Abstract
Indoleamine 2,3-dioxygenase-1 (IDO1; IDO) mediates oxidative cleavage of tryptophan, an amino acid essential for cell proliferation and survival. IDO1 inhibition is proposed to have therapeutic potential in immunodeficiency-associated abnormalities, including cancer. Here, we describe INCB024360, a novel IDO1 inhibitor, and investigate its roles in regulating various immune cells and therapeutic potential as an anticancer agent. In cellular assays, INCB024360 selectively inhibits human IDO1 with IC50 values of approximately 10nM, demonstrating little activity against other related enzymes such as IDO2 or tryptophan 2,3-dioxygenase (TDO). In coculture systems of human allogeneic lymphocytes with dendritic cells (DCs) or tumor cells, INCB024360 inhibition of IDO1 promotes T and natural killer (NK)–cell growth, increases IFN-γ production, and reduces conversion to regulatory T (Treg)–like cells. IDO1 induction triggers DC apoptosis, whereas INCB024360 reverses this and increases the number of CD86high DCs, potentially representing a novel mechanism by which IDO1 inhibition activates T cells. Furthermore, IDO1 regulation differs in DCs versus tumor cells. Consistent with its effects in vitro, administration of INCB024360 to tumor-bearing mice significantly inhibits tumor growth in a lymphocyte-dependent manner. Analysis of plasma kynurenine/tryptophan levels in patients with cancer affirms that the IDO pathway is activated in multiple tumor types. Collectively, the data suggest that selective inhibition of IDO1 may represent an attractive cancer therapeutic strategy via up-regulation of cellular immunity.
Introduction
Indoleamine 2,3-dioxygenase-1 (IDO1; IDO) is a heme-containing, monomeric oxidoreductase that catalyzes the degradation of the essential amino acid tryptophan (trp) to N-formyl-kynurenine, which can be subsequently metabolized through a series of steps to form nicotinamide adenine dinucleotide (NAD+).1 The biologic relevance of IDO to peripheral immune tolerance was first proposed when it was shown that treating mice with 1-methyl-tryptophan (1MT), a small-molecule inhibitor of IDO, could break the tolerogenic state that protects allogeneic concepti from the maternal immune system.2 The role of IDO in immunomodulation has since been corroborated in studies with numerous animal models, including models of allograft tolerance, inflammation, and cancer.1
Recent interest has focused on the role of IDO in the induction of tumor immune tolerance. It has been shown that tumor cells can evade immune destruction despite displaying recognizable antigens on their surface and the presence of high-avidity T cells that are specific for the antigens.3,4 Further, histologic evaluation of most human tumor tissues has shown extensive infiltration by various proinflammatory and immune cells,5 suggesting that the immune system responds to malignancy, but the response is nevertheless ineffective in eliminating tumor cells in most cases. These observations have led to the hypothesis that dominant mechanisms of immune suppression may be responsible for the inability of the immune system to effectively respond to tumor-associated antigens in a way that consistently results in tumor rejection.6
Previous studies suggest that IDO may be an important regulator of the immunosuppressive mechanisms responsible for tumor escape from host immune surveillance. Several groups have demonstrated that blockade of IDO activity can directly increase the ability of tumor-bearing mice to reject tumors.7-9 For example, ectopic expression of IDO1 in tumor cells has been shown to inhibit T-cell responses, resulting in immune escape and growth of grafted tumors in mice preimmunized against a specific tumor antigen.8 In addition, studies with 1MT in mouse tumor models demonstrate that IDO inhibition significantly increases the antitumor activities of various chemotherapeutic (eg, platinum compounds, taxane derivatives, cyclophosphamide) and immunotherapeutic agents without increased toxicity.9-11 Based on studies examining serum levels of trp and kynurenine (kyn), IDO appears to be chronically activated in patients with cancer, and IDO activation correlates with more extensive disease.12,13 IDO1 is overexpressed by a wide variety of human tumor types as well as by the dendritic cells (DCs) that localize to the tumor-draining lymph nodes (TDLNs).8,14 Increased expression of IDO1 has been shown to be an independent prognostic variable for reduced survival in patients with acute myeloid leukemia (AML), small-cell lung, melanoma, ovarian, colorectal, pancreatic, and endometrial cancers.14-21
The aforementioned findings clearly support the role of IDO1 in immunosuppression and tumor escape. However, the interpretation of some experiments conducted primarily with 1MT has been questioned.22 Recent studies suggest that the L stereoisomer of 1MT inhibits the IDO1 enzyme and blocks trp to kyn conversion in vitro, whereas the D isomer of 1MT has been shown to be more selective for IDO2 and to exhibit better activity than L-1MT in vivo in murine models. IDO2 is a recently discovered protein that shares structural similarity (43% identity) to IDO1.23 In mice, IDO2 is expressed in the kidney, liver, and male and female reproductive systems; this enzyme is thought to catalyze the oxidative cleavage of a broad range of indole-containing substrates. Emerging data, however, indicate that whereas human IDO1 can efficiently convert trp to kyn, human IDO2 may not do so effectively.24,25 Given the controversy surrounding the 1MT data and a lack of solid evidence of IDO2 biologic function, a truly potent and selective IDO inhibitor with favorable pharmaceutical properties would have great value for clarifying the exact roles of IDO1 and IDO2 and for further exploring the potential utility of IDO1 inhibition in the clinic. In this report, we describe the discovery and characterization of INCB024360, a novel, potent, and highly selective small-molecule IDO1 enzyme inhibitor, as a potential immunotherapeutic agent for the treatment of cancer.
Methods
Reagents
Cell lines were purchased from the ATTC and routinely maintained according to the recommended culture conditions. PAN02 murine pancreatic ductal adenocarcinoma cells were purchased from the Division of Cancer Treatment and Diagnosis (DCTD) Tumor Repository (National Cancer Institute [NCI]) and routinely maintained as recommended. INCB024360 was synthesized at Incyte Corporation. 1MT (D, L, and DL isomers) were purchased from Sigma-Aldrich. All other signaling pathway inhibitors were purchased from EMD Chemicals, and were stored and used according to the manufacturer's recommendations.
Human samples
Blood samples were obtained from healthy volunteers or patients with cancer after informed consent was obtained in accordance with the Declaration of Helsinki and Institutional Review Board approval was received from Incyte. Bone marrow mononuclear cells of patients with AML were purchased from ALLCELLS and maintained according to the recommended culture conditions.
IDO enzyme assays
Cell-based IDO and TDO assays
The HeLa cell–based kyn assay to determine inhibitory activity of INCB024360 was performed as described previously.27 For the DC-based kyn assay, DCs were differentiated from human monocytes (see “Lymphocyte and DC or HeLa cocultures” for details), and stimulated with 50 ng/mL human recombinant IFN-γ (R&D Systems) and 5 μg/mL lipopolysaccharide (LPS) from Salmonella typhimurium (Sigma-Aldrich) in complete RPMI 1640 for 2 days. Established and primary AML cells were also stimulated with 50 ng/mL human recombinant IFN-γ and 5 μg/mL LPS before kyn measurement. The determination of INCB024360 activity was performed similarly to the HeLa cell assay.
To determine INCB024360 activity against IDO in recombinant cells, HEK293/MSR cells were transiently transfected with full-length human or mouse IDO1, or mouse IDO2 cDNA, with Transit-293 transfection reagent (Mirius Bio Corp) or Lipofectamine 2000 reagents (Invitrogen). INCB024360 at different concentrations was added to the recovered transfected cells seeded at 2 × 104 cells per well in a 96-well plate (200 μL/well). The cells were incubated for 2 days, and kyn in the supernatants was measured as described in the HeLa cell assay. The tryptophan 2,3-dioxygenase (TDO) assay was performed similarly with HEK293/MSR cells transfected with a human TDO expression vector.
3H-Trp uptake assay
The 3H-Trp uptake assay to assess trp transporter function in cells was performed as described previously.28
Lymphocyte and DC or HeLa cocultures
Human peripheral blood mononuclear cells (PBMCs) isolated from peripheral blood of healthy donors by Ficoll-Hypaque density gradient were subjected to centrifugal elutriation (Avanti J-20XPI; Beckman Coulter) to obtain monocytes and T cells. Highly purified (> 90%) monocytes, CD4+ or CD8+ T cells were acquired by immunosorting with anti-CD14, anti-CD4, or anti-CD8 microbeads (Miltenyi Biotec). Highly purified natural killer (NK) cells were obtained from PBMCs using anti–human CD56 microbeads.
To generate DCs, purified monocytes were treated with 10 ng/mL human recombinant IL-4 and 40 ng/mL human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems) in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 50 U/mL penicillin/streptomycin (Mediatech Inc), and 40 ng/mL gentamicin (Sigma-Aldrich) for 5 to 6 days. The floating and loosely attached cells were considered immature DCs. Matured DCs were obtained by treating immature DCs with 50 ng/mL IFN-γ, 1 μg/mL LPS, and/or 10 μg/mL peptidoglycan (PGN; Staphylococcus aureus; Sigma-Aldrich) for 1 or 2 additional days.
Cocultures were conducted in 96-well flat-bottom ultra-low attachment plates (Corning) with a total volume of 200 μL per well. To initiate the coculture, LPS- or PGN-treated DCs were washed and resuspended in the DC culture medium at 2 × 106/mL. The DCs (2 × 105) were then cocultured with allogeneic CD4+ T cells, CD8+ T cells, or NK cells at different ratios for different periods of time. Where indicated, INCB024360 or 1MT was added to cocultures and maintained during the culture period. For the T-HeLa cell coculture experiments, DCs were replaced with HeLa cells. After a 2-day incubation, cell proliferation was measured as previous described,29 using a colorimetric Cell Proliferation ELISA kit per manufacturer's instructions (Roche Molecular Biochemicals).
To measure T- and NK-cell proliferation, 100 ng/mL OKT3 (anti-CD3; eBioscience) and 10 ng/mL human recombinant IL-2 (R&D Systems) were added to the cocultures. At 1 to 5 days later, 0.037 MBq/well (1 μCi/well) [3H]thymidine (PerkinElmer) was added to the cell culture and incubated overnight. The cells were then harvested onto a GF/C filter plate and washed with water extensively. Radioactivity retained on the filter plate was measured.
Cytokine assays
Cytokines in the supernatants from DC and T-cell cocultures were determined using the FastQuant System (Whatman) according the manufacturer's instructions. Slide scanning and data analysis were performed by FastQuant scanning service.
DC apoptosis assay
DC apoptosis was examined using the Annexin V–FITC Apoptosis Detection kit (Sigma-Aldrich) per the manufacturer's instructions. The data were acquired and analyzed by flow cytometry on a FACSCalibur (BD Biosciences).
DC surface marker analysis
DCs were stained with either FITC- or PE-conjugated mAbs (BD Biosciences) against human CD80, CD83, CD86, CD40, CD43, or the appropriate isotype controls according to manufacturer's instructions. After incubation in the dark for 30 minutes, the percentage of DCs stained positive for CD80, CD83, CD86, CD40, or CD43 was determined by fluorescence-activated cell sorter (FACS).
Treg cell experiments
Purified T cells and mature DCs were prepared as described. CD4+CD25− T cells were then isolated through consecutive rounds of magnetic-activated cell sorter (MACS) separations with CD4 (positive selection) and CD25 (depletion) MicroBeads per the manufacturer's recommendation (Miltenyi Biotec). The isolated CD4+CD25− T cells were recovered overnight in RPMI 1640 medium supplemented with 10% FBS and appropriate antibiotics. On the next day, CD4+CD25− T cells (2 × 106/well) were seeded alone or mixed with undifferentiated monocytes (4 × 105/well) or mature DCs (4 × 105/well) in a 6-well plate, in RPMI 1640 medium supplemented with 100 U/mL IL-2 (for all samples), and 5 μg/mL LPS and 25 ng/mL recombinant human IFN-γ (for samples with DCs) and/or INCB024360 at 1μM or L-1MT at 500μM (for samples with DCs). After incubation for 6 days, floating T cells were collected and costained with FITC-conjugated anti–human CD4 antibody (Invitrogen) and APC-conjugated anti–human FOXP3 antibody (eBioscience) according to manufacturers' protocols. Appropriate isotype controls were included. All FACS analyses were performed with FACSCalibur, and data were acquired and analyzed with CellQuest software per manufacturer's instructions (Becton Dickinson).
Western blot analysis
HeLa cells or DCs were treated with either IFN-γ or LPS plus inhibitors at the indicated concentrations for 2 days in 6-well plates, and the cells were lysed and analyzed by Western blot as previously described.30 Anti-IDO and anti-GAPDH antibodies were purchased from Millipore and FitzGerald Industrial, respectively, and used according to manufacturers' recommendations.
In vivo studies
Experiments in the PAN02 syngeneic tumor model were performed in C57BL/6 mice and Balb/c nu/nu mice as described previously.31 Tumor growth control (TGC), expressed in a percentage, is calculated using the formula 1 − [(treated (day X) − treated (day Y)) / (vehicle (day X) − vehicle (day Y)], where X is the day of last or interim measurement and Y is the day when dosing commenced.
Results
INCB024360 is a potent and selective IDO1 inhibitor
The ability of INCB02436027 to inhibit human IDO1 was first determined in an in vitro enzymatic assay using purified recombinant IDO1 protein. The conversion of trp, a de novo substrate for the IDO1 enzyme, to kyn was measured spectrophotometrically. In this assay, INCB024360 was shown to inhibit kyn generation with an average IC50 value of 71.8nM plus or minus 17.5nM (Table 1). Additional kinetic analyses suggest that INCB024360 is a reversible and competitive inhibitor of IDO1, and that it is not a substrate of the IDO1 enzyme (data not shown).
INCB024360 is a potent and selective inhibitor of IDO1
| Assay . | Enzyme source/cell . | IC50, nM ± SD . |
|---|---|---|
| Enzyme assay | His-tagged human IDO1 | 71.8 ± 17.5 (n = 21) |
| Cell assays | ||
| Tryptophan-to-kynurenine conversion | HeLa | 7.1 ± 0.6 (n = 56) |
| Human DCs | 12.7 ± 1.1 (n = 3) | |
| 293/MSR-human IDO1 | 15.0 ± 3.3 (n = 4) | |
| OCI-M2 human AML cells | 3.4 (n = 2) | |
| THP-1 human AML cells | 22.6 (n = 2) | |
| Primary human AML BMMCs | > 90% at 100nM (n = 2) | |
| 293/MSR-mouse IDO1 | 52.4 ± 15.7 (n = 8) | |
| 293/MSR-mouse IDO2 | > 5000 (n = 8) | |
| 293/MSR-human TDO | > 10 000 (n = 2) | |
| Tryptophan transport | THP-1 | > 30 000 (n = 2) |
| Assay . | Enzyme source/cell . | IC50, nM ± SD . |
|---|---|---|
| Enzyme assay | His-tagged human IDO1 | 71.8 ± 17.5 (n = 21) |
| Cell assays | ||
| Tryptophan-to-kynurenine conversion | HeLa | 7.1 ± 0.6 (n = 56) |
| Human DCs | 12.7 ± 1.1 (n = 3) | |
| 293/MSR-human IDO1 | 15.0 ± 3.3 (n = 4) | |
| OCI-M2 human AML cells | 3.4 (n = 2) | |
| THP-1 human AML cells | 22.6 (n = 2) | |
| Primary human AML BMMCs | > 90% at 100nM (n = 2) | |
| 293/MSR-mouse IDO1 | 52.4 ± 15.7 (n = 8) | |
| 293/MSR-mouse IDO2 | > 5000 (n = 8) | |
| 293/MSR-human TDO | > 10 000 (n = 2) | |
| Tryptophan transport | THP-1 | > 30 000 (n = 2) |
IDO1 indicates Indoleamine 2,3-dioxygenase-1; DC, dendritic cell; AML, acute myeloid leukemia; and BMMC, bone marrow mononuclear cell.
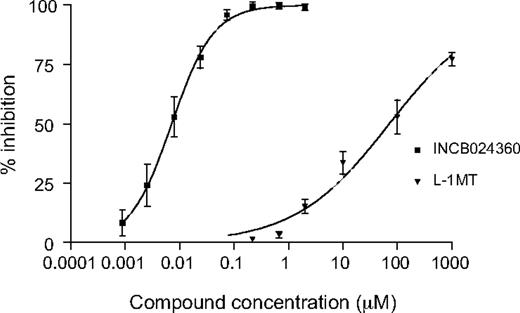
Both HeLa human cervical carcinoma cells and primary human DCs can be induced by proinflammatory cytokines to express endogenous IDO1, consequently resulting in kyn generation in the cell culture supernatant. The ability of INCB024360 to inhibit kyn production after treatment of these cells with human recombinant IFN-γ and/or bacterial LPS was therefore determined. In the HeLa cell-based assay, only IFN-γ was used as the stimulus because the expression of IDO1 cannot be induced by LPS. Under this condition, the average IC50 value for INCB024360 was calculated to be 7.1nM plus or minus 0.6nM (Table 1; Figure 1). It should be noted that IFN-γ only induces expression of IDO1 in HeLa cells and not IDO2 or TDO (data not shown), 2 other known enzymes that may also catabolize trp and produce kyn.32,33 Further, INCB024360 does not affect IDO1 protein level as determined by Western blot analysis, suggesting that INCB024360 inhibits IDO1 enzymatic activity and not its expression in cells. In addition, HeLa cells treated with INCB024360 for 24 hours, washed and replated in the absence of the compound, were fully capable of producing kyn at levels equivalent to DMSO-treated control cells, confirming that the inhibitory effect of the compound is reversible. When the previously reported IDO inhibitor 1MT was tested in this system, it showed a much weaker activity, with an IC50 value for the L stereoisomer (L-1MT) of approximately 120μM (Figure 1) and an IC50 value for the D-1MT of greater than 2.5mM (data not shown), consistent with a previous report.34
INCB024360 potently inhibits kynurenine production in IFN-γ–treated human HeLa cells. HeLa cells were treated with 25 ng/mL IFN-γ and INCB024360 or L-1MT at various concentrations for 2 days. The kyn levels in culture supernatants were measured spectrophotometrically. The data from a composite curve derived from multiple independent assays is presented. Mean values are shown with error bars representing SD.
INCB024360 potently inhibits kynurenine production in IFN-γ–treated human HeLa cells. HeLa cells were treated with 25 ng/mL IFN-γ and INCB024360 or L-1MT at various concentrations for 2 days. The kyn levels in culture supernatants were measured spectrophotometrically. The data from a composite curve derived from multiple independent assays is presented. Mean values are shown with error bars representing SD.
The cellular potency of INCB024360 in blocking kyn production was further determined using several additional human cell types, including IFN-γ and LPS-treated DCs and HEK293/MSR cells transiently expressing human IDO1, as well as established cancer cell lines or primary bone marrow mononuclear cells from patients with hematologic malignances such as AML. As shown in Table 1, INCB024360 inhibited kyn production in these cell types with potencies comparable with that observed in HeLa cells, suggesting that INCB024360 effectively inhibits IDO1 enzyme activity regardless of cell source. The more potent activity of INCB024360 observed in cells compared with that obtained with the purified enzyme (IC50 = 7-15nM vs 71.8nM, respectively) may be attributed to the complicated regeneration system required in the biochemical assay. INCB024360 also exhibited significant activity toward mouse IDO1, with an IC50 value of 52.4nM plus or minus 15.7nM, in a similar assay using mouse IDO1-transfected HEK293/MSR cells (Table 1).
To evaluate the selectivity of INCB024360, assays were developed to measure its activity against TDO and IDO2, 2 other known enzymes that may catalyze the same enzymatic reaction as IDO1, as well as its potential activity against trp transporters, which could also affect trp catabolism in cells.28 As shown in Table 1, INCB024360 demonstrated little inhibition of TDO or IDO2 activity at 10 or 5μM, respectively, and no effect on trp uptake at 30μM. These data indicate that INCB024360 does not cross-react with other proteins that may affect trp catabolism. Further profiling of INCB024360 against a panel of 50 G protein–coupled receptors, ion channels, transporters, and enzymes at concentrations of 1 and 10μM has yielded no appreciable activity against any of the enzymes/proteins tested (data not shown). Collectively, the data suggest that INCB024360 is a potent and selective IDO1 inhibitor.
IDO1 inhibition affects proliferation, survival, and function of T and NK cells
Previous studies suggest that IDO-expressing cells can inhibit T-cell proliferation, and that high concentrations of 1MT can reverse this effect.14,35-37 Thus, IDO-mediated T-cell suppression has been hypothesized to be at least in part responsible for the ability of IDO to promote tumor escape from host immune surveillance. Experiments were therefore undertaken to examine the effects of IDO1 inhibition by INCB024360 on T-cell proliferation and cytokine production, as well as its effects on NK-cell proliferation and DC function because both NK cells and DCs are involved in tumor immunity. Because both human tumor cells and DCs that localize to TDLNs have been identified as the common sources of IDO activity in the immunosuppressive tumor microenvironment, HeLa cells and human DCs were used in these in vitro experiments.
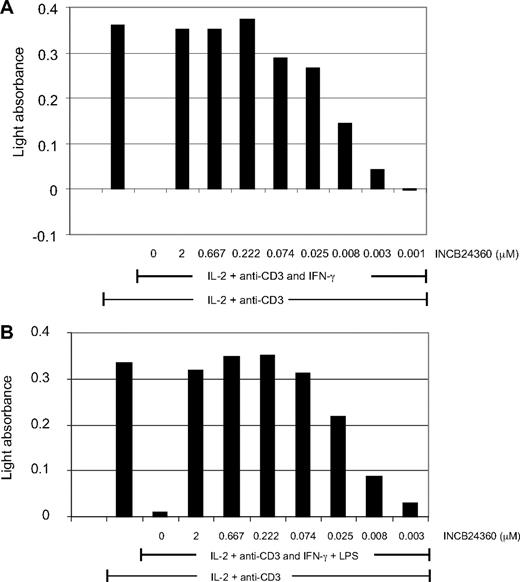
To test whether INCB024360 could reverse the inhibitory effect of IDO1 on T-cell proliferation, either IFN-γ–treated IDO+ HeLa cells (Figure 2A) or IDO+ DCs (Figure 2B) were cocultured with human T cells in the presence of a soluble anti-CD3 antibody and human recombinant IL-2. DCs treated with IFN-γ, LPS, or PGN had significantly increased IDO1 expression as measured by Western blot analysis (Figure 6C,E) and quantitative polymerase chain reaction (PCR; P.W., unpublished data, March 2009), and were used as the source of IDO1+ DCs in these experiments. IDO1 induction significantly suppressed T-cell proliferation in both coculture systems, and the suppression was effectively reversed by INCB024360 with potency similar to that obtained in the cellular assays measuring trp to kyn conversion (Figure 2; Table 1). When T cells alone were treated with IL-2 and anti-CD3 antibody, they proliferated to the same extent as T cells cocultured with DCs and addition of either IFN-γ or LPS had little impact on T-cell proliferation in the absence of IDO+ DCs or HeLa cells (data not shown), suggesting that the suppression of T-cell proliferation in the cocultures was mediated by IDO-expressing DCs or HeLa cells. In addition, the T-cell suppression could also be reversed with an excess amount of trp added into the cultures (data not shown), suggesting that the suppression was primarily due to trp depletion. Only approximately 50% reversal of T-cell suppression could be achieved when 250μM racemic DL-1MT was added to the T-DC cocultures (data not shown), consistent with the finding that 1MT is a much weaker IDO1 inhibitor.
IDO1 inhibition reverses T-cell suppression mediated by IDO-expressing HeLa cells or DCs. Appropriate numbers of HeLa cells, or dendritic cells (DCs) and T cells, were mixed in 96-well plates, and treated with 100 U/mL IL-2 and 100 ng/mL anti-CD3 antibody, as well as 50 ng/mL IFN-γ and 5 μg/mL LPS (only in the DC-T coculture) and various concentrations of INCB024360 as indicated for 2 days. Cell proliferation was measured using the BrdU incorporation cell proliferation ELISA kit. Representative data of 3 independent experiments are presented. Data are from HeLa-T coculture (A) and DC-T coculture (B).
IDO1 inhibition reverses T-cell suppression mediated by IDO-expressing HeLa cells or DCs. Appropriate numbers of HeLa cells, or dendritic cells (DCs) and T cells, were mixed in 96-well plates, and treated with 100 U/mL IL-2 and 100 ng/mL anti-CD3 antibody, as well as 50 ng/mL IFN-γ and 5 μg/mL LPS (only in the DC-T coculture) and various concentrations of INCB024360 as indicated for 2 days. Cell proliferation was measured using the BrdU incorporation cell proliferation ELISA kit. Representative data of 3 independent experiments are presented. Data are from HeLa-T coculture (A) and DC-T coculture (B).
Further examination of the effects of IDO1 expression on purified CD4+ or CD8+ T cells showed that although the proliferation of both CD4+ and CD8+ T cells was suppressed by IDO1, these cells responded to INCB024360 treatment in a quantitatively different manner. Although INCB024360 treatment could restore to some degree the growth of CD4+ T cells cocultured with the IDO+ DCs (Figure 3A), the number of CD8+ T cells cultured in the presence of INCB024360 was even higher than that seen when the cells were cultured with unstimulated (IDO−) DCs (Figure 3B). Differences in the sensitivity between CD4+ and CD8+ T cells to IDO inhibition were also noted in a previous study.38 In addition, as shown in Figure 3C, an approximately 5-fold increase of IFN-γ levels was seen in the INCB024360 containing medium of LPS-treated DCs or PGN-treated DCs cocultured with either CD4+ or CD8+ T cells compared with DMSO control. These results suggest that INCB024360 treatment of IDO1-expressing cells, such as DCs or tumor cells, can increase cytokine production as well as the growth of the neighboring T cells.
IDO1 inhibition increases the proliferation and functional activity of CD4+ T cells, CD8+ T cells, and NK cells. Indoleamine 2,3-dioxygenase-1 (IDO) in DCs differentiated from human monocytes were induced by LPS or PGN. The IDO-positive or -negative DCs were cocultured with primary human CD4+ (A) or CD8+ (B) T cells (2 × 105 cells/well; 1:1) in the presence of OKT3 (100 ng/mL) with or without INCB024360 (1μM) for 4 days. The cell cultures were then pulsed with 3H-thymidine overnight, and the amount of radioactivity incorporated into the cells were measured. The radioactivity from IDO− DCs and T-cell cocultures were normalized to 100%. (C) Supernatants were harvested from these cultures before 3H-thymidine addition and assayed for IFN-γ levels. mDC indicates mature DCs. (D) NK cells (2 × 105/well) purified from allogeneic PBMCs with anti-CD56 microbeads and magnetic sorting were cocultured with IDO-positive or -negative DCs (2 × 105/well) for 3 days in the presence of IL-2 with or without INCB024360 (1μM). The cell cultures were then pulsed with 3H-thymidine as above. The data represent the average of duplicate wells from 4 independent experiments. Error bars represent SD.
IDO1 inhibition increases the proliferation and functional activity of CD4+ T cells, CD8+ T cells, and NK cells. Indoleamine 2,3-dioxygenase-1 (IDO) in DCs differentiated from human monocytes were induced by LPS or PGN. The IDO-positive or -negative DCs were cocultured with primary human CD4+ (A) or CD8+ (B) T cells (2 × 105 cells/well; 1:1) in the presence of OKT3 (100 ng/mL) with or without INCB024360 (1μM) for 4 days. The cell cultures were then pulsed with 3H-thymidine overnight, and the amount of radioactivity incorporated into the cells were measured. The radioactivity from IDO− DCs and T-cell cocultures were normalized to 100%. (C) Supernatants were harvested from these cultures before 3H-thymidine addition and assayed for IFN-γ levels. mDC indicates mature DCs. (D) NK cells (2 × 105/well) purified from allogeneic PBMCs with anti-CD56 microbeads and magnetic sorting were cocultured with IDO-positive or -negative DCs (2 × 105/well) for 3 days in the presence of IL-2 with or without INCB024360 (1μM). The cell cultures were then pulsed with 3H-thymidine as above. The data represent the average of duplicate wells from 4 independent experiments. Error bars represent SD.
Like T cells, NK-cell function may also be inhibited by IDO-mediated trp catabolism.39 The effect of INCB024360 on the proliferation of NK cells that were cocultured with LPS- or PGN-activated DCs in the presence of IL-2 was also examined by measuring 3H-thymidine uptake. As shown in Figure 3D, INCB024360 treatment resulted in a 4- to 5-fold increase in 3H-thymidine uptake by NK cells. LPS- or PGN-treated DCs cultured in the absence of T cells did not proliferate in response to IL-2 treatment.
IDO1 inhibition affects DC viability and CD86 expression
Because the proliferation of both T and NK cells is suppressed by IDO+ DCs, the direct impact of IDO1 expression and inhibition on DCs per se was also examined. In the DC and CD4+ T-cell cocultures, we found that IDO1 induction by either LPS or PGN led to an approximately 50% decrease in the percentage of viable DCs and a concomitant increase in the percentage of apoptotic cells based on FACS analysis (Table 2). These effects of LPS or PGN treatment on DCs could be effectively reversed by INCB024360, suggesting that IDO1 activity was responsible for the changes detected in the number of apoptotic DCs. In addition, the ability of LPS or PGN to induce DC apoptosis could be suppressed by addition of excess trp to the cultures (data not shown).
IDO1 inhibition reduces DC apoptosis and increases CD86high DCs
| . | Percentage viable, mean ± SD . | Percentage apoptotic, mean ± SD . | Percentage CD86high, mean ± SD . |
|---|---|---|---|
| DC | 49.9 ± 25.6 | 28.6 ± 11.3 | 32.9 ± 23.6 |
| DC + LPS | 18.9 ± 5.5 | 59.1 ± 3.3 | 32.3 ± 17.7 |
| DC + LPS + INCB024360 | 54.2 ± 5.9 | 33.7 ± 6.3 | 47.1 ± 15.9 |
| DC + PGN | 29.7 ± 3.3 | 43.7 ± 4.9 | 16.3 ± 7.5 |
| DC + PGN + INCB024360 | 68.1 ± 10.1 | 21.9 ± 9.6 | 42.1 ± 2.9 |
| . | Percentage viable, mean ± SD . | Percentage apoptotic, mean ± SD . | Percentage CD86high, mean ± SD . |
|---|---|---|---|
| DC | 49.9 ± 25.6 | 28.6 ± 11.3 | 32.9 ± 23.6 |
| DC + LPS | 18.9 ± 5.5 | 59.1 ± 3.3 | 32.3 ± 17.7 |
| DC + LPS + INCB024360 | 54.2 ± 5.9 | 33.7 ± 6.3 | 47.1 ± 15.9 |
| DC + PGN | 29.7 ± 3.3 | 43.7 ± 4.9 | 16.3 ± 7.5 |
| DC + PGN + INCB024360 | 68.1 ± 10.1 | 21.9 ± 9.6 | 42.1 ± 2.9 |
Values are mean ± SD of 3 independent experiments. INCB024360 was used at 1μM.
DC indicates dendritic cell; LPS, lipopolysaccharide; and PGN, peptidoglycan.
The ability of IDO to affect the expression of several DC surface molecules that are important for DC function and T-cell activation, including CD86, CD80, CD83, CD40, and CD43, was also examined. When cocultured with CD4+ T cells, LPS- or PGN-treated IDO+ DCs did not appear to have a higher percentage of CD86high cells than untreated DCs, and in the case of PGN-treated cells there was a reduction in the CD86high population. The addition of INCB024360 to the cocultures, however, consistently increased the number of CD86high cells within the LPS- or PGN-treated DC populations (Table 2). In these experiments, INCB024360 treatment had no effect on the levels of CD80, CD83, CD43, or CD40 (data not shown). These results indicate that inhibition of IDO1 by INCB024360 can increase CD86 expression and consequently may promote the ability of DCs to activate T cells and further explain at least in part the growth promoting effects on CD4+ and CD8+ T cells observed after compound treatment.
IDO1 inhibition reduces the conversion of Treg-like cells
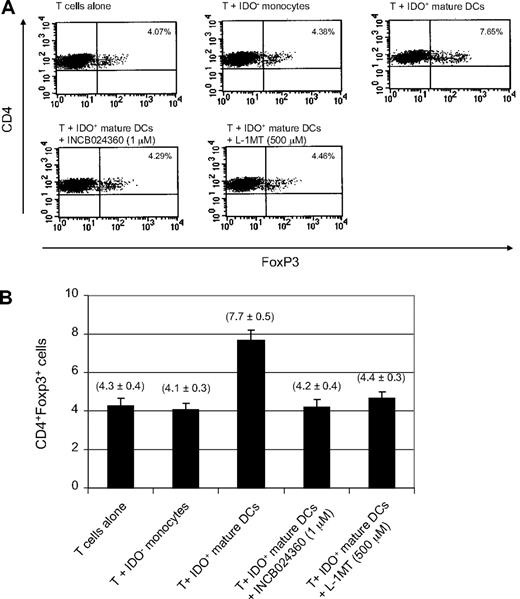
The potential for IDO activity to promote the conversion of naive CD4+CD25− T cells to cells with a regulatory T (Treg) cell phenotype has recently been described.40,41 Because increased Treg cell activity is associated with tumor growth while Treg cell depletion enhances antitumor immune responses,42 it was of interest to determine whether INCB24360 could influence the Treg cell conversion. The ability of IDO+ DCs to promote Treg cell conversion as well as the potential of INCB024360 to reverse this effect was examined in an in vitro culture system similar to those previously reported.40,41 In these studies, CD4+CD25− T cells were cocultured with IDO+ DCs in the presence or absence of INCB024360. After 6 days of culture, T cells were collected and costained with FITC-conjugated anti–human CD4 antibody and APC-conjugated anti–human Foxp3 antibody, a specific marker for Treg cells. As shown in Figure 4, coculture of naive CD4+CD25− T cells with IDO+ DCs resulted in an approximately 2-fold increase in the number of CD4+CD25+ Treg cells as defined by Foxp3 staining when the cells were grown in IL-2–containing medium. Addition of INCB024360 to the cultures reversed this effect, as did treatment with 500μM L-1MT.
IDO1 inhibition reduces the conversion of CD4+Foxp3− T cells to CD4+Foxp3+ Treg-like cells. Primary human CD4+ CD25− T cells were cultured in the presence of IDO+ human DCs, 100 U/mL IL-2, and 1μM INCB024360 or 500μM L-1MT for 6 days. Cells were costained for CD4 and Foxp3 expression. (A) A representative plot of FACS analysis is presented. (B) Average values of 3 independent experiments are shown in the graph. Error bars represent SD.
IDO1 inhibition reduces the conversion of CD4+Foxp3− T cells to CD4+Foxp3+ Treg-like cells. Primary human CD4+ CD25− T cells were cultured in the presence of IDO+ human DCs, 100 U/mL IL-2, and 1μM INCB024360 or 500μM L-1MT for 6 days. Cells were costained for CD4 and Foxp3 expression. (A) A representative plot of FACS analysis is presented. (B) Average values of 3 independent experiments are shown in the graph. Error bars represent SD.
IDO1 inhibition suppresses tumor growth in a lymphocyte-dependent manner
We have shown that inhibition of IDO1 reverses suppression of immune function in multiple in vitro systems. To investigate whether IDO1 inhibition would similarly reverse immune escape in vivo, we treated mice bearing IDO1-expressing PAN02 pancreatic carcinomas orally with INCB024360. The growth of tumors in syngeneic immunocompetent C57BL/6 mice was inhibited in a dose-dependent fashion, with 37% and 57% TGC, respectively, for 25 and 100 mg/kg INCB024360 (Figure 5A; P < .01). However, tumors growing in immunodeficient Balb/c nu/nu mice were not affected by similar doses of INCB024360 (Figure 5B). The inability of INCB024360 to elicit an antitumor response in the immunodeficient mice was not due to lesser impact on kyn generation, as the compound levels were similar between the 2 strains and kyn-to-trp ratios were, in fact, more affected in the immunodeficient mice (Figure 5C). Therefore, consistent with the proposed mechanism of action, INCB024360 suppresses kyn generation in vivo, and its antitumor activity is mediated by lymphocytes.
IDO1 inhibition suppresses tumor growth in immunocompetent, but not immunodeficient, mice. Female C57BL/6 (A) or Balb/c nu/nu (B) mice bearing PAN02 pancreatic cancer cells were treated with 25 mg/kg (▴) or 100 mg/kg (●) INCB024360 orally twice a day or vehicle (■). Mean tumor volumes (mm3) ± SEM (n = 9-11 mice/group) are shown from the initiation of dosing (∼ 90-120 mm3). Both groups are significantly significant (*P < .01) compared with vehicle on the last day of the C57BL/6 study. (C) Plasma was harvested and trp and kyn levels were determined by liquid chromatography–dual mass spectrometry (LC-MS/MS). Kyn/trp ratios and associated percentage of inhibition were calculated.
IDO1 inhibition suppresses tumor growth in immunocompetent, but not immunodeficient, mice. Female C57BL/6 (A) or Balb/c nu/nu (B) mice bearing PAN02 pancreatic cancer cells were treated with 25 mg/kg (▴) or 100 mg/kg (●) INCB024360 orally twice a day or vehicle (■). Mean tumor volumes (mm3) ± SEM (n = 9-11 mice/group) are shown from the initiation of dosing (∼ 90-120 mm3). Both groups are significantly significant (*P < .01) compared with vehicle on the last day of the C57BL/6 study. (C) Plasma was harvested and trp and kyn levels were determined by liquid chromatography–dual mass spectrometry (LC-MS/MS). Kyn/trp ratios and associated percentage of inhibition were calculated.
Differential involvement of selected signaling pathways in the regulation of IDO1 expression and activity
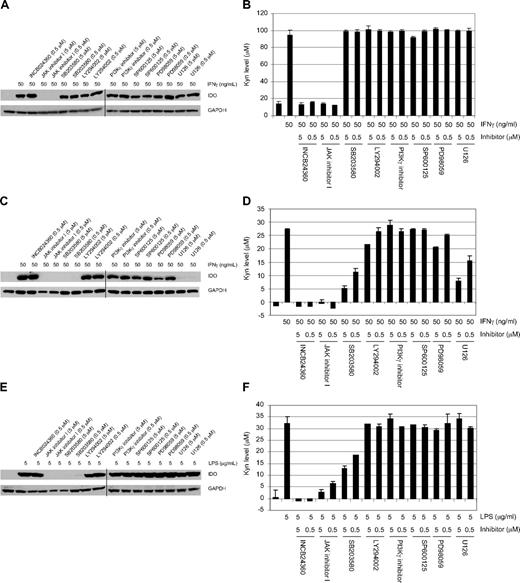
Because the IDO pathway can be activated by a variety of proinflammatory cytokines, as well as other cellular and pathogenic stimuli, we sought to understand which signaling pathways could potentially be involved in regulating the expression and activity of IDO1 in response to IFN-γ or LPS in both HeLa cells and DCs. In other systems, the JAK-STAT pathway has been shown to be an important signaling pathway downstream of IFN-γ, whereas p38 and ERK are activated by LPS. We therefore used commonly studied pathway inhibitors to assess their impact on IDO1 induction. As shown in Figure 6, in both IFN-γ–treated HeLa cells and LPS- or IFN-γ–treated DCs, INCB024360 completely blocked IDO1 enzymatic activity but not expression, whereas a pan-JAK inhibitor, JAK inhibitor I,43 suppressed IDO1 expression and consequently IDO1 activity. No other pathway inhibitors examined significantly affected either IDO1 expression or its activity in HeLa cells. By contrast, the p38 kinase inhibitor SB203580 also blocked IDO1 expression and subsequently IDO1 activity in LPS-treated DCs, whereas both the p38 and MEK kinase (U0126 and PD98059) inhibitors blocked IDO1 expression and activity in IFN-γ–treated DCs. Overall, these data suggest that there is a differential involvement of JAK(s), p38, and MEK kinases in regulating IDO1 expression and activation in different cell types in response to different stimuli.
Signaling pathways involved in the regulation of IDO1 expression and activity induced by IFNγ or LPS in HeLa cells and DCs. HeLa cells or DCs were treated with either IFN-γ or LPS with or without the inhibitors at the indicated concentrations for 2 days, and the cells were lysed and analyzed by Western blot using an anti-IDO or anti-GAPDH antibody. Representative data of 3 independent experiments are shown: IFN-γ–treated HeLa cells (A) and DCs (C), and LPS-treated DCs (E). Kyn levels in the supernatants from these treated cells were determined spectrophotometrically. Representative data of 3 independent experiments are used in the graphs: IFN-γ–treated HeLa cells (B) and DCs (D), and LPS-treated DCs (F). In panels A, C, and E, the vertical lines represent where 2 separate images were aligned. Error bars represent SD.
Signaling pathways involved in the regulation of IDO1 expression and activity induced by IFNγ or LPS in HeLa cells and DCs. HeLa cells or DCs were treated with either IFN-γ or LPS with or without the inhibitors at the indicated concentrations for 2 days, and the cells were lysed and analyzed by Western blot using an anti-IDO or anti-GAPDH antibody. Representative data of 3 independent experiments are shown: IFN-γ–treated HeLa cells (A) and DCs (C), and LPS-treated DCs (E). Kyn levels in the supernatants from these treated cells were determined spectrophotometrically. Representative data of 3 independent experiments are used in the graphs: IFN-γ–treated HeLa cells (B) and DCs (D), and LPS-treated DCs (F). In panels A, C, and E, the vertical lines represent where 2 separate images were aligned. Error bars represent SD.
Kyn levels and kyn/trp ratio are increased in patients with cancer with various tumor types
Increased IDO expression and/or activity have been reported previously in cancer patients.12,13,20,44 As a consequence, increased kyn levels and/or kyn/trp ratios were detected in the circulation of patients with cancer and in some cases have been associated with poor prognosis.20,44 Here, we analyzed plasma samples from 40 patients with breast, colorectal, head and neck, lung, and prostate cancers, as well as hematologic malignancies such as AML. Compared with the healthy donors, patients with all studied cancer types consistently showed increased kyn levels (Table 3). The increases in the kyn/trp ratio range from 1.7-fold in AML to 2.5-fold in head and neck cancer. Although the sample size is relatively small in this analysis, the overall trend clearly suggests that IDO activity is elevated in many patients with cancer. This result is consistent with the findings from other studies, further underscoring the potential clinical relevance of IDO activation in patients with cancer.
Kyn levels and kyn/trp ratios in patients with various tumor types
| Tumor type . | Sample no. . | Trp, mean nM ± SD . | Kyn, mean nM ± SD . | Kyn/trp ratio, nM/μM . |
|---|---|---|---|---|
| AML | 3 | 67 233.3 ± SD 19 728.7 | 4000.0 ± SD 890.2 | 59.5 |
| Breast | 13 | 43 530.8 ± SD 9183.5 | 2641.5 ± SD 961.3 | 60.7 |
| Colorectal | 15 | 54 213.3 ± SD 10 533.0 | 3449.3 ± SD 1210.5 | 63.6 |
| Head and neck | 3 | 55 866.7 ± SD 28 252.8 | 4976.7 ± SD 4094.7 | 89.1 |
| Prostate | 8 | 58 075.0 ± SD 9957.2 | 3493.8 ± SD 1493.8 | 60.2 |
| Lung | 1 | 42 900 | 3010 | 70.2 |
| Healthy person | 12 | 65 416.7 ± SD 8710.3 | 2327.5 ± SD 473.7 | 35.6 |
| Tumor type . | Sample no. . | Trp, mean nM ± SD . | Kyn, mean nM ± SD . | Kyn/trp ratio, nM/μM . |
|---|---|---|---|---|
| AML | 3 | 67 233.3 ± SD 19 728.7 | 4000.0 ± SD 890.2 | 59.5 |
| Breast | 13 | 43 530.8 ± SD 9183.5 | 2641.5 ± SD 961.3 | 60.7 |
| Colorectal | 15 | 54 213.3 ± SD 10 533.0 | 3449.3 ± SD 1210.5 | 63.6 |
| Head and neck | 3 | 55 866.7 ± SD 28 252.8 | 4976.7 ± SD 4094.7 | 89.1 |
| Prostate | 8 | 58 075.0 ± SD 9957.2 | 3493.8 ± SD 1493.8 | 60.2 |
| Lung | 1 | 42 900 | 3010 | 70.2 |
| Healthy person | 12 | 65 416.7 ± SD 8710.3 | 2327.5 ± SD 473.7 | 35.6 |
AML indicates acute myeloid leukemia.
Discussion
IDO1, one of the enzymes mediating the catabolism and degradation of the essential amino acid trp, has been hypothesized to be a drug target for treating various immunodeficiency associated abnormalities, including cancer. This has been primarily supported by studies involving 1MT,8,9 but also by experiments using biochemical and genetic approaches such as siRNA and IDO1-null mice.45-47 More recent studies suggest that the L-1MT isomer selectively inhibits the IDO1 enzyme and blocks trp to kyn conversion in vitro, whereas D-1MT, which is more selective for IDO2, shows better in vivo activity than L-1MT in mouse models. However, IDO2, unlike IDO1, was recently found to be ineffective in converting trp to kyn in human cells in vitro.23,24 These conflicting data, together with the poor cellular activity of 1MT, have hampered efforts to better understand the biologic functions of IDO1 and IDO2.33,48 Thus, a truly potent and selective IDO inhibitor with favorable pharmaceutical properties would be of great value for determining the roles of IDO1 and IDO2 and for further exploring the potential utility of IDO inhibition in the clinic.
To this end, we have identified and characterized INCB024360, a potent and selective inhibitor of IDO1 enzyme activity. Based on the published data of other IDO inhibitors and the results from various experiments presented in this report, INCB024360 is the most potent inhibitor of human IDO1 reported to date, with a potency of 7nM in a HeLa cell–based assay measuring IDO1-mediated trp to kyn conversion, which is more than 10 000-fold more potent than L-1MT when tested in the same assay. The compound also exhibits more than 100-fold selectivity over TDO, IDO2, and trp transporters and shows no appreciable activity against a panel of 50 other proteins, including G protein–coupled receptors, ion channels, transporters, and enzymes.
In our coculture systems using either IDO+ tumor cells or DCs, we have shown that that the removal of IDO1 enzymatic activity by INCB024360 can significantly promote the regrowth of T cells and NK cells, consistent with previous studies using L-1MT. We also noted that in HeLa cells, only IDO1 expression is induced by IFN-γ, whereas in IFN-γ– or LPS-treated DCs, both IDO1 and IDO2 expression are up-regulated (data not shown). The fact that INCB024360, an IDO1 selective compound, can completely inhibit trp to kyn conversion at high concentrations in DCs, suggests that in human DCs only IDO1 appears to be required and sufficient for the conversion of trp to kyn and also appears to be primarily responsible for the restoration of T-cell proliferation. These results are consistent with previous findings using the L-1MT as well as with the possibility that IDO2 may be inactive in mediating trp degradation in humans.24,25,49
In addition to its effects on T-cell proliferation, INCB024360 inhibition of IDO1 can stimulate IFN-γ production from T cells cocultured with IDO+ DCs. In a similar coculture study, Agaugué et al50 showed that the effect of 1MT on IFN-γ release from T cells was dependent on whether IDO1 in the DCs was induced by LPS or PGN. Our results showing IDO1 inhibition by INCB024360 in DCs could increase IFN-γ release from both CD4+ and CD8+ T cells regardless of the stimulus used. The concentrations of 1MT used in the previous studies, however, were very high (1mM), and thus potential off-target effects cannot be excluded. In addition, our data show that IDO1 inhibition by INCB024360 is apparently more effective in reversing IDO-mediated suppression of CD8+ T-cell proliferation. More studies are needed to determine the exact mechanism(s) that may contribute to the differences in the effects of INCB024360 on CD4+ versus CD8+ cells.
Our finding that INCB024360 inhibition of IDO1 could increase viability and decrease apoptosis of DCs is both novel and biologically relevant. Given the importance of DCs in the activation of T cells, the ability of INCB024360 to increase the numbers of functional DCs may represent another mechanism to explain the observed enhancement of IFN-γ production and proliferation of T cells in the DC and T-cell cocultures as shown in this study, in addition to the mechanisms previously suggested by others, such as inhibition of trp depletion. Although our data cannot rule out the possibility that either LPS or PGN induces DC apoptosis through an IDO-independent mechanism, we found that the ability of LPS or PGN to induce DC apoptosis could be suppressed by the addition of an excessive amount of trp, suggesting a potential direct involvement of IDO. We also found that IDO1 inhibition by INCB024360 could increase the number of CD86high DCs, whose engagement by T cells has been suggested to be necessary for T-cell activation.51 These results further support the argument that IDO1 in DCs could negatively regulate T-cell activation by suppressing DC function, which can be effectively prevented by treatment with INCB024360.
Although IDO expression and activity are frequently detected in human tumor cells and immunoregulatory DCs, the biochemical pathways involved in its up-regulation are not completely understood. In this report, we deployed various commonly used inhibitors of relevant signaling pathways and probed their roles in regulating IDO1 expression and activity in response to proinflammatory stimuli such as IFN-γ and LPS in both human HeLa cancer cells and DCs. Although IDO1 expression can be induced by IFN-γ in both HeLa cells and DCs, our data show that there is a differential involvement of JAK(s), p38, and MEK kinases. Only JAK(s) are required for IDO1 expression in both cell types, while p38 and MEK kinases also regulate IDO1 expression in DCs. Although IFN-γ signals predominantly through the JAK/STAT pathway, it may not be surprising that the MAP kinases also regulate IDO1 expression in DCs because these kinases have frequently been shown to play important roles in regulating immune cell function. It is, however, unclear at this point which JAK family kinase(s) is involved in IDO1 regulation, because JAK inhibitor I inhibits multiple JAKs.43 Selective inhibitors or genetic silencing of individual JAKs will be useful to address this question. Differential regulation of IDO expression by LPS and IFN-γ has also been reported in mouse bone marrow–derived DCs,52 in which LPS-induced IDO expression was shown to be JAK-independent and instead involved PI3 kinase and JNK. The apparent differences in the data obtained by this group and our findings could be due to different DC and LPS sources, and different pathway inhibitors and concentrations used. Nevertheless, our data suggest that there is a differential involvement of the JAKs, p38 and MEK kinases in regulating IDO1 expression and activity in response to different stimuli in different cell types. Importantly, the data further demonstrate that INCB024360 is indeed a selective IDO1 enzyme inhibitor devoid of activity to suppress IDO1 expression and that it can completely reverse the induction of IDO1 activity independent of cell type. From a clinical perspective, INCB024360 could have an advantage over other potential IDO intervention approaches by avoiding any off-target toxicities.
In summary, INCB024360 is a novel, highly potent, and selective IDO1 inhibitor that has demonstrated significant activities in promoting proliferation, survival, and functions of various immune (T and NK) and immunoregulatory (DC and Treg) cells via ablation of IDO1 enzyme activity. Together with its ability to inhibit IDO1 activity from tumor cells themselves, including both solid and hematologic malignancies like AML, as well as other host-derived sources, these pleiotropic effects of INCB024360 could work in concert, resulting in significantly enhanced antitumor immunity in vivo. Indeed, this is supported by our in vivo studies demonstrating the significant single-agent antitumor activity of INCB024360 in an immunocompetent mouse tumor model. Thus, INCB024360 could represent a novel immunotherapeutic agent that may help to break the immune tolerance within the tumor microenvironment, and prevent tumor escape from immune surveillance and destruction. Clinical studies to test this hypothesis are clearly warranted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.L. designed the research, performed experiments, analyzed data, and wrote the paper; N.S., H.K.K., G.Y., Q.W., K.W., L.L., M.J.H., B.T., M.R., P.W., K.J.B., Y.L., R.W., J.S.F., and T.C.B. performed experiments and analyzed data; P.P., R.B.S., E.W.Y., and A.P.C. synthesized INCB024360; R.C.N. reviewed the paper; and P.A.S. designed the research and wrote the paper.
Conflict-of-interest disclosure: All authors are employees of and/or own stock in Incyte Corporation.
Correspondence: Peggy Scherle, Incyte Corporation, Experimental Station, Rte 141 and Henry Clay Rd, Wilmington, DE 19880;e-mail: pscherle@incyte.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal