Abstract
Natural killer (NK)–cell alloreactivity in recipients of hematopoietic stem cell grafts from HLA-identical siblings is intriguing and has suggested breaking of NK-cell tolerance during the posttransplantation period. To examine this possibility, we analyzed clinical outcomes in a cohort of 105 patients with myeloid malignancies who received T cell–replete grafts from HLA-matched sibling donors. Presence of inhibitory killer cell immunoglobulin-like receptors (KIRs) for nonself HLA class I ligands had no effect on disease-free survival, incidence of relapse, or graft-versus-host disease. A longitudinal analysis of the NK-cell repertoire and function revealed a global hyporesponsiveness of NK cells early after transplantation. Functional responses recovered at approximately 6 months after transplantation. Importantly, NKG2A− NK cells expressing KIRs for nonself HLA class I ligands remained tolerant at all time points. Furthermore, a direct comparison of NK-cell reconstitution in T cell–replete and T cell–depleted HLA-matched sibling stem cell transplantation (SCT) revealed that NKG2A+ NK cells dominated the functional repertoire early after transplantation, with intact tolerance of NKG2A− NK cells expressing KIRs for nonself ligands in both settings. Our results provide evidence against the emergence of alloreactive NK cells in HLA-identical allogeneic SCT.
Introduction
Transplantation over HLA barriers may trigger natural killer (NK)–cell alloreactivity based on “missing-self” recognition. This can occur when the recipient lacks 1 or more of the major inhibitory killer cell immunoglobulin-like receptor (KIR)–binding HLA motifs present in the donor.1,2 In haploidentical stem cell transplantation (SCT), such alloreactivity is associated with reduced relapse risk and improved survival rates in patients with acute myeloid leukemia (AML).1 Similar effects have been observed in unrelated HLA-mismatched SCT, although the results from different centers vary.3-7 In addition, Hsu et al have reported that patients with AML and myelodysplastic syndromes (MDS) who lacked HLA class I ligands for donor inhibitory KIR had a better disease-free survival (DFS) after HLA-identical T cell–depleted SCT.8 The latter result is surprising, because no “missing-self”–driven alloreactivity is expected when donors and recipients are fully HLA-matched.1,9
New insights into mechanisms controlling NK-cell tolerance have revealed that NK cells lacking inhibitory receptors for self MHC class I ligands are hyporesponsive.10-12 To explain the emergence of an alloreactive NK-cell repertoire in HLA-identical settings, one may speculate that NK cells hyporesponsive in the donor become aberrantly activated and functionally competent in the recipient, thereby providing alloreactivity. Indeed, it has been reported that NK cells expressing KIR for nonself HLA ligands were functionally responsive early after T cell–depleted HLA-identical transplantation and became tolerized after approximately 4 months.13 Given the possibility that T cells may influence NK-cell reconstitution in unrelated donor transplantation,14 we here studied clinical outcomes in 105 patients with myeloid malignancies undergoing T cell–replete HLA-matched sibling SCT. Furthermore, we performed a side-by-side comparison of the NK-cell recovery in T cell–replete and T cell–depleted HLA-matched SCT. Our results revealed no benefit of KIR-HLA mismatch and demonstrate that NKG2A+KIR− NK cells dominate the functional repertoire early after transplantation and that NK cells lacking inhibitory receptors for self HLA class I ligands remain tolerant at all time points in both settings.
Methods
Patients and cell processing
This study was approved by the regional ethics committees in Stockholm, Sweden, and the National Heart, Lung and Blood Institute, National Institutes of Health (NIH), Bethesda, MD. All patients studied underwent SCT with HLA-identical sibling donors. Patients at the Karolinska University Hospital underwent SCT with unmanipulated G-CSF–mobilized peripheral blood (n = 70) or bone marrow (n = 35) after myeloablative (n = 76) or reduced-intensity conditioning (n = 29). Posttransplantation graft-versus-host disease (GVHD) prophylaxis was with methotrexate and cyclosporine A at goal levels of 100 to 200 ng/mL for approximately 1 month and then tapered over 2 to 3 months in the absence of GVHD. Further details on the patients included in the retrospective analysis of the clinical effects of KIR-HLA mismatch are described in Table 1. NIH patients underwent G-CSF–mobilized SCT that was depleted of T cells to a T-cell dose of 2 × 104 CD3 cells/kg using the Miltenyi CliniMACS system. Cyclosporine (goal plasma level 100-200 ng/mL) was given from days −6 to +21 after transplantation and from days 90 to 120 after a donor lymphocyte infusion of 5 × 106 CD3 cells/kg on day 90. Peripheral blood, bone marrow, or samples from peripheral stem cell grafts were separated by density gradient centrifugation (Ficoll-Hypaque; GE Healthcare Bio-Sciences AB). Cells were frozen in FCS (HyClone; Thermo Scientific) supplemented with 10% DMSO (Sigma-Aldrich) and stored in liquid nitrogen until use. For functional experiments, 7 patients undergoing T cell–replete SCT and 5 patients undergoing T cell–depleted allogeneic SCT with an HLA-identical sibling donor were selected. Patients with full donor marrow chimerism at all timepoints were chosen. Lymphocytes were collected from the donor, before transplantation, and from the recipient at 4 to 5 subsequent time points up to 6 months after transplantation. Thawed cells were rested overnight at 37°C in RPMI (GIBCO, Invitrogen) supplemented with 10% fetal calf serum (FCS) and l-glutamine (GIBCO, Invitrogen).
Pretransplantation characteristics
| . | KIR ligand match . | KIR ligand mismatch . | All . |
|---|---|---|---|
| Diagnosis, no. (% of total) | |||
| All | 38 (100) | 67 (100) | 105 (100) |
| AML | 28 (74) | 53 (79) | 81 (77) |
| MDS | 10 (26) | 14 (21) | 24 (23) |
| Recipient age, y (range) | |||
| All | 46 (2.5-65) | 40 (1-64) | 42 (1-65) |
| AML | 46 (8-65) | 40 (1-64) | 41 (1-65) |
| MDS | 54 (2.5-63) | 34 (1-67) | 42 (1-67) |
| Donor age, y (range) | 47 (0.5-71) | 36 (3-66) | 43 (0.5-71) |
| AML remission, no. (%) | |||
| First | 17 (45) | 41 (61) | 58 (55) |
| Second | 6 (16) | 6 (9) | 12 (11) |
| Third | 1 (3) | 1 (1) | |
| Refractory | 4 (11) | 3 (4) | 7 (7) |
| Relapse | 1 (3) | 4 (6) | 5 (5) |
| Conditioning, no. (%) | |||
| TBI based | 8 (21) | 14 (21) | 22 (21) |
| Busulfan + cyclophosphamide | 15 (39) | 41 (61) | 56 (53) |
| Busulfan + fludarabine | 15 (39) | 12 (18) | 27 (26) |
| RIC, no. (%) | 16 (42) | 13 (19) | 29 (28) |
| ATG, no. (%) | |||
| Rabbit-ATG | 8 (21) | 8 (12) | 16 (15) |
| Campath | 4 (11) | 3 (4) | 7 (7) |
| Cell no./kg, median (range) | |||
| CD34 (×106) | 7.6 (0.5-18) | 6.8 (1.5-22) | 7.5 (0.5-22) |
| CD3 (×106) | 283 (63-723) | 244 (14-919) | 254 (14-919) |
| TNC (×108) | 11 (1.6-24) | 8,5 (0.7-47) | 10 (0.7-47) |
| GVHD prophylaxis, no. (%) | |||
| MTX + cyclosporine | 37 (97) | 62 (93) | 99 (94) |
| Other | 1 (3) | 7 (10) | 8 (8) |
| Stem cell source, no. (%) | |||
| BM | 10 (26) | 25 (37) | 35 (33) |
| PBSC | 28 (74) | 42 (63) | 70 (67) |
| DLI, no. (%) | 13 (34) | 15 (22) | 28 (27) |
| Recipient/donor CMV status, no. (%) | |||
| Positive/positive | 23 (61) | 29 (43) | 52 (50) |
| Positive/negative | 8 (21) | 22 (33) | 30 (29) |
| Negative/negative | 3 (8) | 8 (12) | 11 (10) |
| Negative/positive | 4 (11) | 6 (9) | 10 (10) |
| Recipient/donor sex, no. (%) | |||
| M/M | 15 (39) | 22 (33) | 37 (35) |
| M/F | 6 (16) | 16 (24) | 22 (21) |
| F/M | 10 (26) | 16 (24) | 26 (25) |
| F/F | 7 (18) | 13 (19) | 20 (19) |
| Follow-up, y (range) | 5.4 (0.3-16) | 4.7 (0.2-20) | 5.7(0.2-20) |
| . | KIR ligand match . | KIR ligand mismatch . | All . |
|---|---|---|---|
| Diagnosis, no. (% of total) | |||
| All | 38 (100) | 67 (100) | 105 (100) |
| AML | 28 (74) | 53 (79) | 81 (77) |
| MDS | 10 (26) | 14 (21) | 24 (23) |
| Recipient age, y (range) | |||
| All | 46 (2.5-65) | 40 (1-64) | 42 (1-65) |
| AML | 46 (8-65) | 40 (1-64) | 41 (1-65) |
| MDS | 54 (2.5-63) | 34 (1-67) | 42 (1-67) |
| Donor age, y (range) | 47 (0.5-71) | 36 (3-66) | 43 (0.5-71) |
| AML remission, no. (%) | |||
| First | 17 (45) | 41 (61) | 58 (55) |
| Second | 6 (16) | 6 (9) | 12 (11) |
| Third | 1 (3) | 1 (1) | |
| Refractory | 4 (11) | 3 (4) | 7 (7) |
| Relapse | 1 (3) | 4 (6) | 5 (5) |
| Conditioning, no. (%) | |||
| TBI based | 8 (21) | 14 (21) | 22 (21) |
| Busulfan + cyclophosphamide | 15 (39) | 41 (61) | 56 (53) |
| Busulfan + fludarabine | 15 (39) | 12 (18) | 27 (26) |
| RIC, no. (%) | 16 (42) | 13 (19) | 29 (28) |
| ATG, no. (%) | |||
| Rabbit-ATG | 8 (21) | 8 (12) | 16 (15) |
| Campath | 4 (11) | 3 (4) | 7 (7) |
| Cell no./kg, median (range) | |||
| CD34 (×106) | 7.6 (0.5-18) | 6.8 (1.5-22) | 7.5 (0.5-22) |
| CD3 (×106) | 283 (63-723) | 244 (14-919) | 254 (14-919) |
| TNC (×108) | 11 (1.6-24) | 8,5 (0.7-47) | 10 (0.7-47) |
| GVHD prophylaxis, no. (%) | |||
| MTX + cyclosporine | 37 (97) | 62 (93) | 99 (94) |
| Other | 1 (3) | 7 (10) | 8 (8) |
| Stem cell source, no. (%) | |||
| BM | 10 (26) | 25 (37) | 35 (33) |
| PBSC | 28 (74) | 42 (63) | 70 (67) |
| DLI, no. (%) | 13 (34) | 15 (22) | 28 (27) |
| Recipient/donor CMV status, no. (%) | |||
| Positive/positive | 23 (61) | 29 (43) | 52 (50) |
| Positive/negative | 8 (21) | 22 (33) | 30 (29) |
| Negative/negative | 3 (8) | 8 (12) | 11 (10) |
| Negative/positive | 4 (11) | 6 (9) | 10 (10) |
| Recipient/donor sex, no. (%) | |||
| M/M | 15 (39) | 22 (33) | 37 (35) |
| M/F | 6 (16) | 16 (24) | 22 (21) |
| F/M | 10 (26) | 16 (24) | 26 (25) |
| F/F | 7 (18) | 13 (19) | 20 (19) |
| Follow-up, y (range) | 5.4 (0.3-16) | 4.7 (0.2-20) | 5.7(0.2-20) |
TBI indicates total body irradiation; RIC, reduced-intensity conditioning; TNC, total nuclear cell count; MTX, methotrexate; BM, bone marrow; PBSC, peripheral blood stem cell; DLI, donor lymphocyte infusion; M, male; and F, female.
KIR and HLA genotyping
Donor and recipients were typed with allele level resolution for HLA-A, HLA-B, HLA-C, and HLA-DRB1 using polymerase chain reaction with sequence-specific primers (PCR-SSP) technology (Olerup-SSP). KIR ligands were also determined using the KIR-ligand kit (Olerup-SSP) for detecting the HLA-Bw4, HLA-C1, and HLA-C2 motifs. KIR typing was performed as previously described using PCR-SSP and a KIR typing kit (Olerup-SSP).15
Antibodies and flow cytometry
The antibody panel used in the present study was slightly modified from that described in detail elsewhere.16 The specific conjugations used are depicted in Figure 3A. Data were acquired on a CyAn ADP LX 9-color flow cytometer (Dako) as previously described.17 Acquired data were compensated and analyzed with the software-based compensation platform in FlowJo 8.8 (TreeStar).
CD107a assay
Peripheral blood mononuclear cells (PBMCs; 1.5 × 106 cells/mL) were mixed with K562 cells at a ratio of 10:1 in a final volume of 200 μL in V-bottomed 96-well plates and incubated for 2 hours at 37°C and 5% CO2 as previously described.18 Cells were stained with NK-cell markers and CD107a on ice for 20 minutes in cold fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS] supplemented with 2% FCS and 2mM EDTA [GIBCO, Invitrogen]). Cells were washed 3 times and analyzed by flow cytometry.
Statistics
Data were analyzed as of December 2008. Disease-free survival (DFS) was estimated using the Kaplan-Meier method and compared with the log-rank test. The incidence of relapse, transplantation-related mortality (TRM), and acute GVHD (aGVHD) were estimated by the proportional subdistribution hazard regression model of Fine and Gray in a competing risks setting.19 The competing events were relapse (for TRM), death without aGVHD within 100 days after transplantation (for aGVHD), and death without relapse (for relapse). For the multivariate analysis the following factors were considered: conditioning regimen, total body irradiation (TBI), antithymocyte globulin (ATG), serologic cytomegalovirus (CMV) status of the donor and recipient, age, reduced-intensity conditioning (RIC), transplantation year, and donor and recipient age. Factors differing in distribution between the examined groups with a P value less than .10 were included in the final models.
One-way analysis of variance (ANOVA) or repeated measures 1-way ANOVA were used for groups of experimental data passing normality test. For comparisons of groups failing normality test, the nonparametric Kruskal-Wallis test was applied, followed by the Tukey or Dunn post-test. All P values are 2-sided with type I error rate fixed at .05 (significant values are indicated in the figures as follows: *P < .05; **P < .01; ***P < .001). Statistical analyses were performed with GraphPad Prism 5 for Mac OS X and Splus 6.0 for Windows (TIBCO Software Inc) software packages.
Results
Presence of nonself KIRs does not influence the outcome in T cell–replete HLA-matched SCT
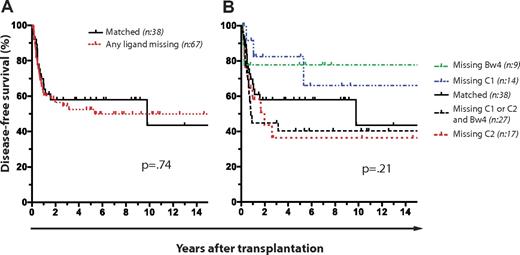
We performed a retrospective analysis of 105 patients with AML (n = 81) and MDS (n = 24) who had undergone T cell–replete SCT from HLA-matched sibling donors at the Karolinska University Hospital between the years 1988 and 2008. Patient characteristics, conditioning, and GVHD prophylaxis are described in Table 1. Expression of KIRs for nonself HLA class I ligands (hereafter referred to as nonself KIRs) had no effect on DFS (Figure 1A-B). Furthermore, the presence of nonself KIRs had no influence on the DFS regardless of whether the analysis was restricted to patients with AML or MDS or those receiving mobilized peripheral blood stem cells or bone marrow, use of ATG, and myeloablative or nonmyeloablative conditioning regimes (Table 2).
No effect of KIR and HLA genetics on DFS in T cell–replete HLA-matched sibling SCT for AML and MDS. Kaplan-Meier plots with P values obtained from log-rank tests for difference. (A) Effect of missing KIR ligand on DFS. Number (n) of patients: matched (n = 38), mismatched (n = 67). (B) Effect of missing KIR ligand on DFS. Number (n) of patients: matched (n = 38), missing HLA-C1 (n = 14), missing HLA-C2 (n = 17), missing HLA-Bw4 (n = 9), and missing HLA-C1, HLA-C2, and HLA-Bw4, (n = 27).
No effect of KIR and HLA genetics on DFS in T cell–replete HLA-matched sibling SCT for AML and MDS. Kaplan-Meier plots with P values obtained from log-rank tests for difference. (A) Effect of missing KIR ligand on DFS. Number (n) of patients: matched (n = 38), mismatched (n = 67). (B) Effect of missing KIR ligand on DFS. Number (n) of patients: matched (n = 38), missing HLA-C1 (n = 14), missing HLA-C2 (n = 17), missing HLA-Bw4 (n = 9), and missing HLA-C1, HLA-C2, and HLA-Bw4, (n = 27).
Effect of missing KIR ligands on transplantation outcome
| Group . | Cohort size . | Relapse* . | aGVHD (grades II-IV)* . | TRM* . | DFS† . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| AML and MDS | 105 | ||||||||
| All KIR-L present | 38 | 1.00 (reference) | — | 1.00 (reference) | — | 1.00 (reference) | — | 1.00 (reference) | — |
| Missing 1 or more KIR-L | 67 | 0.79 (0.40-1.59) | .52 | 1.56 (0.79-3.08) | .20 | 2.19 (0.61-7.80) | .23 | 0.91 (0.50-1.65) | .74 |
| Missing HLA-C1 | 14 | 0.66 (0.20-2.16) | .49 | 1.68 (0.57-4.91) | .34 | 0.26 (0.03-2.43)‡ | .24‡ | 1.81 (0.66-4.95) | .25 |
| Missing HLA-C2 | 17 | 0.73 (0.27-1.96) | .53 | 2.87 (1.29-6.37) | .01 | 4.01 (0.98-16.40) | .05 | 0.68 (0.29-1.57) | .36 |
| Missing HLA-Bw4 | 9 | 0.68 (0.14-3.32) | .63 | 0.61 (0.12-3.05) | .55 | 0.29 (0.02-3.74)‡ | .36‡ | 1.71 (0.53-5.49) | .37 |
| Missing HLA-Bw4 and HLA-C1 or HLA-C2 | 27 | 0.90 (0.41-1.95) | .78 | 1.26 (0.51-3.12) | .62 | 0.43 (0.15-1.24) | .12 | 0.66 (0.32-1.36) | .25 |
| AML | 81 | ||||||||
| All KIR-L present | 28 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 53 | 0.91 (0.40-2.08) | .84 | 1.52 (0.65-3.57) | .33 | 1.05 (0.26-4.17) | .94 | 0.91 (0.45-1.86) | .80 |
| MDS | 24 | ||||||||
| All KIR-L present | 10 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 14 | 0.38 (0.10-1.45) | .15 | 1.80 (0.59-5.51) | .30 | 3.57 (0.71-17.9)‡ | .08‡ | 0.80 (0.27-2.40) | .69 |
| Subgroups of AML and MDS | |||||||||
| Patient with ATG treatment | 23 | ||||||||
| All KIR-L present | 12 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 11 | 1.11 (0.34-3.58) | .86 | 0.36 (0.04-3.35) | .37 | 1.14 (0.08-16.05) | .92 | 1.00 (0.25-4.03) | 1.00 |
| Patient without ATG treatment | 82 | ||||||||
| All KIR-L present | 26 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 56 | 0.78 (0.33-1.84) | .57 | 1.63 (0.76-3.51) | .22 | 2.41 (0.52-11.12) | .26 | 0.86 (0.42-1.77) | .69 |
| Patient receiving BM grafts | 35 | ||||||||
| All KIR-L present | 10 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 25 | 0.51 (0.21-1.25) | .14 | 1.15 (0.32-4.11) | .83 | 2.01 (0.23-17.39) | .52 | 1.45 (0.57-3.68) | .43 |
| Patient receiving PBSC grafts | 70 | ||||||||
| All KIR-L present | 28 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 42 | 1.17 (0.43-3.19) | .76 | 1.67 (0.75-3.72) | .21 | 2.23 (0.46-10.68) | .32 | 0.71 (0.31-1.59) | .40 |
| Patient with MAC | 76 | ||||||||
| All KIR-L present | 22 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 54 | 0.68 (0.29-1.59) | .38 | 1.32 (0.62-2.84) | .48 | 2.14 (0.46-9.86) | .33 | 0.98 (0.47-2.06) | .97 |
| Patient with RIC | 29 | ||||||||
| All KIR-L present | 16 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 13 | 1.31 (0.40-4.25) | .66 | 0.81 (0.15-4.46) | .81 | 1.31 (0.09-18.83) | .84 | 0.74 (0.23-2.34) | .61 |
| Group . | Cohort size . | Relapse* . | aGVHD (grades II-IV)* . | TRM* . | DFS† . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| AML and MDS | 105 | ||||||||
| All KIR-L present | 38 | 1.00 (reference) | — | 1.00 (reference) | — | 1.00 (reference) | — | 1.00 (reference) | — |
| Missing 1 or more KIR-L | 67 | 0.79 (0.40-1.59) | .52 | 1.56 (0.79-3.08) | .20 | 2.19 (0.61-7.80) | .23 | 0.91 (0.50-1.65) | .74 |
| Missing HLA-C1 | 14 | 0.66 (0.20-2.16) | .49 | 1.68 (0.57-4.91) | .34 | 0.26 (0.03-2.43)‡ | .24‡ | 1.81 (0.66-4.95) | .25 |
| Missing HLA-C2 | 17 | 0.73 (0.27-1.96) | .53 | 2.87 (1.29-6.37) | .01 | 4.01 (0.98-16.40) | .05 | 0.68 (0.29-1.57) | .36 |
| Missing HLA-Bw4 | 9 | 0.68 (0.14-3.32) | .63 | 0.61 (0.12-3.05) | .55 | 0.29 (0.02-3.74)‡ | .36‡ | 1.71 (0.53-5.49) | .37 |
| Missing HLA-Bw4 and HLA-C1 or HLA-C2 | 27 | 0.90 (0.41-1.95) | .78 | 1.26 (0.51-3.12) | .62 | 0.43 (0.15-1.24) | .12 | 0.66 (0.32-1.36) | .25 |
| AML | 81 | ||||||||
| All KIR-L present | 28 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 53 | 0.91 (0.40-2.08) | .84 | 1.52 (0.65-3.57) | .33 | 1.05 (0.26-4.17) | .94 | 0.91 (0.45-1.86) | .80 |
| MDS | 24 | ||||||||
| All KIR-L present | 10 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 14 | 0.38 (0.10-1.45) | .15 | 1.80 (0.59-5.51) | .30 | 3.57 (0.71-17.9)‡ | .08‡ | 0.80 (0.27-2.40) | .69 |
| Subgroups of AML and MDS | |||||||||
| Patient with ATG treatment | 23 | ||||||||
| All KIR-L present | 12 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 11 | 1.11 (0.34-3.58) | .86 | 0.36 (0.04-3.35) | .37 | 1.14 (0.08-16.05) | .92 | 1.00 (0.25-4.03) | 1.00 |
| Patient without ATG treatment | 82 | ||||||||
| All KIR-L present | 26 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 56 | 0.78 (0.33-1.84) | .57 | 1.63 (0.76-3.51) | .22 | 2.41 (0.52-11.12) | .26 | 0.86 (0.42-1.77) | .69 |
| Patient receiving BM grafts | 35 | ||||||||
| All KIR-L present | 10 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 25 | 0.51 (0.21-1.25) | .14 | 1.15 (0.32-4.11) | .83 | 2.01 (0.23-17.39) | .52 | 1.45 (0.57-3.68) | .43 |
| Patient receiving PBSC grafts | 70 | ||||||||
| All KIR-L present | 28 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 42 | 1.17 (0.43-3.19) | .76 | 1.67 (0.75-3.72) | .21 | 2.23 (0.46-10.68) | .32 | 0.71 (0.31-1.59) | .40 |
| Patient with MAC | 76 | ||||||||
| All KIR-L present | 22 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 54 | 0.68 (0.29-1.59) | .38 | 1.32 (0.62-2.84) | .48 | 2.14 (0.46-9.86) | .33 | 0.98 (0.47-2.06) | .97 |
| Patient with RIC | 29 | ||||||||
| All KIR-L present | 16 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Missing 1 or more KIR-L | 13 | 1.31 (0.40-4.25) | .66 | 0.81 (0.15-4.46) | .81 | 1.31 (0.09-18.83) | .84 | 0.74 (0.23-2.34) | .61 |
KIR-L indicates KIR ligand; PBSC, peripheral blood stem cell; and MAC, myeloablative conditioning.
Values obtained by proportional subdistribution hazard regression model of Fine and Gray in a competing risks setting. Competing events were relapse (for TRM), death without aGVHD within 100 days after the transplantation (for aGVHD), and death without relapse (for relapse).
Hazard ratio and confidence interval (CI) obtained by the Mantel-Haenszel method, P values by log-rank test.
Values obtained by the Mantel-Haenszel method, P values by log-rank test via GraphPad Prism software. (Fine and Gray analysis could not be used due to zero events in one of the groups.)
Hence, in contrast to the results from a previous study of subjects undergoing T cell–depleted HLA-identical sibling SCT,8 we found no influence on DFS in the T cell–replete SCT setting by HLA and KIR genotypes.
Reconstitution of NK-cell repertoires during the first 6 months after SCT
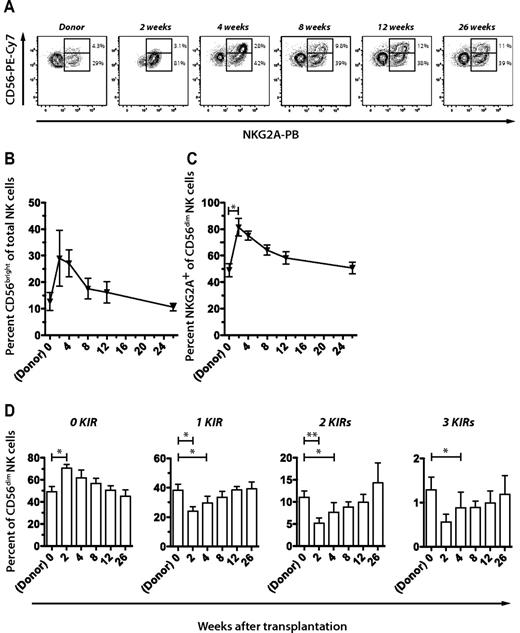
Next, we performed a longitudinal assessment of the phenotypic and functional recovery of the NK-cell repertoire by multiparametric flow cytometry. As previously reported, we found that NK cells engrafted early after transplantation, and the population consisted of increased frequencies of CD56bright NK cells (Figure 2A-B).20-24 During the first weeks after transplantation, most of the CD56dim NK cells expressed NKG2A (Figure 2A,C). During weeks 2 to 8, NK cells expressed fewer KIRs than in the graft, reverting back to donor repertoires within approximately 12 weeks (Figure 2D).
Reconstitution of NK-cell repertoires during the first 6 months after T cell–replete HLA-identical sibling SCT. (A) Representative example of FACS staining and gating strategy to assess frequencies of CD56bright NK cells and expression of NKG2A in the donor and at multiple time points after SCT. Percentage of (B) CD56bright NK cells and (C) NKG2A+ CD56dim NK cells in the donor before SCT and in the recipient at the indicated time points after SCT. (D) Frequencies of CD56dim NK cells expressing 0 to 3 KIRs in the donor and at the indicated time points after SCT. Mean values of 7 donor-recipient pairs are depicted. All error bars represent SEM.
Reconstitution of NK-cell repertoires during the first 6 months after T cell–replete HLA-identical sibling SCT. (A) Representative example of FACS staining and gating strategy to assess frequencies of CD56bright NK cells and expression of NKG2A in the donor and at multiple time points after SCT. Percentage of (B) CD56bright NK cells and (C) NKG2A+ CD56dim NK cells in the donor before SCT and in the recipient at the indicated time points after SCT. (D) Frequencies of CD56dim NK cells expressing 0 to 3 KIRs in the donor and at the indicated time points after SCT. Mean values of 7 donor-recipient pairs are depicted. All error bars represent SEM.
Responses to stimulation by K562 cells were significantly reduced in all examined NK-cell subsets during the first 12 weeks after SCT compared with those in the donors (Figure 3A-B). Overall functional responses recovered gradually over time and were fully restored at 26 weeks. Noteworthy, NKG2A− NK cells expressing KIRs for nonself HLA ligands (NKG2A− nonself KIR+) were hyporesponsive at all time points (Figure 3B). In this respect, the response of NKG2A− nonself KIR+ NK cells did not differ significantly from that of the immature NKG2A−KIR− subset (Figure 3B).20 Hence, uneducated NK cells remained tolerant during the early and late recovery periods after T cell–replete, HLA-identical SCT.
Maintained tolerance of NK cells expressing inhibitory KIR for nonself HLA class I ligands. (A) Gating scheme for analysis of CD107a on NK-cell subsets expressing KIR2DL1, KIR2DL3, KIR3DL1, and/or NKG2A after stimulation with K562 cells. Double events, CD14+, CD3+, and dead cells were excluded. Boolean gating of CD56dim NK cells with and without NKG2A and expressing combinations of KIRs and CD107a was performed by FlowJo software. (B) Shown are functional responses of CD56dim NK cells expressing self or nonself KIRs with or without NKG2A in the donor before SCT, and in the recipient at the indicated time points during the immune reconstitution. Self and nonself KIR refers to the presence or absence, respectively, of the corresponding ligand in the host. Mean values of 6 donor-recipient pairs are shown. Error bars represent SEM.
Maintained tolerance of NK cells expressing inhibitory KIR for nonself HLA class I ligands. (A) Gating scheme for analysis of CD107a on NK-cell subsets expressing KIR2DL1, KIR2DL3, KIR3DL1, and/or NKG2A after stimulation with K562 cells. Double events, CD14+, CD3+, and dead cells were excluded. Boolean gating of CD56dim NK cells with and without NKG2A and expressing combinations of KIRs and CD107a was performed by FlowJo software. (B) Shown are functional responses of CD56dim NK cells expressing self or nonself KIRs with or without NKG2A in the donor before SCT, and in the recipient at the indicated time points during the immune reconstitution. Self and nonself KIR refers to the presence or absence, respectively, of the corresponding ligand in the host. Mean values of 6 donor-recipient pairs are shown. Error bars represent SEM.
Direct comparison of NK-cell tolerance in T cell–replete and T cell–depleted HLA-matched SCT
Our results disagree with the results from a recent report showing breaking of NK-cell tolerance in HLA-matched sibling transplantation.13 Possible explanations for this discrepancy in results are the kinetics and patterns of immune reconstitution due to the presence of T cells in the graft and the use of posttransplantation immune suppression.5,14 To examine these possibilities, we performed a side-by-side comparison of the NK-cell reconstitution in T cell–replete and T cell–depleted settings. Our analysis revealed that patterns of immune reconstitution were similar in the 2 studied settings. Hence, CD56bright NK cells and NKG2A+ CD56dim NK cells were abundant early and appeared with similar kinetics after T cell–depleted SCT (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Next, functional NK-cell responses in patients receiving T cell–replete and T cell–depleted stem cell grafts were compared. To facilitate the interpretation of the results in relation to previously reported data, we plotted the response of NK-cell subsets expressing nonself or self KIRs relative to the response of the immature NKG2A−KIR− NK-cell subset (Figure 4). Corroborating our previous analyses, NK cells expressing nonself KIRs remained tolerant at all time points in T cell–replete SCT (Figure 4 left panel). Although the available sample series of patients undergoing T cell–depleted HLA-matched SCT for AML were limited, direct comparison of the 2 settings did not reveal any significant differences (Figure 4). Hence, we conclude that NK cells expressing only nonself KIRs are hyporesponsive in both T cell–depleted and T cell–replete settings. Of note, in 1 of 5 patients undergoing T cell–depleted transplantation, we observed a general activation of NKG2A−KIR− and NKG2A− nonself KIR+ NK cells at week 4 only, associated with persisting recipient CD3+ cells. However, even at this time point, the response was higher in NKG2A− NK cells expressing a self KIR, suggesting that the underlying principles for NK-cell education remained valid during the posttransplantation immune recovery period.
Tolerance of uneducated NK cells in both T cell–replete and T cell–depleted SCT. CD107a expression by NKG2A−CD56dim NK cells expressing self or nonself KIRs relative to the response of NKG2A−KIR− cells in the donor before SCT and in the recipient at the indicated time points after SCT. Shown are mean values of 6 (T cell–replete) and 5 (T cell–depleted) donor-recipient pairs. Error bars represent SEM.
Tolerance of uneducated NK cells in both T cell–replete and T cell–depleted SCT. CD107a expression by NKG2A−CD56dim NK cells expressing self or nonself KIRs relative to the response of NKG2A−KIR− cells in the donor before SCT and in the recipient at the indicated time points after SCT. Shown are mean values of 6 (T cell–replete) and 5 (T cell–depleted) donor-recipient pairs. Error bars represent SEM.
NKG2A+ NK cells predominate functional responses early after T cell–replete and T cell–depleted HLA-matched SCT
Next, we sought to examine the relative contributions of individual NK-cell subsets to the overall NK-cell response at several time points after SCT. As clearly documented, NKG2A expression appears early after SCT and is sequentially followed by inhibitory KIRs regardless of the transplantation setting.20-24 NKG2A is known to buffer NK-cell responses at a single-cell level and in repertoires characterized by low KIR expression frequencies.25,26 Indeed, NKG2A+KIR− NK cells represented the major responding cell population during the first month after transplantation in both T cell–replete and T cell–depleted SCT (Figure 5). Furthermore, this analysis revealed that NKG2A− nonself KIR+ NK cells contributed to less than 3% of the total degranulation response at all time points after transplantation in both settings (Figure 5). These results highlight the pivotal role of NKG2A in maintaining a functional but tolerant NK-cell repertoire early after transplantation.
NKG2A+ NK cells predominate functional responses early after T cell–replete and T cell–depleted SCT. Gates were set on CD107a+ NK cells and the relative contribution of the indicated NK-cell subset to the total degranulation response was assessed in the donor before SCT and in the recipient at the indicated time points after SCT in T cell–replete (left panel) and T cell–depleted (right panel) settings. Shown are mean values of 6 (T cell–replete) and 5 (T cell–depleted) donor-recipient pairs. Error bars represent SEM.
NKG2A+ NK cells predominate functional responses early after T cell–replete and T cell–depleted SCT. Gates were set on CD107a+ NK cells and the relative contribution of the indicated NK-cell subset to the total degranulation response was assessed in the donor before SCT and in the recipient at the indicated time points after SCT in T cell–replete (left panel) and T cell–depleted (right panel) settings. Shown are mean values of 6 (T cell–replete) and 5 (T cell–depleted) donor-recipient pairs. Error bars represent SEM.
Discussion
Insights into the molecular specificities of NK cells have spurred an interest in developing strategies to exploit NK-cell alloreactivity in the treatment of human cancer. Allogeneic SCT over HLA barriers using partially mismatched unrelated donors or haploidentical sibling donors represents a unique clinical setting in which “missing-self”–driven NK-cell alloreactivity may occur.2 Although promising results have been presented, in particular in haploidentical transplantation for AML,1 the discrepant results in several studies highlight the difficulties associated with linking a single biologic factor to clinical outcome.5,6,22,24,27,28 Further studies are warranted, in particular such where parameters of clinical outcome are coupled to biologic evaluation of the immune system in the donor and during the functional reconstitution in the recipient. Indeed, longitudinal studies of the immunologic reconstitution from stem cells represent a unique setting for studying NK-cell repertoire formation and the perturbation of it by multiple biologic and environmental factors.
Here, we initially performed a retrospective analysis of clinical outcomes for patients undergoing T cell–replete HLA-matched SCT for myeloid malignancies. In contrast to a previous study of subjects undergoing HLA-matched T cell–depleted SCT,8 we found no evidence for a beneficial effect of having a donor expressing KIRs for nonself HLA class I ligands. In a stratified analysis of recipients lacking any of the 3 major KIR motifs (HLA-Bw4, HLA-C1, or HLA-C2), missing HLA-C2 was associated with increased hazard ratio of aGVHD and TRM (Table 2). Importantly, however, the borderline significance for high aGVHD in recipients lacking HLA-C2, as revealed in a multivariate analysis, could not be explained by NK-cell alloreactivity, because recipients lacking 2 ligands (HLA-Bw4 and HLA-C1 or HLA-C2) had a similar risk to develop GVHD as those with all ligands present.
Our clinical data were supported by a longitudinal functional evaluation of NK-cell repertoires during the first 6 months after transplantation. In agreement with a previous study, we found that NK-cell responses were generally depressed immediately after transplantation,24 and recovered slowly to normal levels by week 26. Importantly, educated NK cells expressing inhibitory KIRs for self HLA class I ligands responded better at all examined time points than uneducated NK cells lacking self-specific receptors. In fact, we found that the response of NK cells expressing nonself KIRs was comparable with that of hyporesponsive immature NKG2A−KIR− NK cells throughout the immune reconstitution period. Hence, NK cells expressing nonself KIRs remained tolerant at all time points during the first 6 months after transplantation. This outcome differs from a recent investigation of a heterogenous group of patients receiving T cell–depleted, HLA-matched hematopoietic stem cell grafts.13 In that study, it was suggested that NK cells expressing inhibitory KIRs for nonself HLA ligands were aberrantly activated during the first 3 months after transplantation. The authors concluded that NK-cell tolerance was broken, which could explain the previously reported clinical data of a beneficial effect of missing KIR ligands in HLA-matched SCT.8 The results indicating breaking of NK-cell tolerance in HLA-matched transplantation are intriguing and must be studied further to shed light on mechanisms underlying NK-cell education and tolerance.
Key differences between Yu's studies and ours were the presence of T cells in the graft and use of posttransplantation immunosuppressive treatment in our setting.13 The presence/absence of T cells and/or use of ATG for depletion of T cells in vivo have been discussed as major factors influencing NK-cell alloreactivity in allogeneic SCT.29 This is based on the tenet that the reconstitution and acquisition of full effector function of NK cells is faster in the absence of competition from T cells. Indeed, transplantation of T cell–replete grafts has been associated with delayed KIR acquisition in unrelated SCT.14 However, it is important to point out that the potential interference of T cells on NK-cell function in HLA-mismatched and HLA-matched settings may differ. In the first setting it makes sense to hypothesize that delayed KIR reconstitution may alter the repertoire of alloreactive NK cells capable of sensing “missing self.” However, in HLA-matched settings, T cells must somehow interfere with the mechanisms that underlie NK-cell education with the assumption that uneducated NK cells become unleashed in the absence of T cells.
To investigate this further, we performed a side-by-side comparison of the NK-cell recovery in patients receiving T cell–replete and T cell–depleted grafts. Remarkably, the patterns of NK-cell reconstitution and functional maturation were very similar in the 2 settings. After an initial period of increased frequencies of CD56bright NK cells and NKG2A+CD56dim NK cells, lasting for approximately 8 to 12 weeks, NK-cell repertoires gradually returned to those of the donors. In the 2 settings compared here, KIR repertoires were nearly normalized already at week 8. Importantly, the longitudinal functional recovery of uneducated and educated NK cells was similar in T cell–replete and T cell–depleted settings, suggesting that NK-cell subsets expressing inhibitory receptors for nonself HLA class I molecules remain hyporesponsive after HLA-matched SCT for patients with AML. Furthermore, our results demonstrate that the presence or absence of T cells cannot explain the discrepancies between this study and the previously reported data on NK-cell alloreactivity in T cell–depleted HLA-matched sibling SCT.13
A second major difference between the T cell–depleted and T cell–replete settings studied here was the duration of the posttransplantation immune suppression. One possible explanation for the emergence of an alloreactive NK-cell repertoire in T cell–depleted but not in T cell–replete settings is that NK-cell responses are suppressed by the prolonged use of immunosuppressive treatment after transplantation. Cyclosporine A has been shown to influence NK-cell differentiation and function30 and may have contributed to the general depression of NK-cell responses observed early after T cell–replete transplantation (Figure 2B). In the T cell–depleted series, cyclosoprine A was given only from conditioning to day 21 after transplantation in low doses (100-200 ng/mL) and was not reinstituted until day 90 after transplantation. Of note, all patients included in the initial report by Hsu et al on NK-cell alloreactivity in HLA-matched SCT received GVHD prophylaxis with cyclosporine A,8 whereas no such treatment was given in the series evaluated for breaking of NK-cell tolerance.13 In the present study, differences in posttransplantation GVHD prophylaxis had no major influence on the tolerance of NK cells expressing nonself KIRs. Although larger prospective studies are needed, our data do not suggest that immune suppressive drugs determine the kinetics of the transition from CD56bright to CD56dim subsets, the loss of NKG2A expression, and sequential acquisition of KIRs.
One important novel observation in the present study was the major contribution of NKG2A+ NK cells to the functional response early after transplantation. NKG2A receptors are expressed early during NK-cell differentiation and are expressed on most CD56bright NK-cells.31 Upon differentiation from CD56bright to CD56dim NK subsets, NK cells gradually lose expression of NKG2A and gain expression of KIRs.20 At steady state, KIR and NKG2A expression are complementary at the single-cell and population levels.16,25,26,32,33 Moreover, work from several laboratories has shown that NKG2A+KIR− and NKG2A+ nonself KIR+ NK cells are fully educated,16,20,25 and that NKG2A may “buffer” functional responses to achieve a balanced “missing-self” response in donors with different types of NK cell repertoires.25,26 Hence, NKG2A+ NK cells predominate the functional responses in donors with low overall frequencies of KIR expression.26 Similarly, we now show that NKG2A+ subsets contribute the most to the functional responses early after transplantation before KIR expression has returned to that of the donor. Importantly, this observation was made in both T cell–replete and T cell–depleted settings, suggesting that T cells and/or immune suppression does not interfere with the education of NKG2A+ NK cells. The subtle differences observed between the 2 settings were attributed to differences in the functional repertoires of the donors rather than differences in immune reconstitution. Similarly, frequent expression of NKG2A on NK cells, examined at early time points after haploidentical transplantation, was associated with impaired lysis of HLA-E–expressing AML blasts, thus limiting graft-versus-leukemia effects.22 These results are in sharp contrast to the report by Yu et al, in which NKG2A+ cells were hyporesponsive and in which this receptor was not considered in the majority of the performed experiments.13 We conclude that it is vital to analyze education of NKG2A+ and NKG2A− NK cells separately at early time points after transplantation.
The present study was based on analyses of degranulation responses in educated and uneducated NK cells after coincubation with K562 cells. This strategy allowed us to properly evaluate functional responses of rare NK-cell subsets expressing given combinations of KIRs in limited samples from different transplantation settings, but leaves several questions unanswered that require further studies. First, it will be important to study if NK-cell tolerance is preserved in patients who develop aGVHD or in those with reactivation of CMV, known to imprint human NK-cell repertoires.34 Furthermore, the role of CD56bright NK cells during the early immune recovery after transplantation should be considered. How is the function of CD56bright NK cells affected by presence or absence of T cells and use of immunosuppressive treatments, and how does this relate to clinical outcome? When possible, it should also be interesting to assess multiple functional modalities in response to a broader range of stimuli, including cytokines and physiologically relevant cellular targets.
Our retrospective analysis of patients undergoing T cell–replete, HLA-identical sibling transplantation revealed no benefit of KIR-HLA mismatch. The clinical data were supported by a longitudinal functional evaluation of NK cells, revealing maintained tolerance of NK cells expressing inhibitory KIRs for nonself HLA class I ligands during the posttransplantation immune reconstitution phase. Large multicenter studies in which NK-cell repertoires are studied in detail in the donor and at multiple time points after transplantation are warranted before algorithms for donor selection can be refined to include the KIR genetics of the donor. Such studies should ideally consider KIR haplotypes, gene polymorphism, and receptor expression as well as multiple effector functions in an effort to couple biologic readouts to clinical endpoints.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Swedish Foundation for Strategic Research, the Swedish Research Council, the Swedish Cancer Society, the Swedish Children's Cancer Foundation, the Cancer Society of Stockholm, the Royal Swedish Academy of Sciences, the Tobias Foundation, the Söderberg Foundation, The Belvén Foundation, the Åke Wiberg Foundation, an ALF-project grant from the Stockholm County Council, and the Karolinska Institutet.
National Institutes of Health
Authorship
Contribution: A.T.B. designed and performed experiments, analyzed and interpreted data, performed statistical analysis, and wrote the paper; M.S. and C.F. designed and performed experiments, analyzed and interpreted data, and contributed to the writing of the paper; M.R. collected and interpreted clinical data and performed statistical analyses; O.R., P.L., and H.-G.L. interpreted data and contributed to the writing of the paper; C.H. processed clinical samples; A.J.B. provided vital clinical samples, interpreted data, and contributed to the writing of the paper; and K.-J.M. designed the study, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karl-Johan Malmberg, Center for Infectious Medicine (CIM), F59, Department of Medicine, Karolinska Institutet, Karolinska University Hospital, 141 86 Stockholm, Sweden; e-mail: kalle.malmberg@ki.se.