Abstract
Alveolar macrophages (AMs) are the predominant effector cell in the lungs and contribute to a critical first line of defense against bacterial pathogens through recognition by pattern recognition receptors such as Toll-like receptor 4 (TLR4). TLR4-mediated tumor necrosis factor α (TNFα) release is significantly impaired in HIV+ macrophages, but whether HIV impairs myeloid differentiation factor 88 (MyD88)–dependent and/or MyD-independent TLR4 signaling pathways in human macrophages is not known. Comparing human U937 macrophages with HIV+ U1 macrophages (HIV-infected U937 subclone), the current study shows that HIV infection is associated with impaired macrophage TLR4-mediated signaling, specifically targeting the MyD88-dependent TLR4-mediated signaling pathway (reduced MyD88–interleukin-1 receptor–associated kinase [IRAK] interaction, IRAK phosphorylation, nuclear factor [NF]–κB nuclear translocation, and TNFα release) while preserving the MyD88-independent TLR4-mediated signaling pathway (preserved STAT1 phosphorylation, interferon regulatory factor [IRF] nuclear translocation, and interleukin-10 [IL-10] and RANTES release). Extracellular TLR4 signaling complex was intact (similar levels of CD14 and MD2), and similar patterns of response were observed in clinically relevant AMs from healthy and asymptomatic HIV+ persons at high clinical risk of pneumonia. Taken together, these data support the concept that chronic HIV infection is associated with specific and targeted disruption of critical macrophage TLR4 signaling, which in turn may contribute to disease pathogenesis of bacterial pneumonia.
Introduction
Bacterial pneumonia remains a frequent and serious complication in asymptomatic HIV+ persons, despite relatively preserved peripheral blood CD4+ T-lymphocyte counts1 and use of highly active antiretroviral therapy (HAART) with undetectable plasma viral loads.2 These persons have up to 25-fold greater risk of bacterial pneumonia than their healthy cohorts.3 However, the mechanism contributing to this increased risk is not well understood. Toll-like receptor 4 (TLR4) represents a critical pattern recognition receptor in the innate immune host cell response to bacterial infection. Functional deficiency or genetic deletion of TLR 4 increases susceptibility to Haemophilus influenza, Streptococcus pneumoniae, and Klebsiella pneumoniae respiratory tract infections in murine models.4,5 Another indication of its importance is that TLR4 polymorphisms are associated with increased susceptibility to lung infection.6 Our laboratory has reported impaired TLR4-mediated tumor necrosis factor α (TNFα) release in alveolar macrophages from asymptomatic HIV+ persons at increased clinical risk of bacterial pneumonia.7 Increased susceptibility may be in part related to reduced alveolar macrophage extracellular signal-regulated kinase 1/2 (ERK1/2) mitogen-activated protein (MAP) kinase phosphorylation attributed to elevated MAP kinase phosphatase 1 (MKP-1) activity,7 and constitutive phosphoinositol-3 kinase (PI3K) activation and heightened PI3K signaling in response to TLR4 activation.8 These data suggest that the host cell proinflammatory cytokine may be suboptimal in the lungs of HIV+ persons and may in part contribute to increased bacterial pneumonia susceptibility and pathogenesis.
TLR4 is the most studied of the TLR family of innate receptors.9 It is a unique member of the TLR family of mammalian receptors in that TLR4 is capable of both adaptor molecule myeloid differentiation factor 88 (MyD88)–dependent and MyD88-independent signaling.10-12 TLR4-mediated MyD88-dependent recognition of bacterial cell wall occurs at the cell surface,11,13 does not require receptor internalization,11 involves interleukin-1 receptor–associated kinase (IRAK) phosphorylation, and activation of TNF receptor-associated factor 6 (TRAF6), nuclear factor-κB (NF-κB), and MAP kinases (such as ERK1/2, p38, and Jun kinase), with the subsequent release of proinflammatory cytokines such as TNFα14 that may contribute to an effective host defense response to bacteria. In contrast, TLR4-mediated MyD88-independent signaling requires receptor internalization and is mediated through Toll/interleukin-1 receptor (TIR) domain–containing adaptor inducing interferon-β (IFNβ; TRIF),11 involves activation of IFN regulatory factor 3 (IRF3) and STAT115 with release of type-1 IFNs,16 IL-10,15 RANTES15 and may be involved in antiviral host defense.17 Recent work in our laboratory has shown impaired TLR4-mediated signaling response in alveolar macrophages from asymptomatic HIV+ persons at high clinical risk of bacterial pneumonia.7,8 However, whether both MyD88-dependent and MyD88-independent pathways are impaired by HIV infection is not known. With the use of human macrophage cell lines and clinically relevant human alveolar macrophages, the purpose of this study was to further examine the influence of HIV infection on TLR4-mediated intracellular signaling in greater detail, focusing on MyD88-dependent and MyD88-independent TLR4 signaling pathways.
Methods
Study subjects
Recruited healthy and asymptomatic HIV+ subjects showed no evidence of active pulmonary disease with normal spirometry. Healthy persons were confirmed to be HIV-negative by enzyme-linked immunoabsorbent assay (ELISA; Abbot Diagnostics) and to have no known risk factors for HIV infection. Demographic characteristics for all participants were recorded on standardized forms and included age, sex, smoking status, HIV risk factor, medical history, and prescribed antiretroviral medications.
Reagents
Dynasore, lipid A (the biologically active component of lipopolysaccharide, and specific TLR4 ligand) from Escherichia coli F583 Rd mutant, protease inhibitor cocktail, and phorbol myristic acid (PMA), were purchased from Sigma-Aldrich.
Antibodies
Anti–phospho-IRAK, anti-MyD88, anti–inhibitor of NF-κB, anti-IRF3, anti–phospho STAT1, anti-STAT1, anti-ERK1/2, anti-p65 antibodies were purchased from Cell Signaling, cytokine ELISA kits were from R&D Systems, and anti–β-actin antibody was from Sigma-Aldrich. Neutralizing anti–human TLR4 antibody was purchased from eBioscience. Purified mouse immunoglobulin G was used as isotype control (PharMingen).
