Abstract
Iron maldistribution has been implicated in multiple diseases, including the anemia of inflammation (AI), atherosclerosis, diabetes, and neurodegenerative disorders. Iron metabolism is controlled by hepcidin, a 25-amino acid peptide. Hepcidin is induced by inflammation, causes iron to be sequestered, and thus, potentially contributes to AI. Human hepcidin (hHepc) overexpression in mice caused an iron-deficient phenotype, including stunted growth, hair loss, and iron-deficient erythropoiesis. It also caused resistance to supraphysiologic levels of erythropoiesis-stimulating agent, supporting the hypothesis that hepcidin may influence response to treatment in AI. To explore the role of hepcidin in inflammatory anemia, a mouse AI model was developed with heat-killed Brucella abortus treatment. Suppression of hepcidin mRNA was a successful anemia treatment in this model. High-affinity antibodies specific for hHepc were generated, and hHepc knock-in mice were produced to enable antibody testing. Antibody treatment neutralized hHepc in vitro and in vivo and facilitated anemia treatment in hHepc knock-in mice with AI. These data indicate that antihepcidin antibodies may be an effective treatment for patients with inflammatory anemia. The ability to manipulate iron metabolism in vivo may also allow investigation of the role of iron in a number of other pathologic conditions.
Introduction
Precise control of iron absorption, storage, and transport are required to prevent iron deficiency while avoiding oxidative damage caused by free iron. Loss of this control has been implicated in an array of diseases, including anemia of inflammation (AI),1 atherosclerosis,2 diabetes,3 and multiple neurodegenerative disorders such as Alzheimer disease, Parkinson disease, and Friedreich ataxia.4 Genetic and clinical observational studies5,6 have highlighted that aberrant iron localization in cells or tissues may be more important than overall body iron content in iron-related disease. Despite this, therapeutic control of iron metabolism has only involved manipulation of total body iron levels either by iron administration, chelation therapy, or phlebotomy. Targeting control of iron distribution in the body may therefore represent an attractive therapeutic approach to treat disease.
Hepcidin, a 25-amino acid peptide expressed mainly in the liver, is the central mediator of iron homeostasis.7,8 Hepcidin stimulates internalization and degradation of the iron-export protein ferroportin to control dietary iron absorption, iron release from storage sites, and ultimately iron bioavailability in the body.9 Inactivating mutations in human hepcidin (hHepc) result in juvenile-onset hemochromatosis, a severe form of iron overload.10 Conversely, in patients with hepatic adenomas overproducing hepcidin, a severe iron-deficiency anemia was observed that was corrected upon surgical resection of the tumor.11 In agreement with the human mutations, disruption of mouse hepcidin 1 (mHepc1: the ortholog of the hHepc gene HAMP) resulted in iron loading,12,13 and transgenic hepcidin overexpression resulted in iron deficiency.14,15
In addition to its role in iron homeostasis, hepcidin also is recognized as an acute-phase reactant induced by inflammation in mice and humans.9,16-18 Inflammatory hepcidin induction is accompanied by impaired dietary iron uptake, iron sequestration, and anemia,18 implicating hepcidin as a controlling factor in AI. AI (also sometimes referred to as the anemia of chronic disease) has been reported in numerous patient populations19-24 and is characterized by a relative resistance to erythropoiesis-stimulating agent (ESA; epoetin alfa or darbepoetin alfa) therapy.25-30 In the current work, neutralization of hepcidin in vitro and in vivo illustrated that an antihepcidin therapy substantially modulated iron transport and effectively treated anemia in a mouse model of AI.
Methods
Hepcidin and antihepcidin antibody generation
hHepc was either produced synthetically and refolded31 (shHepc) or expressed recombinantly in Escherichia coli as a propeptide, refolded, and cleaved to produce mature hepcidin (recombinant hHepc [rhHepc]; described previously as ehHepc32 ). rhHepc was conjugated to keyhole limpet hemocyanin (KLH) by the use of standard 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride chemistry (EDC; Thermo Fisher Scientific). The conjugate was emulsified in a 1:1 ratio with Complete Freund Adjuvant (Thermo Fisher Scientific) or RIBI (Sigma-Aldrich) and PBS (Gibco Invitrogen). C57BL/6 mice were immunized subcutaneously with the equivalent of 50 μg of rhHepc starting material in Complete Freund Adjuvant. Fourteen days later, a second immunization of 25 μg of rhHepc-KLH in RIBI adjuvant was delivered subcutaneously and intraperitoneally. At 10 days after this immunization, anti-hHepc serum levels were determined, and candidate mice were selected by screening for the ability to bind to biotinylated rhHepc immobilized on Neutravidin-coated plates. In brief, microtiter plates were coated with Neutravidin (Thermo Fisher Scientific) at 100 ng/mL, blocked with 1% bovine serum albumin, 1% goat serum, 0.5% Tween 20 solution in PBS, then used to capture 1 ng of biotinylated hepcidin per well (rhHepc conjugated to biotin with the use of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride chemistry as described previously for KLH conjugation). After mouse serum incubation and washing, a polyclonal goat antimurine immunoglobulin G (IgG) Fc horseradish peroxidase (HRP)–labeled secondary antibody (Thermo Fisher Scientific) was used to detect the murine IgG-hHepc complexes. TMB (Thermo Fisher Scientific) was used to visualize the complexes. Approximately 2 weeks after the test bleeds, mice were boosted intraperitoneally with 75 μg of rhHepc suspended in PBS. Five days later, spleen cells were used to generate hybridomas with SP2/0.AG14 myeloma cells. Antibodies were purified from hybridoma supernatant by the use of standard techniques or cloned and expressed transiently in 293-6E cells. Ab2.7, a mouse IgG1 antibody that binds to rhHepc with 110-pM affinity (Biacore analysis: data not shown), was used for all studies shown.
Western analysis
Reduced or nonreduced samples were loaded onto a NuPage 4% to 12% Bis-Tris gel, separated in Invitrogen MES SDS running buffer with or without 50mM dithiothreitol for reduced and nonreduced gels, respectively (Invitrogen), blotted onto a nitrocellulose membrane, stained with Ponceau S solution (Sigma-Aldrich) to visualize protein loading, and blocked with Tris-buffered saline, 0.1% Tween 20 (TBST), 5% powdered milk. Blots were probed with 1 μg/mL antihepcidin Ab2.7 in TBST 2.5% powdered milk. After washing, primary antibody treatment was followed by 0.25 μg/mL horse anti–mouse HRP–conjugated secondary antibody (Cell Signaling Technology) in TBST, 1.25% powdered milk. All washes were with TBST. The HRP conjugate was detected with the use of Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific).
Knockin mouse development
Targeting vectors were designed to remove either mHepc1 (designated Hep1 mice) or mHepc1 and 2 (designated Hep2 mice). In either case, the vector contained a 6.6-kb long homology arm, a 2.2-kb short homology arm, a 16-kb hHepc genomic coding sequence flanked by 2 loxP sites (floxed), and drug selection cassettes for selection in embryonic stem cells by homologous recombination. The floxed Neo cassette was removed after electroporation of a cre recombinase plasmid and confirmed by Southern analysis. Targeted 129 embryonic stem cell lines were microinjected into C57BL/6 blastocyst embryos to generate chimeric male mice, which were bred with Black Swiss females (Taconic) to obtain germline transmission. F1 heterozygous mice were interbred to generate homozygous animals expressing the hHepc gene. Wild-type littermates obtained from heterozygote breeding were used to generate control animals for each strain knock-in strain. Genotyping was conducted by Southern analysis and confirmed by detection of mouse or hHepc in serum. All experimental findings were confirmed by the use of both knock-in strains and their relevant controls.
Adeno-associated virus-mediated hepcidin overexpression
For initial long-term expression studies, adeno-associated virus serotype 8 (AAV8) capsid serotype virus expressing human or mouse hepcidin was administered to 4-week-old C57BL/6 male mice via the hepatic portal vein with empty vector administration as the control. Subsequent studies in which we examined ESA response or serum iron levels were carried out by the use of AAV5 capsid serotype introduced by tail vein injection. In these studies, control groups comprised mice treated with virus overexpressing either green fluorescent protein (GFP) or β-galactosidase as nonrelevant protein expression controls. After approximately 2 weeks, mice demonstrated stable levels of hepcidin protein in the serum. To detect response to ESA and antibody treatment, mice were treated with 100 μg/kg darbepoetin alfa subcutaneously 2 weeks or more after virus treatment with or without concurrent subcutaneous antibody treatment. Hemoglobin (Hb) was measured 6 to 7 days after ESA treatment. Control antibodies were isotype-matched IgGs raised against KLH.
Brucella abortus AI model
Heat-killed Brucella abortus (BA; strain 1119-3; US Department of Agriculture, Animal and Plant Health Inspection Service, National Veterinary Services Laboratories) was ultracentrifuged at 17 700g for 15 minutes and resuspended in PBS at 5 × 109 particles/mL, then stored in aliquots at −70°C. Optimal particle dose (intraperitoneally in 200 μL of PBS) was determined for each mouse strain by investigating the dose required to blunt ESA response (as in Figure 2D) in the majority of animals but not cause serious illness. At this dose 10% to 20% of animals did not show signs of inflammation or develop anemia. BA administration 1 to 8 days before ESA treatment was shown to result in a blunted Hb response 6 to 7 days later. For experiments characterizing BA and shRNA-mediated hepcidin neutralization (described in next section), C57BL/6 mice (Charles River Laboratories) were injected with BA (5 × 108 particles/mouse) and 1 day later treated with ESA (100 μg/kg subcutaneous darbepoetin alfa). Blood was then analyzed 7 days later to assess Hb response and serum iron levels. For experiments characterizing antibody treatment response, Hep1 or Hep2 mice were injected with BA (3 × 108 particles/mouse) and Hb was measured 6 to 7 days later to determine which mice had developed anemia.
Animals with a Hb value greater than the 95% confidence interval of the mean for all BA-treated animals were excluded from the study, resulting in fewer than 5 mice in some groups. This exclusion process was carried out to lessen the possibility of false-positive results produced by including animals that did not have sufficient inflammation (measured by insufficient anemia) to blunt ESA response. After the exclusion process, ESA was administered on day 8 relative to BA treatment, with or without antibody treatment as specified in figure legends. Response to ESA and antibody therapy was measured 6 to 7 days later.
AAV-mediated antihepcidin shRNA treatment
Multiple siRNA constructs were tested for the ability to knock down hepcidin in vitro. siRNA6 (CUACAGAGCUGCAGCCUUUdTdT) and siRNA10 (ACAGAUGAGACAGACUACAdTdT) showed 95% and 96% knockdown, respectively, and retained the ability to knock down hepcidin when converted to shRNAs (H6 sequence sense loop antisense are underlined, respectively: ACCG CTACAGAGCTGCAGCCTTT TTCATGAGA AAAGGCTGCAGCTCTGTAG CTTTTT. H10 sequence sense loop antisense: ACCG ACAGATGAGACAGACTACA TTCATGAGA TGTAGTCTGTCTCATCTGT CTTTTT). Luciferase control siRNA (Luc: ACGUACGCGGAAUACUUCGdTdT) showed no knockdown of hepcidin (shRNA sequence: ACCG ACGTACGCGGAATACTTCG TTCATGAGA CGAAGTATTCCGCGTACGT CTTTTT). AAV8 capsid serotype virus expressing antihepcidin shRNA constructs or antiluciferase-negative control shRNA was administered to 4-week-old C57BL/6 male mice via the hepatic portal vein (n = 5 per group). BA was administered 18 days after shRNA treatment because optimization experiments indicated that this timing allowed adequate stabilization of shRNA expression but minimized toxicities caused by extreme iron loading over time. Complications from hepatic portal vein delivery caused death in some animals, resulting in fewer than 5 animals in some treatment groups.
mRNA measurement
mRNA levels were determined by bDNA (branched DNA assay; QuantiGene Panomics Inc). Because of inflammatory modulation of all housekeeping genes tested in mouse studies (cyclophilin, glyceraldehyde 3-phosphate dehydrogenase, and actin), normalization against a housekeeping gene was not used but instead absolute hepcidin values/10 μg RNA were reported.
Cellular hepcidin response assay
Hepcidin activity was measured with an intracellular iron retention assay. This assay was performed on the basis of an established hepcidin-responsive assay9 but with the addition of a β-lactamase reporter gene controlled by the ferritin 5′IRE.33 Cells were plated at 2.5 × 105 cells/well and treated with 10 μg/mL doxycycline overnight to induce ferroportin expression. The following day, cells were treated with 16.3 μg/mL ferric citrate and increasing amounts of hepcidin with or without antibody added, then incubated overnight. β-lactamase activity was detected with the use of GeneBlazer CCF4 A/M development reagent (Invitrogen). Blue/green signal was read at 447 nm/520 nm (intracellular-iron driven β-lactamase activity/cell viability dye). The resulting assay yielded a highly sensitive measure of intracellular iron in live cells with the ability to normalize against cell viability.
Blood and serum analyte measurement
hHepc levels were determined by solid-phase extraction followed by tandem liquid chromatography-mass spectrometry detection.31 The lower limit of quantitation (LLOQ) in the present study was 10 ng/mL. mHepc1 levels also were determined by tandem liquid chromatography-mass spectrometry relative to an internal hHepc standard denoted as IS (Figure 3B) or in a fully quantitative mouse hepcidin assay for all other figures (LLOQ 25 ng/mL). Values represent a total of both full-length mature hepcidin (hepc25) and hepc24 caused by partial mHepc1 degradation in serum. Values less than LLOQ for mHepc1 or hHepc were represented as 0. Serum iron was measured with an Olympus AU400 clinical chemistry analyzer (Olympus Diagnostics). Cytokine levels were determined with a Biosource Multiplex assay (Biosource). Blood cell parameters were determined with a Bayer Advia 2120 hematology analyzer (Bayer Instruments).
Statistics
Statistics were generated with GraphPad Prism software Version 4.0 (GraphPad Software). All results were expressed as the mean plus or minus SE. Statistics shown in the figures represent analysis of variance with Bonferroni post-hoc test (used to assess the significance all groups against each other) with *P < .05, **P < .01, and ***P < .001 and NS denoting no statistical significance. All statistically significant differences between groups were shown unless otherwise stated in the figure legends. Comparisons between experimental groups included in the body of the text were conducted with the use of Student t tests, with statistical significance indicated by P value.
Results
Hepcidin overexpression induced iron-limited erythropoiesis
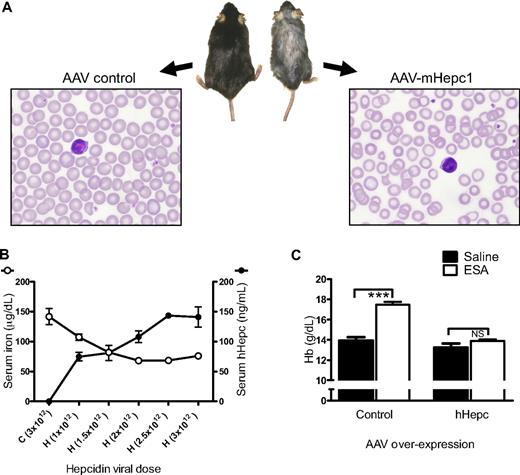
To determine the long-term consequences of elevated hepcidin expression, AAV was used to overexpress hepcidin constitutively in mice. Anemia, as defined by a decrease in Hb of 1 g/dL or more, was observed approximately 1 month after transduction with AAV expressing mouse hepcidin 1 (AAV-mHepc1). After expression of mHepc1 for approximately 4 months, mice displayed hypoferremia (serum iron concentrations of 79 ± 16 μg/dL in mHepc1-expressing mice compared with 200 ± 25 μg/dL in empty vector-treated control animals; P < .01). This finding was consistent with previous preclinical findings that used transgenic or tumor-driven expression of hepcidin in mice14,15,34 and clinical observations of hepcidin overexpression from human hepatic adenomas.11 Also in common with previous findings, mice were anemic (Hb values of 10.7 ± 0.8 g/dL compared with 13.1 ± 0.5 g/dL for the control mice; P < .05). A representative animal and blood smear from each group is shown in Figure 1A. Mice overexpressing mHepc1 were smaller and had substantial hair loss compared with control mice, consistent with the known effects of iron deficiency. Blood smears exhibited signs of iron-limited erythropoiesis (microcytosis and hypochromicity).
Hepcidin overexpression caused iron-limited erythropoiesis. (A) Photograph of gross appearance and Wright-Giemsa–stained peripheral blood smear of representative C57BL/6 mice 4 months after viral transduction (AAV8) with AAV-mHepc1 (1.3 × 1013 particles/mouse) or AAV control (empty-vector: 8 × 1012 particles/mouse). Overexpression of mHepc1 resulted in runted growth, hair loss, and microcytic, hypochromic anemia. Gross appearance of mice was captured using a standard digital camera and brightness adjusted using Microsoft PowerPoint. Peripheral blood smears were visualized using a Nikon ECLIPSE E600 microscope with a Nikon Plan Fluor 100×/1.30 NA oil objective. Images were captured using a Nikon Digital Camera DXM 1200f (Nikon) and processed using Nikon's ACT-1 Version 2.62 software. (B) AAV-hHepc expression in mice (AAV5; n = 4/group) caused an increase in serum levels of hHepc and a dose-dependent decrease in serum iron 2 weeks after viral transduction. H indicates AAV-hHepc; C, AAV-control (AAV-GFP). Infectious units administered via tail vein injection are shown in parentheses. (C) Control animals (AAV-GFP) responded to suprapharmacologic ESA treatment, whereas animals overexpressing hHepc did not show a significant Hb response (ESA administered 4 weeks after virus treatment as in panel B and Hb measured 1 week later; n = 4-5/group). Horizontal bars indicate groups compared for statistical analysis: ***P < .001; NS, no significance. Results in all figures are shown as mean ± SEM.
Hepcidin overexpression caused iron-limited erythropoiesis. (A) Photograph of gross appearance and Wright-Giemsa–stained peripheral blood smear of representative C57BL/6 mice 4 months after viral transduction (AAV8) with AAV-mHepc1 (1.3 × 1013 particles/mouse) or AAV control (empty-vector: 8 × 1012 particles/mouse). Overexpression of mHepc1 resulted in runted growth, hair loss, and microcytic, hypochromic anemia. Gross appearance of mice was captured using a standard digital camera and brightness adjusted using Microsoft PowerPoint. Peripheral blood smears were visualized using a Nikon ECLIPSE E600 microscope with a Nikon Plan Fluor 100×/1.30 NA oil objective. Images were captured using a Nikon Digital Camera DXM 1200f (Nikon) and processed using Nikon's ACT-1 Version 2.62 software. (B) AAV-hHepc expression in mice (AAV5; n = 4/group) caused an increase in serum levels of hHepc and a dose-dependent decrease in serum iron 2 weeks after viral transduction. H indicates AAV-hHepc; C, AAV-control (AAV-GFP). Infectious units administered via tail vein injection are shown in parentheses. (C) Control animals (AAV-GFP) responded to suprapharmacologic ESA treatment, whereas animals overexpressing hHepc did not show a significant Hb response (ESA administered 4 weeks after virus treatment as in panel B and Hb measured 1 week later; n = 4-5/group). Horizontal bars indicate groups compared for statistical analysis: ***P < .001; NS, no significance. Results in all figures are shown as mean ± SEM.
Results with hHepc overexpression were consistent with those observed after mHepc1 overexpression. hHepc overexpression caused hypoferremia in a dose-dependent manner evident at 2 weeks after transduction (Figure 1B) and development of anemia over time (data not shown). For all subsequent hepcidin overexpression experiments, each new viral preparation was titrated and viral particle dose selected to generate a serum hHepc concentration of 30 to 100 ng/mL. This concentration was sufficient to mediate all the described effects.
One of the clinical features of AI is a relative resistance to administered ESAs. To determine whether resistance to ESA treatment was a consequence of elevated hepcidin levels, mice overexpressing hHepc were treated with large doses of ESA (Figure 1C). Normal mice treated with 10 to 1000 μg/kg darbepoetin alfa show a Hb increase of approximately 3 g/dL in 7 days.35 Similarly, animals overexpressing control protein showed an appropriate response to supramaximal ESA treatment (100 μg/kg darbepoetin alfa), demonstrating both that an appropriate erythropoietic response was possible and that sufficient iron stores existed in these mice. In contrast, animals expressing hHepc did not show a significant Hb response to the same supramaximal ESA dose. This agreed with previous findings that mice overexpressing hepcidin were not able to respond to increased levels of endogenous erythropoietin15 and also demonstrated that the hepcidin effect was able to blunt the response to even suprapharmacologic doses of ESA. Hepcidin-induced erythropoietic suppression, as evidenced by a substantially blunted ESA response, was apparent 2 weeks after viral transduction and before the onset of anemia. Similar ESA hyporesponse was observed as a consequence of mHepc1 expression but not mHepc2 (data not shown), confirming previous observations that mHepc2 did not appear to regulate iron metabolism.36 Thus, these experiments demonstrated that hepcidin overexpression caused iron-limited erythropoiesis with associated systemic features of iron deficiency and substantially impaired ESA response.
Development of a novel mouse AI model
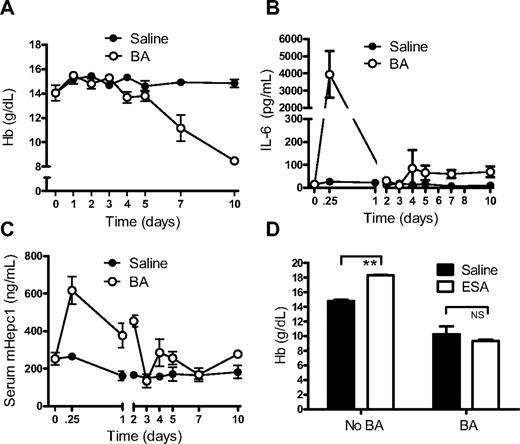
To examine the contribution of hepcidin to inflammatory anemia, a mouse model of AI was required. Heat-inactivated BA, which had previously been shown to induce chronic fatigue in mice,37 was investigated as a potential mouse model of AI. C57BL/6 mice treated with a single dose of BA developed anemia within 1 week (Figure 2A). The anemia reached a nadir by 10 to 14 days, after which Hb gradually increased (termed the recovery phase) and returned to normal after 3 to 5 weeks (data not shown). Interleukin-6 and hepcidin were both elevated within 6 hours of BA exposure (Figure 2B-C). Serum erythropoietin levels remained unchanged initially, despite the development of anemia. A modest elevation in serum erythropoietin was observed by day 7 (eg, an increase from 53 ± 11 pg/mL to 112 ± 16 pg/mL; P < .05).
BA treatment caused an inflammatory anemia. (A) Administration of BA caused rapid development of anemia (n = 5/group/time point). (B) Interleukin-6 (IL-6) levels were elevated within 6 hours of BA treatment (n = 5/group/time point). (C) Serum hepcidin levels in mice from panel B were elevated by BA treatment. (D) BA treatment blunted response to ESA treatment. ESA (or saline control) was administered 1 day after BA (or saline control) and Hb response measured 1 week later. Control mice showed a significant increase in Hb, whereas BA-treated mice did not (n = 5/group). Horizontal bars indicate groups compared for statistical analysis. **P < .01; NS, no significance.
BA treatment caused an inflammatory anemia. (A) Administration of BA caused rapid development of anemia (n = 5/group/time point). (B) Interleukin-6 (IL-6) levels were elevated within 6 hours of BA treatment (n = 5/group/time point). (C) Serum hepcidin levels in mice from panel B were elevated by BA treatment. (D) BA treatment blunted response to ESA treatment. ESA (or saline control) was administered 1 day after BA (or saline control) and Hb response measured 1 week later. Control mice showed a significant increase in Hb, whereas BA-treated mice did not (n = 5/group). Horizontal bars indicate groups compared for statistical analysis. **P < .01; NS, no significance.
Consistent with findings in anemic mice expressing a hepcidin transgene15 and similar to the clinical presentation of AI, reticulocyte production was normal or decreased despite the anemia and elevated erythropoietin levels. No evidence of substantial red cell lysis was detected (data not shown), but a low level of inflammation-induced hemolysis was not ruled out. Inflammation-induced shortening of red cell lifespan also was not investigated. Mean cell volume (MCV) and mean cell hemoglobin (MCH) were normal during onset of anemia but began to decrease in the recovery phase, presumably as the result of ongoing iron limitation as reticulocyte production rebounded (eg, MCV 14 days after BA treatment of 49.6 ± 0.9 fL compared with 51.9 ± 0.2 fL for control mice, P < .05; MCH of 12.5 ± 0.2 pg compared with 15.0 ± 0.1 pg for control mice, P < .001). Similar to the situation with hepcidin overexpression and consistent with the clinical condition, the anemia was hyporesponsive to ESA treatment (Figure 2D), although control animals were able to adequately respond to ESA treatment.
Hepcidin mRNA suppression effectively treated anemia in mice with AI
To determine whether modulating hepcidin might impact anemia, shRNA constructs were developed to selectively knock down mHepc1 mRNA. Two antihepcidin shRNAs (H6 and H10) were used, and virus was titrated to allow examination of moderate and more profound hepcidin suppression in vivo. Introduction of these constructs into BA-treated mice by the use of AAV transduction (experimental scheme shown in Figure 3A) led to a decrease of mHepc1 liver mRNA and a decrease in serum hepcidin (Figure 3B). Moderate suppression of hepcidin (mediated by shRNA H10) decreased serum hepcidin in inflammatory animals to achieve levels similar to normal levels in control animals (luciferase shRNA-treated animals without BA treatment). More profound hepcidin suppression by shRNA H6 led to further reduction of mRNA and serum hepcidin levels. Both moderate and profound hepcidin suppression resulted in an increase in serum iron compared with BA-treated shRNA control mice (Figure 3C). These data suggest that hepcidin suppression mobilized iron effectively in animals with inflammation.
Hepcidin suppression by shRNA mobilized iron and corrected anemia in mice treated with BA. (A) Experimental scheme detailing administration of shRNA (control = antiluciferase, H10 or H6 = antihepcidin: 2 × 1012 particles/mouse via the hepatic portal vein) relative to BA (5 × 108 particles/mouse, intraperitoneally) or saline control and ESA (100 μg/kg darbepoetin alfa, subcutaneously) or saline control (no BA). Serum iron, serum hepcidin, liver hepcidin mRNA, and red cell parameters were measured at sample collection (n = 4-5/group). (B) Liver hepcidin mRNA (■, measured in RLU/10 μg total RNA) and serum hepcidin (▩, measured relative to hHepc internal standard in relative units [RU]). BA treatment increased serum hepcidin. Antihepcidin shRNA (H6 or H10) decreased both liver hepcidin mRNA and serum hepcidin. (C) Serum iron was increased by antihepcidin shRNAs H10 and H6. (D) Treatment with BA induced an anemia that was reversed with antihepcidin shRNA. The combination of hepcidin suppression and ESA treatment was more effective than hepcidin suppression alone. All statistical differences against control without inflammation (▩) and against BA-control (■) are indicated. Each shRNA subset was also compared with and without ESA administration and any significant differences shown (■ vs □ for each shRNA tested). (E-F) MCV and MCH, respectively, were increased by hepcidin suppression. All statistical differences compared with control group with no inflammation (▩) are shown. (G) Reticulocyte count in BA-treated animals was not increased by either hepcidin suppression or ESA treatment. Combination treatment showed a synergistic increase in reticulocytes. All statistical differences compared with control group with no inflammation (▩) are shown. No significant differences were seen between shRNA groups treated with BA alone (■). Significant differences between shRNA treatments caused by ESA administration (□) are shown. For statistical comparisons, *P < .05; **P < .01; *** P < .001.
Hepcidin suppression by shRNA mobilized iron and corrected anemia in mice treated with BA. (A) Experimental scheme detailing administration of shRNA (control = antiluciferase, H10 or H6 = antihepcidin: 2 × 1012 particles/mouse via the hepatic portal vein) relative to BA (5 × 108 particles/mouse, intraperitoneally) or saline control and ESA (100 μg/kg darbepoetin alfa, subcutaneously) or saline control (no BA). Serum iron, serum hepcidin, liver hepcidin mRNA, and red cell parameters were measured at sample collection (n = 4-5/group). (B) Liver hepcidin mRNA (■, measured in RLU/10 μg total RNA) and serum hepcidin (▩, measured relative to hHepc internal standard in relative units [RU]). BA treatment increased serum hepcidin. Antihepcidin shRNA (H6 or H10) decreased both liver hepcidin mRNA and serum hepcidin. (C) Serum iron was increased by antihepcidin shRNAs H10 and H6. (D) Treatment with BA induced an anemia that was reversed with antihepcidin shRNA. The combination of hepcidin suppression and ESA treatment was more effective than hepcidin suppression alone. All statistical differences against control without inflammation (▩) and against BA-control (■) are indicated. Each shRNA subset was also compared with and without ESA administration and any significant differences shown (■ vs □ for each shRNA tested). (E-F) MCV and MCH, respectively, were increased by hepcidin suppression. All statistical differences compared with control group with no inflammation (▩) are shown. (G) Reticulocyte count in BA-treated animals was not increased by either hepcidin suppression or ESA treatment. Combination treatment showed a synergistic increase in reticulocytes. All statistical differences compared with control group with no inflammation (▩) are shown. No significant differences were seen between shRNA groups treated with BA alone (■). Significant differences between shRNA treatments caused by ESA administration (□) are shown. For statistical comparisons, *P < .05; **P < .01; *** P < .001.
As mentioned previously, animals treated with BA were anemic and did not show a significant Hb response to ESA treatment. This fact did not alter when control shRNA was expressed (Figure 3D). Moderate hepcidin suppression alone (shRNA 10) did not significantly increase Hb. In combination with ESA treatment, however, moderate hepcidin suppression resulted in a significant increase in Hb and a complete correction of anemia. Consistent with these findings, more profound hepcidin suppression in the absence of any ESA treatment was sufficient to correct the anemia and was as effective as the combination of moderate hepcidin suppression and ESA treatment (Figure 3D).
Presumably as a response to altered iron kinetics, hepcidin suppression resulted in a significant increase in MCV in most groups and MCH in all groups (Figure 3E and F, respectively). These data suggested that iron was mobilized by hepcidin suppression and enhanced iron-dependent red cell parameters.
As expected, no appreciable reticulocyte response was observed in BA-control animals despite the presence of profound anemia (Figure 3G). This result was true even when animals were treated with suprapharmacologic doses of ESA. This dampened reticulocyte response to anemia was consistent with an inflammation-driven erythropoietic suppression. Equally, hepcidin suppression did not increase reticulocyte number. Surprisingly, the combination of both hepcidin suppression and ESA treatment did increase reticulocyte numbers, demonstrating synergism between the 2 therapies. These data, taken together, suggest that hepcidin suppression enhanced iron-dependent red cell parameters such as MCV and MCH by increasing bioavailable iron and that increased bioavailable iron, in combination with ESA treatment, induced reticulocytosis.
High-affinity antibodies neutralized hepcidin in vitro and in vivo
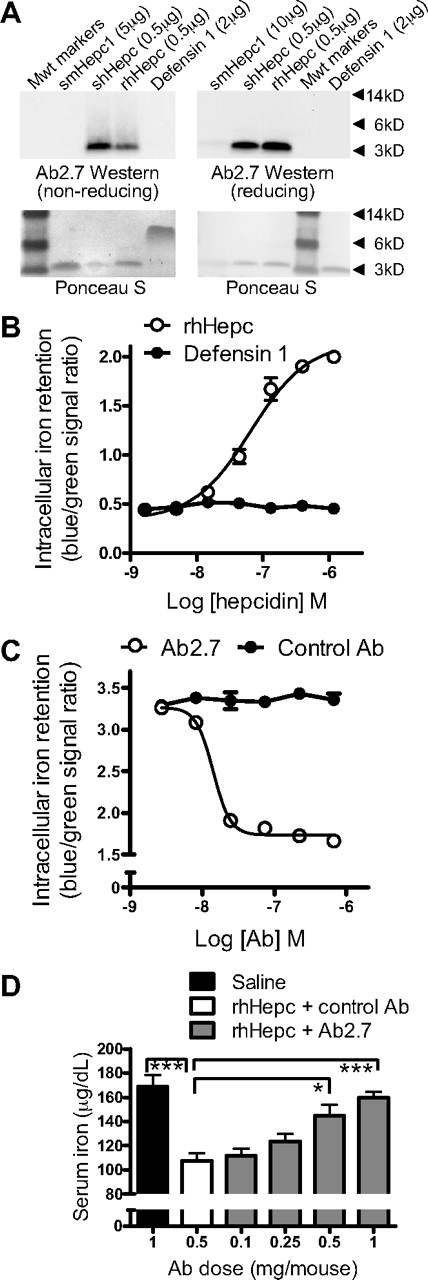
To allow efficient antibody generation and testing, rhHepc of high purity and structural integrity was produced, quality controlled by the use of biophysical techniques,32 and used to generate antibodies in mice. Affinity of the anti-rhHepc antibodies for hHepc varied from low picomolar to mid-nanomolar by Biacore or KinExA assessment. Although all antibodies bound hepcidin in solution, only a subset were able to recognize purified rhHepc by Western analysis. No monoclonal antibodies tested were sufficiently effective in Western analysis to detect unpurified rhHepc from hepcidin-overexpressing cell lysates, nor could these antibodies detect hepcidin or any other protein in human liver lysates by Western analysis (data not shown).
The mouse monoclonal IgG1 antibody Ab 2.7 recognized shHepc in solution with an affinity of 110pM (95% confidence interval, 80-150pM) and synthetic mouse hepcidin (smHepc1) with an affinity of 40nM (95% confidence interval, 27-44nM). By Western analysis, Ab2.7 was able to detect purified preparations of shHepc and rhHepc and weakly detected purified smHepc1 (Figure 4A).
Antihepcidin antibody treatment neutralized hepcidin in vitro and in vivo. (A) By Western analysis, Ab2.7 detected purified shHepc and rhHepc, weakly detected smHepc1, and did not detect a defensin 1 control (Sigma-Aldrich). Ponceau S staining of blots was conducted to demonstrate protein loading. Some precipitation of smHepc1 occurred in reducing conditions, evident by decreased Ponceau S staining. (B) Hepcidin treatment (rhHepc) increased intracellular iron, measured as blue/green ratio with an iron-responsive β-lactamase reporter gene; EC50 40nM (n = 3 wells/point). (C) Ab2.7 neutralized hepcidin activity measured as in panel B (37nM rhHepc); IC50 14nM. (D) Ab2.7 prevented hepcidin-mediated serum iron decrease in mice. Subcutaneous Ab2.7 administered 3 days before 25 μg of hepcidin (IP); serum iron measured 2 hours later (n = 5/group). All statistical differences from rhHepc and control antibody group (□) are shown: *P < .05; ***P < .001.
Antihepcidin antibody treatment neutralized hepcidin in vitro and in vivo. (A) By Western analysis, Ab2.7 detected purified shHepc and rhHepc, weakly detected smHepc1, and did not detect a defensin 1 control (Sigma-Aldrich). Ponceau S staining of blots was conducted to demonstrate protein loading. Some precipitation of smHepc1 occurred in reducing conditions, evident by decreased Ponceau S staining. (B) Hepcidin treatment (rhHepc) increased intracellular iron, measured as blue/green ratio with an iron-responsive β-lactamase reporter gene; EC50 40nM (n = 3 wells/point). (C) Ab2.7 neutralized hepcidin activity measured as in panel B (37nM rhHepc); IC50 14nM. (D) Ab2.7 prevented hepcidin-mediated serum iron decrease in mice. Subcutaneous Ab2.7 administered 3 days before 25 μg of hepcidin (IP); serum iron measured 2 hours later (n = 5/group). All statistical differences from rhHepc and control antibody group (□) are shown: *P < .05; ***P < .001.
A cellular assay was used to test for hepcidin activity (Figure 4B). A previously described assay9 was modified by the addition of an iron-responsive β-lactamase reporter gene to provide a high-throughput, sensitive, and internally controlled readout representative of intracellular iron retention. Ab2.7 neutralized the effects of hepcidin in this assay (Figure 4C). Ab2.7 also neutralized hepcidin in vivo (Figure 4D). As demonstrated previously,38 injection of hHepc produced a statistically significant decrease in serum iron that reached its nadir 2 hours after injection. This serum iron decrease could be prevented by pretreatment of mice with Ab2.7. Thus, Ab2.7 was effective at blocking the effects of hHepc in vitro and in vivo.
Ab2.7 neutralized hHepc overexpressed in mice
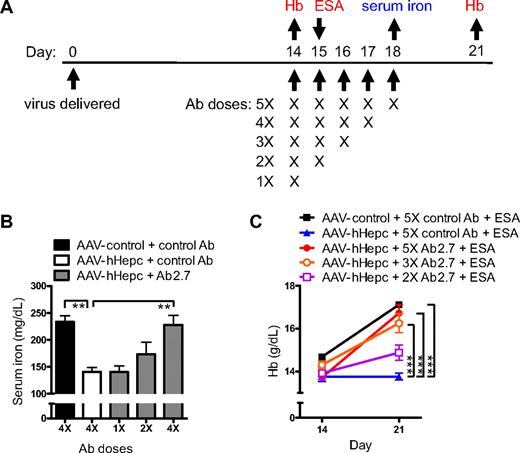
To determine whether an antihepcidin antibody would be able to reverse the effects of constitutively produced hepcidin, neutralization in AAV-mediated hHepc overexpression mice was tested (experimental scheme shown in Figure 5A). Treatment with Ab2.7 reversed hepcidin-induced hypoferremia in AAV-hHepc mice (Figure 5B). The effect of hepcidin neutralization was dose dependent, with no effect on serum iron observed with a single dose of antibody and the greatest effect seen with daily antibody dosing.
hHepc constitutively overexpressed in mice was neutralized by Ab2.7. (A) Experimental scheme detailing administration time of hepcidin (or control) virus (AAV5) with dosing schemes for antihepcidin (or control) antibody (1 mg subcutaneously: n = 5/group). Sampling for panel B (serum iron) is shown in blue. Extra treatment (ESA: 100 μg/kg subcutaneous darbepoetin alfa) and samplings for panel C are shown in red. (B) AAV-hHepc (1.5 × 1012 particles/mouse) caused a serum iron decrease compared with control virus treatment (AAV-β-galactosidase), which was prevented by Ab2.7 treatment in a dose-responsive manner. Statistical differences compared with AAV-hHepc + control antibody (□) are shown. (C) Control virus-treated mice (AAV-GFP; 5 × 1012 particles/mouse) showed a normal response to ESA, whereas those expressing AAV-hHepc did not. Ab2.7 restored response to ESA in AAV-hHepc mice in a dose-responsive manner. Statistics represent comparison of Hb values at day 21. Significant differences from AAV-hHepc + 5X control Ab + ESA (blue line) are shown. For statistical comparisons, **P < .01; ***P < .001.
hHepc constitutively overexpressed in mice was neutralized by Ab2.7. (A) Experimental scheme detailing administration time of hepcidin (or control) virus (AAV5) with dosing schemes for antihepcidin (or control) antibody (1 mg subcutaneously: n = 5/group). Sampling for panel B (serum iron) is shown in blue. Extra treatment (ESA: 100 μg/kg subcutaneous darbepoetin alfa) and samplings for panel C are shown in red. (B) AAV-hHepc (1.5 × 1012 particles/mouse) caused a serum iron decrease compared with control virus treatment (AAV-β-galactosidase), which was prevented by Ab2.7 treatment in a dose-responsive manner. Statistical differences compared with AAV-hHepc + control antibody (□) are shown. (C) Control virus-treated mice (AAV-GFP; 5 × 1012 particles/mouse) showed a normal response to ESA, whereas those expressing AAV-hHepc did not. Ab2.7 restored response to ESA in AAV-hHepc mice in a dose-responsive manner. Statistics represent comparison of Hb values at day 21. Significant differences from AAV-hHepc + 5X control Ab + ESA (blue line) are shown. For statistical comparisons, **P < .01; ***P < .001.
Ab 2.7 also was evaluated for its ability to restore ESA response in AAV-hHepc mice (Figure 5C). AAV-hHepc mice treated with ESA showed no increase in Hb, confirming again the hepcidin-mediated hyporesponse to ESA therapy. In contrast, AAV-hHepc mice treated with Ab2.7 and ESA showed a change in Hb similar to that observed in ESA-treated control mice. Again, the Hb response was dependent on the dose of Ab2.7. These data indicated that the effects of hepcidin overexpression had been neutralized by Ab2.7.
Ab2.7 was effective in combination therapy for AI
Experiments were performed to evaluate the utility of the antihepcidin antibody in a mouse model of AI. Because anti-hHepc antibodies recognized mouse hepcidin with low affinity, hHepc knock-in mice were generated. Two strains of mice were derived and tested. In Hep1 mice, the hHepc locus replaced the mhepc1 locus. In Hep2 mice, the hHepc locus replaced the mHepc1 and mHepc2 loci.
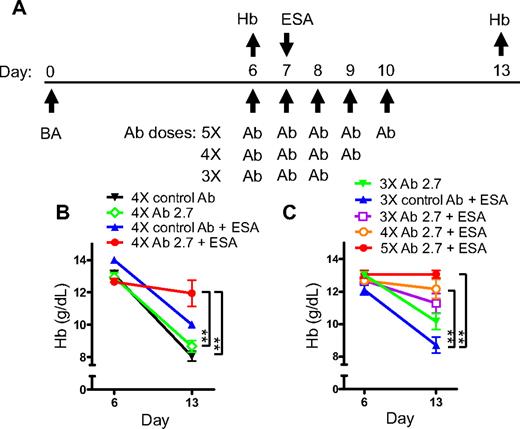
An increased baseline serum iron concentration was observed in hHepc knock-in animals, which contrasted with control animals (Table 1). In both mouse strains hHepc was produced in place of mouse hepcidin and was induced appropriately by inflammation (Table 1). In common with C57BL/6 wild-type mice, both knock-in strains developed anemia in response to BA. Mice were treated with BA and their Hb was measured 6 days later to determine which animals developed anemia (experimental scheme shown in Figure 6A). Anemic animals were treated with ESA plus the antihepcidin antibody, and the effect of therapy on anemia progression was monitored at day 13 relative to BA. Hep1 knock-in mice treated with ESA showed further progression of anemia: Hb values decreased between onset of treatment and day 13 (ESA and control Ab group: Figure 6B). Thus, as was the case for wild-type C57BL/6 mice treated with BA, ESA treatment was not effective. Treatment with Ab2.7 in Hep1 mice did not significantly inhibit progression of anemia. However, antibody treatment did cause a similar improvement of iron-dependent red cell characteristics as was seen in the antihepcidin shRNA experiments and did not appreciably affect reticulocyte production (data not shown).
Comparison of mouse strain characteristics
| Mouse strain . | Hb, g/dL . | Serum iron, μg/dL . | Baseline hepcidin, ng/mL . | BA-induced hepcidin, ng/mL . | ||
|---|---|---|---|---|---|---|
| mHepc1 . | hHepc . | mHepc1 . | hHepc . | |||
| C57BL/6 | 15.0 ± 0.1 | 167 ± 6 | 175 ± 9 | 0 | 617 ± 73 | 0 |
| Hep1 knock-in | 15.0 ± 0.1 | 340 ± 6 | 0 | 113 ± 16 | 0 | 329 ± 26 |
| Hep1 control | 15.2 ± 0.3 | 207 ± 16 | 171 ± 25 | 0 | ND | 0 |
| Hep2 knock-in | 15.8 ± 0.1 | 330 ± 15 | 0 | 106 ± 35 | 0 | 428 ± 49 |
| Hep2 control | 14.9 ± 0.2 | 260 ± 6 | 210 ± 25 | 0 | 368 ± 64 | 0 |
| Mouse strain . | Hb, g/dL . | Serum iron, μg/dL . | Baseline hepcidin, ng/mL . | BA-induced hepcidin, ng/mL . | ||
|---|---|---|---|---|---|---|
| mHepc1 . | hHepc . | mHepc1 . | hHepc . | |||
| C57BL/6 | 15.0 ± 0.1 | 167 ± 6 | 175 ± 9 | 0 | 617 ± 73 | 0 |
| Hep1 knock-in | 15.0 ± 0.1 | 340 ± 6 | 0 | 113 ± 16 | 0 | 329 ± 26 |
| Hep1 control | 15.2 ± 0.3 | 207 ± 16 | 171 ± 25 | 0 | ND | 0 |
| Hep2 knock-in | 15.8 ± 0.1 | 330 ± 15 | 0 | 106 ± 35 | 0 | 428 ± 49 |
| Hep2 control | 14.9 ± 0.2 | 260 ± 6 | 210 ± 25 | 0 | 368 ± 64 | 0 |
Mean ± SEM values for mice illustrating serum iron, Hb, and serum hepcidin. BA-induced hepcidin values were measured 6 hours after BA administration.
Hb indicates hemglobulin; BA, Brucella abortus; Hep, hepcidin; hHepc, human hepcidin; mHepc, mouse hepcidin; and ND, values not determined experimentally.
Ab 2.7 restored response to ESA treatment in hHepc knockin AI mice. (A) Experimental scheme detailing administration time of BA (3 × 108 particles/mouse, intraperitoneally), antihepcidin (or control) antibody (1 mg subcutaneously), and ESA (100 μg/kg darbepoetin alfa, subcutaneously) or saline control. Hb was measured before and after treatment (n = 3-5/group). (B) Hep1 AI mice showed declining Hb when treated with 4× control antibody. Treatment with either ESA or Ab2.7 had no effect but combination treatment was effective at preventing the Hb decline. (C) Hep1 mice showed a dose-response to Ab2.7. Statistics represent comparison of Hb values at day 13: **P < .01.
Ab 2.7 restored response to ESA treatment in hHepc knockin AI mice. (A) Experimental scheme detailing administration time of BA (3 × 108 particles/mouse, intraperitoneally), antihepcidin (or control) antibody (1 mg subcutaneously), and ESA (100 μg/kg darbepoetin alfa, subcutaneously) or saline control. Hb was measured before and after treatment (n = 3-5/group). (B) Hep1 AI mice showed declining Hb when treated with 4× control antibody. Treatment with either ESA or Ab2.7 had no effect but combination treatment was effective at preventing the Hb decline. (C) Hep1 mice showed a dose-response to Ab2.7. Statistics represent comparison of Hb values at day 13: **P < .01.
Hep1 mice treated with ESA and Ab2.7 showed relatively stable Hb between days 6 and 13 (Figure 6B). Hence, Ab2.7 was able to prevent progression of hepcidin-induced AI when administered in combination with ESA. Similar results were seen in Hep2 mice (data not shown). The effect of Ab2.7 was dose dependent (Figure 6C). Control animals expressing mouse hepcidin were unable to respond to Ab2.7 treatment (data not shown), presumably because of the substantial difference in affinity of Ab2.7 for human and mouse hepcidin. Thus there was concordance between the independently derived Hep1 and Hep2 knock-in mouse strains and lack of activity with a hHepc-specific antibody in the absence of the hHepc gene. There was also similarity between antibody-mediated hepcidin neutralization and the shRNA-mediated suppression described previously.
To establish whether intravenous iron supplementation increased bioavailable iron in a similar manner to hepcidin neutralization, several doses of iron were tested in combination with ESA in BA mice (data not shown). Intravenous iron treatment did not restore response to ESA at doses equivalent to those used in humans (1 mg/kg) or at substantially increased doses (1 mg per mouse: equivalent to 40 mg/kg), which produced a noticeable increase in iron staining in spleen and liver. At very high doses (2.5 mg per mouse: equivalent to ∼ 100 mg/kg intravenous iron supplementation) a partial effect was observed similar to low-dose Ab2.7 treatment. This level of intravenous iron treatment was accompanied by substantial iron deposition in the liver. The limited utility of intravenous iron observed was supported by data in humans demonstrating that hepcidin overexpression limited the efficacy of parenteral iron administration.11 The inflammatory increase in hepcidin in the BA mouse model presumably led to sequestration of the majority of the intravenous iron dose, with a relatively minor proportion becoming bioavailable. Taken together, the aforementioned data illustrated that Ab2.7 neutralized hHepc and in combination with ESA was an effective treatment for AI in mice.
Discussion
The aim of the current work was to explore the importance of iron sequestration in AI and ask whether interfering with hepcidin function could modulate this response. It had been previously demonstrated that hepcidin expression alone was sufficient to cause iron-deficiency anemia14 and resistance to endogenous erythropoietin.15 In the present work, it was demonstrated that overexpression of hepcidin also caused resistance to high-dose exogenous ESA treatment and that neutralization of hepcidin reversed this effect. To further explore the role of hepcidin in inflammatory anemia, a model of mouse AI was developed. Hepcidin modulation in AI was first examined by mRNA suppression. Moderate-dose shRNA treatment led to a reduction in hepcidin to preinflammatory levels and, in combination with ESA, to effective anemia treatment. In the presence of near complete suppression of hepcidin signal via high-dose shRNA as a single-agent treatment, effective correction of Hb was possible. An antihepcidin antibody was then developed and tested in the hHepc knock-in AI mice. This antihepcidin antibody had limited activity alone but was an effective anemia treatment when used in combination with ESA.
It was interesting that more complete reduction of hepcidin activity was observed by mRNA suppression than by Ab2.7-mediated neutralization of circulating hHepc peptide. This difference may be simply a result of the limits of the antibody used such as affinity or dose, or to intrinsic difficulties in neutralizing an abundant serum peptide that is produced at a high rate in mice. Hepcidin levels are approximately 100 to 200 ng/mL in normal or human-hepcidin knockin mice fed on a standard iron diet. This is at least 10- to 20-fold greater than baseline levels in normal human donors in which the same hepcidin detection method is used.31 Hepcidin has a short half-life and is cleared primarily through the kidney.38 The faster glomerular filtration rate in mice (∼ 5 times that of humans), together with the high serum levels suggest that the production rate of hepcidin may be very high, requiring relatively large doses of antibody for effective neutralization in the mouse system.
Hepcidin neutralization in AI mice substantially improved iron-dependent red cell parameters. Although the time of detection (day 7 after ESA stimulus) was not an ideal point to study reticulocyte response, it appeared that neither hepcidin suppression alone nor ESA treatment alone induced a reticulocyte response. In contrast, combination treatment did induce reticulocytosis, demonstrating synergy between the 2 treatments. This finding suggested the intriguing possibility that by improving effective hemoglobinization of progenitors, hepcidin neutralization may have created permissive conditions to allow an effective reticulocyte response to erythropoietin. Inflammation is known to suppress bone marrow response to erythropoietin, perhaps suggesting why supraphysiologic levels of ESA were necessary to see this effect. Hence mobilization of iron and erythropoietic stimulus are both deficient in inflammation and fixing either one alone has limited value compared with supplying both.
On the basis of the data presented here, hepcidin mRNA suppression or antibody-mediated neutralization was able to overcome AI in a mouse model. In patients with AI, limited activity of ESA treatment and evidence of iron-deficient erythropoiesis has led to the practice of using intravenous iron treatment to transiently increase iron availability. A therapeutic such as an antihepcidin antibody may be capable of redistributing iron from storage sites as well as allowing normal dietary iron absorption. Such a therapy would be anticipated to reduce the requirement for intravenous iron and therefore offer a means to assess the potential risk associated with intravenous iron treatment,2,39 while still effectively treating anemia in patients with inflammation. Furthermore, an antihepcidin antibody may enable investigation of the role of iron metabolism in a wide array of diseases where iron maldistribution is implicated in disease initiation or progression.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jeanne Sloan and Adrienne Augustic for in vivo experiments; Yvonne Connell, Lynn Tran, and Patrice Lincoln for Western blot analysis; Jacob Corcoran for in vitro hepcidin assays; Ching Chen for antibody affinity determination; Mark Daris for virus production and testing; Yun Lin for virus production and bDNA analysis; Amro Shehabeldin for bDNA analysis; Yu Sun for cell line construction; Hiko Kohno and Noi Nuanmanee for E coli–derived hepcidin production; Jingwen Zhang for synthetic hepcidin production; and Linh Tran for hepcidin measurement.
Authorship
Contribution: B.J.S. designed and interpreted the work, drafted the paper, and is responsible for the integrity of the work as a whole; K.S.C. designed and interpreted the work, performed experiments and interpreted data, and drafted the paper; T.L.A., C.P., and J.S. designed and interpreted the work; A.R.E., A.W., and H.L. performed experiments and interpreted data; T.J. designed and interpreted the work and supervised knock-in mouse generation; and C.G.B. and G.M. made intellectual comments on the study and aided in interpretation and context.
Conflict-of-interest disclosure: All authors are employees of Amgen Inc.
Correspondence: Barbra J. Sasu, Mailstop 15-2-A, 1 Amgen Center Dr, Thousand Oaks, CA 91320; e-mail: bajohnso@amgen.com.

![Figure 3. Hepcidin suppression by shRNA mobilized iron and corrected anemia in mice treated with BA. (A) Experimental scheme detailing administration of shRNA (control = antiluciferase, H10 or H6 = antihepcidin: 2 × 1012 particles/mouse via the hepatic portal vein) relative to BA (5 × 108 particles/mouse, intraperitoneally) or saline control and ESA (100 μg/kg darbepoetin alfa, subcutaneously) or saline control (no BA). Serum iron, serum hepcidin, liver hepcidin mRNA, and red cell parameters were measured at sample collection (n = 4-5/group). (B) Liver hepcidin mRNA (■, measured in RLU/10 μg total RNA) and serum hepcidin (▩, measured relative to hHepc internal standard in relative units [RU]). BA treatment increased serum hepcidin. Antihepcidin shRNA (H6 or H10) decreased both liver hepcidin mRNA and serum hepcidin. (C) Serum iron was increased by antihepcidin shRNAs H10 and H6. (D) Treatment with BA induced an anemia that was reversed with antihepcidin shRNA. The combination of hepcidin suppression and ESA treatment was more effective than hepcidin suppression alone. All statistical differences against control without inflammation (▩) and against BA-control (■) are indicated. Each shRNA subset was also compared with and without ESA administration and any significant differences shown (■ vs □ for each shRNA tested). (E-F) MCV and MCH, respectively, were increased by hepcidin suppression. All statistical differences compared with control group with no inflammation (▩) are shown. (G) Reticulocyte count in BA-treated animals was not increased by either hepcidin suppression or ESA treatment. Combination treatment showed a synergistic increase in reticulocytes. All statistical differences compared with control group with no inflammation (▩) are shown. No significant differences were seen between shRNA groups treated with BA alone (■). Significant differences between shRNA treatments caused by ESA administration (□) are shown. For statistical comparisons, *P < .05; **P < .01; *** P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/17/10.1182_blood-2009-09-245977/4/m_zh89991049560003.jpeg?Expires=1767736658&Signature=Ge0oWu6DbHqHM~fSbBqWmA6v4QdbBZ4yTTFgyJerPLNg8IjxessLInq4La4x~xwI5duGkHZ8dPrbo5nN2hfjqAXkPoetrStReIoDWAPedQeCfYBB9hKxlyaMfNDLJm53krl-IUgcuc9XuQQc~2SPN6iO5ntW-QtTK1EptjYJpwyabDaPM6CSwD4UvkLsHVNM0vgLptEWUyFHbIr1x2lmo1ldPtwsZbV197-z3lCzVREJ4oKtzRp3xNtZAKnWnAgSMtN-ym~hN14gdRIlOLcFt9aZ9-wLUgrlL7R6pHcm1WAKUjgoEkvc2ZGpnlRWZBlBBT7B3x3qtvjwacwqW-j-CA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal