Abstract
T cell–depleted haploidentical hematopoietic stem cell transplantation (haploHSCT) is an option to treat children with very high-risk acute lymphoblastic leukemia (ALL) lacking an HLA-identical donor. We analyzed 127 children with ALL who underwent haploHSCT in first (n = 22), second (n = 48), or third (n = 32), complete remission or in relapse (n = 25). The 5-year leukemia-free survival (LFS) was 30%, 34%, 22%, and 0%, respectively. A risk-factor analysis was performed for patients who underwent transplantation in remission (n = 102). Five-year nonrelapse mortality (NRM), relapse incidence (RI), and LFS were 37%, 36%, and 27%, respectively. A trend of improved LFS rate and decreased RI was observed for children given a graft with higher number of CD34+ cells (adjusted P = .09 and P = .07, respectively). In a multivariate analysis, haploHSCT performed in larger centers (performing ≥ 231 allotransplantations in the studied period) was associated with improved LFS rate and decreased RI (adjusted P = .01 and P = .04, respectively), adjusting for different patient-, disease-, and transplant-related factors such as number of previous autotransplantations, cytomegalovirus serology status, type of T-cell depletion, and use of total body irradiation and antithymocyte globulin. In conclusion, higher CD34+ cell dose and better patient selection may improve outcomes of children with ALL who undergo a haploHSCT. Transplant centers initiating programs on haploHSCT for children may collaborate with more experienced centers.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is being increasingly used for pediatric patients with acute lymphoblastic leukemia (ALL) in second or subsequent complete remission (CR) after marrow relapse, as well as in patients in first CR but with high-risk characteristics.1-7 However, HLA-identical sibling donors are not available for approximately 75% of the patients, and unrelated donors, matched at the allelic level, cannot be found in time for all patients who need an allograft. Therefore, transplantations using alternative donor sources such as unrelated cord blood8-12 or haploidentical stem cells are increasingly used.13-15 The Perugia Group has first shown, in adult patients with hematologic malignancies who received a transplant from an HLA-disparate relative, that the infusion of a large number of extensively T cell–depleted CD34+ cells ensures sustained engraftment of donor hematopoiesis and minimizes the risk of both acute and chronic graft-versus-host disease (GVHD).15,16 The feasibility of haploHSCT was reproduced also in children, in particular in patients with ALL, transplanting high numbers of positively selected stem cells.17 However, data on the role of haploHSCT in childhood ALL are still limited and the reports analyzing outcomes and risk factors that are available in the literature include a limited number of children.
With the goal to evaluate the role of haploHSCT in childhood ALL, we have studied in a retrospective, multicenter, registry-based analysis the outcomes of 127 children given a T cell–depleted allograft from an HLA-disparate relative and risk factors of 102 patients who underwent transplantation in remission in European transplant centers and reported to the Pediatric diseases and the Acute Leukemia working parties European Group for Blood and Marrow Transplantation (EBMT) registry.
Methods
Selection criteria
The study used data reported to the EBMT registry and selected the patients according to the following criteria: (1) children with a diagnosis of ALL aged 16 years or younger; (2) T cell–depleted bone marrow or peripheral blood allografts from a relative; (3) 2 or 3 antigen HLA differences at loci A, B, or DRB1 in the donor-recipient pair; (4) transplantations performed in EBMT centers from 1995 to 2004. A total of 127 patients who underwent transplantation in 36 EBMT centers met these eligibility criteria. For these patients, the missing information was retrieved by the statistical office of the EBMT from each center providing patients for analysis.
Definitions
High-resolution typing of recipient and donor HLA-C, and low-resolution typing of HLA-B and HLA-A alleles of recipient and donor was used to segregate patients and donors in the following killer immunoglobulin-like receptor (KIR) ligand groups according to the list of HLA-C, group 1 and group 2, HLA-Bw4, and HLA-Bw6 alleles by Ruggeri et al.18 A3/A11 were not included because their role in vitro is controversial. We were unable to study the influence of noninherited maternal antigens and noninherited paternal antigens with outcomes because most of the donors were parents, and there were very few siblings according to definition published by van Rood et al.19
Center size was defined based on the total number of allogeneic HSCTs performed in children (< 16 years old) for all diseases, during the same time period, by the EBMT transplant centers that have included consecutive haploHSCT. In the EBMT megafile, we have found that the median number of allotransplantations performed by the same transplant centers was 231. The median number of transplantations was the best cutoff number that was statistically associated with leukemia-free survival (LFS) and it was used to discriminate large and small centers. Based on this definition, 8 transplant centers performing a median of 8 haploHSCTs and more than 231 allotransplantations were classified as larger centers, whereas 28 transplant centers performing a median of 1 haploHSCT and fewer than 231 allotransplantations were classified as smaller centers.
End points
Myeloid engraftment was defined as the first of 3 consecutive days when the absolute neutrophil count was 0.5 × 109/L or higher with evidence of donor hematopoiesis. Engraftment that occurred after day 60 was considered as nonengraftment. Acute GVHD was diagnosed and graded according to published criteria.20 Chronic GVHD was diagnosed according to standard criteria.21 Criteria for classification of GVHD proposed by National Institutes of Health consensus were not used in this retrospective analysis.22 Children with evidence of donor engraftment who survived more than 90 days from transplantation were evaluated for occurrence of chronic GVHD. Nonrelapse mortality (NRM) was defined as any death without evidence of relapse. Relapse was defined on the basis of morphologic evidence of leukemia in bone marrow or other sites. Survival was calculated from transplantation to death due to any cause, and leukemia-free survival (LFS) was defined as the time from transplantation to either first relapse or death in complete remission, whichever occurred first.
Statistical analysis
The duration of follow-up was the time to the last assessment for survivors. Nonrelapse mortality (NRM) and relapse incidence (RI) were expressed as cumulative incidence curves, to adjust the analysis for competing risks.23 Probabilities of LFS were calculated using the Kaplan-Meier estimate. Univariate analyses were done using log-rank test for LFS, whereas the Gray test was applied for RI and NRM. A risk-factor analysis was restricted to patients who underwent transplantation in remission because all patients who underwent transplantation in relapse died. Patients receiving a second transplant for graft failure were kept in the analysis, and their follow-up was considered after second transplantation until relapse or death. The variables considered in risk-factor analyses for NRM, RI, and LFS were as follows: recipient age, sex, and cytomegalovirus (CMV) serology; disease characteristics (cytogenetic, disease status at transplantation [CR1, CR2, CR3]), donor characteristics (age, sex, female donor to male recipient, CMV serology); transplant characteristics (number of antigen incompatibilities [2 vs > 2 of 6], previous autologous transplantation, dose of CD34+ and CD3+ cells infused, conditioning regimen including total body irradiation [TBI] or not, use of antithymocyte/antilymphocyte globulin [ATG/ALG], year of transplantation, and centers performing ≥ 231 or < 231 alloHSCTs during the period studied). KIR matching was analyzed only for patients with available information. For prognostic analyses, continuous variables were categorized as follows: each variable was first divided into 5 categories at approximately the 20th, 40th, 60th, and 80th percentiles. In the absence of a clear pattern among the 5 categories, the median was used as a cutoff point. This approach allows distinguishing 2 types of centers according to the number of allotransplantations performed during the period studied. Multivariate analyses were performed using Cox proportional-hazard regression model, including all factors associated with a P value less than .2 by univariate analysis and all factors statistically different among the 2 types of centers (P < .10). Then a stepwise backward procedure was used with a cutoff significance level of .05 for removal of factors from the model. P values are 2-sided, with a type I error rate fixed at .05. Statistical analyses were performed with SPSS 17.0 and R 2.9.0 software.
Patient, donor, and disease characteristics
Table 1 lists the characteristics of the 102 children who underwent transplantation in remission. Median age of children at time of transplantation was 8.7 years (range, 0.6-16 years); the median number of white blood cell count at diagnosis was 27 × 109/L (range, 2-517 × 109/L). Twenty-five (29%) of the 86 patients with cytogenetic data available had high-risk ALL defined by the presence of t(9;21) or t(4;11). At transplantation, 22 (22%) patients were in first complete remission (CR1); 48 (47%), in CR2; and 32 (31%), in CR3. In fact, of 22 children who underwent transplantation in CR1, 16 patients had poor cytogenetics (t(9;22) in 10 patients, t(4;11) in 5 patients, and monosomy 7 in 1 patient). Three patients had white blood cell count at diagnosis higher than 190 × 109/L (1 had hypodiploidy), and 3 patients achieved CR after 80 days of induction. For 48 patients who underwent transplantation in CR2, 35% of the patients relapsed before 18 months after achieving first CR; 41%, between 18 and 30 months; and 24% had later first relapse but poor cytogenetics, including more frequently t(9;22) or t(4;11). All patients who obtained CR3 were offered a haploHSCT, independent of the ALL diagnosis characteristics or time to relapse. Among them, 6 patients had been previously autografted.
Patient characteristics: patients in remission (n = 102)
| Characteristic . | No. . |
|---|---|
| Patient-related factors | |
| Age at transplantation, y (range) | 8.7 (0.64-16) |
| Sex, male/female | 70/32 |
| CMV serology | |
| Negative | 40 |
| Positive | 59 |
| Not known | 3 |
| Donor-related factors | |
| Age at transplantation, y (range) | 36.6 (18-53.8) |
| Sex, male/female | 55/47 |
| Female donor to male recipient | |
| No | 71 |
| Yes | 31 |
| CMV serology | |
| Negative | 32 |
| Positive | 61 |
| Not known | 9 |
| Disease-related factors | |
| WBC at diagnosis × 109/L | 27.2 (1.9-517) |
| Immunophenotype | |
| B | 84 |
| T | 11 |
| Biphenotypic | 2 |
| Not known | 5 |
| Cytogenetic | |
| No Ph+ | 63 |
| Ph+ or t(4;11) | 24 |
| Not performed/not available | 15 |
| Status at transplantation | |
| CR1 | 22 (22%) |
| CR2 | 48 (47%) |
| CR3 | 32 (31%) |
| Median time from first diagnosis to transplantation, mo | |
| CR1 | 6 (3-16) |
| CR2 | 32 (6-80) |
| CR3 | 57 (11-141) |
| Previous autologous transplantation | |
| No | 94 |
| Yes | 8 |
| Transplant-related factors | |
| Year of transplantation | |
| 1995 | 4 |
| 1996 | 6 |
| 1997 | 5 |
| 1998 | 14 |
| 1999 | 11 |
| 2000 | 17 |
| 2001 | 10 |
| 2002 | 10 |
| 2003 | 14 |
| 2004 | 11 |
| Median, y | 2000 |
| Donor type | |
| Mother | 44 |
| Father | 51 |
| Brother | 5 |
| Sister | 2 |
| No. of antigen mismatches in HLA-A, -B, -DR | |
| 2 | 34 |
| 3 | 68 |
| KIR mismatches between donor and recipients | |
| Yes | 25 |
| No | 41 |
| Not known | 36 |
| Cell source | |
| BM | 6 |
| PB | 96 |
| In vitro T-cell depletion, device used | |
| CliniMacs | 79 |
| Baxter | 12 |
| Cellpro | 6 |
| Negative T depletion | 5 |
| Serotherapy with ATG or ALG | |
| No | 22 |
| Yes | 76 |
| Not known | 4 |
| Use of monoclonal antibodies | |
| No | 57 |
| Yes | 44 |
| Not known | 1 |
| Total body irradiation | |
| No | 24 |
| Yes | 78 |
| No. of CD34+ × 106/kg body weight | |
| All | 12.33 (1.36-95.2) |
| PB only | 12.4 (1.9-67) |
| No. of CD3+ × 106/kg body weight* | |
| All | 0.05 (0-129) |
| PB only | 0.045 (0-128.7) |
| Donor lymphocyte infusion performed | |
| No | 86 |
| Yes | 16 |
| Characteristic . | No. . |
|---|---|
| Patient-related factors | |
| Age at transplantation, y (range) | 8.7 (0.64-16) |
| Sex, male/female | 70/32 |
| CMV serology | |
| Negative | 40 |
| Positive | 59 |
| Not known | 3 |
| Donor-related factors | |
| Age at transplantation, y (range) | 36.6 (18-53.8) |
| Sex, male/female | 55/47 |
| Female donor to male recipient | |
| No | 71 |
| Yes | 31 |
| CMV serology | |
| Negative | 32 |
| Positive | 61 |
| Not known | 9 |
| Disease-related factors | |
| WBC at diagnosis × 109/L | 27.2 (1.9-517) |
| Immunophenotype | |
| B | 84 |
| T | 11 |
| Biphenotypic | 2 |
| Not known | 5 |
| Cytogenetic | |
| No Ph+ | 63 |
| Ph+ or t(4;11) | 24 |
| Not performed/not available | 15 |
| Status at transplantation | |
| CR1 | 22 (22%) |
| CR2 | 48 (47%) |
| CR3 | 32 (31%) |
| Median time from first diagnosis to transplantation, mo | |
| CR1 | 6 (3-16) |
| CR2 | 32 (6-80) |
| CR3 | 57 (11-141) |
| Previous autologous transplantation | |
| No | 94 |
| Yes | 8 |
| Transplant-related factors | |
| Year of transplantation | |
| 1995 | 4 |
| 1996 | 6 |
| 1997 | 5 |
| 1998 | 14 |
| 1999 | 11 |
| 2000 | 17 |
| 2001 | 10 |
| 2002 | 10 |
| 2003 | 14 |
| 2004 | 11 |
| Median, y | 2000 |
| Donor type | |
| Mother | 44 |
| Father | 51 |
| Brother | 5 |
| Sister | 2 |
| No. of antigen mismatches in HLA-A, -B, -DR | |
| 2 | 34 |
| 3 | 68 |
| KIR mismatches between donor and recipients | |
| Yes | 25 |
| No | 41 |
| Not known | 36 |
| Cell source | |
| BM | 6 |
| PB | 96 |
| In vitro T-cell depletion, device used | |
| CliniMacs | 79 |
| Baxter | 12 |
| Cellpro | 6 |
| Negative T depletion | 5 |
| Serotherapy with ATG or ALG | |
| No | 22 |
| Yes | 76 |
| Not known | 4 |
| Use of monoclonal antibodies | |
| No | 57 |
| Yes | 44 |
| Not known | 1 |
| Total body irradiation | |
| No | 24 |
| Yes | 78 |
| No. of CD34+ × 106/kg body weight | |
| All | 12.33 (1.36-95.2) |
| PB only | 12.4 (1.9-67) |
| No. of CD3+ × 106/kg body weight* | |
| All | 0.05 (0-129) |
| PB only | 0.045 (0-128.7) |
| Donor lymphocyte infusion performed | |
| No | 86 |
| Yes | 16 |
CMV indicates cytomegalovirus; WBC, white blood cell count; CR, complete remission; Ph+, Philadelphia chromosome positive; ATG, antithymocyte globulin; ALG, antilymphocyte globulin; PB, peripheral blood; and BM, bone marrow.
n = 10 not known.
Sixty-eight patients received a graft with 3 HLA antigen disparities (Table 1). In 66 patients, sufficient information on HLA-C, -Bw4, and -Bw6 typing was available to classify patients into KIR ligand matching between donors and recipients. KIR ligand was matched with the donor in the GVHD direction in 41 patients and mismatched in 25 patients.
The donors were mothers in 44 cases, fathers in 51 cases, brothers in 5 cases, and sisters in 2 cases.
Transplantation and graft cell selection
Hematopoietic stem cells were collected either by the transplant center or at the local transfusion service and processed by cellular therapy laboratories. All patients but 6, who received a bone marrow cell transplant, were given T cell–depleted peripheral blood (PB) progenitor cells, which were mobilized in the donor through the use of granulocyte colony-stimulating factor and collected by a cell separator. In most cases, CD34+ cells were positively selected using the CliniMacs one-step procedure (Miltenyi Biotec). The median number of CD34+ and CD3+ cells infused using PB was 12.3 × 106/kg (range, 1.36-95 × 104/kg) and 5.0 × 104/kg (range, 0-12.9 × 104/kg). In vivo T-cell depletion by ATG or ALG was used in 78% of reported transplantations. In vivo use of monoclonal antibodies was added in 44 children. All patients received a myeloablative conditioning regimen; total body irradiation was used in 76% of patients (Table 1).
Results
A total of 127 children with ALL underwent haploHSCT from 1995 to 2004 in 36 EBMT centers. Twenty-two received a transplant in first complete remission (CR1); 48, in second CR (CR2); and 32, in third CR (CR3); and 25 were not in remission. According to disease phase, the 5-year probability of LFS after haploHSCT was 30% (± 10%), 34% (± 7%), 22% (± 7%), and 0%, respectively. Because all 25 children with active disease who underwent transplantation died, description of the population and risk factor analyses were restricted to patients in remission.
Outcomes
Neutrophil recovery.
Engraftment was observed in 92 patients (91%). Median time to reach a neutrophil count higher than 500/mm3 was 15 days (range, 8-55 days). Chimerism data were not obtained from 9 patients who died early or were in aplasia, but data were available for 87 of 93 patients; 9% of the patients experienced autologous reconstitution (n = 8); 10%, mixed chimerism (n = 9); and 80%, full donor chimerism (n = 70) in the 3 months after transplantation. Primary graft failure was observed in 9 and secondary graft failure in 4 children. A second transplantation was performed in 12 patients, and second engraftment was successful in 10.
Acute and chronic graft-versus-host disease.
Acute GVHD was absent or grade 1 in 80 children, grade 2 in 13, grade 3 in 6, and grade 4 in 3 cases, and chronic GVHD was observed in 14 of the 84 patients at risk. Preemptive DLI was given for only 1 child who has not developed GVHD.
Relapse incidence.
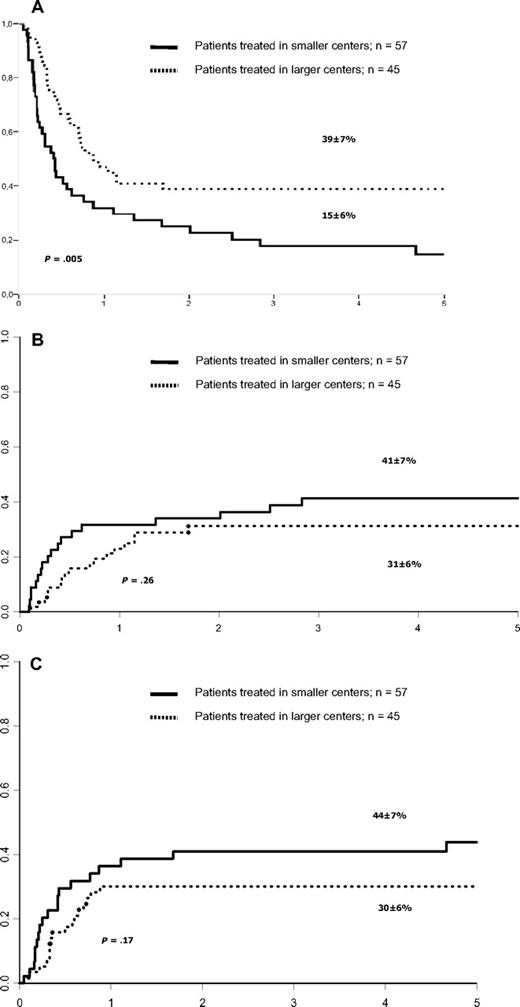
The cumulative incidence of hematologic relapse at 5 years was 36% (± 4%). Table 2 lists the univariate analysis for RI. None of the factors studied in univariate analysis were statistically associated with RI. KIR incompatibilities were not associated with RI (Table 2). Relapse cumulative incidence at 5 years was 32% (± 8%) for patients with no incompatibility and 36% (± 10%) for those with KIR incompatibilities in the GVHD direction (P = .55). Of note, RI was 41% (± 7%) for those children who underwent transplantation in smaller centers and 31% (± 6%) for those who underwent transplantation in larger centers (P = .26, Figure 1B). However, after adjusting for other prognostic factors (Table 2) and the differences between smaller and larger centers (Table 3), RI was decreased in larger centers (hazard ratio [HR]: 0.49, 95% confidence interval [95% CI]: 0.24-0.98; P = .04). In addition, in a multivariate model, there was a trend of decreased RI for children with higher number of CD34+ cells who underwent transplantation (HR: 0.52, 95% CI: 0.25-1.06; P = .07).
Univariate analysis for outcomes after haploHSCT for childhood ALL in remission (n = 102): 5-year results
| . | No. . | LFS, % . | RI, % . | NRM, % . |
|---|---|---|---|---|
| Overall | 102 | 27 ± 5 | 36 ± 4 | 37 ± 4 |
| Patient-related factors | ||||
| Age, y | ||||
| Younger than 8.7 | 51 | 30 ± 7 | 30 ± 6 | 39 ± 6 |
| 8.7 or older | 51 | 24 ± 6 | 42 ± 6 | 34 ± 6 |
| P | .43 | .37 | .93 | |
| Sex | ||||
| Male | 70 | 28 ± 6 | 35 ± 5 | 37 ± 5 |
| Female | 32 | 25 ± 8 | 40 ± 10 | 35 ± 9 |
| P | .96 | .59 | .69 | |
| CMV serology | ||||
| Negative | 40 | 25 ± 8 | 47 ± 8 | 28 ± 8 |
| Positive | 59 | 28 ± 6 | 30 ± 6 | 42 ± 6 |
| P | .34 | .15 | .053 | |
| Donor-related factors | ||||
| Age, y | ||||
| Younger than median | 45 | 31 ± 7 | 34 ± 7 | 35 ± 7 |
| Older than median | 45 | 23 ± 7 | 40 ± 7 | 37 ± 7 |
| P | .48 | .67 | .7 | |
| Sex | ||||
| Male | 55 | 34 ± 7 | 28 ± 6 | 38 ± 7 |
| Female | 47 | 19 ± 6 | 45 ± 7 | 36 ± 8 |
| P | .26 | .08 | .67 | |
| CMV serology | ||||
| Negative | 32 | 24 ± 8 | 42 ± 8 | 35 ± 9 |
| Positive | 61 | 30 ± 6 | 31 ± 6 | 39 ± 6 |
| P | .8 | .31 | .35 | |
| Disease-related factors | ||||
| Status at transplantation | ||||
| CR1 | 22 | 15 ± 12 | 33 ± 10 | 52 ± 18 |
| CR2 | 48 | 34 ± 7 | 36 ± 6 | 30 ± 7 |
| CR3 | 32 | 22 ± 7 | 37 ± 9 | 42 ± 9 |
| P | .26 | .99 | .26 | |
| Cytogenetics | ||||
| No Ph+ | 63 | 29 ± 6 | 38 ± 7 | 33 ± 7 |
| Ph+ and t(4;11) | 24 | 32 ± 10 | 30 ± 10 | 39 ± 9 |
| P | .83 | .71 | .63 | |
| Previous autologous transplantation | ||||
| No | 94 | 29 ± 5 | 38 ± 5 | 34 ± 5 |
| Yes | 8 | 0 (1vv) | 0 | 75 ± 15 |
| P | .12 | .18 | .002* | |
| Transplant-related factors | ||||
| No. of HLA antigen mismatches in -A, -B, -DR | ||||
| 2 | 34 | 18 ± 9 | 44 ± 9 | 39 ± 12 |
| 3 | 68 | 30 ± 6 | 32 ± 6 | 38 ± 6 |
| P | .3 | .18 | .73 | |
| KIR (C and Bw4) mismatch | ||||
| No | 41 | 32 ± 8 | 32 ± 8 | 36 ± 8 |
| Yes | 25 | 36 ± 10 | 36 ± 10 | 28 ± 9 |
| P | .82 | .55 | .59 | |
| Female donor to male recipient | ||||
| No | 71 | 31 ± 6 | 31 ± 5 | 38 ± 6 |
| Yes | 31 | 18 ± 8 | 47 ± 9 | 35 ± 10 |
| P | .58 | .2 | .62 | |
| Year of transplantation | ||||
| 2000 or earlier | 57 | 26 ± 6 | 36 ± 6 | 34 ± 6 |
| Later than 2000 | 45 | 25 ± 7 | 38 ± 8 | 37 ± 7 |
| P | .73 | .98 | .71 | |
| Use of ATG/ALG | ||||
| No | 22 | 32 ± 10 | 32 ± 11 | 36 ± 11 |
| Yes | 76 | 24 ± 6 | 38 ± 5 | 38 ± 6 |
| P | .67 | .61 | .99 | |
| Conditioning with TBI | ||||
| No | 24 | 20 ± 9 | 35 ± 11 | 44 ± 11 |
| Yes | 78 | 30 ± 5 | 36 ± 5 | 34 ± 5 |
| P | .29 | .99 | .36 | |
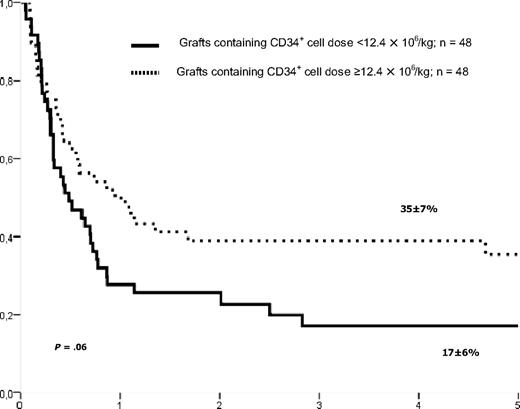
| CD34+ cell dose × 106/kg body weight | ||||
| Lower than 12.4 | 48 | 17 ± 6 | 45 ± 8 | 38 ± 7 |
| 12.4 or higher | 48 | 35 ± 7 | 27 ± 7 | 37 ± 7 |
| P | .06 | .13 | .74 | |
| CD3+ cell dose × 106/kg body weight | ||||
| Lower than 0.05 | 48 | 26 ± 7 | 30 ± 7 | 41 ± 8 |
| 0.05 or higher | 42 | 28 ± 7 | 38 ± 8 | 31 ± 8 |
| P | .98 | .35 | .35 | |
| Center-related factor | ||||
| No. of alloHSCTs performed | ||||
| Fewer than 231 | 45 | 15 ± 6 | 41 ± 7 | 44 ± 7 |
| 231 or more | 57 | 39 ± 7 | 31 ± 6 | 30 ± 6 |
| P | .005* | .26 | .17 |
| . | No. . | LFS, % . | RI, % . | NRM, % . |
|---|---|---|---|---|
| Overall | 102 | 27 ± 5 | 36 ± 4 | 37 ± 4 |
| Patient-related factors | ||||
| Age, y | ||||
| Younger than 8.7 | 51 | 30 ± 7 | 30 ± 6 | 39 ± 6 |
| 8.7 or older | 51 | 24 ± 6 | 42 ± 6 | 34 ± 6 |
| P | .43 | .37 | .93 | |
| Sex | ||||
| Male | 70 | 28 ± 6 | 35 ± 5 | 37 ± 5 |
| Female | 32 | 25 ± 8 | 40 ± 10 | 35 ± 9 |
| P | .96 | .59 | .69 | |
| CMV serology | ||||
| Negative | 40 | 25 ± 8 | 47 ± 8 | 28 ± 8 |
| Positive | 59 | 28 ± 6 | 30 ± 6 | 42 ± 6 |
| P | .34 | .15 | .053 | |
| Donor-related factors | ||||
| Age, y | ||||
| Younger than median | 45 | 31 ± 7 | 34 ± 7 | 35 ± 7 |
| Older than median | 45 | 23 ± 7 | 40 ± 7 | 37 ± 7 |
| P | .48 | .67 | .7 | |
| Sex | ||||
| Male | 55 | 34 ± 7 | 28 ± 6 | 38 ± 7 |
| Female | 47 | 19 ± 6 | 45 ± 7 | 36 ± 8 |
| P | .26 | .08 | .67 | |
| CMV serology | ||||
| Negative | 32 | 24 ± 8 | 42 ± 8 | 35 ± 9 |
| Positive | 61 | 30 ± 6 | 31 ± 6 | 39 ± 6 |
| P | .8 | .31 | .35 | |
| Disease-related factors | ||||
| Status at transplantation | ||||
| CR1 | 22 | 15 ± 12 | 33 ± 10 | 52 ± 18 |
| CR2 | 48 | 34 ± 7 | 36 ± 6 | 30 ± 7 |
| CR3 | 32 | 22 ± 7 | 37 ± 9 | 42 ± 9 |
| P | .26 | .99 | .26 | |
| Cytogenetics | ||||
| No Ph+ | 63 | 29 ± 6 | 38 ± 7 | 33 ± 7 |
| Ph+ and t(4;11) | 24 | 32 ± 10 | 30 ± 10 | 39 ± 9 |
| P | .83 | .71 | .63 | |
| Previous autologous transplantation | ||||
| No | 94 | 29 ± 5 | 38 ± 5 | 34 ± 5 |
| Yes | 8 | 0 (1vv) | 0 | 75 ± 15 |
| P | .12 | .18 | .002* | |
| Transplant-related factors | ||||
| No. of HLA antigen mismatches in -A, -B, -DR | ||||
| 2 | 34 | 18 ± 9 | 44 ± 9 | 39 ± 12 |
| 3 | 68 | 30 ± 6 | 32 ± 6 | 38 ± 6 |
| P | .3 | .18 | .73 | |
| KIR (C and Bw4) mismatch | ||||
| No | 41 | 32 ± 8 | 32 ± 8 | 36 ± 8 |
| Yes | 25 | 36 ± 10 | 36 ± 10 | 28 ± 9 |
| P | .82 | .55 | .59 | |
| Female donor to male recipient | ||||
| No | 71 | 31 ± 6 | 31 ± 5 | 38 ± 6 |
| Yes | 31 | 18 ± 8 | 47 ± 9 | 35 ± 10 |
| P | .58 | .2 | .62 | |
| Year of transplantation | ||||
| 2000 or earlier | 57 | 26 ± 6 | 36 ± 6 | 34 ± 6 |
| Later than 2000 | 45 | 25 ± 7 | 38 ± 8 | 37 ± 7 |
| P | .73 | .98 | .71 | |
| Use of ATG/ALG | ||||
| No | 22 | 32 ± 10 | 32 ± 11 | 36 ± 11 |
| Yes | 76 | 24 ± 6 | 38 ± 5 | 38 ± 6 |
| P | .67 | .61 | .99 | |
| Conditioning with TBI | ||||
| No | 24 | 20 ± 9 | 35 ± 11 | 44 ± 11 |
| Yes | 78 | 30 ± 5 | 36 ± 5 | 34 ± 5 |
| P | .29 | .99 | .36 | |
| CD34+ cell dose × 106/kg body weight | ||||
| Lower than 12.4 | 48 | 17 ± 6 | 45 ± 8 | 38 ± 7 |
| 12.4 or higher | 48 | 35 ± 7 | 27 ± 7 | 37 ± 7 |
| P | .06 | .13 | .74 | |
| CD3+ cell dose × 106/kg body weight | ||||
| Lower than 0.05 | 48 | 26 ± 7 | 30 ± 7 | 41 ± 8 |
| 0.05 or higher | 42 | 28 ± 7 | 38 ± 8 | 31 ± 8 |
| P | .98 | .35 | .35 | |
| Center-related factor | ||||
| No. of alloHSCTs performed | ||||
| Fewer than 231 | 45 | 15 ± 6 | 41 ± 7 | 44 ± 7 |
| 231 or more | 57 | 39 ± 7 | 31 ± 6 | 30 ± 6 |
| P | .005* | .26 | .17 |
LFS indicates leukemia-free survival; RI, relapse incidence; NRM, nonrelapse mortality; CMV, cytomegalovirus; WBC, white blood cell count; CR, complete remission; Ph+, Philadelphia chromosome–positive; ATG, antithymocyte globulin; ALG, antilymphocyte globulin; and TBI, total body irradiation.
Statistically significant.
(A) Leukemia-free survival after haploHSCT in children with ALL according to number of alloHSCTs performed in participating transplant centers. (B) Cumulative incidence of relapse after haploHSCT in children with ALL according to number of alloHSCTs performed in participating transplant centers. (C) Cumulative incidence of nonrelapse mortality in patients with ALL in remission only, according to number of haploHSCTs.
(A) Leukemia-free survival after haploHSCT in children with ALL according to number of alloHSCTs performed in participating transplant centers. (B) Cumulative incidence of relapse after haploHSCT in children with ALL according to number of alloHSCTs performed in participating transplant centers. (C) Cumulative incidence of nonrelapse mortality in patients with ALL in remission only, according to number of haploHSCTs.
Patient-, donor-, disease-, and transplant-related factors in 102 children who underwent haploHSCT for ALL in remission, according to number of transplantations performed during the study period
| . | Fewer than 231 alloHSCTs performed . | 231 or more alloHSCTs performed . | P . |
|---|---|---|---|
| Patient-related factors | |||
| Age, y | 8.8 (0.96-16) | 8.7 (0.64-15.7) | .64 |
| Sex | |||
| Male | 32 | 38 | .63 |
| Female (%) | 13 (29) | 19 (33) | |
| CMV serology | |||
| Negative | 8 | 32 | < .001* |
| Positive (%) | 35 (81) | 24 (43) | |
| Donor-related factors | |||
| Age, y | 36 (18.6-49.5) | 36.9 (18-53.8) | .8 |
| Sex | |||
| Male | 20 | 35 | .09 |
| Female (%) | 25 (56) | 22 (39) | |
| CMV serology | |||
| Negative | 8 | 24 | .02* |
| Positive (%) | 30 (79) | 31 (56) | |
| Disease-related factors | |||
| WBC at diagnosis | 21.25 (2.8-517) | 27.9 (1.9-272.4) | .67 |
| Cytogenetics | |||
| No Ph+ | 27 | 36 | .25 |
| Ph+ or t(4;11) (%) | 7 (21) | 17 (32) | |
| Previous autologous transplantation | |||
| No | 39 | 55 | .07 |
| Yes (%) | 6 (13) | 2 (4) | |
| Status at transplantation (%) | |||
| CR1 | 6 (13) | 16 (28) | .2 |
| CR2 | 23 (51) | 25 (44) | |
| CR3 | 16 (36) | 16 (28) | |
| Transplant-related factors | |||
| Year of transplantation | 2000 (1995-2004) | 2000 (1995-2004) | .8 |
| No. of antigen mismatch in HLA-A, -B, -DR | |||
| 2 | 17 | 17 | .31 |
| 3 (%) | 28 (62) | 40 (70) | |
| Female donor to male recipient | |||
| No | 28 | 43 | .15 |
| Yes (%) | 17 (38) | 14 (25) | |
| Cell source | |||
| BM | 3 | 3 | .77 |
| PB (%) | 42 (93) | 54 (95) | |
| Type of T-cell depletion (%) | |||
| CliniMacs | 30 (68) | 48 (84) | .04* |
| Isolex | 9 (21) | 3 (5) | |
| Cellpro | 4 (9) | 2 (4) | |
| Negative T-depletion | 1 (2) | 4 (7) | |
| No. of CD34+ cells infused ×106/kg body weight | 12.7 (1.36-95.2) | 12.1 (3-33) | .68 |
| TBI in conditioning | |||
| No | 16 | 8 | .01* |
| Yes (%) | 29 (64) | 49 (86) | |
| ATG/ALG in conditioning | |||
| No | 4 | 18 | .01* |
| Yes (%) | 38 (90) | 38 (68) |
| . | Fewer than 231 alloHSCTs performed . | 231 or more alloHSCTs performed . | P . |
|---|---|---|---|
| Patient-related factors | |||
| Age, y | 8.8 (0.96-16) | 8.7 (0.64-15.7) | .64 |
| Sex | |||
| Male | 32 | 38 | .63 |
| Female (%) | 13 (29) | 19 (33) | |
| CMV serology | |||
| Negative | 8 | 32 | < .001* |
| Positive (%) | 35 (81) | 24 (43) | |
| Donor-related factors | |||
| Age, y | 36 (18.6-49.5) | 36.9 (18-53.8) | .8 |
| Sex | |||
| Male | 20 | 35 | .09 |
| Female (%) | 25 (56) | 22 (39) | |
| CMV serology | |||
| Negative | 8 | 24 | .02* |
| Positive (%) | 30 (79) | 31 (56) | |
| Disease-related factors | |||
| WBC at diagnosis | 21.25 (2.8-517) | 27.9 (1.9-272.4) | .67 |
| Cytogenetics | |||
| No Ph+ | 27 | 36 | .25 |
| Ph+ or t(4;11) (%) | 7 (21) | 17 (32) | |
| Previous autologous transplantation | |||
| No | 39 | 55 | .07 |
| Yes (%) | 6 (13) | 2 (4) | |
| Status at transplantation (%) | |||
| CR1 | 6 (13) | 16 (28) | .2 |
| CR2 | 23 (51) | 25 (44) | |
| CR3 | 16 (36) | 16 (28) | |
| Transplant-related factors | |||
| Year of transplantation | 2000 (1995-2004) | 2000 (1995-2004) | .8 |
| No. of antigen mismatch in HLA-A, -B, -DR | |||
| 2 | 17 | 17 | .31 |
| 3 (%) | 28 (62) | 40 (70) | |
| Female donor to male recipient | |||
| No | 28 | 43 | .15 |
| Yes (%) | 17 (38) | 14 (25) | |
| Cell source | |||
| BM | 3 | 3 | .77 |
| PB (%) | 42 (93) | 54 (95) | |
| Type of T-cell depletion (%) | |||
| CliniMacs | 30 (68) | 48 (84) | .04* |
| Isolex | 9 (21) | 3 (5) | |
| Cellpro | 4 (9) | 2 (4) | |
| Negative T-depletion | 1 (2) | 4 (7) | |
| No. of CD34+ cells infused ×106/kg body weight | 12.7 (1.36-95.2) | 12.1 (3-33) | .68 |
| TBI in conditioning | |||
| No | 16 | 8 | .01* |
| Yes (%) | 29 (64) | 49 (86) | |
| ATG/ALG in conditioning | |||
| No | 4 | 18 | .01* |
| Yes (%) | 38 (90) | 38 (68) |
CMV indicates cytomegalovirus; WBC, white blood cell count; Ph+, Philadelphia chromosome–positive; CR, complete remission; BM, bone marrow; PB, peripheral blood; TBI, total body irradiation; ATG, antithymocyte globulin; and ALG, antilymphocyte globulin.
Statistically significant.
Nonrelapse mortality.
The cumulative incidence of NRM at 5 years was 37% (± 4%). Table 2 lists the univariate analysis of factors potentially influencing NRM. A higher cumulative incidence of NRM (75% ± 15%) was observed in the 8 children who had previously received an autologous transplant compared with 34% (± 5%) for the remaining patients (P = .002). In univariate analysis, recipient's positive CMV serology and previous autologous transplantation were associated with higher NRM (Table 2), however in a multivariate analysis, only previous autograft was associated with increased NRM (HR: 3.01, 95% CI: 1.16-7.84; P = .024). No other patient-, disease-, donor-, or transplantation-related variable or size of transplant center was statistically associated with NRM (Figure 1C).
Leukemia-free survival.
The estimate probability of LFS at 5 years of patients who underwent transplantation in CR was 27% (± 5%). Factors such as KIR matching and donor type were not associated with LFS, as shown in Table 2. In univariate (Table 2 and Figure 1A) and in multivariate analyses, size of the center was significantly associated with LFS (HR: 1.9, 95% CI: 1.15-3.11; P = .01). There was also a trend of improved LFS rate in univariate (P = .06, Figure 2) and multivariate analyses in children given a higher CD34+ cell dose than 12.4 × 106/kg (HR: 1.53, 95% CI: 0.93-2.52; P = .09).
Leukemia-free survival of patients with ALL in remission according to CD34+ cell dose in the graft.
Leukemia-free survival of patients with ALL in remission according to CD34+ cell dose in the graft.
Survival and causes of death.
The estimated probability of overall survival (OS) at 5 years was 29% (± 5%) for the whole cohort, 28% (± 10%) for patients who underwent transplantation in CR1, 39% (± 7%) for those in CR2, and 32% (± 8%) for those in CR3. Five-year OS was 38% (± 6%) in larger centers (n = 57, deaths = 34) and 18% (± 6%) in smaller centers (n = 45, deaths = 35; P = .008). Thirty-one patients died of recurrence of the disease, 16 in larger centers and 15 in smaller centers, and 22 died of infection (11 and 11 according to the center size, respectively). Other causes of death were GVHD in 9 cases and interstitial pneumonitis in 5 patients.
Impact of center size on outcomes
The differences in terms of patient-, donor-, and transplantation-related factors between large and small centers are shown in Table 3. In smaller centers, children were more likely to have CMV-positive serology (P < .001), to have previously undergone an autologous HSCT (P = .07), to have received ATG/ALG in the preparative regimen (P = .01), and to have undergone transplantation without TBI (P = .01). In addition, the technique of T-cell depletion was different because larger centers more frequently used CliniMacs device (P = .04). All of these differences were taken into consideration in the multivariate model for outcomes.
Center size, defined as the total number of allotransplantations performed during the studied period in transplant center, was associated in univariate analysis with LFS (Table 2). After statistical adjustments of the differences between larger and smaller centers RI was decreased (HR: 0.49, 95% CI: 0.24-0.98; P = .04) and LFS improved (HR: 1.9, 95% CI: 1.15-3.11; P = .01) in larger transplant centers.
Discussion
Most reports in the literature regarding haploidentical transplantations include a limited number of patients and those reports are heterogeneous because they include patients with malignant and nonmalignant diseases, adolescents and young adults, as well as patients who received a transplant from one antigen–disparate related donor, whose outcome is known to be similar to matched donor transplantations.14,15,17,24-31 All of these factors preclude the possibility of drawing a reliable conclusion on the role of haploHSCT in specific pediatric hematologic disorders. We analyzed a homogenous cohort of children (≤ 16 years) who underwent transplantation over a 10-year period in European Group for Blood and Marrow Transplantation (EBMT) centers using related donors with at least 2 HLA antigen disparities and after a relatively homogenous procedure of T-cell depletion, namely positive selection of CD34+ cells.
The first message of our study is that haploHSCT should not be considered an option if complete remission is not achieved before transplantation. In fact, although the 5-year probability of LFS after haploHSCT was 27% for patients who underwent transplantation in remission, it was 0% for children with active/resistant disease who underwent transplantation.
LFS after other strategies, such as unrelated donors or unrelated cord blood transplantation, for children with ALL lacking a matched sibling donors varies between 30% and 58% in various series.2,4,8 Whether outcomes after haploHSCT are comparable with those strategies is not known and should be further investigated. It must be emphasized that those comparisons are possible only after adjustments for differences among these strategies, such as the waiting time for locating a suitable donor. Importantly, retrospective studies cannot take into consideration the previous selection of patients given that the main advantage of haploHSCT over unrelated donor transplantation is the easy, rapid access to donor and the possibility of using cells for immunotherapy.
The second aim of our study was to analyze risk factors affecting the outcomes of patients who undergo haploHSCT in remission. Interestingly, in our study, LFS did not differ among patients who underwent transplantation in CR1, CR2, or CR3, suggesting that even patients who undergo transplantation in early disease phase represent a very high-risk population. The quite low survival rate of 30% in patients who received a transplant in first CR is a concern. One could argue that the reason for this poor LFS is related to a very high-risk group of patients with a high frequency of poor-risk cytogenetics (16/22 patients had poor cytogenetics [t(9;22) in 10 patients, t(4;11) in 5 patients, and monosomy 7 in 1 patient], 3 patients had white blood cell count at diagnosis higher than 190 × 109/L [1 hypodiploidy], and 3 patients achieved CR after 80 days of induction). Another speculative explanation for the poor result is the lack of graft-versus-leukemia effect needed in this specific group. It is difficult to draw definitive conclusions of the place of haploHSCT in CR1 patients based on this low number of patients, but indication of haploidentical HSCT in CR1 patients should be properly evaluated.
Increased NRM was observed in children who had received a previous autologous transplant. This is not unexpected because patients who already underwent myeloablative therapy have a higher burden of toxicity, eventually resulting in death. In univariate analysis, a recipient's positive CMV serology was associated with higher NRM, but his/her factor was not isolated in the final multivariate model. Of note, none of the graft-related factors, such as type and number of HLA differences in the haplotype, transplant from a female donor to a male recipient, year of transplantation, use of serotherapy, the type of conditioning, and the number of CD3+ cells, had influence on the cumulative incidence of NRM.
Importantly, in the multivariate analysis, we have found that there was a trend of improved LFS rate and decreased RI in patients receiving higher number of CD34+ cells in the graft. This is an important finding, because the number of hematopoietic progenitors is, to some degree, under the control of clinicians. However, the experimental data at the basis of the “megadose” concept and identifying the magnitude of CD34+ cell dose as critical for successfully overcoming the HLA barrier, and in establishing tolerance, provide support to the conclusion that, whenever possible, the maximum number of CD34+ cells should be infused.32,33 Whether a higher CD34+ cell dose infused is associated with a higher graft-versus-leukemia effect has to be further investigated. In accordance with this hypothesis, the Tübingen group has demonstrated that a CD34+ cell dose greater than 20 × 106/kg is associated with a faster immunologic recovery.34
In contrast to other investigators35-40 who did describe a benefit for patients with KIR ligand mismatch and alloreactive natural killer cells in the haploidentical setting, we did not find any impact of KIR mismatch between donor and recipient in our study for ALL. However this finding should be taken with caution because the number of patients with available information on KIR ligand was small. Moreover, some other factors such as use of ATG/ALG could negatively impact the KIR ligand effect. Another donor-related factor that we would expect to be associated with outcomes was type of related donor. Recently, a mother donor was associated with better survival in 118 patients with leukemia.35 Those patients underwent transplantation in 2 centers and were more frequently adults with acute myeloid leukemia. This finding was not observed in our study: LFS was 19% (± 6%) when the donor was a female, and it was 34% (± 7%) when the donor was a male (P = not significant).
An important finding of our study was the association of outcomes, LFS and RI, with the center size. The “center-effect” issue is known in transplantation medicine,41,42 and it has been associated with outcomes after HSCT in adults43 ; however in pediatric HSCT, it has not been appropriately studied. Indeed, the outcome of bone marrow transplantation for acute myeloid leukemia in CR1 was demonstrated to be influenced by the center at which the procedure was performed, even after adjustment for known prognostic risk factors.43 In a first analysis, we have found that patients who underwent transplantation in centers performing more than 9 haploHSCTs during the studied period had improved LFS compared with transplant centers performing fewer (data not shown). To better define center size, we addressed the question of whether those transplant centers performing more allogeneic HSCTs during the period study would change the analysis. Once more, we found that center size (defined as more than or fewer than 231 allotransplantations performed during the period study) was associated with outcomes of haploHSCT.
We found that several variables were differently distributed between larger and smaller centers (Table 3). These differences may have all contributed to the better results obtained in the centers with larger activity; however, the center size differences cannot be explained only by a confounding factor related to patient selection or a specific therapy administrated at the transplant center. In fact, use of TBI, use of ATG, as well as patient's CMV serology or previous autologous transplantation were not associated with outcome in univariate or multivariate analyses, and cannot explain the findings related to center size. This observation indicates that many factors related to the center cannot be measured, such as personal experience of physicians and nurses that lead to good choice of patients, timing and transplantation procedure, infrastructure of laboratories, and infection surveillance. We also think that in future studies of haploHSCT, it is important that center effect should be further considered and used to adjust for prognostic factors. Finally, center size or experience effect is found to be the most important factor associated with outcomes in this setting, independent of any other prognostic factor analyzed. We strongly believe in the importance of this message in helping to improve patient outcomes and, importantly, if transplant centers want to initiate a haploHSCT program, they need training and sharing of knowledge from experienced centers.
Despite the number of patients analyzed, our findings should be confirmed by other groups because of the retrospective and multicenter design of our study; in addition, the time period (1995-2004) may be too long considering the rapid development of transplantation medicine.44 We cannot conclude that factors not reaching statistical significance as prognostic factors had no impact on outcome because this may be due to a lack of power. Their impact should be tested in larger series of patients. Moreover, some more recent data have described the impact of minimal residual disease and use of tyrosine kinase inhibitors before and after transplantation,45-47 but unfortunately it was not possible to evaluate in our retrospective analysis.
In conclusion, haploHSCT is an alternative option to treat children with very high-risk ALL lacking an HLA-identical donor and should be considered whenever there is an indication to allogeneic transplantation,17,30 and not as the last option in relapse of the disease. The final outcome of patients undergoing a haploHSCT may be improved by increasing the number of CD34+ cells infused and performing transplantation in experienced centers with programs dedicated to this type of allograft. Innovative procedures of progenitor cell selection, such as those based on the negative depletion of CD3/CD19 cells,48,49 use of natural killer alloreactive donors,18 as well as strategies of adoptive cell therapy with T-cell lines or clones aimed at enhancing the graft-versus-leukemia and pathogen-specific immune reconstitution may also contribute to ameliorate the outcome of children undergoing a haploHSCT.3,50 Exciting perspectives for haploHSCT may also result from cotransplantation of mesenchymal stem cells.51
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.K. wrote the paper, worked on study design, and contributed patients; J.C. worked on study design, participated in the writing committee, and contributed patients; M.L. designed and performed statistical analysis; F.L., P.D., R.H., A.B., J.O.-L., F.F., R.O., C.P., F.A., and G.D. contributed clinical data and worked on the writing committee; E.P. collected and checked clinical data; and V.R. wrote the paper, worked on study design, verified and confirmed clinical data, and helped M.L. perform statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of participants from the Acute Leukaemia and Paediatric Diseases Working Parties of European Blood and Marrow Transplant Group Registry, see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Thomas Klingebiel, Theodor Stern Kai 7, Klinik für Kinder-und Jugendmedizin III, Johann Wolfgang Goethe Universität, 60590 Frankfurt, Germany; e-mail: thomas.klingebiel@kgu.de.