Human macrophage cell lines
U937 cells are human promonocytic cells (ATCC). U1 cells (HIV-infected subclone of U937 cells; AIDS Research and Reference Reagent Program) contain 2 integrated copies of HIV-1 proviral DNA and are characterized by low levels of constitutive virus expression that can be modulated by cytokines and pharmacologic agents.18 For experiments, U937 and U1 cells were harvested during the exponential growth phase, washed, and then incubated in complete medium (RPMI 1640 containing 10% heat-inactivated fetal calf serum, 2mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin). To allow differentiation to macrophages, U937 and U1 cells were incubated with 100nM PMA at 37°C in a humidified atmosphere containing 5% CO2 for 24 hours. Adherent cells were then washed 3 times with phosphate-buffered saline (PBS; to remove PMA), and incubated in complete media (without PMA) for an additional 24 hours before use in experiments.
Human alveolar macrophages
Select experiments with the use of human alveolar macrophages were carried out to determine clinical relevance of the study. Recruited healthy and asymptomatic HIV+ persons were without evidence of active pulmonary disease and had normal spirometry. Healthy persons were confirmed to be HIV seronegative by ELISA and had no known risk factors for HIV infection. For the HIV+ subjects, peripheral blood CD4 lymphocyte counts were more than 200 cells/mm3, HIV risk factors included intravenous drug use and homosexual exposures, all were prescribed HAART, all had undetectable serum viral load (< 50 HIV-1 RNA copies/mL), and none experienced a prior opportunistic pneumonia. With the use of standard techniques, bronchoalveolar lavage (BAL) was performed to obtain lung immune cells.19 All procedures were performed on consenting adults in accordance with the Declaration of Helsinki, following protocols approved by Beth Israel Deaconess Medical Center Institutional Review Board and Committee for Clinical Investigations. The cells were separated from the pooled BAL fluid and alveolar macrophages (AMs) were isolated as described.20
Western blot analyses
Human macrophages were allowed to adhere, treated with the indicated dose of lipid A, and washed 2 times with ice-cold PBS (pH 7.4). Cells were lysed in lysis buffer containing 25mM Tris-HCl (pH 7.6), 150mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and protease inhibitor cocktail (Sigma-Aldrich) and placed on ice for 20 minutes. Cells were harvested by scraping, followed by centrifugation at 4°C for 15 minutes at 14 000 rpm. Equal amounts of cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide electrophoresis and Western blot analysis with designated antibodies and detected by enhanced chemiluminescence detection system (Amersham Biosciences). Resolved bands were quantified by densitometry (Amersham Biosciences).
Immunoprecipitation
Immunoprecipitation studies were carried out as described.20 Briefly, equivalent amount of proteins were precleared by incubation with protein A/G sepharose (Amersham Biosciences) for 1 hour at 4°C. The supernatant was collected after a brief centrifugation. Antibody was then added to the samples and incubated overnight at 4°C. The immune complexes were precipitated with 50 μL of protein A/G sepharose (50% suspension) and incubated for 1 hour at 4°C. Nonspecific bound proteins were removed by washing the sepharose beads 3 times with modified radioimmune precipitation assay buffer and once with 1× PBS. The immune complexes bound to the beads were solubilized in 40 μL of 2× Laemmli loading buffer and further analyzed by Western blotting as described above.
Targeted gene silencing (RNAi) in macrophages
To determine the functional relevance of MyD88-independent pathway in TLR4 signaling, targeted gene silencing of macrophage TRIF and IRF3 were performed with the use of the synthetic duplex RNA oligonucleotides. We used On-Target plus smart pool short interfering RNA (siRNA) TRIF (Dharmacon). Target sequences were GGAGCCACAUGUCAUUUGG, CCAUAGACCACUCAGCUUU, GGACGAACACUCCCAGAUC, and CCACUGGCCUCCUGAUACA. In addition, we also used On-Target plus smart pool siRNA IRF3 (Dharmacon). The target sequences were CGAGGCCACUGGUGCAUAU, CCAGACACCUCUCCGGACA, GGAGUGAUGAGCUACGUGA, and AGACAUUCUGGAUGAGUUA. On-target plus nontargeting siRNAs were used as controls (Dharmacon). The target sequence was UGGUUUACAUGUCGACUAA. Macrophages were electroporated with 100nM siRNA using Amaxa system following the manufacturer's protocol (Amaxa). TRIF and IRF3 siRNA-mediated knockdown was determined by Western blot probed with anti-TRIF or anti-IRF3 antibody 24 to 48 hours after transfection.
For MyD88-targeted gene silencing undifferentiated human monocytic U937 cells were transfected with a short hairpin RNA (shRNA) vector-specific for human MyD88 (Open Biosystems; GenBank accession no. NM_002468). Plasmid vectors (pSM2) for catalog numbers (1) RHS1764-9690114 and (2) RHS1764-9694563 corresponded to the following shRNA sequences: 5′TGCTGTTGACAGTGAGCGCGGACCCTAAATCCA-ATAGAAATAGTGAAGCCACAGATGTATTTCTATTGGATTTAGGG-TCCTTGCCTACTGCCTCGGA-3′ and 5′TGCTGTTGACAGTGAGCGACCTAACCATGTCCCTGAACAATAGTGAAGCCACAGATGTAT-TGTTCAGGGACATGGTTAGGCTGCCTACTGCCTCGGA-3′. Bacteria transformed with each vector were grown in chloramphenicol-containing (25 μg/mL) LB broth, and plasmids were isolated with the use of PureLink HiPure Plasmid Maxiprep (Invitrogen) according to the manufacturer's protocol. In addition, a vector containing a scrambled shRNA sequence was also cloned and isolated. Plasmid purity was confirmed with the use of restriction enzyme analysis as recommended by the manufacturer. Plasmid vectors were transfected into undifferentiated U937 cells with the use of the Lipofectamine LTX and Plus reagents according to the manufacturer's protocols. Cells were allowed to grow for 72 hours after transfection and then were selected for stable transfection with puromycin (0.4:mu]g/mL; InvivoGen). Cells were differentiated with PMA (100nM) for 24 hours before assay for MyD88 expression and were compared with cells transfected with a scrambled shRNA vector. U937 cells transfected with vector 1 showed best knockdown and were used for subsequent experiments.
Flow cytometric analysis
Human AM CD14 receptor surface expression was analyzed in BAL cell suspension specimens with Epics XL flow cytometer (Beckman Coulter) with laser power of 5.76 mW. The instrument was calibrated before each measurement with standardized fluorescent particles (Immunocheck; AMAC). Fluorescent signals of the cells were measured simultaneously by 3 photomultiplier tubes and optical filters and shown as the mean of the log fluorescence intensity of the cell population within gate. AMs were labeled with primary phycoerythrin (PE)–conjugated antibody to CD14 receptor (Beckman Coulter) for 1 hour at 4°C in the dark, washed twice with balanced salt solution with calcium and magnesium in 0.1% bovine serum albumin, fixed with 250 μL of Optilyse C buffer (Beckman Coulter) diluted with PBS, and analyzed by flow cytometry. AMs were first identified by the characteristic forward and side scatter parameters on unstained AMs, and the population was confirmed by staining with PE-conjugated primary anti–human leukocyte antigen–DR (Beckman Coulter). Results were expressed as a mean relative fluorescence units and the percentage of cells staining positive. Isotype primary conjugated antibodies served as a negative control. Samples were prepared and analyzed in duplicate, and a minimum of 5000 cells was counted for each sample.
Enzyme-linked immunoabsorbent assay
Cells were stimulated for 24 hours, and cultured supernatants were collected, centrifuged to remove cellular debris, and assayed immediately or stored at −80°C until assayed. Cytokine measurements were performed with the commercially available ELISA (R&D Systems) following the manufacturer's instructions, and absorbance was measured at 450 nm on a Bio kinetic ELISA reader (Bio-Tek Instruments). The detection limit for TNFα was 4.4 pg/mL. All measurements were performed in duplicate, and mean values of the 2 measurements were used for statistical analysis.
Statistical analysis
Group comparisons were performed with the Student t test (2-sample test) or 1-way analysis of variance. Calculations were performed with StatView (SAS Institute) and INSTAT2 (GraphPad Software) software packages. Results are given as mean plus or minus SEM. Statistical significance was accepted for P less than .05.
Results
Reduced TLR4-mediated TNFα release associated with impaired MyD88-dependent signaling pathway in HIV+ human macrophages
In general, ligation of mammalian TLRs (with the exception of TLR3) promotes activation of a MyD88-dependent intracellular signal transduction pathway that includes IRAK-1, nuclear translocation of NF-κB, and release of cytokines such as TNFα.21 Reduced TNFα release from lipopolysaccharide-tolerant cells is associated with impaired MyD88-IRAK interaction, reduced IRAK expression, and reduced NF-κB activation.22,23 Previously, we demonstrated reduced lipid A–mediated TNFα release in HIV+ human macrophages,7 although whether the MyD88-dependent TLR4 signaling pathway is intact was not determined. To further examine the influence of HIV on the MyD88-dependent TLR4 signaling pathway, human U937 macrophages showed a robust release of TNFα in response to TLR4 ligand challenge (lipid A, 10 μg/mL), and macrophage TNFα release was significantly reduced in the presence of anti–TLR4-neutralizing antibody (Figure 1A left). Similarly, TNFα release by human HIV+ U1 macrophages was also significantly reduced in the presence of anti–TLR4-neutralizing antibody (Figure 1A right), although overall the levels of TNFα release were significantly lower compared with human U937 macrophages, consistent with our previous observations.7
Reduced TLR4-mediated TNFα release associated with impaired MyD88-dependent signaling pathway in HIV+ human macrophages. (A) Human macrophage TNFα release is mediated by TLR4. Human macrophage U937 and U1 cells were incubated with lipid A (10 μg/mL) in the presence or absence of neutralizing anti-TLR4 antibody (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for TNFα by ELISA. Data shown are mean ± SEM of 4 independent experiments done in triplicate. (*P < .01 compared with U937 with lipid A alone; **P < .05 compared with U1 with lipid A alone). (B) Reduced TLR4-mediated MyD88-IRAK interaction and IRAK phosphorylation in HIV+ alveolar macrophages (AMs). Healthy AM (B left) and HIV+ AM (B right) were incubated with lipid A (10 μg/mL) up to 60 minutes, detergent soluble extracts were immunoprecipitated with anti-MyD88 antibody and immunoblotted with anti–p-IRAK antibody. Densitometric analysis for each p-IRAK band is displayed beneath Western blot. Western blot is a representative of 3 independent experiments with similar results (n = 3). *P < .01 unstimulated control; **P < .01 compared with healthy in the presence of lipid A with time. (C) Reduced TLR4-mediated NF-κB nuclear translocation in HIV+ macrophages. U937 and U1 cells were incubated with lipid A (10 μg/mL) up to 120 minutes, nuclear and cytoplasmic extracts were isolated and probed with anti-p65 antibody. Densitometric analysis of p65 bands for each lane is displayed beneath Western blot. Western blot is a representative of 4 independent experiments with similar results. *P < .01 compared with unstimulated control; **P < .01 compared with U937 cells in the presence of lipid A with time. (D) Functional silencing of human MyD88 leads to marked diminution of TLR4-mediated TNFα release in U937 cells. Western blot analysis of human MyD88 after gene silencing with the use of MyD88 shRNA and scrambled shRNA. β-Actin was used to monitor protein loading after stripping the membrane. A representative blot shows results from 1 experiment with similar results of 3 independent experiments (left). U937 cells were pretreated with shRNA MyD88 and scrambled shRNA. Cells were differentiated with phorbol ester, challenged with lipid A and incubated for 24 hours. Cell-free supernatant was assayed for TNFα by ELISA. Results are representative of 3 independent experiments in triplicate.
Reduced TLR4-mediated TNFα release associated with impaired MyD88-dependent signaling pathway in HIV+ human macrophages. (A) Human macrophage TNFα release is mediated by TLR4. Human macrophage U937 and U1 cells were incubated with lipid A (10 μg/mL) in the presence or absence of neutralizing anti-TLR4 antibody (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for TNFα by ELISA. Data shown are mean ± SEM of 4 independent experiments done in triplicate. (*P < .01 compared with U937 with lipid A alone; **P < .05 compared with U1 with lipid A alone). (B) Reduced TLR4-mediated MyD88-IRAK interaction and IRAK phosphorylation in HIV+ alveolar macrophages (AMs). Healthy AM (B left) and HIV+ AM (B right) were incubated with lipid A (10 μg/mL) up to 60 minutes, detergent soluble extracts were immunoprecipitated with anti-MyD88 antibody and immunoblotted with anti–p-IRAK antibody. Densitometric analysis for each p-IRAK band is displayed beneath Western blot. Western blot is a representative of 3 independent experiments with similar results (n = 3). *P < .01 unstimulated control; **P < .01 compared with healthy in the presence of lipid A with time. (C) Reduced TLR4-mediated NF-κB nuclear translocation in HIV+ macrophages. U937 and U1 cells were incubated with lipid A (10 μg/mL) up to 120 minutes, nuclear and cytoplasmic extracts were isolated and probed with anti-p65 antibody. Densitometric analysis of p65 bands for each lane is displayed beneath Western blot. Western blot is a representative of 4 independent experiments with similar results. *P < .01 compared with unstimulated control; **P < .01 compared with U937 cells in the presence of lipid A with time. (D) Functional silencing of human MyD88 leads to marked diminution of TLR4-mediated TNFα release in U937 cells. Western blot analysis of human MyD88 after gene silencing with the use of MyD88 shRNA and scrambled shRNA. β-Actin was used to monitor protein loading after stripping the membrane. A representative blot shows results from 1 experiment with similar results of 3 independent experiments (left). U937 cells were pretreated with shRNA MyD88 and scrambled shRNA. Cells were differentiated with phorbol ester, challenged with lipid A and incubated for 24 hours. Cell-free supernatant was assayed for TNFα by ELISA. Results are representative of 3 independent experiments in triplicate.
Next, to determine whether downstream MyD88-dependent TLR4-mediated signaling molecules were impaired, clinically relevant human AMs from healthy and asymptomatic HIV+ persons at risk of bacterial pneumonia were incubated with TLR4 ligand (lipid A, 10 μg/mL), and then detergent soluble cellular extracts were immunoprecipitated with anti-MyD88 antibody and immunoblotted with anti–p-IRAK antibody. Experiments show evidence for MyD88-IRAK interaction in AMs from both groups by Western blot (Figure 1B). Although the pattern for IRAK phosphorylation was similar for both groups, quantitative densitometric analysis showed marked reduction in the level of IRAK phosphorylation in AMs from HIV+ persons at all time points examined (Figure 1B right) compared with healthy persons (Figure 1B left). This data suggest that the activity of IRAK in HIV+ macrophages is diminished in response to TLR4 activation and is in agreement with a previous report.24 Recognizing the importance of NF-κB in the TLR-mediated signaling pathway,25 human U937 and U1 macrophages were incubated with lipid A (10 μg/mL) up to 120 minutes; nuclear and cytoplasmic extracts were isolated and probed by Western blot with the antibody directed against the NF-κB p65 component. Quantitative densitometric analysis showed marked reduction of p65 nuclear translocation in HIV+ U1 macrophages compared with U937 macrophages (Figure 1C). To formally show whether MyD88 mediates TLR4-driven TNFα release, functional assays were carried out with targeted functional gene silencing of MyD88. A reduced MyD88 protein level was shown in U937 macrophages after MyD88 silencing by 70% to 80% (Figure 1D left). TLR4-mediated TNFα release was robust in U937 with scrambled shRNA, whereas in MyD88-silenced cells, TLR4-mediated TNFα release was markedly diminished (Figure 1D right), suggesting that TLR4-mediated TNFα release in human macrophages is predominantly MyD88 dependent. Taken together, these data show that human macrophage TNFα release is in part TLR4 dependent, MyD88 dependent, and that reduced TLR4-mediated TNFα release in HIV+ macrophages is associated with impaired MyD88-dependent signaling (as determined by reduced MyD88-IRAK interaction, reduced IRAK phosphorylation, and reduced NF-κB p65 nuclear translocation).
Preserved TLR4-mediated MyD88-independent pathway in HIV+ human macrophages
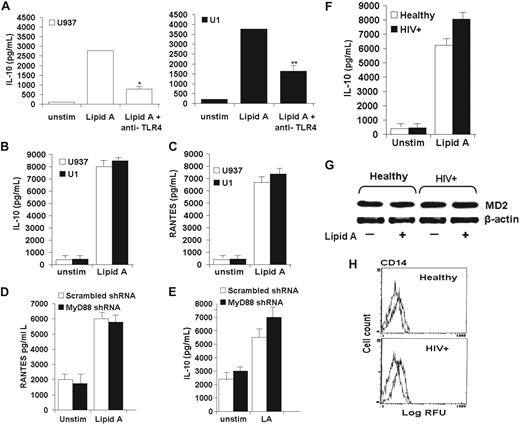
Next, to examine human macrophage TLR4-mediated MyD88-independent signaling pathway, studies focused on prototypic markers of the MyD88-independent signaling pathway, RANTES and IL-10.26-29 Similar to the pattern of TNFα release in human macrophages described above, macrophage IL-10 release was significantly reduced in the presence of anti–TLR4-neutralizing antibody in both human U937 and HIV+ U1 macrophages in response to the TLR4 ligand lipid A, suggesting that IL-10 release is mediated by TLR4 signaling pathway (Figure 2A). However, in contrast to TLR4-mediated TNFα release, release of IL-10 (Figure 2B) and RANTES (Figure 2C) was similar in magnitude in both U937 and HIV+ U1 human macrophages as measured by ELISA, suggesting that TLR4-mediated MyD88-independent pathway is preserved in HIV+ macrophages. Furthermore, targeted gene silencing of MyD88 did not influence IL-10 or RANTES release in response to lipid A (Figure 2D-E), suggesting that in contrast to TLR4-mediated TNFα release (which is MyD88-dependent as above), TLR4-mediated IL-10 and RANTES release is predominantly MyD88 independent in human macrophages. The pattern of IL-10 release was confirmed in clinically relevant human AMs, showing similar levels of lipid A–mediated IL-10 release comparing AMs from healthy persons to AMs from asymptomatic HIV+ persons (Figure 2F). The observed differences in TLR4-mediated TNFα and IL-10 release were not related to differences in macrophage expression of essential TLR4 modulator molecule MD230 (Figure 2G) or surface expression of TLR4 coreceptor CD14 (Figure 2H), consistent with our prior studies showing similar TLR4 surface expression comparing U937 and HIV+ U1 macrophages7,31 and comparing AMs from healthy and HIV+ persons.8 In contrast to impaired TLR4-mediated MyD88-dependent release of TNFα, collectively these data show that TLR4-mediated IL-10 and RANTES release in human macrophages is predominantly MyD88 independent and suggest that HIV may specifically target the MyD88-dependent TLR4 signaling pathway, while preserving the MyD88-independent TLR4 signaling pathway.
Preserved TLR4-mediated MyD88-independent pathway in HIV+ human macrophages. (A) Human macrophage IL-10 release is mediated by TLR4. U937 and U1 cells were incubated with lipid A (10 μg/mL) in the presence or absence of neutralizing anti-TLR4 antibody (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for IL-10 by ELISA. Data shown are mean ± SEM of 4 independent experiments done in triplicate. *P < .01 compared with U937 with lipid A alone; **P < .05 compared with U1 with lipid A alone. (B-C) Lipid A–mediated release of IL-10 and RANTES is preserved in HIV+ macrophages. U937 and U1 cells were incubated with or without lipid A (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for IL-10 (B) or RANTES (C) by ELISA. Data shown are mean ± SEM of 4 independent experiments done in triplicate. (D-E) Functional silencing of human MyD88 did not affect TLR4-mediated release of RANTES (D) and IL-10 (E). Cells were challenged with lipid A and incubated for 24 hours. Cell-free supernatant was assayed for RANTES and IL-10 by ELISA. Results are representative of 3 independent experiments done in triplicate. (F-H) Healthy and HIV+ AMs show similar levels of IL-10 release (F), similar levels of the TLR4 adaptor molecule MD2 (J), and surface expression of TLR4 coreceptor CD14 (H). (F) Healthy AMs and HIV+ AMs were incubated with or without lipid A (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for IL-10 by ELISA. Data shown are mean ± SEM from 4 independent experiments in triplicate (n = 4 subjects for each group). (G) Total cell lysates from healthy AMs and HIV+ AMs were probed with specific anti-MD2 antibody by Western blot, with β-actin probed for protein loading. (H) Healthy AMs and HIV+ AMs were incubated with PE-conjugated anti-CD14 antibody or isotype control antibody, and surface expression was determined by flow cytometry. Representative blot and profiles were similar of 3 independent experiments (n = 3 subjects for each group).
Preserved TLR4-mediated MyD88-independent pathway in HIV+ human macrophages. (A) Human macrophage IL-10 release is mediated by TLR4. U937 and U1 cells were incubated with lipid A (10 μg/mL) in the presence or absence of neutralizing anti-TLR4 antibody (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for IL-10 by ELISA. Data shown are mean ± SEM of 4 independent experiments done in triplicate. *P < .01 compared with U937 with lipid A alone; **P < .05 compared with U1 with lipid A alone. (B-C) Lipid A–mediated release of IL-10 and RANTES is preserved in HIV+ macrophages. U937 and U1 cells were incubated with or without lipid A (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for IL-10 (B) or RANTES (C) by ELISA. Data shown are mean ± SEM of 4 independent experiments done in triplicate. (D-E) Functional silencing of human MyD88 did not affect TLR4-mediated release of RANTES (D) and IL-10 (E). Cells were challenged with lipid A and incubated for 24 hours. Cell-free supernatant was assayed for RANTES and IL-10 by ELISA. Results are representative of 3 independent experiments done in triplicate. (F-H) Healthy and HIV+ AMs show similar levels of IL-10 release (F), similar levels of the TLR4 adaptor molecule MD2 (J), and surface expression of TLR4 coreceptor CD14 (H). (F) Healthy AMs and HIV+ AMs were incubated with or without lipid A (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for IL-10 by ELISA. Data shown are mean ± SEM from 4 independent experiments in triplicate (n = 4 subjects for each group). (G) Total cell lysates from healthy AMs and HIV+ AMs were probed with specific anti-MD2 antibody by Western blot, with β-actin probed for protein loading. (H) Healthy AMs and HIV+ AMs were incubated with PE-conjugated anti-CD14 antibody or isotype control antibody, and surface expression was determined by flow cytometry. Representative blot and profiles were similar of 3 independent experiments (n = 3 subjects for each group).
TLR4-mediated IRF3 nuclear translocation and STAT1 phosphorylation is preserved in HIV+ macrophages
To further examine the influence of HIV on TLR4-mediated MyD88-independent signaling pathway, studies focused on the activation of IRF3 and STAT1, important signaling molecules of the MyD88-independent pathway.26,27 Human U937 and HIV+ U1 macrophages were incubated with lipid A (10 μg/mL) up to 240 minutes, and then nuclear and cytoplasmic extracts were isolated and probed with specific anti-IRF3 antibody by Western blot. Experiments showed limited constitutive IRF3 nuclear translocation in unstimulated U937 macrophages, followed by nuclear translocation within 15 minutes in response to lipid A, peaking over 30 to 120 minutes followed by gradual decline by 240 minutes (Figure 3A). In comparison, HIV+ U1 macrophages showed elevated constitutive IRF3 nuclear translocation in unstimulated macrophages, with further increased levels of IRF3 nuclear translocation in response to lipid A that continued to increase up to 240 minutes (Figure 3B). Recognizing that TLR4-mediated MyD88-independent signaling through TRIF and IRF3 requires STAT1 transcription factors,32 we studied STAT1 phosphorylation in untreated or lipid A–treated U937 and HIV+ U1 cells. As shown in Figure 3C, time kinetics for STAT1 phosphorylation showed low constitutive STAT1 phosphorylation in unstimulated human U937 macrophages but increased by 15 minutes in response to lipid A. In comparison, HIV+ U1 macrophages showed elevated constitutive STAT1 phosphorylation, with further marked increased phosphorylation in response to lipid A (Figure 3C). Taken together, in contrast to impaired TLR4-mediated MyD88-dependent signaling, these data suggest a preserved TLR4-mediated MyD88-independent signaling pathway in HIV+ macrophages. In the context of HIV infection, divergent regulation of MyD88-dependent and MyD88-independent TLR4 signaling pathways could underlie the differential gene expression and host cell responses in macrophages.
TLR4-mediated IRF3 nuclear translocation and STAT1 phosphorylation is preserved in HIV+ macrophages. Lipid A–mediated nuclear translocation of IRF3 is intact comparing U937 human macrophages and HIV+ U1 cells. (A) U937 and (B) U1 macrophages were incubated with lipid A (10 μg/mL) over time, and then nuclear and cytoplasmic extract was isolated and probed with anti-IRF3 antibody by Western blot. Western blot is a representative of 4 independent experiments with similar results. (C) Preserved lipid A–mediated phosphorylation of STAT1 in HIV+ human macrophages. U937 and U1 macrophages were incubated with lipid A (10 μg/mL) over time, and detergent-soluble cell extracts were probed with anti–phosphorylated STAT1 antibody by Western blot. Membranes were stripped and probed with anti–β-actin for protein loading. Western blot is a representative experiment of 3 independent experiments with similar results. Quantitative densitometric analysis of IRF3 nuclear extract bands (A-B) and phosphorylated-STAT1 bands (C) are displayed directly beneath the blots. Error bars indicate SEM.
TLR4-mediated IRF3 nuclear translocation and STAT1 phosphorylation is preserved in HIV+ macrophages. Lipid A–mediated nuclear translocation of IRF3 is intact comparing U937 human macrophages and HIV+ U1 cells. (A) U937 and (B) U1 macrophages were incubated with lipid A (10 μg/mL) over time, and then nuclear and cytoplasmic extract was isolated and probed with anti-IRF3 antibody by Western blot. Western blot is a representative of 4 independent experiments with similar results. (C) Preserved lipid A–mediated phosphorylation of STAT1 in HIV+ human macrophages. U937 and U1 macrophages were incubated with lipid A (10 μg/mL) over time, and detergent-soluble cell extracts were probed with anti–phosphorylated STAT1 antibody by Western blot. Membranes were stripped and probed with anti–β-actin for protein loading. Western blot is a representative experiment of 3 independent experiments with similar results. Quantitative densitometric analysis of IRF3 nuclear extract bands (A-B) and phosphorylated-STAT1 bands (C) are displayed directly beneath the blots. Error bars indicate SEM.
Targeted gene silencing of MyD88-independent signaling pathway molecules TRIF or IRF3 in human macrophages reduces TLR4-mediated RANTES release (but not TNFα release)
TLR4-mediated MyD88-independent signal transduction is mediated through TRIF.12 To determine whether TRIF regulates TLR4-mediated signaling in human macrophages, release of RANTES and TNFα was examined in the presence and absence of targeted functional gene silencing of TRIF with the use of the RNAi method. In human U937 and HIV+ U1 macrophages, TRIF protein was constitutively expressed in the presence of nonsilencing RNAi, but TRIF protein levels are markedly reduced with TRIF-targeted gene silencing (Figure 4A). Furthermore, targeted gene silencing of TRIF was associated with reduction in lipid A–mediated RANTES release in both U937 macrophages (Figure 4B) and HIV+ U1 macrophages (Figure 4C). In contrast, lipid A–mediated TNFα release was not influenced by targeted gene silencing of TRIF, showing specificity of MyD88-independent pathway (Figure 4D).
Targeted gene silencing of MyD88-independent signaling pathway molecules TRIF or IRF3 in human macrophages reduces TLR4-mediated RANTES release (but not TNF release). RNAi-based targeted gene silencing of TRIF (A-D) or IRF3 (E-H) in human macrophages was performed as detailed in “Targeting gene silencing (RNAi) in macrophages.” (A) Western blot analysis of human U937 and HIV+ U1 macrophages after pretreatment with RNAi targeted to TRIF (TRIF siRNA) or nonsilencing RNAi (N.S. siRNA) and probed with anti-TRIF antibody. Anti–β-actin antibody was used to monitor protein loading. The blot is representative of 3 independent experiments with similar results. (B-D) Human macrophage U937 and U1 cells, pretreated with either TRIF siRNA or N.S. siRNA, were then incubated with or without lipid A (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for RANTES (B-C) or TNFα (D) by ELISA. Data shown are mean ± SEM of 3 independent experiments done in triplicate with similar results. (E) Western blot analysis of human U937 and U1 macrophages after pretreatment with RNAi targeted to IRF3 (IRF3 siRNA) or N.S. siRNA and probed with anti-IRF3 antibody. Anti–β-actin antibody was used to monitor protein loading. The blot is representative of 3 independent experiments with similar results. (F-H) U937 and U1 cells, pretreated with either IRF3 siRNA or N.S. siRNA, were then incubated with or without lipid A (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for RANTES (F-G) or TNF (H) by ELISA. Data shown are mean ± SEM of 3 independent experiments done in triplicate with similar results.
Targeted gene silencing of MyD88-independent signaling pathway molecules TRIF or IRF3 in human macrophages reduces TLR4-mediated RANTES release (but not TNF release). RNAi-based targeted gene silencing of TRIF (A-D) or IRF3 (E-H) in human macrophages was performed as detailed in “Targeting gene silencing (RNAi) in macrophages.” (A) Western blot analysis of human U937 and HIV+ U1 macrophages after pretreatment with RNAi targeted to TRIF (TRIF siRNA) or nonsilencing RNAi (N.S. siRNA) and probed with anti-TRIF antibody. Anti–β-actin antibody was used to monitor protein loading. The blot is representative of 3 independent experiments with similar results. (B-D) Human macrophage U937 and U1 cells, pretreated with either TRIF siRNA or N.S. siRNA, were then incubated with or without lipid A (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for RANTES (B-C) or TNFα (D) by ELISA. Data shown are mean ± SEM of 3 independent experiments done in triplicate with similar results. (E) Western blot analysis of human U937 and U1 macrophages after pretreatment with RNAi targeted to IRF3 (IRF3 siRNA) or N.S. siRNA and probed with anti-IRF3 antibody. Anti–β-actin antibody was used to monitor protein loading. The blot is representative of 3 independent experiments with similar results. (F-H) U937 and U1 cells, pretreated with either IRF3 siRNA or N.S. siRNA, were then incubated with or without lipid A (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for RANTES (F-G) or TNF (H) by ELISA. Data shown are mean ± SEM of 3 independent experiments done in triplicate with similar results.
To further investigate the MyD88-independent TLR4-mediated macrophage release of RANTES and TNFα, experiments were performed in the presence or absence of targeted gene silencing of IRF3, transcription factor downstream of TRIF signaling. In human U937 and HIV+ U1 macrophages, IRF3 protein was constitutively expressed in the presence of nonsilencing RNAi, but IRF3 protein levels are markedly reduced after IRF3-targeted gene silencing (Figure 4E). Furthermore, targeted gene silencing of IRF3 was associated with reduction in lipid A–mediated RANTES release in both U937 macrophages (Figure 4F) and HIV+ U1 macrophages (Figure 4G). In contrast, lipid A–mediated TNFα release was not influenced by targeted gene silencing of IRF3 (Figure 4H), suggesting that TLR4-mediated RANTES and IL-10 release by human macrophages is predominantly MyD88 independent. Taken together, these data suggest that TLR4-mediated release of RANTES and IL-10 in human macrophages is mediated through a MyD88-independent process involving TRIF and IRF3, whereas TLR4-mediated TNFα release in human macrophages is mediated through a MyD88-dependent process and does not involve TRIF and IRF3 signaling molecules.
TLR4-mediated macrophage release of IL-10 and RANTES (but not TNFα) and IRF3 phosphorylation (but not ERK1/2 phosphorylation) depend on TLR4 endocytosis
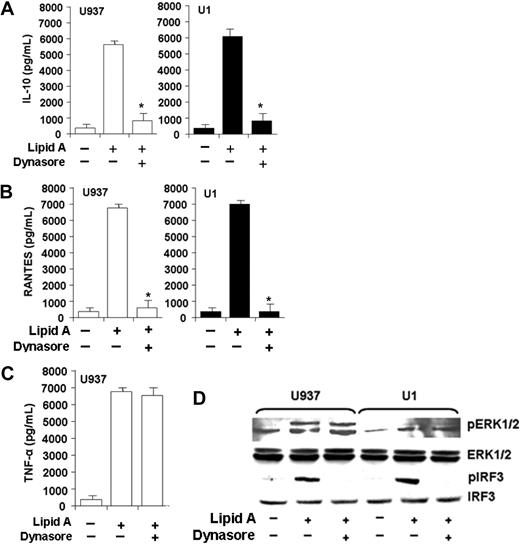
TLR4-mediated MyD88-independent signaling requires endocytosis, whereas TLR4-mediated MyD88-dependent signaling does not require endocytosis.11 We investigated the role of endocytosis in TLR4-mediated MyD88-dependent and MyD88-independent signaling pathways in human macrophages, measuring macrophage IL-10, RANTES, and TNFα release in response to lipid A in the presence and absence of an inhibitor of guanosine triphosphatase (GTPase) dynamin (dynasore). Inhibition of endocytosis (with dynasore) was shown to inhibit TLR4-mediated MyD88-independent dynamin-mediated endocytosis.11 As a measure of TLR4-mediated MyD88-independent signaling, incubation of human U937 or HIV+ U1 macrophages with lipid A in the presence of dynasore markedly reduced macrophage IL-10 release (Figure 5A) and RANTES release (Figure 5B). In contrast, as a measure of TLR4-mediated MyD88-dependent signaling, lipid A–mediated TNFα release was not influenced by dynasore (Figure 5C). Furthermore, pretreatment of human U937 or HIV+ U1 macrophages with dynasore impaired lipid A–mediated IRF3 phosphorylation (MyD88-independent signaling molecule) but did not influence ERK1/2 phosphorylation (MyD88-dependent signaling molecule; Figure 5D). These data show that, in human macrophages, TLR4-mediated MyD88-independent signaling requires endocytosis (in contrast to TLR4-mediated MyD88-dependent signaling) and is not influenced by HIV infection.
TLR4-mediated macrophage release of IL-10 and RANTES (but not TNFα) and IRF3 phosphorylation (but not ERK phosphorylation) depend on TLR4 endocytosis. MyD88-independent TLR4-mediated IL-10 and RANTES release requires endocytosis. (A-C) U937 and U1 macrophages were pretreated with a highly specific inhibitor of the endocytosis regulator dynamin GTPase (dynasore, 50μM) for 1 hour and then incubated in the presence or absence of lipid A (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for IL-10 (A), RANTES (B), or TNFα (C) by ELISA. Data reflect representative experiments (performed in triplicate) of 3 independent experiments with similar results. *P < .01 compared with lipid A alone. (A-C) Error bars indicate SEM. (D) MyD88-independent TLR4-mediated IRF3 phosphorylation requires endocytosis. U937 and U1 macrophages were pretreated with a highly specific dynamin GTPase inhibitor (dynasore, 50μM) for 1 hour and then incubated in the presence or absence of lipid A (10 μg/mL) for 15 minutes, and cell lysates were probed with specific antibodies to phosphorylated ERK1/2 and phosphorylated IRF3 antibody. Protein loading controls used antibody to total ERK1/2 and total IRF3 protein. Representative Western blot from 1 of 3 independent experiments are shown with similar results.
TLR4-mediated macrophage release of IL-10 and RANTES (but not TNFα) and IRF3 phosphorylation (but not ERK phosphorylation) depend on TLR4 endocytosis. MyD88-independent TLR4-mediated IL-10 and RANTES release requires endocytosis. (A-C) U937 and U1 macrophages were pretreated with a highly specific inhibitor of the endocytosis regulator dynamin GTPase (dynasore, 50μM) for 1 hour and then incubated in the presence or absence of lipid A (10 μg/mL) for 24 hours, and cell-free supernatants were analyzed for IL-10 (A), RANTES (B), or TNFα (C) by ELISA. Data reflect representative experiments (performed in triplicate) of 3 independent experiments with similar results. *P < .01 compared with lipid A alone. (A-C) Error bars indicate SEM. (D) MyD88-independent TLR4-mediated IRF3 phosphorylation requires endocytosis. U937 and U1 macrophages were pretreated with a highly specific dynamin GTPase inhibitor (dynasore, 50μM) for 1 hour and then incubated in the presence or absence of lipid A (10 μg/mL) for 15 minutes, and cell lysates were probed with specific antibodies to phosphorylated ERK1/2 and phosphorylated IRF3 antibody. Protein loading controls used antibody to total ERK1/2 and total IRF3 protein. Representative Western blot from 1 of 3 independent experiments are shown with similar results.
Discussion
This study shows that HIV infection is associated with impaired human macrophage TLR4-mediated signaling, specifically targeting the MyD88-dependent TLR4-mediated signaling pathway while preserving the function of the MyD88-independent TLR4-mediated signaling pathway. The impaired function of the MyD88-dependent pathway may in part contribute to reduce TNFα release in AMs from HIV+ persons in response to lipid A. Compared with human U937 macrophages, MyD88-dependent TLR4-mediated signaling in HIV+ U1 human macrophages showed impaired MyD88-IRAK protein interaction, reduced IRAK phosphorylation, reduced NF-κB nuclear translocation, and reduced TNFα release in response to the TLR4 ligand stimulation. In marked contrast, MyD88-independent TLR4-mediated signaling in HIV+ U1 human macrophages showed preserved STAT1 phosphorylation, preserved IRF3 nuclear translocation, and preserved IL-10 and RANTES release in response to TLR4 agonist stimulation compared with human U937 macrophages. Similar patterns of response were observed in select experiments with the use of clinically relevant human AMs from healthy and asymptomatic HIV+ persons. Taken together, these data support the concept that chronic HIV infection is associated with specific and targeted disruption of critical macrophage innate immune TLR4 signaling, which in turn may contribute to susceptibility and disease pathogenesis of bacterial pneumonia.
The finding in the current study that TLR4-mediated impairment of TNFα release was significantly reduced in HIV+ macrophages is consistent with our prior observations7,8,20 and observations by other investigators.31,33 TLR4 is unique among the TLR family, supporting both MyD88-dependent and MyD88-independent signaling pathways,11 whereas TLR3 exhibits MyD88-independent signaling, and all other TLRs exhibit MyD88-dependent signaling.34 Activation of the MyD88-dependent signaling pathway promotes proinflammatory cytokine release such as TNFα and IL-12,35 whereas activation of the MyD88-indepenent pathway promotes release of IFN-inducible genes such as type-1 IFN, IL-10, and RANTES.21 In the current study, demonstration that macrophage release of IL-10 and RANTES was significantly reduced after targeted gene silencing of macrophage IRF3 and TRIF (signaling components of MyD88-independent TLR4 signaling) and demonstration of intact IRF3 nuclear translocation and STAT1 phosphorylation suggest that the MyD88-independent signaling pathway represents that principal mechanism for IL-10 and RANTES release in HIV+ macrophages. In addition, the finding in the current study that MyD88-independent TLR4-mediated signaling requires endocytosis, whereas MyD88-dependent TLR4-mediated signaling does not require endocytosis, is consistent with data from other investigators11 and extends the concept of distinct molecular mechanisms for TLR4-mediated signaling to human macrophages.
The current study provides an additional mechanism for the observed reduction in TLR4-mediated TNFα release in HIV+ macrophages.7,8,20 Our prior work has shown that the observed reduction in macrophage TLR4-mediated TNFα release in HIV+ macrophages may in part be attributed to activation of macrophage cellular phosphatase MKP-1,7 and may in part be attributed to constitutive activation of the PI3K pathway.8 The current study extends the mechanisms to include the impaired TLR4-mediated MyD88-dependent signaling pathway, suggesting that chronic HIV infection may influence macrophage innate immune signaling pathways at several molecular levels. Importantly, our prior observations that pharmacologic agents can partially restore macrophage TLR4-mediated immune functions in AMs from HIV+ persons7 suggest the potential for immunomodulation of macrophage innate immune function to restore host cell response to bacterial challenge.
Recognizing that HIV infection of macrophages is generally nonproductive,36 the preserved or elevated levels of IRF3 and pSTAT1 in HIV+ macrophages may in part represent an antiviral mechanism to limit or control HIV replication in macrophages. IRF3 signaling in general is MyD88 independent and may involve TRAF3 self-ubiquitination.37 A consequence of preserved or elevated IRF3 signaling to limit viral replication may be impaired MyD88-dependent signaling, although it was not specifically established in the current study and represents an area of active investigation.
The mechanism for the select impairment of MyD88-dependent TLR4 signaling (while preserving MyD88-independent signaling) was not established in the current study. In the current study, the observed differences in TLR4-mediated signaling comparing healthy and HIV+ human macrophages was not related to differences in macrophage expression of the TLR4-signaling complex, because MD2 levels and surface expression of CD14 were similar. Our prior publications have shown similar levels of macrophage TLR4 surface expression and TLR4 mRNA,7,8 confirmed by other independent investigators.31 Taken together, these data suggest that the TLR4-MD2-CD14 signaling complex is intact in both AMs from healthy persons and AMS from asymptomatic HIV+ persons and suggest that impairment of signaling in HIV+ macrophages represents events downstream of the TLR4 receptor complex. Our prior investigations have shown that HIV nef protein is sufficient to induce macrophage MKP-1,7 activate PI3K,8 and impair TLR4-mediated TNFα release8 and suggest that HIV nef protein may selectively influence MyD88-dependent TLR4-mediated signaling, although this mechanism was not specifically investigated in the current study. Our prior observation that HIV nef was sufficient to impair TLR4-mediated TNFα release while simultaneously promoting IL-10 release8 is consistent with findings in the current study, suggesting targeted effects of MyD88-dependent TLR4-mediated signaling.
Other limitations of the current study include whether the findings are specific to TLR4 or whether MyD88-dependent signaling was affected in other macrophage TLRs was not investigated. In addition, whether these findings apply in general to the other family members of the Toll/interleukin-1 receptor–containing domains38 was not established. Finally, the in vitro observations may not accurately reflect in vivo function, but the use of clinically relevant human AMs may allow for more direct translation of observed results to human disease.
This is the first study to report that the MyD88-dependent signaling pathway is specifically targeted by HIV that resulted in diminished TLR4-mediated TNFα release in response to lipid A. Importantly, the TLR4-mediated MyD88-independent pathway is preserved in HIV+ human macrophages leading to robust IL-10 and RANTES release in response to lipid A. Furthermore, it has been recently shown that HIV induces down-regulation of IRAK-4 and inhibits TNFα in response to TLR4 activation and supports the present study.24 Taken together, these observations support the concept that HIV infection is associated with targeted and specific impairment of macrophage innate immune function rather than a global impairment of macrophage function.
In conclusion, this study shows that the impaired TLR4-mediated macrophage response shown in HIV-infected human macrophages is targeted to the MyD88-dependent signaling pathway, whereas the TLR4-mediated MyD88-independent signaling pathway is preserved. Recognizing that the MyD88-dependent signaling pathway is critical to the acute inflammatory response that promotes clearance of various pathogens,39-42 impaired MyD88-dependent TLR4-mediated response in AMs from HIV+ persons may contribute to bacterial pneumonia pathogenesis. In general, this study supports the concept that chronic HIV infection is associated with altered macrophage innate immune function. Moreover, the influence of chronic HIV infection on macrophage innate immune function is targeted and specific, with evidence of impaired innate immune pathways (eg, MyD88-dependent TLR4 signaling) while other innate immune pathways remain preserved (eg, MyD88-independent signaling). Identifying specific abnormalities in lung macrophages from HIV+ persons may provide potential novel therapeutic targets aimed to restore or rescue innate immune function against potential pathogens and to augment current antimicrobial therapy in HIV+ persons with pneumonia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all study participants who consented to research bronchoscopy. We also thank Robert Garland, Lorraine Gryniuk, Renee Andwood, and Michael McBride for technical support.
This work was supported by the National Institutes of Health (grant NIH R01-HL092811, S.D.T.; grant R01-HL063655, H.K.; and grant K08-AI064014, N.R.P.).
National Institutes of Health
Authorship
Contribution: S.D.T. designed and performed research, analyzed data, and wrote the paper; X.L. and M.B. performed research; A.A. and N.R.P. analyzed data; K.S. contributed reagents; and H.K. designed research, analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Souvenir D. Tachado, Pulmonary, Critical Care and Sleep Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Dana Bldg, Rm 652, Boston, MA 02215; e-mail: stachado@bidmc.harvard.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal