Abstract
Variants of fibrinogen A α-chain (AFib) cause the most common type of hereditary renal amyloidosis in Europe and, possibly, the United States as well. Variant fibrinogen is produced in the liver, and solitary renal allografts fail within 1 to 7 years with recurrent amyloidosis. We assessed 22 AFib patients for combined liver and kidney transplantation (LKT) and report the clinical features and outcome. Twenty-one had E526V and 1, the R554L variant. Coronary atherosclerosis was identified in 68% and systemic atheromatosis in 55%. Vascular atheroma excised at endarterectomy and endomyocardial biopsies contained purely variant fibrinogen amyloid. Half had autonomic neuropathy. Six of 9 patients who underwent LKT are alive (67%), with good allograft function and no amyloidosis at median 67 months (range, 33-155 months) of follow-up. Serial technetium-99m–labeled dimercaptosuccinic acid (99mTc-DMSA) renal scintigraphy in 2 cases of preemptive LKT demonstrated preserved native kidney residual function at 5 years. Four explanted livers were used successfully for domino transplantation. Fibrinogen amyloidosis is a systemic amyloid disease with visceral, vascular, cardiac, and neurologic involvement. LKT is curative; however, cardiovascular amyloidosis may preclude this option. Our data encourage evaluation of preemptive solitary liver transplantation early in the course of amyloid nephropathy to prevent hemodialysis and kidney transplantation.
Introduction
Amyloidosis is a protein misfolding disorder, in which normally soluble proteins undergo conformational changes and are deposited in the extracellular space as abnormal insoluble fibrils that progressively disrupt tissue structure and function.1
The clinical syndromes of autosomal dominant hereditary renal amyloidoses were first described by Ostertag in 1932.2 To date, more than 25 different mutations in lysozyme,3 apolipoprotein AI (ApoAI),4,5 ApoAII,6,7 and fibrinogen A α-chain genes8-11 have been identified that share in common the manifestation of amyloid nephropathy in middle age. Given the absence of overt peripheral neurologic disease, these forms are collectively referred to as nonneuropathic hereditary renal amyloidoses.12
Fibrinogen amyloidosis due to mutations in the fibrinogen α-chain gene (AFib), first described by Benson et al in 1993,8 is emerging as the most common type of all hereditary renal amyloid diseases in the United Kingdom and Europe,11,13,14 whereas our data (M.D.B.) from a tertiary US amyloid reference center suggest AFib is the leading cause of hereditary renal amyloidosis associated with nephrotic syndrome in the United States.8,15,16
There are currently no treatments that can lead to resolution of amyloid deposits. Management is restricted to attempting to interrupt further supply of precursor amyloidogenic proteins, in combination with supportive care of failing organs, including transplantation. The role of transplantation may be either potentially curative, as in liver replacement to eliminate the source of the variant transthyretin in familial amyloid polyneuropathy, or merely supportive to restore failing organ function.1,17,18
Fibrinogen production is exclusively hepatic.19 Isolated renal transplantation as a treatment for renal failure in AFib amyloidosis is of limited value. Kidney amyloid recurrence and subsequent allograft loss is almost universal, and occurs within 1 to 7 years. Ten-year graft survival among the 18 reported kidney transplants for AFib to date is 5.5%.11,20-23 This outcome compares poorly with the current half-life of a cadaveric renal transplant for all causes of chronic kidney disease (CKD) of at least 10 to 12 years, or 10-year graft and patient survival of 64% and 68%, respectively.24,25
The lack of success of isolated kidney transplantation in fibrinogen amyloidosis in the current climate of organ shortage prompted us to evaluate hepatorenal transplantation at the Amyloidosis Treatment Center at King's College Hospital to manage the underlying disorder and prevent disease recurrence. Twenty-one of the cases presented here were initially evaluated at the UK National Amyloidosis Center, Royal Free Hospital, and appeared to have exclusively renal disease.22
We report major additions to the current phenotypic description of fibrinogen amyloidosis disease, previously unrecognized disease manifestations and risks, and the first systematic evaluation for liver transplantation in this series of 22 patients with fibrinogen amyloidosis managed in our center; and we present the long-term outcome of 9 combined hepatorenal transplantations and 4 sequential (domino) liver transplantations using the explanted livers from patients with AFib who underwent transplantation.
Methods
Twenty-two patients with fibrinogen amyloidosis and stage III-5 CKD with median creatinine clearance of 0.26 mL/s/m2 (range, 0-0.86 mL/s/m2, SI system) and median glomerular filtration rate (GFR) of 16 mL/min (range, 0-52 mL/min) were assessed for combined liver and kidney transplantation between 1996 and 2007. Three patients had been misdiagnosed as primary systemic AL (light-chain) amyloidosis in association with B-cell dyscrasias13 : 1 had received inappropriate chemotherapy and 2 had each received 2 renal allografts that all failed within 58 months. Twenty patients were British and 2, New Zealanders; 21 had the E526V and 1, the R554L AFib variant.22 None were diabetic. Median age at presentation was 55 years (range, 33-63 years), and at assessment 57 years (range, 49-68 years). Patient demographics are summarized in Table 1.
Patient demographics, clinical characteristics, presenting features, diagnosis, and course of renal disease
| Pt no. . | Age, y . | Sex . | Presenting features . | Medical history . | Symptoms history to diagnosis, mo . | Cr Cl, mL/s/m2 . | Urinary protein, g/24 h . | Mode of diagnosis . | AFib variant . | Renal family history . | Cardiovascular family history . | Diagnosis to ESRF, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | M | Proteinuria | HTN | 12 | 0.82 | 7.5 | Renal bx | E526V | Negative | Positive | 10 |
| 2* | 36 | F | HTN, proteinuria | Renal Tx | 156 | 0 | 0 | Renal bx | E526V | Positive | Positive | 6 |
| 3* | 42 | M | HTN, proteinuria | CVA | 127 | 0.45 | 1.7 | Graft bx | E526V | Negative | Positive | 6 |
| 4 | 57 | M | HTN, proteinuria | CVA, IHD | 46 | 0.46 | 11.8 | Renal bx | E526V | Unknown | Unknown | 8 |
| 5 | 57 | M | TIA, HTN | IHD | 44 | 0.75 | 4.8 | Renal bx | E526V | Negative | Negative | 8 |
| 6 | 63 | M | Retinopathy HTN | TIA | 8 | 0.30 | 7.8 | Renal bx | E526V | Negative | Positive | 8 |
| 7 | 45 | M | Proteinuria | None | 58 | 1.16 | 9.4 | Renal bx | E526V | Negative | Positive | 70 |
| 8 | 53 | F | Fatigue, edema | HTN | 24 | 0.36 | 8.0 | Renal bx | E526V | Negative | Negative | 5 |
| 9 | 53 | F | Edema | HTN | 24 | 0.41 | 7.9 | Renal bx | E526V | Negative | Positive† | 5 |
| 10 | 59 | M | Retinal bleed | HTN | 3 | 0.16 | 7.25 | Renal bx | E526V | Negative | Positive | 0 |
| 11 | 54 | M | Fatigue, HTN | MI | 4 | 0.33 | 6.7 | Renal bx | E526V | Negative | Negative | 4 |
| 12 | 56 | M | Proteinuria | HTN | 5 | 0.41 | 9.8 | Renal bx | E526V | Negative | Positive | 5 |
| 13 | 61 | F | Proteinuria | HTN | 6 | 0.61 | 3.8 | Renal bx | E526V | Negative | Positive | 26 |
| 14 | 52 | F | HTN | IHD | 7 | 0.46 | 4.6 | Renal bx | E526V | Positive | Positive | 6 |
| 15 | 53 | F | Proteinuria | None | 22 | 1.50 | 2.8 | Renal bx | E526V | Positive | Positive | 11 |
| 16 | 51 | M | Proteinuria | HTN | 18 | 0.78 | 9.8 | Renal bx | E526V | Negative | Positive‡ | 42 |
| 17 | 61 | M | TIA, HTN | None | 33 | 0.90 | 11.6 | Renal bx | E526V | Negative | Positive | 10 |
| 18 | 51 | F | Proteinuria | Angina | 32 | 1.40 | 3.5 | Renal bx | R554L | Negative | Positive | 14 |
| 19 | 56 | F | Headaches, HTN | Edema | 6 | 0.41 | 8.0 | Renal bx | E526V | Negative | Negative | 8 |
| 20 | 59 | M | Proteinuria | MI, IHD | 28 | 1.72 | 2.2 | Renal bx | E526V | Positive | Positive§ | 18 |
| 21 | 59 | M | Known carrier | MI, IHD | — | 1.53 | 4.8 | Renal bx | E526V | Positive | Positive§ | At CKD 3 |
| 22 | 53 | M | HTN, proteinuria | None | 12 | 1.50 | 2.9 | Renal bx | E526V | Negative | Positive | At CKD 4 |
| Pt no. . | Age, y . | Sex . | Presenting features . | Medical history . | Symptoms history to diagnosis, mo . | Cr Cl, mL/s/m2 . | Urinary protein, g/24 h . | Mode of diagnosis . | AFib variant . | Renal family history . | Cardiovascular family history . | Diagnosis to ESRF, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | M | Proteinuria | HTN | 12 | 0.82 | 7.5 | Renal bx | E526V | Negative | Positive | 10 |
| 2* | 36 | F | HTN, proteinuria | Renal Tx | 156 | 0 | 0 | Renal bx | E526V | Positive | Positive | 6 |
| 3* | 42 | M | HTN, proteinuria | CVA | 127 | 0.45 | 1.7 | Graft bx | E526V | Negative | Positive | 6 |
| 4 | 57 | M | HTN, proteinuria | CVA, IHD | 46 | 0.46 | 11.8 | Renal bx | E526V | Unknown | Unknown | 8 |
| 5 | 57 | M | TIA, HTN | IHD | 44 | 0.75 | 4.8 | Renal bx | E526V | Negative | Negative | 8 |
| 6 | 63 | M | Retinopathy HTN | TIA | 8 | 0.30 | 7.8 | Renal bx | E526V | Negative | Positive | 8 |
| 7 | 45 | M | Proteinuria | None | 58 | 1.16 | 9.4 | Renal bx | E526V | Negative | Positive | 70 |
| 8 | 53 | F | Fatigue, edema | HTN | 24 | 0.36 | 8.0 | Renal bx | E526V | Negative | Negative | 5 |
| 9 | 53 | F | Edema | HTN | 24 | 0.41 | 7.9 | Renal bx | E526V | Negative | Positive† | 5 |
| 10 | 59 | M | Retinal bleed | HTN | 3 | 0.16 | 7.25 | Renal bx | E526V | Negative | Positive | 0 |
| 11 | 54 | M | Fatigue, HTN | MI | 4 | 0.33 | 6.7 | Renal bx | E526V | Negative | Negative | 4 |
| 12 | 56 | M | Proteinuria | HTN | 5 | 0.41 | 9.8 | Renal bx | E526V | Negative | Positive | 5 |
| 13 | 61 | F | Proteinuria | HTN | 6 | 0.61 | 3.8 | Renal bx | E526V | Negative | Positive | 26 |
| 14 | 52 | F | HTN | IHD | 7 | 0.46 | 4.6 | Renal bx | E526V | Positive | Positive | 6 |
| 15 | 53 | F | Proteinuria | None | 22 | 1.50 | 2.8 | Renal bx | E526V | Positive | Positive | 11 |
| 16 | 51 | M | Proteinuria | HTN | 18 | 0.78 | 9.8 | Renal bx | E526V | Negative | Positive‡ | 42 |
| 17 | 61 | M | TIA, HTN | None | 33 | 0.90 | 11.6 | Renal bx | E526V | Negative | Positive | 10 |
| 18 | 51 | F | Proteinuria | Angina | 32 | 1.40 | 3.5 | Renal bx | R554L | Negative | Positive | 14 |
| 19 | 56 | F | Headaches, HTN | Edema | 6 | 0.41 | 8.0 | Renal bx | E526V | Negative | Negative | 8 |
| 20 | 59 | M | Proteinuria | MI, IHD | 28 | 1.72 | 2.2 | Renal bx | E526V | Positive | Positive§ | 18 |
| 21 | 59 | M | Known carrier | MI, IHD | — | 1.53 | 4.8 | Renal bx | E526V | Positive | Positive§ | At CKD 3 |
| 22 | 53 | M | HTN, proteinuria | None | 12 | 1.50 | 2.9 | Renal bx | E526V | Negative | Positive | At CKD 4 |
Pt no. indicates patient number (number sequence represents the same patients in Table 1 and Table 2, at presentation and during evaluation for LKT, respectively); Cr Cl, creatinine clearance; ESRF, end-stage renal failure (glomerular filtration rate 15 mL/min or less); HTN, hypertension; TIA, transient ischemic attack; CVA, cerebrovascular attack; IHD, ischemic heart disease; MI, myocardial infarct; renal bx, renal biopsy; Tx, transplantation; CKD, chronic kidney disease; and –, nonapplicable because the patient was diagnosed at the presymptomatic stage during family screen.
Patient no. 2, who presented initially with hypertension and proteinuria, is the only AFib patient reported to date with amyloid-related liver failure and she received LKT for the indication of both liver and kidney failure. She was anuric and on hemodialysis and creatinine clearance 0 mL/s/m2 at assessment for LKT.26 Patient no. 3 had liver amyloid deposits with normal function.
Patient no. 9 had a strong family history of IHD and aortic aneurysms including mother, maternal uncle, and grandmother, none of whom had renal disease. Computed tomography scan excluded aortic aneurysms in the patient before LKT. On subsequent family screen, a maternal aunt was diagnosed as heterozygous for the E526V variant, thus suggesting the just-mentioned family members as obligatory carriers.
Patient no. 16 had no family history of renal disease; his father had IHD and died in his late 50s of cerebrovascular attack. Patient had extensive carotid, systemic, and coronary disease at age 55 years. His sister was diagnosed with IHD requiring bypass graft in her early 40s with normal renal function. She was identified as carrier of the AFib E526V variant during subsequent family screen, and has during follow-up developed renal amyloid and ESRF at age 53 years. Two further siblings are noncarriers and remain free of cardiac events at ages 48 to 60 years.
Patient nos. 20 and 21 are related and both have paternal history of renal amyloidosis. Patient no. 20 presented with IHD and acute MI at age 52 years with normal kidney function; he noticed foaming urine and was tested positive for trace proteinuria 7 years later, whereas GFR was 102 mL/min and blood pressure, normal. Nephrotic range proteinuria was documented 2 years later; renal biopsy revealed amyloid. Patient no. 21 presented with MI and normal renal function, as did a further sibling, requiring angioplasty/stenting for IHD 5 years earlier. Patient no. 21 is at stage 3 CKD; sibling is a carrier and was recently diagnosed with nonnephrotic range proteinuria and GFR 105 mL/min. A further 2 members of the same kindred have long-standing IHD and amyloid nephropathy; noncarrier siblings have no history of IHD.
Four patients aged 58 to 63 years with hepatitis C, cryptogenic cirrhosis, or alcoholic liver cirrhosis complicated by hepatocellular carcinoma underwent sequential (domino) liver transplantation in which we used as liver grafts the functionally and morphologically normal explanted livers from AFib patients who underwent combined liver and kidney transplantation. The domino recipients received full information and comprehensive pretransplantation counseling regarding the potential risks of transmission of AFib through domino transplantation and consented to the procedure as well as the requirements for regular long-term follow-up.
Patients were managed in accordance with the Declaration of Helsinki. The King's College Hospital Research Ethics Committee had sight of the study and indicated approval. Unrelated liver transplant regulatory authority (ULTRA, UK transplant) approval was obtained for all domino donors and recipients prior to placement on the transplant list. Written informed consent was obtained for diagnostic investigations, for tissue transfer, and for use of material in this publication.
Clinical assessment
Cardiovascular investigations comprised 12 lead electrocardiogram and transthoracic or transesophageal echocardiography at baseline. Patients with one or more risk factors of ischemic heart disease subsequently underwent coronary angiography. Endomyocardial biopsies were performed in the R554L case, and in 3 E526V patients with abnormal echocardiography. Iliac and carotid vessels were assessed with Doppler ultrasound. Cardiac autonomic function was assessed by serial measurements of heart rate variability and blood pressure monitoring at rest and after stress challenges, and 24-hour blood pressure monitoring. Peripheral neurologic assessment comprised sensory topographic mapping and the modified polyneuropathy disability score for evaluation of motor function. Patients who were accepted for transplantation and placed on the liver transplant list underwent re-evaluation at 6- to 12-month intervals during waiting time.
Routine histology was carried out in all explanted livers from AFib patients undergoing combined liver and kidney transplantation before being sequentially used for domino transplant, to exclude parenchymal amyloid deposition and to assess the degree of possible steatosis and graft suitability.
Posttransplantation follow-up comprised 3 monthly dual allograft function, creatinine clearance, and 24-hour urine protein levels. Neurologic evaluation, allograft Doppler ultrasound scans, and echocardiography were performed at least annually. Two AFib patients who had residual native kidney function with GFR of 15 and 12 mL/min, respectively, at the time of dual organ transplantation underwent dynamic and static 99mTc-DMSA renograms annually postoperatively.
Immunohistochemical characterization of cardiac amyloid deposits
Immunohistochemistry was performed by standard technique. Sections were prepared and incubated sequentially in 1.5% goat serum for 30 minutes, rabbit antihuman fibrinogen 1:200 (Dako Cytomation Inc) for 30 minutes, biotinylated goat anti–rabbit immunoglobulin G (1:200; Vector Laboratories) for 30 minutes, ABC reagent (Vector Laboratories) for 45 minutes, and substrate for 3 to 7 minutes. Horseradish peroxidase substrate was prepared using FAST diaminobenzidine and urea H2O2 tablets (Sigma-Aldrich). Tissues were counterstained with hematoxylin. Representative sections were photographed on a Nikon Microphot-SA microscope with a SPOT RT-KE digital camera (Diagnostic Instruments).
Biochemical analysis of amyloid atheromatous plaque
Fibrils were isolated from an atheroma excised at endarterectomy from the carotid artery of a fibrinogen A α-chain E526V patient by hand homogenization in 2 mL of 0.1M sodium citrate, 0.15M sodium chloride, and centrifugation. The pellet was homogenized and centrifuged again 2 more times. The final pellet was solubilized in 1 mL of 8M guanidine hydrochloride, 0.5M tris(hydroxymethyl)aminomethane, pH 8.2, containing 10 mg of dithiothreitol/mL with magnetic stirring at room temperature overnight. The sample was alkylated with iodoacetic acid (25 mg/mL) and centrifuged. The supernatant was chromatographed on a Sepharose CL6B column (0.90 × 40 cm) equilibrated and eluted with 4M guanidine hydrochloride, 50mM tris(hydroxymethyl)aminomethane, pH 8.2. Fractions from approximately 25 kDa to the column volume elution area were pooled for analysis, exhaustively dialyzed in Spectra Por 6 dialysis tubing (Spectrum Laboratories) against water, and lyophilized. The pool was digested with trypsin in 0.1M ammonium bicarbonate overnight at room temperature, and the resulting peptides were fractionated by reverse-phase high-performance liquid chromatography on a Synchropak RP8 column (0.46 × 25 cm; Eprogen) equilibrated with 0.1% trifluoracetic acid in water and eluted with a 0% to 60% acetonitrile gradient over 90 minutes. High-performance liquid chromatography fractions were dried in a Speed Vac concentrator (Savant Instruments) and analyzed by Edman degradation27 on an Applied Biosystems Model 491 cLC protein sequencer using the manufacturer's standard cycles and methods.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 for Windows software (SPSS Inc) for estimation of median and mean values and bivariate analysis for correlation of variables. Survival was estimated by Kaplan-Meier analysis.
Results
Proteinuria was the most common presenting feature, either identified on routine medical screen or diagnosed during investigations for edema and fatigue in 55% of cases. Hypertension or complications of headaches or retinal bleed were the initial presentation in 40%, and one patient was diagnosed during family screen. All patients were subsequently placed under renal follow-up and investigated with renal biopsy for the indications of declining renal function or increasing proteinuria. Mode of diagnosis was renal biopsy revealing amyloidosis in all cases. Median GFR at diagnosis was 32.5 mL/min (range, 0-103 mL/min) and 24-hour urine protein, 7.2 g (range, 0-11.8 g). Median time from initial presentation to diagnosis of fibrinogen amyloidosis was 23 months (range, 3-156 months), and time from diagnosis to end-stage renal failure (GFR < 10 mL/min) was 8 months (0-70 months). At the time of assessment in our institution, median GFR was 16 mL/min (0-52 mL/min). One patient had end-stage liver disease in association with extensive hepatic amyloidosis,23 and a second patient had liver amyloidosis with preserved liver function (Tables 1–2). Fibrinogen concentration determined by the Clauss (functional) method and thrombin clotting times were within the normal range in all plasma samples.
Findings during assessment for LKT, patient selection, and transplantation outcome
| Pt no. . | Duration of dialysis at assessment for LKT, mo . | CrCl, mL/s/m2 . | Autonomic neuropathy . | Echo findings (IVS and LVPW in mm) . | Coronary disease . | Systemic vascular disease . | LKT listing decision . | Duration of dialysis at LKT, mo . | ITU stay, d . | Hospital stay, d . | LKT outcome survival, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0.38 | GI | Normal | None | None | Listed | 0 | 2 | 21 | Alive 74 |
| 2 | Failed 2nd Tx† | 0 | GI | Normal | None | None | Listed | 36 | 2 | 19 | Alive 155 |
| 3 | Failed 2nd Tx† | 0.45 | Cardiac, GI | Dilated LA | IHD | Aorta | Rejected | — | — | — | — |
| 4 | 42 | 0 | Cardiac, GI | EF 45%, IVS 13 | Previous MI | Iliac | Rejected | — | — | — | — |
| 5 | 36 | 0 | GI | Dilated RA, LA | IHD | Carotid | Rejected | — | — | — | — |
| 6 | 48 | 0 | GI | Normal | IHD | Carotid | Rejected | — | — | — | — |
| 7 | 0 | 0.86 | None | Normal | None | None | Listed | 0 | 12 | 21 | Alive 68 |
| 8 | 2 | 0.16 | GI | Normal | None | Femoral | Listed | 26 | 63 | 112 | Alive 33 |
| 9 | 0 | 0.26 | Cardiac, GI | Normal | IHD | None | Listed | 8 | 45 | 71 | Dead 2.5 |
| 10 | 26 | 0 | Cardiac, GI | Normal | None | Aorta | Listed | 34 | 35 | 40 | Dead 1.3 |
| 11 | 33 | 0 | Cardiac, GI | Normal | IHD, MI | Carotid | Listed | 42 | 43 | 91 | Alive 66 |
| 12 | 0 | 0.26 | GI | Normal | None | None | Listed | 4 | 2 | 18 | Alive 51 |
| 13 | 0 | 0.29 | Cardiac, GI | Normal | IHD | Carotid | Listed § | — | — | — | — |
| 14 | 0 | 0.16 | GI | EF 45%, LVH | IHD, MI | Iliac | Listed § | — | — | — | — |
| 15 | 0 | 0.29 | Cardiac | IVS, LVW 13 | IHD | None | Listed § | — | — | — | — |
| 16 | 0 | 0.55 | Cardiac | Restrictive | IHD | Carotid | Listed § | — | — | — | — |
| 17 | 0 | 0.26 | Cardiac | IVS 13, AV 18 | IHD | Aorta | Rejected | — | — | — | — |
| 18 | 0 | 1.03 | Cardiac | Dilated CMP | IHD | Carotid | Rejected | — | — | — | — |
| 19 | 0 | 0.15 | Cardiac | IVS, LVW 14 | None | None | Listed | 8 | 130 | 130 | Dead 4.3 |
| 20 | 0 | 0.86 | None | IVS, LVW 12.5 | MI-CABG | None | Rejected | — | — | — | — |
| 21* | 0 | 0.75 | GI | Wall akinesis* | MI-stent | None | Rejected | — | — | — | — |
| 22‡ | 0 | 1.16 | Cardiac, GI | Impaired relaxation | Mild | None | Listed | — | — | — | Awaiting LKT |
| Pt no. . | Duration of dialysis at assessment for LKT, mo . | CrCl, mL/s/m2 . | Autonomic neuropathy . | Echo findings (IVS and LVPW in mm) . | Coronary disease . | Systemic vascular disease . | LKT listing decision . | Duration of dialysis at LKT, mo . | ITU stay, d . | Hospital stay, d . | LKT outcome survival, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0.38 | GI | Normal | None | None | Listed | 0 | 2 | 21 | Alive 74 |
| 2 | Failed 2nd Tx† | 0 | GI | Normal | None | None | Listed | 36 | 2 | 19 | Alive 155 |
| 3 | Failed 2nd Tx† | 0.45 | Cardiac, GI | Dilated LA | IHD | Aorta | Rejected | — | — | — | — |
| 4 | 42 | 0 | Cardiac, GI | EF 45%, IVS 13 | Previous MI | Iliac | Rejected | — | — | — | — |
| 5 | 36 | 0 | GI | Dilated RA, LA | IHD | Carotid | Rejected | — | — | — | — |
| 6 | 48 | 0 | GI | Normal | IHD | Carotid | Rejected | — | — | — | — |
| 7 | 0 | 0.86 | None | Normal | None | None | Listed | 0 | 12 | 21 | Alive 68 |
| 8 | 2 | 0.16 | GI | Normal | None | Femoral | Listed | 26 | 63 | 112 | Alive 33 |
| 9 | 0 | 0.26 | Cardiac, GI | Normal | IHD | None | Listed | 8 | 45 | 71 | Dead 2.5 |
| 10 | 26 | 0 | Cardiac, GI | Normal | None | Aorta | Listed | 34 | 35 | 40 | Dead 1.3 |
| 11 | 33 | 0 | Cardiac, GI | Normal | IHD, MI | Carotid | Listed | 42 | 43 | 91 | Alive 66 |
| 12 | 0 | 0.26 | GI | Normal | None | None | Listed | 4 | 2 | 18 | Alive 51 |
| 13 | 0 | 0.29 | Cardiac, GI | Normal | IHD | Carotid | Listed § | — | — | — | — |
| 14 | 0 | 0.16 | GI | EF 45%, LVH | IHD, MI | Iliac | Listed § | — | — | — | — |
| 15 | 0 | 0.29 | Cardiac | IVS, LVW 13 | IHD | None | Listed § | — | — | — | — |
| 16 | 0 | 0.55 | Cardiac | Restrictive | IHD | Carotid | Listed § | — | — | — | — |
| 17 | 0 | 0.26 | Cardiac | IVS 13, AV 18 | IHD | Aorta | Rejected | — | — | — | — |
| 18 | 0 | 1.03 | Cardiac | Dilated CMP | IHD | Carotid | Rejected | — | — | — | — |
| 19 | 0 | 0.15 | Cardiac | IVS, LVW 14 | None | None | Listed | 8 | 130 | 130 | Dead 4.3 |
| 20 | 0 | 0.86 | None | IVS, LVW 12.5 | MI-CABG | None | Rejected | — | — | — | — |
| 21* | 0 | 0.75 | GI | Wall akinesis* | MI-stent | None | Rejected | — | — | — | — |
| 22‡ | 0 | 1.16 | Cardiac, GI | Impaired relaxation | Mild | None | Listed | — | — | — | Awaiting LKT |
LKT indicates liver and kidney transplantation; Cr Cr, creatinine clearance; Echo, echocardiography; IVS, intraventricular septum; LVPW, left ventricular posterior wall (thickness in millimeters); GI, gastrointestinal; EF, ejection fraction; CAGB, coronary artery bypass graft; MI, myocardial infarct; LA, left atrium; RA, right atrium; restrictive, restrictive physiology with impaired relaxation; IHD, ischemic heart disease; CMP, cardiomyopathy; and —, nonapplicable because the patients did not receive LKT.
Patient no. 21 underwent echocardiography in another facility and echo findings are excluded from analysis.
Both patients received hemodialysis at end-stage renal disease before kidney transplantation and for varying periods between subsequent failed kidney transplants.
Patient no. 22 was initially listed for preemptive isolated liver transplantation, but transplantation status was changed to listing for LKT when GFR fell below 50 mL/min during follow-up on the waiting list.
Patient nos. 13, 14, 15, and 16 were initially assessed as suitable candidates and were listed for LKT, but were removed from the waitlist due to deteriorating cardiac function or progressive IHD 11 to 34 months later.
Of note, only 24% of patients had family history of renal disease, but 81% had family history of ischemic heart disease or systemic vascular disease with aortic aneurysms or cerebrovascular events. Further family screening with DNA analysis for the AFib gene revealed that the propensitus or the family members who had cardiovascular history were indeed either the confirmed or obligatory carriers of the fibrinogen gene mutations in each evaluable case. (Table 1).
Cardiovascular findings
Coronary atherosclerotic disease was documented in 15 patients (68%). Of those, 8 had a diagnosis of coronary artery disease or previous myocardial infarction, in half of cases predating evolution of proteinuria or kidney impairment by 5 to 7 years. Six patients were diagnosed with asymptomatic 40% to 80% stenosis of the left or right coronary arteries during transplant assessment, and one had triple vessel atherosclerotic disease with limited plaque burden (Tables 1–2).
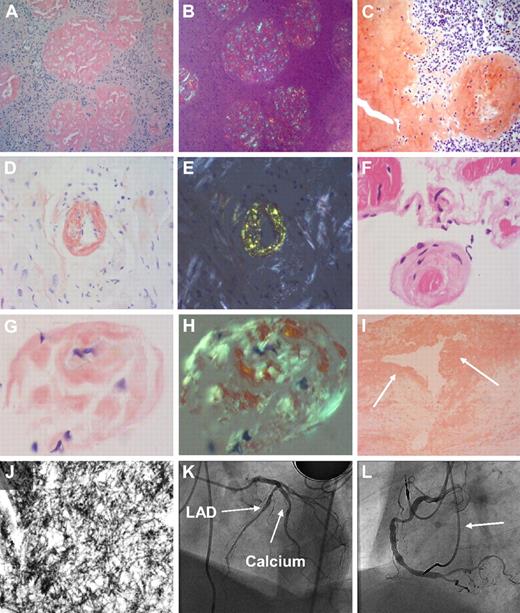
Twelve patients (55%) had severe systemic vascular disease including mobile atheromatous plaques in the atria, aorta, splanchnic vessels, or carotids. Two patients underwent carotid endarterectomy for 80% stenosis. The excised atheroma contained amyloid purely consistent of mutant fibrinogen A α-chain. Arteries and veins of the hilum of explanted livers contained traces of birefringent fibrinogen amyloid (Figure 1).
Morphologic findings in the common fibrinogen A α-chain amyloidosis E526 variant. (A) Congo red stain in renal biopsy (×100 magnification) in fibrinogen A α-chain amyloidosis. Extensive amyloid infiltrate with glomerular enlargement and replacement of the normal glomerular architecture by amyloid deposition, with almost no amyloid in the interstitium. (B) Same section as panel A viewed between crossed polars shows apple green birefringence typical of amyloid deposits. (C) Congo red stain in histologic sample from a ruptured spleen (×100 magnification) in AFib E526V exhibits extensive splenic amyloid deposits with predominantly trabecular and subcapsular distribution. (D) Endomyocardial biopsy specimen (×100 magnification) in a patient with AFib E526V and renal failure, with abnormal echocardiography (patient 19, Tables 1 and 2). Cardiac histology stained with Congo red demonstrates extensive, diffuse amyloid deposition in the myocardium. An endomyocardial vessel is demonstrated at the center of the section, showing complete replacement of its entire wall thickness by amyloid deposition. (E) Bright apple green birefringence of the amyloid deposits in myocardium and endocardial vessels of section in panel D under cross-polarized light. (F) Endomyocardial biopsy (×40 magnification) hematoxylin and eosin stain, in a patient with AFib E526V variant (patient 16, tables), on hemodialysis for 18 months, who had severe carotid atherosclerosis, and whose echocardiography showed normal wall thickness with impaired left ventricular relaxation. (G) Congo red stain demonstrates amyloid deposition at the periphery of an atrophic muscle fiber from the same sample (×100 magnification). (H) Apple green birefringence under polarized light in previous section. (I) Arterial intima atheroma (×100 magnification) excised during endarterectomy for the indication of 80% carotid stenosis in the same patient as in panels D and E. Congo red stain demonstrates extensive amyloid deposition within the intima and atheromatous plaque (arrows). The Congo red material exhibited strong apple green birefringence. Immunostaining with antifibrinogen antibodies was not performed because normal (wild-type) fibrinogen is expected to be part of the thrombus, and therefore a positive immunohistochemistry could not be reliably positive for a diagnosis of variant fibrinogen amyloidosis. (J) Transmission electron microscopy (×150 000 magnification) images of the atheroma in panel F demonstrates fibrillar material with fibril diameter of approximately 10 nm, compatible with amyloid. Fibril extraction and characterization revealed the amyloid atheromatous plaque to consist wholly of variant E526V fibrinogen. (K) Images from subsequent coronary angiography in the same patient, carried out to exclude significant asymptomatic coronary atherosclerosis as part of generalized amyloid angiopathy, in context of findings in panels F through I. Left anterior oblique cranial angiogram of left coronary artery shows diffuse atheroma in the left anterior descending coronary artery (LAD) with heavy calcification (arrows). (L) Left anterior oblique angiographic projection of the right coronary artery (same patient as in panel K) shows diffuse atheroma throughout its course. Leads from dual chamber pacemaker inserted for the indication of bradyarrhythmias are also shown (arrow).
Morphologic findings in the common fibrinogen A α-chain amyloidosis E526 variant. (A) Congo red stain in renal biopsy (×100 magnification) in fibrinogen A α-chain amyloidosis. Extensive amyloid infiltrate with glomerular enlargement and replacement of the normal glomerular architecture by amyloid deposition, with almost no amyloid in the interstitium. (B) Same section as panel A viewed between crossed polars shows apple green birefringence typical of amyloid deposits. (C) Congo red stain in histologic sample from a ruptured spleen (×100 magnification) in AFib E526V exhibits extensive splenic amyloid deposits with predominantly trabecular and subcapsular distribution. (D) Endomyocardial biopsy specimen (×100 magnification) in a patient with AFib E526V and renal failure, with abnormal echocardiography (patient 19, Tables 1 and 2). Cardiac histology stained with Congo red demonstrates extensive, diffuse amyloid deposition in the myocardium. An endomyocardial vessel is demonstrated at the center of the section, showing complete replacement of its entire wall thickness by amyloid deposition. (E) Bright apple green birefringence of the amyloid deposits in myocardium and endocardial vessels of section in panel D under cross-polarized light. (F) Endomyocardial biopsy (×40 magnification) hematoxylin and eosin stain, in a patient with AFib E526V variant (patient 16, tables), on hemodialysis for 18 months, who had severe carotid atherosclerosis, and whose echocardiography showed normal wall thickness with impaired left ventricular relaxation. (G) Congo red stain demonstrates amyloid deposition at the periphery of an atrophic muscle fiber from the same sample (×100 magnification). (H) Apple green birefringence under polarized light in previous section. (I) Arterial intima atheroma (×100 magnification) excised during endarterectomy for the indication of 80% carotid stenosis in the same patient as in panels D and E. Congo red stain demonstrates extensive amyloid deposition within the intima and atheromatous plaque (arrows). The Congo red material exhibited strong apple green birefringence. Immunostaining with antifibrinogen antibodies was not performed because normal (wild-type) fibrinogen is expected to be part of the thrombus, and therefore a positive immunohistochemistry could not be reliably positive for a diagnosis of variant fibrinogen amyloidosis. (J) Transmission electron microscopy (×150 000 magnification) images of the atheroma in panel F demonstrates fibrillar material with fibril diameter of approximately 10 nm, compatible with amyloid. Fibril extraction and characterization revealed the amyloid atheromatous plaque to consist wholly of variant E526V fibrinogen. (K) Images from subsequent coronary angiography in the same patient, carried out to exclude significant asymptomatic coronary atherosclerosis as part of generalized amyloid angiopathy, in context of findings in panels F through I. Left anterior oblique cranial angiogram of left coronary artery shows diffuse atheroma in the left anterior descending coronary artery (LAD) with heavy calcification (arrows). (L) Left anterior oblique angiographic projection of the right coronary artery (same patient as in panel K) shows diffuse atheroma throughout its course. Leads from dual chamber pacemaker inserted for the indication of bradyarrhythmias are also shown (arrow).
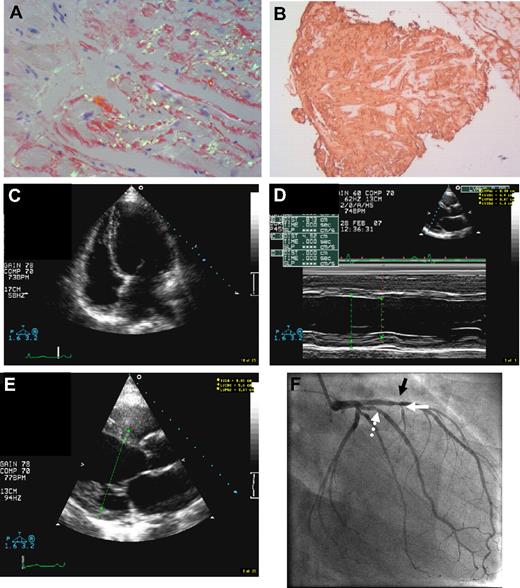
Echocardiography was abnormal in 11 of 21 patients (52%), demonstrating impaired relaxation pattern, increased wall thickness, and/or reduced ejection fraction, findings consistent with amyloidosis, and in 1 case dilated cardiomyopathy (Table 2). Three of the 4 endomyocardial biopsies revealed substantial amyloid deposition in endomyocardium, interstitium, and within the walls of endocardial vessels (Figures 1–2). The R554L patient defies nomenclature criteria for definition of cardiac amyloidosis which include restrictive physiology,28 and is the first AFib case diagnosed with dilated amyloid cardiomyopathy in association with coronary disease and myocardial amyloidosis (Figure 2).
Dilated amyloid cardiomyopathy in fibrinogen A α-chain amyloidosis R554L variant. (A) Endomyocardial biopsy (×100 magnification) in a 55-year-old patient with the AFib R554L variant and stage II CKD, whose echocardiography during evaluation for combined LKT showed dilated cardiomyopathy with ejection fraction of only 25% (patient 18, tables). Endomyocardial histology shows bright apple green birefringence of diffuse Congo red–positive endocardial and interstitial amyloid deposits. No additional pathology or potential causes for dilated cardiomyopathy other than amyloidosis were identified. (B) Strongly positive fibrinogen immunohistochemistry using rabbit anti–human fibrinogen 1:200 (Dako Cytomation Inc), in the same section of panel A. (C) Apical 4-chamber 2-dimensional echocardiographic view at end diastole, demonstrating a dilated and globular left ventricular cavity. (D) Imaging from M-mode echocardiogram in the same patient, demonstrating increased left ventricular dimensions and reduced function. (E) Parasternal long axis echocardiographic images of increased left ventricular end diastolic dimensions. (F) Invasive angiogram of the left coronary artery (right anterior oblique projection), showing a 40% stenosis in the proximal anterior descending artery (black arrow), lumen irregularity, and more minor narrowing in the intermediate vessel (dotted white arrow) and narrowing in the first septal perforator (white arrow). The patient's right coronary had minor irregularity (not shown).
Dilated amyloid cardiomyopathy in fibrinogen A α-chain amyloidosis R554L variant. (A) Endomyocardial biopsy (×100 magnification) in a 55-year-old patient with the AFib R554L variant and stage II CKD, whose echocardiography during evaluation for combined LKT showed dilated cardiomyopathy with ejection fraction of only 25% (patient 18, tables). Endomyocardial histology shows bright apple green birefringence of diffuse Congo red–positive endocardial and interstitial amyloid deposits. No additional pathology or potential causes for dilated cardiomyopathy other than amyloidosis were identified. (B) Strongly positive fibrinogen immunohistochemistry using rabbit anti–human fibrinogen 1:200 (Dako Cytomation Inc), in the same section of panel A. (C) Apical 4-chamber 2-dimensional echocardiographic view at end diastole, demonstrating a dilated and globular left ventricular cavity. (D) Imaging from M-mode echocardiogram in the same patient, demonstrating increased left ventricular dimensions and reduced function. (E) Parasternal long axis echocardiographic images of increased left ventricular end diastolic dimensions. (F) Invasive angiogram of the left coronary artery (right anterior oblique projection), showing a 40% stenosis in the proximal anterior descending artery (black arrow), lumen irregularity, and more minor narrowing in the intermediate vessel (dotted white arrow) and narrowing in the first septal perforator (white arrow). The patient's right coronary had minor irregularity (not shown).
Neurologic manifestations
Cardiac parasympathetic dysfunction and risk of bradycardia were identified in 12 patients (55%). Seven patients who fulfilled Mayo Clinic criteria29 had pacemaker insertion. Autonomic involvement of the gastrointestinal tract with constipation or diarrhea, early satiety and nausea, or delayed gastric emptying was a feature in 15 patients (68%). Polyneuropathy disability scores and sensory mapping were normal in all cases. Results are summarized in Table 2.
Morphologic findings
Kidneys.
Extensive amyloid deposits were present in all renal biopsies, showing enlarged glomeruli replaced by amyloid with minimal interstitial involvement.
Spleen.
Spontaneous splenic rupture occurred in 1 patient during hemodialysis and in 3 further cases during transplantation. The excised spleens demonstrated widespread amyloid with predominantly trabecular and subcapsular distribution.
Atheromatous plaque.
The atheromatous carotid lesion exhibited Congo red–positive deposits that on transmission electron microscopic examination revealed fibrillar material with a fibril diameter of approximately 10 nm, compatible with amyloid. Edman analysis of tryptic peptides from the amyloid protein yielded the sequence TFPGFFSPMLGEFVSETVSR, which corresponds to the residue 509-528 peptide of mature fibrinogen A α-chain with the V526 at position 18. No residue 509-528 peptide containing the normal E526 or peptides from apolipoprotein A1 or medin were found.
Cardiac histology.
Endomyocardial amyloid deposits stained strongly positive on immunohistochemistry with rabbit antihuman fibrinogen antibodies (Dako) in both the E526V and R554L variants (Figure 1).
Transplantation assessment outcome
Fourteen patients were accepted for listing for combined liver and kidney transplantations. Eight patients were declined as the assessment suggested a greater than 50% 5-year mortality risk due to poor ventricular function (ejection fraction less than 50%), or cardiovascular/systemic atheromatous disease not amenable to reconstructive treatment. Four patients originally listed were removed from the wait list because of deterioration in cardiorespiratory function 11 to 34 months later, and 1 patient remains on the active waiting list. Time spent on hemodialysis correlated with frequency of identified conventional cardiovascular exclusion criteria (P < .05).
Outcome of hepatorenal transplantation and domino liver transplantation
Nine patients received combined liver and kidney allografts between January 1996 and September 2009 in our center. Transplantation procedures were complicated by spontaneous perioperative splenic rupture in 3 and postoperative deep vein thrombosis in 2 cases. At median follow-up of 67 months (range, 33-155 months), 6 of 9 patients are alive and well (cumulative survival, 67%) with normal liver function. Five patients have good renal function, creatinine 120μM (range, 87-159μM), and median GFR of 54 mL/min (range, 45-69 mL/min). The sixth patient developed renal failure after 68 months and renal biopsy demonstrated chronic allograft nephropathy. All patients who underwent LKT before or within 6 months of initiating renal replacement therapy are alive and well (survival 100%), but outcome of LKT was successful in only 50% of patients who were on long-term hemodialysis at the time transplantation. Two fatal transplantation outcomes occurred in long-term hemodialysis patients and were due to biliary dyskinesia and acute necrotizing pancreatitis complicated by fatal bradyarrhythmia in a 62-year-old man, and biliary leak, sepsis, subendocardial infarct, and multiorgan failure in a 55-year-old woman. A further patient on peritoneal dialysis, previously misdiagnosed and inappropriately treated as AL amyloidosis in another facility, developed hepatic artery thrombosis requiring retransplantation but died 4 months after the initial transplantation. Median intensive treatment unit and overall hospital stay was 35 days (range, 2-130 days) and 40 days (range, 18-130 days), respectively. Patients received standard immunosuppression with tacrolimus and steroids and additionally mycophenolate mofetil. There were no episodes of rejection.
Twenty-three echocardiography examinations in 8 patients after LKT have documented stable cardiac indices and no evidence of progressive or de novo cardiac amyloidosis, at up to 12 years of follow-up. Symptoms of gut dysmotility had continued in the 2 patients who had received previous isolated kidney transplants, but resolved in all patients after hepatorenal transplantation. All patients have resumed normal everyday activities and those of nonretiring age have been able to return to full employment.
After elimination of the source of amyloid production through hepatorenal transplantation, whole-body 123I-labeled serum amyloid P component (SAP) scintigraphy demonstrated regression of systemic visceral amyloid deposits as early as at first annual follow-up scan after transplantation. None of the AFib patients who underwent transplantation presented evidence of de novo or progressive amyloid deposition at up to 13 years of follow-up as previously reported.23 In contrast, SAP scintigraphy documented progressive systemic amyloidosis and amyloid deposition in the kidney grafts in 2 AFib patients in this series who had previously undergone isolated kidney transplantation.22,23 One domino recipient had SAP scans and echocardiography examinations for up to 5 years with no evidence of de novo amyloid deposition. The remaining 3 patients did not have SAP scans. One has normal liver and renal function at 2.5 years, and 2 were lost to follow-up after returning to their country of origin after successful liver transplantation (LT).
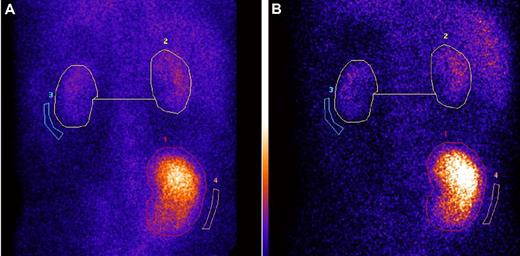
Serial dynamic and static 99mTc-DMSA renal scintigraphy after preemptive LKT in 2 cases demonstrated stable native renal function, with consistent contribution to the overall kidney function of 14% and 20% at up to 5 years of follow-up (Figure 3).
Serial 99mTc-DMSA scintigraphy shows stable renal graft: native kidneys divided function at 1 and 4 years after preemptive combined liver and kidney transplantation, in AFib E526. (A-B) Serial 99mTc-DMSA scintigraphy in an AFib E526V patient who received preemptive combined liver and kidney transplantation at stage 5 CKD, 1 month before scheduled commencement of hemodialysis. (A) Posterior view of dynamic renal scintigraphy at 1 year after combined liver and kidney function, 3 hours after injection of 80 MBq 99mTc-DMSA. Regions of interest (ROI; circles) shown are used in the calculation of divided function. The percentage of divided function is as follows: transplant kidney 86% and total native 14%. (B) DMSA scintigraphy in the same patient at 4 years after LKT. The panel shows posterior view scintigraphy 3 hours after injection of 80 MBq 99mTc-DMSA, and same regions of interest are used in the calculation of divided function. The percentage of divided function is as follows: transplant kidney 87% and total native kidney function 13%. We have confirmed by mass spectrometry19 in this patient that variant fibrinogen A α-chain has been completely eliminated from the plasma and is replaced by normal (wild-type) fibrinogen A α-chain after LKT.
Serial 99mTc-DMSA scintigraphy shows stable renal graft: native kidneys divided function at 1 and 4 years after preemptive combined liver and kidney transplantation, in AFib E526. (A-B) Serial 99mTc-DMSA scintigraphy in an AFib E526V patient who received preemptive combined liver and kidney transplantation at stage 5 CKD, 1 month before scheduled commencement of hemodialysis. (A) Posterior view of dynamic renal scintigraphy at 1 year after combined liver and kidney function, 3 hours after injection of 80 MBq 99mTc-DMSA. Regions of interest (ROI; circles) shown are used in the calculation of divided function. The percentage of divided function is as follows: transplant kidney 86% and total native 14%. (B) DMSA scintigraphy in the same patient at 4 years after LKT. The panel shows posterior view scintigraphy 3 hours after injection of 80 MBq 99mTc-DMSA, and same regions of interest are used in the calculation of divided function. The percentage of divided function is as follows: transplant kidney 87% and total native kidney function 13%. We have confirmed by mass spectrometry19 in this patient that variant fibrinogen A α-chain has been completely eliminated from the plasma and is replaced by normal (wild-type) fibrinogen A α-chain after LKT.
Discussion
Fibrinogen is a plasma protein with a crucial role in the coagulation cascade through its conversion to fibrin, and is composed of 2 identical sets of 3 polypeptide chains termed A α, B β, and γ, joined by disulfide bridging. Each polypeptide is encoded by a distinct gene, FGA, FGB, and FGG. The gene for the fibrinogen A α-chain with 610 amino acid residues is localized on chromosome 4 and has 6 exons.30 Mutations in any of the 3 genes encoding for fibrinogen polypeptides can cause dysfibrinogenemias, and recently identified mutations in the A α-chain gene can lead to hereditary systemic amyloidosis.12
Six amyloidogenic mutations in the fibrinogen A α-chain gene have been described to date. The first variant, identified in a Peruvian-Mexican family and 2 unrelated African-American and French kindreds, is caused by a point mutation in the α-chain gene encoding for substitution of arginine to leucine (R554L) in the fibrinogen molecule.8,16 The E526V and E540V variants, 2 frameshift mutations (4904delG and 4897delT), and more recently an insertion/deletion (1636-1650del, 1649-1650insCA) variant in the fibrinogen α-chain gene were identified in kindreds of Irish, British, Portuguese, French, German, and Far Eastern origin with amyloid nephropathy due to variant fibrinogen.9-12,31
Renal amyloidosis in AFib universally progresses to complete ESRF and the focus of clinical intervention has been to provide adequate renal replacement therapy including kidney transplantation.13,22 Age of onset varies with different mutations, from childhood in the insertion deletion mutation to middle life in the most common E526V variant.8-10,31 The onset of the disease is usually heralded by the development of renal amyloidosis, and penetrance is reportedly low.13,22
Our data provide valuable insights into the disease phenotype and suggest that hereditary fibrinogen amyloidosis is neither solely nephropathic nor solely nonneuropathic, but is a disease with a diverse and complex phenotype. All reported cases to date have been diagnosed through renal pathology, during investigation for hypertension, kidney impairment, and proteinuria, frequently identified during routine medical screening. However, in light of the findings in this series, corroborated by another recent report,32 a bias toward underdiagnosing cases with predominantly cardiovascular amyloidosis, in the absence of readily accessible tissue for histologic sampling, cannot be excluded and may account for the apparent reduced penetrance.
We have identified a high incidence of cardiovascular atheromatous disease among our patients, often predating evolution of proteinuria or renal impairment by many years. There was also a strong family history for coronary/vascular disease among carriers even in the absence of overt renal disease (Table 1). Our findings are unlikely to be due solely to the vascular effects of renal failure,33 because the specific form of fibrinogen amyloid was present in the vascular walls and atheromatous plaques. Biochemical analysis indeed revealed that amyloid atheromatous plaques, and cardiac and vascular amyloid deposits in AFib were composed wholly of variant fibrinogen, and excluded the presence of other amyloid precursor proteins with inherent atherogenetic properties such as senile transthyretin or apolipoprotein ApoAI.34
This is the first report of a link between variant fibrinogen amyloidosis and atheromatosis. A plausible explanation for the syndrome of systemic amyloid angiopathy in AFib lies principally with direct amyloid deposition in vascular walls and myocardial vessels. The associated, but likely not necessarily prerequisite, manifestations of nephrotic syndrome, hyperlipidemia, hypertension, and declining renal function facilitate atheroma formation on a background of vascular amyloid deposition and impaired endothelial function, further accelerated by commencement of renal replacement therapy.33,35,36 The well-described predilection for renal amyloid localization to the glomerulus rather than interstitium largely represents a form of amyloid vascular disease within the kidney, and is in accord with our observation of vascular deposition of variant fibrinogen.13,22
We are further investigating the mechanisms through which vascular amyloidosis may be proatherogenic in AFib and other types of systemic amyloidosis associated with nephrotic syndrome such as systemic AL and AA amyloidosis, as well as the possibility that coronary amyloid atheromatous lesions may per se mimic atherosclerotic appearances on conventional angiography. The presence of amyloid deposits in the vascular walls of endomyocardial vessels, as well as systemically, suggests that a specific type of global coronary amyloid cardiomyopathy may exist in fibrinogen amyloidosis. This syndrome can result in clinical presentation with dilated amyloid cardiomyopathy as in the R554L case presented here, rather than the typical features of restrictive hypertrophic cardiomyopathy universally seen in the systemic amyloidoses.28 Cautious interpretation of echocardiographic findings as well as coronary angiography imaging findings is thus required to avoid misdiagnosis or underdiagnosis of disease features.
Our observation emphasizes the importance of awareness regarding cardiovascular symptoms for those at potential risk such as AFib carriers and nonscreened family members, and of counseling regarding risk factors such as smoking, obesity, hyperlipidemia, and hypertension. Conversely, screening for proteinuria is inexpensive and readily available, and we recommend its routine use in the primary care monitoring of families with history of cardiovascular events.
Neuropathic features have been observed in association with uremia, and may be reversible after successful renal transplantation. The clinical pattern of cardiac parasympathetic and systemic autonomic neuropathy in this series, corroborated by previous demonstration of cardiac denervation on iodine-131-meta-iodobenzylguanidine scintigraphy and resolution of symptoms after hepatorenal but not after kidney transplantation, suggest this is a true amyloid-related autonomic manifestation.20,37
Splenic involvement in AFib may be clinically significant as manifested by resistant anemia,20 in the absence of demonstrable splenomegaly. Spontaneous or intraoperative splenic rupture in 4 cases due to extensive splenic amyloid is a specific risk, and has led us to alter the transplantation surgical technique to using superior mesenteric venous bypass, to avoid even transient rises in portal pressure. AFib patients receive prophylactic triple vaccination for meningitis, pneumococcal pneumonia, and influenza upon placement on the waiting list.
Liver transplantation is the standard treatment for a number of genetic hepatic metabolic disorders including Wilson disease, hemochromatosis, homozygous hypercholesterolemia, ornithine transcarboxylase deficiency, and primary hyperoxaluria type I, and more than 1200 liver transplantations have been performed worldwide for transthyretin-related familial amyloid polyneuropathy.38,39
Fibrils isolated from amyloid deposits in AFib have consistently been shown to contain exclusively variant fibrinogen. Circulating total fibrinogen in AFib consists of a mixture of wild-type variant fibrinogen in a ratio of 1:1 to 3:2; however, only the variant fibrinogen molecule is incorporated in the amyloid fibrils, suggesting that unlike transthyretin, wild-type fibrinogen does not perpetuate amyloid disease.9,20
We have previously shown by mass spectrometry (Table 2, patients 2, 7, and 11) that after liver transplantation the variant fibrinogen is eliminated and promptly replaced by wild-type fibrinogen.19 The long-term AFib E526V hepatorenal transplant recipients have no amyloid progression at up to 12 years of follow-up, suggesting that liver transplantation for fibrinogen amyloidosis may be truly curative.19-21,23,26 We have used 4 of the explanted AFib livers for sequential (domino) transplantation in patients with end-stage liver disease and hepatocellular carcinoma, without evidence of AFib disease transmission at up to 5 years of follow-up.
The unexpected salvage of residual native kidney function in the 2 patients who received LKT preemptively at stage IV CKD is of particular interest. Stable contribution of 15% to 20% of total (native and graft) renal function is maintained at 4 and 5 years after LKT. We suggest that the apparent long-term stabilization of residual native amyloidotic kidney function in both cases is attributed to arresting the disease through liver replacement.
In conclusion, we have shown that AFib is a systemic amyloid disease with multivisceral and neurologic involvement, and is associated with cardiac amyloid deposition and amyloid angiopathy and atheromatosis. Hepatic amyloidosis is rare but can lead to liver failure. The addition of liver transplantation to kidney transplantation in AFib with ESRF is curative. We support AFib as a new indication for liver transplantation, and suggest that low-cardiovascular-risk patients are a favorable group for combined liver and kidney transplantation. Long-term hemodialysis patients, or those cardiovascularly unsuitable for the combined approach, could undergo kidney transplantation as the best form of renal replacement therapy.40
The characterization of the disease phenotype as systemic amyloid disease justifies earlier intervention to arrest amyloid disease progression, and renal outcomes of preemptive hepatorenal transplantation encourage evaluation of isolated liver transplantation early in the course of amyloid nephropathy to prevent systemic and renal progression to ESRF and requirements for dialysis and kidney transplantation. Amyloidotic kidneys may be exceptionally vulnerable to perioperative hemodynamic changes and possible nephrotoxicity of immunosuppression. It is thus recommended that AFib patients who are listed for preemptive isolated LT should be monitored monthly while on the waiting list, to ensure that GFR is maintained at levels greater than 50 mL/min at the time of a suitable liver graft being available for LT. The transplantation status of patients whose GFR falls below the safety cutoff of 50 mL/min on the LT waiting list should be altered to LKT, as in case 22 in this series. The explanted liver grafts can be used in domino transplantation, thus neutralizing the impact on the supply of liver allografts.
We encourage all centers involved in the management of amyloid disease to report transplantation and domino procedures for fibrinogen A α-chain amyloidosis to the Familial Amyloid Polyneuropathy World Transplant Registry (FAPWTR, www.FAPWTR.org), to enable international centralized data collection and meaningful analysis of long-term outcomes in this novel indication.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge and thank the scientists and staff in Merrill Benson's Amyloid Research Group, Pathology and Laboratory Medicine, Indiana University School of Medicine; Mr Hisham Rashid, Vascular Surgery Department; Mr Benjamin Corcoran, Nuclear Medicine Department at King's College Hospital; Mr Bart Wagner in the Electron Microscopy Department, Northern General Hospital; Mrs Katharine Bleasdale-Barr and technicians in the Neurovascular Medicine Department, NHNN, Queen Square; Dr Margaret Burke and Dr Alex Bell, Histopathology Department, Harefield Hospital; Profs Ian Simpson and Edward Gane, Auckland City Hospital; Profs Mark Pepys and Philip Hawkins and Staff in the National Amyloidosis Center, Royal Free Hospital; the Nephrology Departments of the Queen Elizabeth, Heartlands, and Wolverhampton Hospitals; the Glasgow Western Infirmary Hospital, the Moriston Hospital; Wirral Hospital; the Preston and Blackpool Victoria Hospitals; the Bournemouth Hospital; the John Radcliffe Hospital; and Nuffield Department of Surgery.
Authorship
Contribution: A.J.S., J.O., N.D.H., and M.D.B. are responsible for the conception, design, organization, and execution of the study; A.J.S. wrote the paper; J.O. and N.D.H. contributed to editing of the paper; N.R.B. contributed to the design and organization of the study and drafting and editing of the paper and carried out some of the cardiovascular investigations; B.M.H. contributed to the renal management of the patients and the editing of the paper; M.R. devised the surgical technique for liver transplantation in fibrinogen amyloidosis and performed the combined liver and kidney transplantations; B.P. examined and typed the histologic samples, and contributed to editing of the paper; J.W. has been responsible for expert intensive care management and has seen and approved the paper; M.M. and P.M. contributed to the cardiovascular investigations; M.B.-T. was responsible for the nuclear medicine investigations; C.J.M. has been responsible for all neurologic investigations and has seen and approved the paper; J.J.L. and M.D.B. carried out the amyloid fibril characterization and typing of amyloid deposits; and M.D.B. contributed to drafting and editing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arie J. Stangou, Institute of Liver Studies and Amyloidosis Treatment Center, King's College Hospital, Denmark Hill St, London, SE5 9RS, United Kingdom; e-mail: arie.stangou@kcl.ac.uk.