Abstract
Execution of physiologic cell death known as apoptosis is tightly regulated and transfers immunologically relevant information. This ensures efficient clearance of dying cells and shapes the phenotype of their “captors” toward anti-inflammatory. Here, we identify a mechanism of sphingosine-1-phosphate production by apoptotic cells. During cell death, sphingosine kinase 2 (SphK2) is cleaved at its N-terminus in a caspase-1–dependent manner. Thereupon, a truncated but enzymatically active fragment of SphK2 is released from cells. This step is coupled to phosphatidylserine exposure, which is a hallmark of apoptosis and a crucial signal for phagocyte/apoptotic cell interaction. Our data link signaling events during apoptosis to the extracellular production of a lipid mediator that affects immune cell attraction and activation.
Introduction
Sphingosine-1-phosphate (S1P), a ligand for a family of 5 G protein–coupled receptors,1 regulates cellular functions, including cell migration, proliferation, and survival.2 In particular, its role in immune regulation and tumor biology attracted considerable interest.3,4
S1P is produced by phosphorylation of sphingosine by sphingosine kinases. The 2 isozymes, sphingosine kinase 1 (SphK1) and SphK2, although facilitating the same reaction, may have distinct or even antithetic biologic virtues.5 Although a considerable amount of data concerning activation of SphKs and production of S1P exists, mechanistic explanations of how S1P, after being produced inside cells, surmounts the plasma membrane to activate its specific receptors are limited. Once activated, SphK1 translocates and binds to the plasma membrane. This ensures S1P formation in proximity to, for example, ABC family transporters that facilitate the release of S1P from mast cells6 and platelets.7 Alternatively, a variant of SphK1 (SphK1a) is exported from cells, provoking extracellular production of S1P, which contributes to the high intravascular S1P concentration observed in mice.8 Red blood cells are the major cellular constituent for maintaining high S1P levels in the blood,9 although the vascular endothelium may also participate in this process.10 S1P abundance in other tissues is normally low, but can increase during tissue injury or inflammation.2 However, the sources for S1P under such pathologic conditions are largely unknown.
S1P is produced and released during apoptotic cell death,11,12 a phenomenon that likely enhances its biologic importance, because apoptosis plays a fundamental role in the pathogenesis of many human diseases. Previously, we reported that S1P release from apoptotic cells (ACs) was attenuated when SphK2 was absent.11 Mechanistic explanations how SphK2 accomplishes S1P release from dying (ie, apoptotic cells) remained obscure.
Methods
Cells
Human Jurkat T cells and THP-1 monocytes were maintained in RPMI 1640. HEK293 human embryonal kidney cells and NIH 3T3 mouse fibroblasts were cultured in Dulbecco modified Eagle medium (DMEM) high glucose, supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal calf serum (FCS; PAA Laboratories).
Mice
Mice with ablation of Casp1,13 backcrossed into the C57BL/6 genetic background, were kindly provided by Dr H. D. Beer (ETH Zurich, Switzerland). Spleens were isolated from homozygous caspase-1–deficient and wild-type littermates at an age of 6 to 8 weeks. Single-cell suspensions were generated by processing the spleens with a Medimachine System (BD Biosciences), and the cellular composition was analyzed with CD3-FITC, CD4-APC, CD19-APC (Immunotools), and F4/80-APC (eBioscience) antibodies using flow cytometry. Animal experiments followed the guidelines of the Hessian Animal Care and Use Committee.
Induction and detection of apoptosis
Jurkat cells were seeded in RPMI 1640 without FCS, treated with 0.5 μg/mL staurosporine (Sts; Sigma) for the times indicated in the individual experiments or for 2 hours, washed, and further incubated for 4 hours in full medium. Primary spleen cells isolated from mice were killed accordingly. Alternatively, cell death in Jurkat cells was triggered with 100μM etoposide or ultraviolet (UV) irradiation (10 mJ/cm2). Apoptosis in HEK293 was induced with 0.5 μg/mL Sts for 6 hours, followed by washing and further incubation for 6 hours, unless indicated otherwise. NIH 3T3 cells were treated with 0.5 μg/mL Sts for the times indicated. Alternatively, HEK293 cells were killed with 100 ng/mL human recombinant TNF-α for 24 hours. Cell death was routinely controlled and quantified by staining with annexin V/propidium iodide (PI; Immunotools) using a fluorescence-activated cell sorter (FACS).
Generation of SphK2 knockdown cells
For SphK2 knockdown, pSilencer-siSphK2 was transfected into Jurkat cells (Jurkat-siSphK2) using Nucleofector technology (Lonza AG) as described previously for MCF-7 cells.14
S1P quantification in cell-culture supernatants using LC-MS/MS
Quantification of S1P from cell-culture supernatants with liquid chromatography tandem mass-spectrometry (LC-MS/MS) was performed as described previously.11 Cells were incubated with Sts in serum-free media for the times indicated, and supernatants were harvested and analyzed.
Macrophage survival assay
THP-1 cells were plated at a density of 2 × 105 cells/mL, treated with 50nM tetradecanoyl-phorbol-13-acetate (TPA) for 24 hours, washed, and cultured for another 24 hours without TPA. Afterward, supernatants from apoptotic Jurkat cells (apoptotic cell–conditioned medium [ACM]), produced as described,11 were added for 16 hours. Apoptosis in THP-1 macrophages was induced with 250μM etoposide for 8 hours as described previously11 and quantified as cleavage of Ac-DEVD-AMC, indicating effector caspase activity.
Caspase activity assays
Caspase-3/7–like or caspase-1–like activity was quantified after the cleavage of Ac-DEVD-AMC or Ac-YVAD-AMC (both from Alexis), as described previously.11
Fluorescence-based sphingosine kinase activity assay
Activity of sphingosine kinases was measured as phosphorylation of NBD-sphingosine to NBD-S1P (Avanti) after incubating 15 μL of cell culture supernatant, generated by centrifugation at 16 000g (30 minutes at 4°C), or 10 μL of whole-cell lysate in 100 μL of assay buffer for 1 hour, as described.15 For specificity of SphK2, the assay buffer contained 1M KCl, which blocks SphK1 activity.5
Immunoprecipitation and Western analysis
Immunoprecipitation was performed using μMACS Protein A or G Kits according to the manufacturer's instructions (Miltenyi Biotec). Western blot analysis was performed as described.11 Polyclonal SphK2 antibodies, recognizing either the C-terminus (Exalpha Biologicals) or the N-terminus (ABGENT) of both SphK2 isoforms, were used. Polyclonal antibodies against human IL-1β precursor and mature IL-1β, pro–caspase-1 and the p20 subunit, pro–caspase-3 and the p17 subunit (all from Cell Signaling), and murine caspase-1 p10 subunit (Santa Cruz Biotechnology), as well as monoclonal α-V5 and α-HA antibodies, were used. IRDye infrared secondary antibodies were visualized with the Odyssey infrared imaging system from LI-COR. Western blots were quantified using Odyssey software Version 2.1. Cellular fractions were obtained by lysis of cell pellets in fractionation buffer (20mM Tris-HCl, 2mM EDTA, 5mM EGTA, 1mM DTT, and 1× protease inhibitor mix [pH 7.5]), followed by incubation on ice for 1 hour, with regular vortexing. Lysates were passed 40 times through 25-gauge needles. Nuclei were pelleted by centrifugation (300g for 10 minutes at 4°C), supernatants were removed, and the membrane and cytosolic fractions were separated by centrifugation at 16 000g (30 minutes at 4°C).
Transfections
Caspase-1 was knocked down with siRNA from QIAGEN in Jurkat cells using Nucleofector technology. Nucleofection efficiency was around 80% as verified by FACS after nucleofection of Jurkat cells with pmaxGFP (Lonza AG; data not shown). Caspase-1 kd was controlled by siCONTROL nontargeting Duplex no. 1 from Dharmacon. After transfection of caspase-1 siRNA, Jurkat cells were cultured for another 24 hours before apoptosis induction. To overexpress pro–IL-1β, Jurkat cells were nucleofected with the pCAGGS-pIL-1B vector (LMBP 4441), obtained from the Belgian Co-ordinated Collections of Micro-organisms (BCCM)/Laboratory of Molecular Biology–Plasmid Collection (LMBP). For expression of HA-hSphK2, SphK2 mutants, or different HA-tagged caspase-1 constructs, HEK293 cells were transiently transfected by CaPO4 precipitation. Expression of V5-mSphK2 in NIH 3T3 cells was performed with Rotifect (Carl Roth).
Site-directed mutagenesis
Site-directed mutagenesis to generate point mutations was performed using the QuickChange XLII kit from Stratagene as described previously.16 The following primers were used (changed nucleotides are italicized): D138A, 5′-CGCACCTTCCGGGCAGCTGGGGCCGCCACCTACG-3′ and 5′-CGTAGGTGGCGGCCCCAGCTGCCCGGAAGGTGCG-3′; D552A, 5′-GCCACCTAGGCGCTGCCCTGGTGGCAGCTCCGC-3′ and 5′-GCGGAGCTGCCACCAGGGCAGCGCCTAGGTGGC-3′; T219A 5′-CAACCTCATCCAGGCAGAACGACAGAACCACG-3′ and 5′-CGTGGTTCTGTCGTTCTGCCTGGATGAGGTTG-3′. The HA-hSphK2/pCMV5 construct was used as a template. Correct sequence of the generated vectors was verified by sequencing.
Immunofluorescence staining
HEK293 cells were seeded directly onto slides and transiently transfected by CaPO4 precipitation with HA-SphK2 or the mutated constructs. At 24 hours after transfection, cells were used for experiments. Fixation and staining was performed essentially as described.16 Cells were incubated for 2 hours with mouse α-HA antibody and rabbit α–caspase-1 antibody. Secondary antibodies (2 hours) were goat α–rabbit Alexa Fluor 546 and goat α-mouse Alexa Fluor 488. Cells were counterstained with DAPI (1 μg/mL in phosphate-buffered saline [PBS] for 15 minutes). After a final 5-minute washing step, cells were covered with Vectashield mounting medium (Vector Laboratories) and a cover slip. Localization of wild-type and mutated HA-SphK2 as well as caspase-1 was analyzed using an AxioScope fluorescence microscope with the ApoTome upgrade (lens 63×/0.6 NA; ocular 10) at room temperature, documented by a charge-coupled device camera and the AxioVision Software, all from Carl Zeiss MicroImaging.
Statistical analysis
For Western analysis, 1 representative experiment of at least 3 is displayed. P values were calculated using 1-factor analysis of variance (ANOVA) modified by the Bonferroni multiple comparison test.
Results
A truncated SphK2 is released during apoptosis
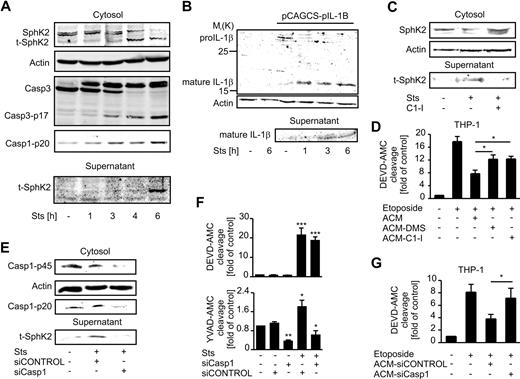
To approach the mechanism of S1P production by ACs, subcellular expression, and activity of SphK2 in Jurkat cells, prior and subsequent to apoptosis induction with Sts was investigated (Figure 1A-C). Corroborating a previous report,5 we noticed reduced expression of both SphK2 isoforms17 in intracellular fractions during apoptosis (Figure 1A). New data emerged when we observed a protein of lower molecular weight in the supernatant of Sts-treated cells (Figure 1A) when using an antibody recognizing the C-terminus of SphK2, but not with an antibody recognizing the N-terminus. Using a fluorescence-based activity assay,15 we measured decreased intracellular SphK2 activity in apoptotic Jurkat cells, whereas extracellular SphK2 activity was significantly elevated (Figure 1B). In Jurkat cells transfected with a vector encoding shRNA against human SphK2, basal expression of SphK2 was reduced, and expression of the extracellular protein fragment after apoptosis induction was likewise diminished, indicating that it was derived from SphK2 (Figure 1C). SphK2 cleavage was further observed in UV-irradiated Jurkat cells, concomitant with the release of the truncated fragment (Figure 1D), thus indicating that SphK2 cleavage occurs in response to different death stimuli.
A truncated active SphK2 (t-SphK2) is released into the supernatant of apoptotic Jurkat cells. (A-B) Jurkat cells remained as controls or were treated with Sts as described in “Induction and detection of apoptosis.” (A) Western analysis of SphK2 in subcellular fractions (nuclear [Nuc], membrane [Mem], cytosolic [Cyt]) or the cell supernatant (Sup). The histogram shows quantification of Western data as means ± SEM from 3 independent experiments. (B) SphK2 activity in Jurkat cell lysates (■) and cell supernatants (□). Data are means ± SEM from 4 independent experiments. *P < .05 compared with unstimulated controls. (C) Western analysis of SphK2 expression in whole-cell lysate (L) and supernatant (S) from Jurkat and SphK2-deficient Jurkat cells that were untreated or treated with Sts. (D) Blots show expression of SphK2 in cytosol and supernatants of control or UV-irradiated (10 mJ/cm2) Jurkat cells.
A truncated active SphK2 (t-SphK2) is released into the supernatant of apoptotic Jurkat cells. (A-B) Jurkat cells remained as controls or were treated with Sts as described in “Induction and detection of apoptosis.” (A) Western analysis of SphK2 in subcellular fractions (nuclear [Nuc], membrane [Mem], cytosolic [Cyt]) or the cell supernatant (Sup). The histogram shows quantification of Western data as means ± SEM from 3 independent experiments. (B) SphK2 activity in Jurkat cell lysates (■) and cell supernatants (□). Data are means ± SEM from 4 independent experiments. *P < .05 compared with unstimulated controls. (C) Western analysis of SphK2 expression in whole-cell lysate (L) and supernatant (S) from Jurkat and SphK2-deficient Jurkat cells that were untreated or treated with Sts. (D) Blots show expression of SphK2 in cytosol and supernatants of control or UV-irradiated (10 mJ/cm2) Jurkat cells.
Caspase-1 cleavage sites in SphK2
Because cleavage of SphK2 was not previously reported, we used PeptideCutter on the ExPASy Proteomics Server (Swiss Institute of Bioinformatics) to identify potential protease cleavage sites. With a focus on apoptosis-associated proteases, 2 putative caspase-1 cleavage sites (at D138 and D552) were identified in human SphK2, both of them being well conserved in mammals (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Furthermore, the N-terminal cleavage site showed homology to a caspase-1 cleavage site in pro–IL-1β,18 with phenylalanine in the P4 and glycine in the P1′ position being highly preferred substrates of caspase-1 (supplemental Figure 1).19 Next, we asked for potential consequences of SphK2 cleavage regarding its subcellular distribution. We used the online software WoLF PSORT20 to predict localization of full-length as well as cleaved SphK2. The theoretical predictions based on established sorting signal motifs and correlative sequence features were as follows: full-length SphK2 should be localized mainly nuclear and cytosolic, as reported before.21 Localization of SphK2 resulting from cleavage at D552, yielding a C-terminally truncated enzyme, should be identical with the full-length protein. Interestingly, the protein generated by N-terminal cleavage at D138 was predicted, among other compartments, to localize in the extracellular space.
Interfering with caspase-1 abrogates SphK2 release
Taking this information into account, we asked whether or not caspase-1 was activated during cell death. First, we monitored time kinetics of SphK2 cleavage, release, and S1P production compared with markers of apoptosis such as caspase-3 activation and phosphatidylserine (PS) externalization. SphK2 processing first occurred 3 hours after treatment of Jurkat cells with Sts, which coincided with accumulation of active caspase-1 (caspase-1–p20 subunit) as well as active caspase-3 (capase-3–p17; Figure 2A). Notably, truncated SphK2 accumulated in the cytosol of Jurkat cells before it was maximally released into the culture supernatant 6 hours after Sts treatment. This release was independent of cell lysis resulting from secondary necrosis, because Jurkat cells were PI− 6 hours after Sts treatment, indicating an intact plasma membrane (supplemental Figure 2). Strikingly, S1P levels in the supernatant of dying Jurkat cells correlated well with the presence of truncated SphK2 (Table 1).
Release of SphK2 depends on activation of caspase-1. (A) Western blots show expression of SphK2, caspase-3, and active caspase-1 (p20) in the cytosol and of SphK2 in the supernatants of Jurkat cells incubated with Sts for the times indicated. (B) Western analysis of pro–IL-1β and mature IL-1β. Jurkat cells were controls or transfected with the pro–IL1β expression vector pCACGS-p-IL-1B and treated with Sts for the times indicated. (C) Western analysis of SphK2 expression in the cytosol and supernatants of Jurkat cells. Cells were controls, treated with Sts, or Sts and 1μM caspase-1 inhibitor Ac-YVAD-CMK (C1-I). (D) THP-1 macrophages were pretreated with supernatants of apoptotic Jurkat cells (ACM), or respective supernatants generated with either DMS (ACM-DMS) or Ac-YVAD-CMK (ACM-C1-I) present. Apoptosis was induced for 8 hours with 250μM etoposide. Caspase-3 activity in THP-1 macrophages normalized to untreated cells is shown. Data are means ± SEM from 4 independent experiments. (E-F) Jurkat cells were controls or transfected with either siCONTROL or siRNA directed against caspase-1 before induction of apoptosis with Sts. (E) Western analysis shows expression of pro–caspase-1 and the caspase p20 subunit in the cytosol and t-SphK2 in the cell supernatant. (F) Histograms display caspase-3/7 (DEVD-AMC cleavage) or caspase-1 (YVAD-AMC cleavage) activity normalized to controls. Data are means ± SEM from 4 independent experiments. (G) THP-1 macrophages were pretreated with supernatants of apoptotic Jurkat cells transfected with siCONTROL (ACM-siControl) or siRNA targeting caspase-1 (ACM-siCasp1). Apoptosis was induced for 8 hours with 250μM etoposide. Caspase-3 activity in THP-1 macrophages was normalized to untreated cells. Data are means ± SEM from 5 independent experiments (*P < .05; **P < .01; ***P < .001).
Release of SphK2 depends on activation of caspase-1. (A) Western blots show expression of SphK2, caspase-3, and active caspase-1 (p20) in the cytosol and of SphK2 in the supernatants of Jurkat cells incubated with Sts for the times indicated. (B) Western analysis of pro–IL-1β and mature IL-1β. Jurkat cells were controls or transfected with the pro–IL1β expression vector pCACGS-p-IL-1B and treated with Sts for the times indicated. (C) Western analysis of SphK2 expression in the cytosol and supernatants of Jurkat cells. Cells were controls, treated with Sts, or Sts and 1μM caspase-1 inhibitor Ac-YVAD-CMK (C1-I). (D) THP-1 macrophages were pretreated with supernatants of apoptotic Jurkat cells (ACM), or respective supernatants generated with either DMS (ACM-DMS) or Ac-YVAD-CMK (ACM-C1-I) present. Apoptosis was induced for 8 hours with 250μM etoposide. Caspase-3 activity in THP-1 macrophages normalized to untreated cells is shown. Data are means ± SEM from 4 independent experiments. (E-F) Jurkat cells were controls or transfected with either siCONTROL or siRNA directed against caspase-1 before induction of apoptosis with Sts. (E) Western analysis shows expression of pro–caspase-1 and the caspase p20 subunit in the cytosol and t-SphK2 in the cell supernatant. (F) Histograms display caspase-3/7 (DEVD-AMC cleavage) or caspase-1 (YVAD-AMC cleavage) activity normalized to controls. Data are means ± SEM from 4 independent experiments. (G) THP-1 macrophages were pretreated with supernatants of apoptotic Jurkat cells transfected with siCONTROL (ACM-siControl) or siRNA targeting caspase-1 (ACM-siCasp1). Apoptosis was induced for 8 hours with 250μM etoposide. Caspase-3 activity in THP-1 macrophages was normalized to untreated cells. Data are means ± SEM from 5 independent experiments (*P < .05; **P < .01; ***P < .001).
S1P release from apoptotic cells
| Sample . | S1P, mean ng/mL (n ≥ 4) . | SEM . |
|---|---|---|
| Jurkat cells, ×106 cells/mL | ||
| Control | 0.23 | ± 0.02 |
| 2 h staurosporine (0.5 ng/mL) | 0.29 | ± 0.02 |
| 4 h staurosporine (0.5 ng/mL) | 0.41 | ± 0.02 |
| 6 h staurosporine (0.5 ng/mL) | 1.11 | ± 0.09 |
| EGTA + 6 h staurosporine (0.5 ng/mL) | 0.33 | ± 0.04 |
| Ac-YVAD-CMK (1μM) + 6 h staurosporine (0.5 ng/mL) | 0.36 | ± 0.03 |
| HEK293 cells, 2 × 105 cells/mL | ||
| Control | ND | ND |
| 12 h staurosporine (0.5 ng/mL) | 0.2 | ± 0.02 |
| HA-SphK2 + 12 h staurosporine (0.5 ng/mL) | 5.37 | ± 0.51 |
| HA-SphK2-D138A + 12 h staurosporine (0.5 ng/mL) | 0.49 | ± 0.05 |
| HA-SphK2-T219A + 12 h staurosporine (0.5 ng/mL) | 0.22 | ± 0.03 |
| Sample . | S1P, mean ng/mL (n ≥ 4) . | SEM . |
|---|---|---|
| Jurkat cells, ×106 cells/mL | ||
| Control | 0.23 | ± 0.02 |
| 2 h staurosporine (0.5 ng/mL) | 0.29 | ± 0.02 |
| 4 h staurosporine (0.5 ng/mL) | 0.41 | ± 0.02 |
| 6 h staurosporine (0.5 ng/mL) | 1.11 | ± 0.09 |
| EGTA + 6 h staurosporine (0.5 ng/mL) | 0.33 | ± 0.04 |
| Ac-YVAD-CMK (1μM) + 6 h staurosporine (0.5 ng/mL) | 0.36 | ± 0.03 |
| HEK293 cells, 2 × 105 cells/mL | ||
| Control | ND | ND |
| 12 h staurosporine (0.5 ng/mL) | 0.2 | ± 0.02 |
| HA-SphK2 + 12 h staurosporine (0.5 ng/mL) | 5.37 | ± 0.51 |
| HA-SphK2-D138A + 12 h staurosporine (0.5 ng/mL) | 0.49 | ± 0.05 |
| HA-SphK2-T219A + 12 h staurosporine (0.5 ng/mL) | 0.22 | ± 0.03 |
S1P contents in supernatants were measured by liquid chromatography tandem mass spectrometry as described in “S1P quantification in cell-culture supernatants using LC-MS/MS.” The reliable lower limit of quantification was 0.2 ng/mL. ND indicates S1P levels below this limit.
To further prove activity of caspase-1 after induction of apoptosis, we transfected Jurkat cells with a plasmid encoding pro–IL-1β22 before apoptosis induction. Indeed, pro–IL-1β was processed starting 1 hour after Sts treatment, and mature IL-1β was released into the supernatant of apoptotic Jurkat cells after 3 hours (Figure 2B). Having shown that caspase-1 was activated during Sts-induced apoptosis of Jurkat cells, we corroborated that inhibition of caspase-1 with 1μM Ac-YVAD-CMK indeed prevented SphK2 cleavage and release of the N-terminally cleaved fragment into the supernatant of Sts-treated cells (Figure 2C), concomitant with reduced extracellular S1P levels (Table 1). In contrast, the caspase-3/7–specific inhibitor Ac-DEVD-CHO did not prevent SphK2 cleavage and release from Sts-treated Jurkat cells, although efficiently inhibiting caspase-3 activation (supplemental Figure 3). Thus, SphK2 cleavage was independent of major apoptotic caspases.
Previously, we showed that supernatants of AC (ACM) attenuated etoposide-induced caspase-3 activation in THP-1 macrophages via S1P.11 This inhibition of caspase-3 activity was reduced when ACM was generated from Jurkat cells treated with Sts in the presence of the caspase-1 inhibitor Ac-YVAD-CMK or the sphingosine kinase inhibitor dimethylsphingosine (DMS; Figure 2D).
To obtain more direct evidence for the involvement of caspase-1 in provoking SphK2 release, we targeted caspase-1 in Jurkat cells with siRNA. Expression of pro–caspase-1 and the p20 subunit were reduced below the level of control and Sts-treated Jurkat cells (Figure 2E). Release of truncated SphK2 into the supernatant was triggered by Sts in Jurkat cells transfected with nontargeting siRNA, but was attenuated in caspase-1 knockdown cells (Figure 2E). Sts increased caspase-1–like activity (cleavage of YVAD-AMC) as well as caspase-3/7–like activity (cleavage of DEVD-AMC), but only caspase-1 activity was reduced in caspase-1 knockdown cells (Figure 2F). Along that line, ACM from caspase-1 knockdown Jurkat cells failed to protect THP-1 macrophages against etoposide-induced cell death compared with ACM from Jurkat cells transfected with nontargeting siRNA (Figure 2G).
For unequivocally proving the involvement of caspase-1, neither the use of inhibitors nor siRNA might be specific enough. Therefore, we investigated the influence of genetic ablation of caspase-1 on SphK2 release during apoptosis. First, we verified whether SphK2 cleavage and release during apoptosis occurred in the murine system. We transfected NIH 3T3 murine fibroblasts with a plasmid encoding murine C-terminal V5-tagged SphK2 and induced apoptosis (supplemental Figure 4). Unlike in the human system, several intracellular V5-tagged fragments appeared during the time course of V5-SphK2 cleavage, which may indicate further digestion by proteases (supplemental Figure 4). However, the truncated V5-tagged SphK2 was observed in the supernatant after Sts treatment.
Having confirmed SphK2 release during apoptosis in a murine cell line, we went on to analyze SphK2 cleavage in spleen cells derived from caspase-1–deficient versus competent mice. Composition of spleen cell populations did not differ between Casp1−/− and Casp1+/+ mice, consisting mainly of T cells, B cells, and F4/80+ myeloid cells, as confirmed by FACS analysis (Figure 3A). The time course of apoptosis in whole spleen cell populations, induced with Sts and quantified as PS exposure with FITC-labeled annexin V, was similar between wild-type and knockout cells, as exemplified for CD4+ cells (Figure 3B). Cells were harvested 6 hours after cell death induction, and SphK2 expression was analyzed compared with untreated cells of the same origin. In line with the cell-culture data on caspase-1 knockdown, SphK2 cleavage was attenuated or completely prevented in Casp1−/− cells, but occurred in wild-type cells (Figure 3C). Furthermore, an increase in extracellular SphK2 activity was only observed in supernatants of apoptotic Casp1+/+ cells, but not in those of apoptotic Casp1−/− cells compared with viable controls (Figure 3D). Both the cleavage of SphK2 and its activity in supernatants of wild-type cells were lower compared with that of Jurkat cells (Figure 3C-D vs Figure 1B). These differences most likely result from lower numbers of ACs in Sts-treated spleen cells. However, these data strongly suggest that caspase-1 is necessary to process SphK2 during apoptotic cell death, thereby preparing the protein for its release.
Caspase-1 knockout mice have defects in SphK2 processing and release during apoptosis. Spleen cells from caspase-1 knockout (Casp1−/−; n = 7) and wild-type (Casp1+/+; n = 7) littermates were left untreated or incubated with Sts. (A) Dot blots show FACS analysis of spleen cell subsets after preparation from whole spleens. CD45+ cells were further stained with CD3-FITC, CD4-APC (T cells), CD19-APC (B cells), F4/80-APC (macrophages), and/or respective isotype antibodies. Representative data from 3 different mice of each genotype are displayed. (B) FACS dot blots display PS exposure visualized with annexin V–FITC in spleen cell conglomerates before and after incubation with Sts for 2 and 4 hours. CD4-APC staining specifies apoptosis mainly in the T-cell compartment. Representative data from 5 different mice of each genotype are displayed. (C) Western analysis of cytosolic SphK2 expression before and after induction of apoptosis with Sts. The histogram shows quantification of Western data as means ± SEM from 5 mice of each genotype. (D) Histogram shows SphK2 activity in the supernatants of control or Sts-treated spleen cell conglomerates. Data are means ± SEM from 7 different mice of each genotype. ***P < .001.
Caspase-1 knockout mice have defects in SphK2 processing and release during apoptosis. Spleen cells from caspase-1 knockout (Casp1−/−; n = 7) and wild-type (Casp1+/+; n = 7) littermates were left untreated or incubated with Sts. (A) Dot blots show FACS analysis of spleen cell subsets after preparation from whole spleens. CD45+ cells were further stained with CD3-FITC, CD4-APC (T cells), CD19-APC (B cells), F4/80-APC (macrophages), and/or respective isotype antibodies. Representative data from 3 different mice of each genotype are displayed. (B) FACS dot blots display PS exposure visualized with annexin V–FITC in spleen cell conglomerates before and after incubation with Sts for 2 and 4 hours. CD4-APC staining specifies apoptosis mainly in the T-cell compartment. Representative data from 5 different mice of each genotype are displayed. (C) Western analysis of cytosolic SphK2 expression before and after induction of apoptosis with Sts. The histogram shows quantification of Western data as means ± SEM from 5 mice of each genotype. (D) Histogram shows SphK2 activity in the supernatants of control or Sts-treated spleen cell conglomerates. Data are means ± SEM from 7 different mice of each genotype. ***P < .001.
SphK2 binds to caspase-1, whereas its cleavage requires active caspase-1
We established a system allowing overexpression of SphK2 to perform mutational analysis of caspase-1 cleavage sites. We used HEK293 cells, which are easy to transfect and show low basal SphK2 expression. Some extracellular SphK2 activity was noticed in supernatants of apoptotic HEK293 cells induced by Sts or TNF-α, which was attenuated by the caspase-1 inhibitor. DMS was used as an internal control to block SphK2 activity in the in vitro assay (Figure 4A). We then transfected a plasmid encoding human N-terminal HA-tagged full-length SphK217 into HEK293 cells. Upon induction of apoptosis with Sts in transfectants, high extracellular SphK2 activity was elicited compared with controls. Again, extracellular SphK2 activity was ablated with the caspase-1 inhibitor Ac-YVAD-CMK (Figure 4B), which was as potent as direct inhibition of SphK2 activity in the supernatant with DMS (Figure 4B).
Active full-length caspase-1 is needed for SphK2 cleavage during cell death. (A) Apoptosis in HEK293 cells was induced with Sts in the presence/absence of 1μM Ac-YVAD-CMK (C1-I), or with TNF-α. SphK2 activity in the supernatant is displayed. DMS was used as an internal control in the kinase assay. Data are means ± SEM from 4 independent experiments. *P < .05. (B) HEK293 cells were transfected with HA-hSPHK2/pCMV5 (HA-SphK2). Cells were left as controls or treated with Sts alone or in combination with Ac-YVAD-CMK. Histograms show SphK2 activity in the supernatant. DMS was used as an internal control. Data are means ± SEM from 5 independent experiments. ***P < .001. (C-E) HEK293 cells were transfected with HA-SphK2 or different HA-tagged caspase-1 constructs as indicated. Transfectants remained untreated or were incubated with Sts for 6 hours. (C) Western analysis showing expression of HA-SphK2, HA-tagged caspase-1, constructs and caspase-1–p20 in the cytosol as well as SphK2 in supernatants. (D) Western analysis showing expression of HA-SphK2 and HA-tagged caspase-1 constructs by staining for the HA-tag. Vertical lines have been inserted to indicate repositioned lanes from the same gel. The histogram shows quantification of SphK2 expression as means ± SEM from 4 independent experiments. *P < .05. (E) Overexpressed HA-Casp1 and HA-Casp1-C285A were precipitated from crude lysates using an anti-HA antibody. Binding of SphK2 to precipitated HA-tagged caspase-1 was detected by Western analysis. As an input control, crude lysates were analyzed for total amounts of overexpressed HA-tagged caspase-1.
Active full-length caspase-1 is needed for SphK2 cleavage during cell death. (A) Apoptosis in HEK293 cells was induced with Sts in the presence/absence of 1μM Ac-YVAD-CMK (C1-I), or with TNF-α. SphK2 activity in the supernatant is displayed. DMS was used as an internal control in the kinase assay. Data are means ± SEM from 4 independent experiments. *P < .05. (B) HEK293 cells were transfected with HA-hSPHK2/pCMV5 (HA-SphK2). Cells were left as controls or treated with Sts alone or in combination with Ac-YVAD-CMK. Histograms show SphK2 activity in the supernatant. DMS was used as an internal control. Data are means ± SEM from 5 independent experiments. ***P < .001. (C-E) HEK293 cells were transfected with HA-SphK2 or different HA-tagged caspase-1 constructs as indicated. Transfectants remained untreated or were incubated with Sts for 6 hours. (C) Western analysis showing expression of HA-SphK2, HA-tagged caspase-1, constructs and caspase-1–p20 in the cytosol as well as SphK2 in supernatants. (D) Western analysis showing expression of HA-SphK2 and HA-tagged caspase-1 constructs by staining for the HA-tag. Vertical lines have been inserted to indicate repositioned lanes from the same gel. The histogram shows quantification of SphK2 expression as means ± SEM from 4 independent experiments. *P < .05. (E) Overexpressed HA-Casp1 and HA-Casp1-C285A were precipitated from crude lysates using an anti-HA antibody. Binding of SphK2 to precipitated HA-tagged caspase-1 was detected by Western analysis. As an input control, crude lysates were analyzed for total amounts of overexpressed HA-tagged caspase-1.
The overexpression system further allowed determination whether caspase-1 catalytic activity was needed for SphK2 cleavage. Therefore, we cotransfected HEK293 cells with HA-SphK2 together with either functional full-length HA-tagged caspase-1 (HA-Casp1) or caspase-1 with a mutation of cysteine 285 to alanine in the catalytic site (HA-Casp1-C285A), rendering the protease nonfunctional. Induction of apoptosis provoked cleavage and release of SphK2 when HA-Casp1 but not HA-Casp1-C285A was coexpressed (Figure 4C). Furthermore, significant accumulation of caspase-1–p20 was only observed in Sts-treated HEK cells transfected with HA-Casp1 (Figure 4C). Thus, active caspase-1 was needed for SphK2 cleavage. A further proof that caspase-1 activation was required to process SphK2 emerged when we overexpressed HA-SphK2 together with full-length HA-Casp1 or HA-tagged p30 or p20. Both p20 and p30 lack the CARD domain that is necessary for activation of caspase-1.23 Basal HA-SphK2 expression was not reduced upon incubation with Sts when HA-tagged p30 or p20 were coexpressed (Figure 4D). Coimmunoprecipitation of endogenous SphK2 in HEK293 cells with HA-Casp1 or HA-Casp1-C285A revealed binding of SphK2 to caspase-1 even when the cells were not stimulated with Sts (Figure 4E). Nevertheless, upon Sts treatment, SphK2 coprecipitated only with HA-Casp1-C285A, not HA-Casp1 (Figure 4E). These data indicate that caspase-1 binds to SphK2 and that both caspase-1 activation and its active site are required for SphK2 cleavage.
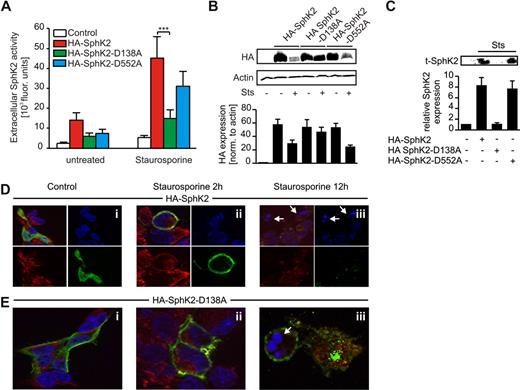
Mutational analysis of caspase-1 cleavage sites in SphK2
To verify N-terminal cleavage of SphK2 by caspase-1, we introduced point mutations in the plasmid encoding HA-SphK2 through site-directed mutagenesis, changing aspartate to alanine in both putative caspase-1 cleavage sites. Mutated or wild-type SphK2 was expressed in HEK293 cells to check basal SphK2 activity. Expression of the naive plasmid versus expression of the mutants produced comparable intracellular SphK2 activity (supplemental Figure 5). We then induced apoptosis with Sts and measured extracellular SphK2 activity. Expression of wild-type HA-SphK2 increased SphK2 activity in the supernatant of apoptotic HEK293 cells and produced high levels of extracellular S1P (Table 1). The same behavior was seen when expressing HA-SphK2-D552A. However, in cells transfected with HA-SphK2-D138A, extracellular SphK2 activity (Figure 5A) and S1P levels (Table 1) were markedly reduced. Analysis of intracellular and extracellular SphK2 protein expression revealed comparable information. Degradation of intracellular HA-tagged constructs was seen when expressing HA-SphK2 or HA-SphK2-D552A, but was absent with the HA-SphK2-D138A mutant (Figure 5B). Release of truncated SphK2 was observed in supernatants of apoptotic HEK293 cells expressing HA-SphK2 or HA-SphK2-D552A, but not in cells transfected with HA-SphK2-D138A (Figure 5C).
Mutation of the N-terminal caspase-1 cleavage site in SphK2 attenuates cleavage and release of SphK2 during apoptosis. (A-C) HEK293 cells were transfected with HA-SphK2 or mutated constructs. (A-B) Transfectants were left as controls or treated with Sts. (A) Extracellular SphK2 activity is shown. Data are means ± SEM from 5 independent experiments. ***P < .001. (B) Western analysis showing intracellular expression of wild-type and mutated HA-SphK2. (C) Western analysis showing extracellular t-SphK2 expression in transfectants stimulated with Sts. (B-C) The histograms show quantification of Western data as means ± SEM from 4 independent experiments. (D-E) HEK293 cells, seeded on cover slips, were transfected with (D) HA-SphK2 or (E) HA-SphK2-D138A. Cells remained as controls or were treated with Sts for 2 or 12 hours. Cells were fixed and stained for HA (green) and caspase-1 (red). Nuclear staining was performed with DAPI (blue). White arrows indicate nuclear fragmentation. Each experiment was performed 4 times, and representative images are shown.
Mutation of the N-terminal caspase-1 cleavage site in SphK2 attenuates cleavage and release of SphK2 during apoptosis. (A-C) HEK293 cells were transfected with HA-SphK2 or mutated constructs. (A-B) Transfectants were left as controls or treated with Sts. (A) Extracellular SphK2 activity is shown. Data are means ± SEM from 5 independent experiments. ***P < .001. (B) Western analysis showing intracellular expression of wild-type and mutated HA-SphK2. (C) Western analysis showing extracellular t-SphK2 expression in transfectants stimulated with Sts. (B-C) The histograms show quantification of Western data as means ± SEM from 4 independent experiments. (D-E) HEK293 cells, seeded on cover slips, were transfected with (D) HA-SphK2 or (E) HA-SphK2-D138A. Cells remained as controls or were treated with Sts for 2 or 12 hours. Cells were fixed and stained for HA (green) and caspase-1 (red). Nuclear staining was performed with DAPI (blue). White arrows indicate nuclear fragmentation. Each experiment was performed 4 times, and representative images are shown.
Caspase-1 and SphK2 colocalize at the plasma membrane
Next, we investigated localization of SphK2 before and after induction of apoptosis. We stained paraformaldehyde-fixed HEK293 cells expressing HA-SphK2 or HA-SphK2-D138A for the HA-tag and caspase-1. Caspase-1 was stained as a marker for the cytosol and to possibly monitor colocalization with SphK2. HA-SphK2 was expressed in the cytosol in HEK293 cells as described previously21 (Figure 5Di). The same holds true for caspase-1. At 2 hours after Sts treatment, HA-SphK2 translocated to the plasma membrane, where it colocalized with caspase-1 (Figure 5Dii). After 12 hours, cell death was visible based on chromatin condensation (Figure 5Dii white arrows), and HA-SphK2 and caspase-1 were hardly detectable (Figure 5Diii). Caspase-1 is known to be shed from dying cells and/or degraded subsequent to its activation. HA-SphK-D138A localized near or at the plasma membrane in untreated HEK293 cells (Figure 5Ei) and remained there when Sts was added for 2 hours (Figure 5Eii). In contrast to HA-SphK2, HA-SphK2-D138A was still detected after 12 hours of Sts treatment and stayed membrane-associated or was cytosolic (Figure 5Eiii). Our data so far indicated colocalization of SphK2 with caspase-1 at the plasma membrane after induction of apoptosis. HA-SphK2-D138A, although located at the plasma membrane, was not cleaved by caspase-1. These observations supported caspase-1–dependent cleavage of SphK2 at its N-terminus as a prerequisite for its release.
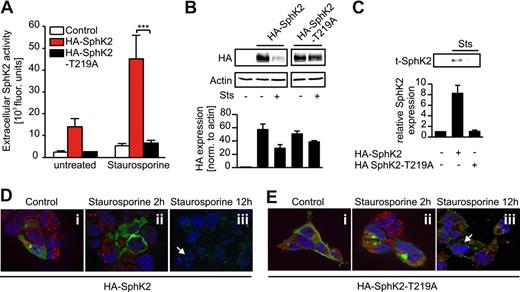
Disruption of PS binding prevents SphK2 release
Cleavage by caspase-1 is not sufficient to explain why truncated SphK2 is released from cells with an intact plasma membrane. Therefore, other apoptosis-associated cellular events might be necessary for specific SphK2 release. Interestingly, SphK2 as well as SphK1 contain PS binding sites, which are well conserved between both isozymes (supplemental Figure 6). PS was shown to stimulate the activity of both isoforms.24 PS exposure on the outer leaflet of ACs is a hallmark of apoptosis and crucial for provoking anti-inflammatory macrophage polarization after their interaction with ACs.25 We therefore questioned whether PS exposure would affect the release or activity of SphK2 during apoptosis. Critical amino acid residues for PS binding were identified in SphK1 and shown to affect SphK1 membrane targeting.26 Therefore, we mutated threonine 219 of SphK2 (analogous to T55 in SphK1) to alanine (supplemental Figure 6).
Mutation of the PS binding site did not significantly affect SphK2 activity compared with the naive plasmid under control conditions in vitro (supplemental Figure 5). However, extracellular SphK2 activity (Figure 6A) and production of S1P (Table 1) were largely reduced upon apoptosis induction in cells expressing HA-SphK2-T219A compared with cells transfected with the wild-type plasmid. Surprisingly, intracellular expression of HA-SphK2-T219A still decreased in apoptotic versus viable HEK293 cells (Figure 6B), although less pronounced compared with the wild-type construct. Nevertheless, release of truncated SphK2 was absent (Figure 6C). Next, we determined intracellular localization of the T219A mutant by staining for the HA-tag and caspase-1 in transfected HEK293 cells. Some basal expression of the T219A mutant was observed at the plasma membrane, which might be due to a SphK2-specific, PS-independent lipid-binding domain.27 However, in contrast to cells expressing HA-SphK2 (Figure 6D) or HA-SphK2-D138A (Figure 5E), the T219A mutant remained mainly in the cytosol upon Sts treatment (Figure 6Ei-iii). These data might suggest that binding of SphK2 to PS at the plasma membrane was critical for its release rather than its cleavage.
Mutation of the PS binding site in SphK2 attenuates release of SphK2 during apoptosis. (A-C) HEK293 cells were transfected with HA-SphK2 or HA-SphK2-T219A. (A-B) Transfectants were left as controls or treated with Sts. (A) Extracellular SphK2 activity is shown. Data are means ± SEM from 5 independent experiments. ***P < .001. (B) Western analysis showing intracellular expression of HA-SphK2 and the T219A mutant from the same gel. (C) Western analysis showing extracellular t-SphK2 expression in transfectants stimulated with Sts. (B-C) The histograms show quantification of Western data as means ± SEM from 4 independent experiments. (D-E) HEK293 cells, seeded on cover slips, were transfected with (D) HA-SphK2 or (E) HA-SphK2-T219A. Cells remained as controls or were treated with Sts for 2 or 12 hours. Cells were fixed and stained for HA (green) and caspase-1 (red). Nuclear staining was performed with DAPI (blue). White arrows indicate nuclear fragmentation. Each experiment was performed 4 times, and representative images are shown.
Mutation of the PS binding site in SphK2 attenuates release of SphK2 during apoptosis. (A-C) HEK293 cells were transfected with HA-SphK2 or HA-SphK2-T219A. (A-B) Transfectants were left as controls or treated with Sts. (A) Extracellular SphK2 activity is shown. Data are means ± SEM from 5 independent experiments. ***P < .001. (B) Western analysis showing intracellular expression of HA-SphK2 and the T219A mutant from the same gel. (C) Western analysis showing extracellular t-SphK2 expression in transfectants stimulated with Sts. (B-C) The histograms show quantification of Western data as means ± SEM from 4 independent experiments. (D-E) HEK293 cells, seeded on cover slips, were transfected with (D) HA-SphK2 or (E) HA-SphK2-T219A. Cells remained as controls or were treated with Sts for 2 or 12 hours. Cells were fixed and stained for HA (green) and caspase-1 (red). Nuclear staining was performed with DAPI (blue). White arrows indicate nuclear fragmentation. Each experiment was performed 4 times, and representative images are shown.
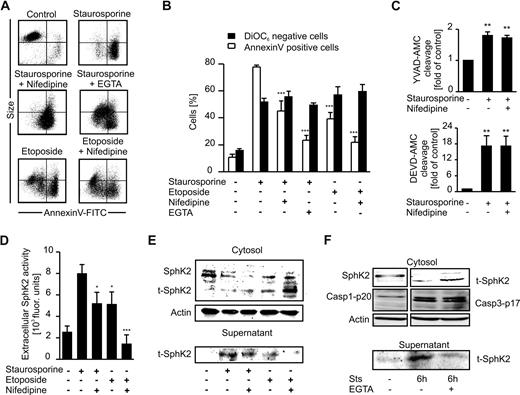
Lowering PS exposure attenuates SphK2 release
To obtain more information on the role of PS in affecting SphK2 release, we modulated PS exposure during apoptosis. First, we killed Jurkat cells with etoposide, which is known to induce apoptosis with only minor PS exposure.28 Furthermore, we attenuated PS exposure during Sts- or etoposide-induced apoptosis with 100μM nifedipine, an inhibitor of L-type calcium channels,29 or by chelating extracellular calcium with 5mM EGTA.30 Apoptosis in Jurkat cells elicited by Sts induced PS exposure, mitochondrial membrane depolarization (ΔΨ), and cell shrinkage (Figure 7A-B). Nifedipine significantly reduced Sts-induced PS exposure, whereas ΔΨ and cell shrinkage remained unchanged (Figure 7A-B). Nifedipine neither blocked Sts-induced cleavage of YVAD-AMC nor cleavage of DEVD-AMC, indicating preserved caspase activitity (Figure 7C). Jurkat cells killed with etoposide showed loss of ΔΨ comparable with that of Sts, but less PS exposure, which was further reduced in the presence of nifedipine (Figure 7B). Strongly reduced PS exposure was further achieved by chelating extracellular calcium with EGTA during Sts-induced cell death in Jurkat cells (Figure 7B). Although cell shrinkage in this setting was largely absent, apoptosis was still the major mode of cell death as indicated by loss of ΔΨ. Thus, we were able to initiate Jurkat cell apoptosis with a variable degree of PS exposure. Importantly, we did not observe enhanced necrosis under these conditions (supplemental Figure 2).
SphK2 release during apoptosis is coupled to PS exposure. Jurkat cells were killed with Sts or etoposide in the absence/presence of 100μM nifedipine or 5mM EGTA. (A) FACS dot blots display PS exposure visualized with annexin V–FITC compared with cell size, with cell shrinkage indicating cell death. Representative data of 4 experiments are shown. (B) Cells were stained with annexin V–FITC (□) or DiOC6 (■) and analyzed by flow cytometry. Data are means ± SEM from 4 independent experiments. (C) Histograms display caspase-3/7 (DEVD-AMC cleavage) or caspase-1 (YVAD-AMC cleavage) activity normalized to controls. Data are means ± SEM from 3 independent experiments. (D) Extracellular SphK2 activity is displayed. Data are means ± SEM from 4 independent experiments. (E) Western analysis of intra- and extracellular SphK2 expression. (F) Western analysis of intra- and extracellular SphK2 and intracellular caspase-1–p20 as well as caspase-3–p17 expression from the same gel. (B-D) Asterisks indicate significant differences compared with cells treated with Sts alone. *P < .05; **P < .01; ***P < .001.
SphK2 release during apoptosis is coupled to PS exposure. Jurkat cells were killed with Sts or etoposide in the absence/presence of 100μM nifedipine or 5mM EGTA. (A) FACS dot blots display PS exposure visualized with annexin V–FITC compared with cell size, with cell shrinkage indicating cell death. Representative data of 4 experiments are shown. (B) Cells were stained with annexin V–FITC (□) or DiOC6 (■) and analyzed by flow cytometry. Data are means ± SEM from 4 independent experiments. (C) Histograms display caspase-3/7 (DEVD-AMC cleavage) or caspase-1 (YVAD-AMC cleavage) activity normalized to controls. Data are means ± SEM from 3 independent experiments. (D) Extracellular SphK2 activity is displayed. Data are means ± SEM from 4 independent experiments. (E) Western analysis of intra- and extracellular SphK2 expression. (F) Western analysis of intra- and extracellular SphK2 and intracellular caspase-1–p20 as well as caspase-3–p17 expression from the same gel. (B-D) Asterisks indicate significant differences compared with cells treated with Sts alone. *P < .05; **P < .01; ***P < .001.
SphK2 activity in supernatants of Sts- or etoposide-treated cells with/without the addition of nifedipine was reduced in parallel to PS exposure (Figure 7D vs 7B). This pattern was reflected by SphK2 expression in whole-cell lysate or supernatants. Attenuating PS exposure decreased the release of truncated SphK2 from ACs, whereas the respective SphK2 fragment accumulated inside the cells (Figure 7E). Furthermore, blocking PS exposure with EGTA prevented release but not cleavage of SphK2 from Sts-treated Jurkat cells, without affecting caspase-1 or caspase-3 activation (Figure 7F). Attenuated SphK2 release correlated well with low S1P levels in supernatants of Jurkat cells killed with Sts in the presence of EGTA (Table 1). These data strongly indicate that SphK2 release and concomitant S1P production required PS externalization.
Discussion
Our data suggest that an enzymatically active SphK2 is released from ACs upon N-terminal truncation, a process demanding caspase-1 activation. Extracellular sphingosine kinases can produce S1P, because degradation of sphingomyelin to sphingosine at the outer leaflet of the plasma membrane is described.31 This was corroborated in mice in vivo, which exhibit a catalytically active SphK1 in the plasma.8 Besides sphingosine, ATP, required for sphingosine phosphorylation, is also known to be secreted by ACs.32 Export of sphingosine kinases from cells might be an elegant way for efficient production of bioavailabe S1P, because it avoids degradation of S1P by intracellular phosphatases or S1P-lyase.5
Recently, it was proposed that S1P production during apoptosis may also involve up-regulation of SphK1.12 Thus, both sphingosine kinases might contribute to S1P production by ACs, depending on the proapoptotic stimulus. A putative caspase-1 cleavage site was predicted in the protein sequences of the known human SphK1 isoforms. However, cleavage at this site would separate the catalytic domain from the ligand-binding domain and thus would render SphK1 inactive.33
SphK2 colocalized with caspase-1 at the plasma membrane during apoptosis in our system. Interfering with SphK2 localization at the plasma membrane attenuated its cleavage by caspase-1 (Figure 6). In HEK293 cells, SphK2 was distributed mainly throughout the cytosol, whereas predominant nuclear localization was described for other cell lines, based on the nuclear localization signal (NLS) in the N-terminus of SphK2.21 We observed this pattern, for example, in Jurkat cells. However, during apoptosis, caspase-1 was reported to enter the nucleus,34 which may allow cleavage of nuclear substrates. Furthermore, a putative nuclear export signal in the sequence of SphK2 was identified recently.35 The export signal and the removal of the NLS by caspase-1–dependent cleavage may prepare SphK2 for nuclear export and release from the cell.
Attenuating PS exposure during apoptosis blocked the release of truncated SphK2 (Figure 6). These findings raise the exciting possibility that PS exposure is directly involved in SphK2 release, or is even the primary mechanism. Indeed, unconventional protein secretion, linked to PS exposure, may require sphingosine kinases.36 Alternatively, it was shown recently that an altered lipid exposure at the plasma membrane of ACs might result from supplying intracellular organelle membranes, namely the endoplasmic reticulum (ER)37 and lysosomes29 in a calcium-dependent manner, which fits with our observations of the calcium channel inhibitor nifedipine, which reduces PS exposure. SphK2 localized in these cellular compartments might be transported to the plasma membrane accordingly. The link between PS exposure and SphK2 release may provide a new mechanistic explanation of how PS exposure on ACs influences immunity. Besides its known anti-inflammatory potential, it might promote the release of SphK2, which results in proximate formation of immunomodulatory S1P in the extracellular compartment.38,39
Increased caspase-1 activity during cell death, without being necessary for its execution, was unexpected. Caspase-1 knockdown did not interfere with apoptotic caspase activity, and inhibition of apoptotic caspases did not block SphK2 cleavage and release. Activation of caspase-1 is critically dependent on the formation of inflammasomes,40 which are not known to be activated during classical apoptosis. In contrast, NLRP3 inflammasomes were demonstrated in necrotic cells, but not in ACs.41 However, this does not rule out the formation of NLRP3-independent inflammasomes. Principally, assembly of inflammasomes is triggered by danger signals. Such signals in the context of cell death might be reactive oxygen species (ROS),42,43 which often provoke and/or accompany apoptosis.44 Thus, activation of caspase-1 during apoptosis may occur due to ROS production and subsequent formation of NLRP3-independent inflammasomes.
Importantly, capase-1 as well as other inflammasome components are not only expressed in leukocytes, but in a variety of tissues or human cell lines (see human protein atlas [HPA]; http://www.proteinatlas.org) that do not primarily regulate innate immune responses. One example are keratinocytes, where stress-induced caspase-1 activation is needed for unconventional secretion of proteins such as growth factors.45 In caspase-1–deficient mice, this protective stress response was blocked, concomitant with impaired wound healing and angiogenesis compared with wild-type littermates.45 This so-far unappreciated phenotype was attributed to defective unconventional protein secretion in response to injury. However, wounding and subsequent healing generally involve inflammation and cell death,46 likely resulting in SphK2 release from dying cells. Taking the proangiogenic function of S1P into consideration,2 impaired wound healing in caspase-1–deficient mice might also be attributed to absent SphK2 release and reduced S1P levels at wound sites. Indirect support for this hypothesis emerged from the recent finding that SphK2-deficient mice show diminished kidney regeneration after ischemia/reperfusion damage.47 Deficiency in either caspase-1 or SphK2 thus impaired tissue regeneration after injury.
Our results indicate a so-far unappreciated role for caspase-1 in modulating immune responses. Besides promoting the maturation of proinflammatory cytokines,40 its activation during apoptosis in combination with PS exposure facilitates cleavage and release of SphK2, which then produces S1P outside cells. S1P is capable of inducing T-helper 2 cell (Th2) responses by polarizing a variety of immune cells.4 Thus, caspase-1, in the context of PS exposure, may actually have anti-inflammatory properties regarding immune cell activation. The proposed mechanism might be an intrinsic negative regulator to limit harmful events resulting from caspase-1–dependent proinflammatory cytokine production. Because activation of a variety of immune cells triggers nonapoptotic PS exposure48 and caspase-1 activation,40 SphK2 secretion might also occur in this context to limit inflammation. Alternatively, because S1P is vital for lymphocyte recruitment,9 a local increase in S1P that occurs at sites of tissue injury as a consequence of apoptotic death may foster immune cell infiltration.
We propose ACs as a local source for S1P under pathologic conditions when apoptotic cell death occurs, rather than contributing to physiologic vascular concentrations of the lipid. However, given the importance of S1P in several human inflammation-associated pathologies, including cancer,3,49 extracellular SphK2 may represent an interesting druggable target.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank H. D. Beer for caspase-1–deficient mice and HA-Casp1-pC constructs, T. Okada for the HA-hSphK2/pCMV5 construct, and S. Spiegel for the V5-mSphK2/pCDNA3.1 construct. We further thank Franz Streb and Sandra Labocha for excellent technical assistance.
This work was supported by grants from Deutsche Forschungsgemeinschaft (Br 999, FOG 784) and the LOEWE Lipid Signaling Forschungszentrum Frankfurt (LiFF).
Authorship
Contribution: A.W. performed experiments and analyzed data; A.W., S.C., and M.V.S. performed animal experiments; C.A. and G.G. performed and designed LC-MS/MS experiments; A.W., A.v.K., and B.B. designed research; and A.W. and B.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Brüne, Institute of Biochemistry I–Pathobiochemistry/ZAFES, Goethe-University Frankfurt, Theodor-Stern-Kai 7, 60590 Frankfurt, Germany; e-mail: bruene@pathobiochemie1.de.

![Figure 1. A truncated active SphK2 (t-SphK2) is released into the supernatant of apoptotic Jurkat cells. (A-B) Jurkat cells remained as controls or were treated with Sts as described in “Induction and detection of apoptosis.” (A) Western analysis of SphK2 in subcellular fractions (nuclear [Nuc], membrane [Mem], cytosolic [Cyt]) or the cell supernatant (Sup). The histogram shows quantification of Western data as means ± SEM from 3 independent experiments. (B) SphK2 activity in Jurkat cell lysates (■) and cell supernatants (□). Data are means ± SEM from 4 independent experiments. *P < .05 compared with unstimulated controls. (C) Western analysis of SphK2 expression in whole-cell lysate (L) and supernatant (S) from Jurkat and SphK2-deficient Jurkat cells that were untreated or treated with Sts. (D) Blots show expression of SphK2 in cytosol and supernatants of control or UV-irradiated (10 mJ/cm2) Jurkat cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/17/10.1182_blood-2009-10-243444/4/m_zh89991051990001.jpeg?Expires=1769157093&Signature=LEXDUtQGdm-eXFkFEHgWpRH5zXuxZoebYS-CAYKltrl4WyWFTfCbtqLxSmUe~uJqS~j6FFStSUqotCmYEEGmgtjViJdOrReiCyuyOwtKXZxUktf2FsTBajT1lnpRBHFg8K26O9MiGGaijYJIN7kIJUUBSj31zKmo-qsDy8d-aofQHmnoWXZnAphIXjXnGIL22lnPEL22OOL6efJ4Qtil4JFVuODxyns2uz4tvB~xxZvJuKWuqE~8aAA8oXsaIXFYuKNQ3TQo4H7N5zbm3xIyG0uUA2IN1QunVJCi1GV54iyGnRwS1YCOebqAw1roc7M5zaFFSAQPWHioQWZMALMe5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)