using the Mann-Whitney test. (C) Levels of regulatory T cells on HO-1 induction in FVIII-deficient mice. Levels of T cells positive for CD4, CD25, and FoxP3 expression were analyzed by flow cytometry in spleens from mice treated twice with PBS (□) or hemin (■) at a 7-day interval, 72 hours after the second treatment. Horizontal bars represent the arithmetic means. All data are from 2 independent experiments.
It is noteworthy that hemorrhages that occur in hemophilia A patients are associated with the release of heme, which should induce HO-1 expression and thus prevent inhibitor development. However, unlike the mouse model of hemophilia A, where HO-1 is induced by a systemic administration of heme, bleedings in patients, and hence heme release, occur at localized sites of the body (eg, joints) and are accompanied by inflammation-prone tissue damage. Furthermore, the fact that some patients develop FVIII inhibitors after replacement therapy may relate in part to different polymorphisms in the promoter region of the HO-1 encoding gene.21,22 Indeed, polymorphisms in the HO-1 gene have been shown to influence the outcome of cardiovascular, pulmonary, and autoimmune diseases, and graft rejection.21,22
Therapies for the eradication of FVIII inhibitors in patients include elimination of FVIII inhibitors by plasmapheresis, depletion of B cells by monoclonal anti-CD20 antibody, or administration of high-dose FVIII to induce specific tolerance.23 Abrogation of the cross-talk between T cells and APCs using anti-CD40L monoclonal antibody or CTLA4-Ig constructs, as well as depletion of T cells using anti-CD3 antibody have shown promising results in FVIII-deficient mice.24-26 Our data suggest that induction of HO-1, or administration of CO,22 before initiation of replacement therapy, represents a novel therapeutic strategy to prevent the onset of FVIII immune response, provided appropriate monitoring of potential side effects, such as opportunistic infections, consecutive to the immunosuppressive properties of HO-1.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Inserm, Centre National de la Recherche Scientifique, and Agence Nationale de la Recherche (ANR-07-JCJC-0100-01, ANR-07-RIB-002-02, ANR-07-MRAR-028-01). J.D.D. and Y.M. are recipients of fellowships from Fondation de la Recherche Médicale. A.-M.N. and M.T. are recipients of fellowships from Région Ile-de-France and Ministère de la Recherche (Paris, France).
Authorship
Contribution: J.D.D., S. Dasgupta, A.-M.N., S. Delignat, J.B., and S.L.-D. designed research; J.D.D., S. Dasgupta, A.-M.N., S. Delignat, Y.R., Y.M., C.P., M.T., and S.V.K. performed research; R.M. contributed vital new reagents or analytical tools; J.D.D., S. Dasgupta, A.-M.N., S. Delignat, Y.R., Y.M., M.T., and S.L.-D. analyzed data; and J.D.D., J.B., S.V.K., and S.L.-D. wrote the paper.
Conflict-of-interest disclosure: R.M. has financial interest with Alfama and hemoCORM. The other authors declare no competing financial interests.
Correspondence: Sébastien Lacroix-Desmazes, Inserm Unite Mixte de Recherche 872 Equipe 16, Centre de Recherche des Cordeliers, Paris, F-75006 France; e-mail: Sebastien.Lacroix-Desmazes@crc.jussieu.fr.
References
Author notes
J.D.D. and S. Dasgupta contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal