Abstract
During embryonic development, lymph sacs form from the cardinal vein, and sprout centrifugally to form mature lymphatic networks. Separation of the lymphatic from the blood circulation by a hitherto unknown mechanism is essential for the homeostatic function of the lymphatic system. O-glycans on the lymphatic endothelium have recently been suggested to be required for establishment and maintenance of distinct blood and lymphatic systems, primarily by mediating proper function of podoplanin. Here, we show that this separation process critically involves platelet activation by podoplanin. We found that platelet aggregates build up in wild-type embryos at the separation zone of podoplanin+ lymph sacs and cardinal veins, but not in podoplanin−/− embryos. Thus, podoplanin−/− mice develop a “nonseparation” phenotype, characterized by a blood-filled lymphatic network after approximately embryonic day 13.5, which, however, partially resolves in postnatal mice. The same embryonic phenotype is also induced by treatment of pregnant mice with acetyl salicylic acid, podoplanin-blocking antibodies, or by inactivation of the kindlin-3 gene required for platelet aggregation. Therefore, interaction of endothelial podoplanin of the developing lymph sac with circulating platelets from the cardinal vein is critical for separating the lymphatic from the blood vascular system.
Introduction
The lymphatic vascular system supplements the blood circulation by draining extravasated cells, proteins, and fluids from the tissues back to the blood circulation, which necessitates separation of the lymphatic from the blood circulatory systems during development.1,2 The lymphatic vasculature starts to develop in mouse embryos at around embryonic day (E) 10.5, when the cardiovascular system is already functioning. Focally, clusters of endothelial cells in the cardinal vein commit to the lymphatic phenotype, and sprout to form the primary lymphatic sacs,1-4 from where part of the peripheral lymphatic vasculature is generated by further centrifugal growth. In addition, lymphatic vessels also may be generated in the periphery from precursor cells5 in embryos, as well as in adult tissues in pathologic conditions.6 The lymphatic circulation subsequently becomes separated from the blood circulation by currently unknown mechanisms, and only the connection between the veins in the neck region and the thoracic ducts is left open in adults4,7 to permit the entrance of lymph into the blood stream.
Endothelial cells of lymphatic vessels are characterized by the expression of specific markers, such as the abundant lymphatic endothelial membrane mucoprotein podoplanin and the CD44 hyaluronan receptor-related protein Lyve-1.1 The former is expressed under the control of the transcription factor Prox-1,8-10 a “master” control gene for lymphatic development. Disruption of the Prox-1 or VEGF-C gene or of certain other genes specific to the lymphatic endothelium10 results in a so called “lymphatic” phenotype characterized by the absence or by severe defects of lymphatic vessels.8,11-14 In contrast, deletion of Lyve-115 had no consequence on lymphatic development. Unexpectedly, however, disruption of Syk or SLP-76,16 genes involved in the development of T lymphocytes17,18 or B lymphocytes19,20 and, importantly, also for the function of platelets21-23 resulted in a “nonseparation” phenotype that was characterized by blood filling of the lymphatic vessels and hemorrhages. A similar phenotype was found in endothelial cell O-glycan deficiency that in turn decreased podoplanin expression.24
Here, we confirm a role for podoplanin25,26 in the separation of the lymphatic from the venous system during development and attribute its role in this process to its interaction with platelets. Podoplanin-deficient mice were first generated in the 129/SvEv background,27 but study of their postnatal vascular development was impossible, because they died at birth from respiratory failure resulting from lack of podoplanin in lung alveolar type I cells.28 These embryos show defects in lymphatic vessel patterning, diminished lymphatic transport, congenital lymphedema, and lymphatic dilatation, but they appeared not to have misconnections between blood and lymphatic vessels.29 Re-evaluation of these mice by Fu et al,24 however, revealed that they had disorganized and blood-filled lymphatic vessels at birth.24
We have generated podoplanin−/− mice in a different background30 and backcrossed them into C57bl/6 mice. We found that a fraction of these mice survive without developing of a pulmonary phenotype, allowing more detailed studies. These podoplanin−/− mice showed a full-blown “nonseparation” phenotype in line with the report by Fu et al.24 The observation that podoplanin induces platelet activation31,32 by interacting with the platelet membrane lectin CLEC-232 allowed us to demonstrate that local podoplanin-induced platelet aggregation and subsequent closure of the junction between the cardinal vein and the developing lymph sacs are responsible for separating of the lymphatic from the blood vessels. This “platelet hypothesis” of vessel separation was corroborated by several different means, indicating that the absence of podoplanin interferes directly with platelet aggregation and results in the “nonseparation” phenotype.
Methods
Generation of knockout mice
Podoplanin-deficient mice in the 129S/v × Swiss background were generated by disrupting the coding region of the podoplanin gene, and were characterized as described.30 These mice were backcrossed onto the C57BL/6 background for 6 generations and also bred into the VEGFR-3 gene–targeted strain that directs β-galactosidase expression into lymphatic endothelial cells.33 Kindlin-3–deficient mice were generated as described.34 Animal care and all experimental procedures were approved by the Animal Experimental Committee of the Medical University of Vienna, and by the Austrian Ministry of Science (License No. 1321/115, and 66.009/0103-C/Gl/2007).
Immunohistochemistry
Tissue samples were snap-frozen in liquid N2-cooled OCT (Miles), and 10-μm cryostat sections were prepared. For immunofluorescence, the following primary antibodies were used: rabbit polyclonal anti–mouse Lyve-1 (Abcam Ltd), rat anti–mouse monoclonal CD31 (Becton Dickinson), hamster monoclonal anti–mouse podoplanin antibody (Acris), rabbit polyclonal anti–Prox-1 antibody (AngioBio Co), rat anti–mouse monoclonal Ter119 antibody (eBioscience), and monoclonal rat anti–mouse antibody directed against the activated form of mouse platelet integrin αIIbβ3 (clone JON/A-PE; Emfret-Analytics). The nuclei were counterstained with DAPI (Sigma-Aldrich). Secondary reagents were goat anti–rabbit IgG (Invitrogen) labeled with Alexa Fluor 488 or 568, goat anti–mouse IgG labeled with Alexa Fluor 488 or 647 (Invitrogen), conjugated biotinylated rabbit anti–hamster polyclonal IgG (Acris), and streptavidin Alexa Fluor 488 or 568 (Invitrogen). Fluorescent imaging was performed on a Zeiss LSM 510 META confocal system in multitrack mode, using a 40×/1.3 plan Neofluar oil objective, pinhole sizes between 0.7 and 1.5 μm and the appropriate standard laser-filter combinations (images were handled with the Zeiss AIM software package; Zeiss), or on a motorized Olympus AX70 microscope by 10× and 20× UPlanApo air objective lenses and using a cooled F-View II Camera and the CellP imaging software (Olympus Soft Imaging Solutions). Some tissue samples were also fixed in 4% formaldehyde, and processed for standard paraffin embedding, followed by immunohistochemical detection of Lyve-1 or podoplanin, as described.26 β-galactosidase activity in transgenic podoplanin+/+ and podoplanin−/− mice was detected as described.33
In vitro platelet adhesion assay
Podoplanin-expressing NIH-3T3 cells were generated by retroviral transfection, using human podoplanin cDNA cloned into pBMN-Z plasmid (kindly provided by Gerry Nolan, Stanford University) and transfected into the Phoenix-Eco packaging cell line (Gentaur). Retrovirus-transduced cells were purified by fluorescence-activated cell sorter (FACS)–sorting and single-cell cloning. Cells transfected with empty vector were used as controls. Human umbilical vein endothelial cells (Technoclone) that were devoid of podoplanin and human dermal lymphatic endothelial cells were prepared as described.35
For the purification of mouse platelets, blood was drawn from the periorbital sinus of mice into heparinized capillaries and centrifuged at 230g for 7 minutes, and the platelet-rich fraction was collected. Human platelet concentrates were obtained from the Department of Blood Grouping and Transfusion Medicine, Medical University of Vienna. A total of 3 × 106 platelets were suspended in 70 μL of phosphate-buffered saline (PBS), and injected via microinjection needles at a flow rate of 3 μL/minute for 20 minutes onto confluent monolayers of NIH-3T3 cells or lymphatic or blood endothelial cells cultured in 24-well plates that contained 2 mL of medium. For inhibition experiments, rabbit IgG directed against human podoplanin36 was added at a concentration of 50 μg/mL to the incubation media. In other experiments, the isolated platelets were preincubated in 500 μg/mL acetyl salicylic acid for 3 hours at room temperature before infusion into the culture dishes. Platelet movement and aggregate formation were recorded by video microscopy with an F-View camera (Olympus) and an Olympus IX70 microscope using the AnalySIS software (Soft Imaging System).
In vivo inhibition of formation of platelet aggregates
A total of 2 mg of rabbit anti–human podoplanin IgG or control preimmune IgG from the same rabbit were injected intravenously into ketamine/xylazine-anesthetized pregnant podoplanin+/+ females at E10.5, and the embryos were harvested at E13.5. In other experiments, the drinking water of pregnant podoplanin+/+ mothers was supplemented with acetyl salicylic acid (500 mg/L, 250 mg/L, or 75 mg/L) starting from approximately E8.5 until E16.5, and embryos were collected at E13.5, E14.5, and E16.5. The amount of acetyl salicylic acid consumed by a mouse was estimated to be 45 to 70 mg/kg/d, 25 to 33 mg/kg/d, and 6 to 9 mg/kg/d, respectively.
Functional tracer experiments
Podoplanin+/+ and podoplanin−/− mice aged 3 days and 8 weeks were injected with 50 μL or 250 μL of a 1% Chicago sky blue 6B (Sigma-Aldrich) in PBS into the periorbital sinus under ketamine/xylazine anesthesia. At 1 minute after injection, the abdominal wall was dissected and fixed in 4% formalin, and micrographs were taken with a stereo microscope SZ40 (Olympus).
Double-labeling experiments were performed in 8-week-old podoplanin+/+ and podoplanin−/− mice. We injected 30 μL of 1% Chicago sky blue 6B in PBS intradermally into the hind footpad, followed 2 minutes later by intravenous injection of Indian ink (Winsor & Newton) into the periorbital sinus. After 1 minute, the skin of the hind limb and the hips were peeled off, and micrographs were taken.
Podoplanin+/+ and podoplanin−/− mice aged 3 days were also injected with 50 μL of FITC-labeled Bandeiraea simplicifolia lectin (1 μg/μL in PBS; Sigma-Aldrich) into the periorbital sinus under ketamine/xylazine anesthesia. At 1 minute after injection, the abdominal skin was snap-frozen in liquid N2-cooled OCT, and FITC-lectin–perfused tissue sections were additionally stained for Lyve-1 and Ter119.
Blood chemistry
Blood samples were collected by decapitation of postnatal day (P) 1 to P5 mice. Activated partial thromboplastin time was determined with a commercial kit (Technoclone). Hematocrit was determined by standard procedure. Triglycerides were analyzed enzymatically, using a commercial kit (Roche Diagnostics).
Statistical analysis
Results are given as means plus or minus SD. Statistical significance was calculated by paired and unpaired t test, or by analysis of variance(ANOVA) as indicated. Significance was assigned to P values less than .05. Experiments were performed at least in triplicate if not stated otherwise.
Results
Generation and gross phenotype of podoplanin−/− mice
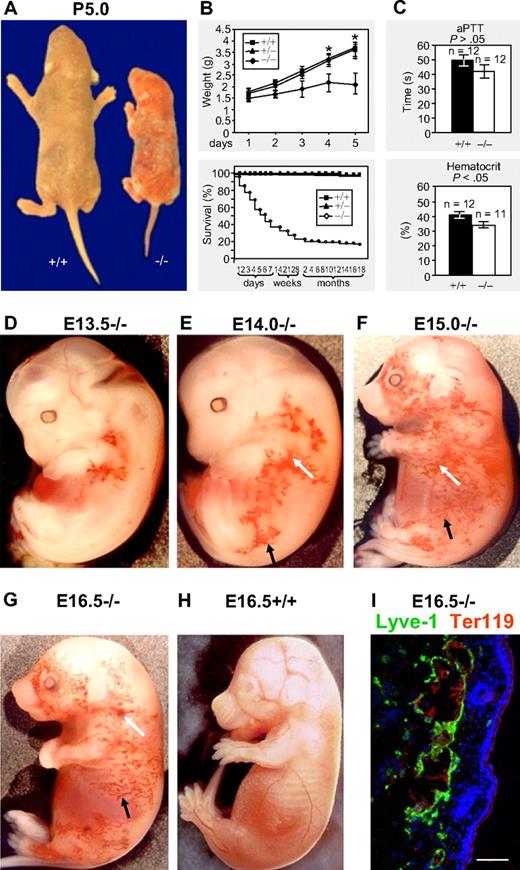
We generated podoplanin-deficient mice in a mixed 129S/v × Swiss background by disrupting the entire coding region of the podoplanin gene. Our strategy inactivated the podoplanin gene, which has been previously shown by Northern and Western blotting.30 We subsequently backcrossed the mice for 6 generations onto the C57Bl/6 background. The phenotype of these backcrossed podoplanin−/− mice resembled the one observed in the mixed 129S/v × Swiss background. Podoplanin-deficient neonates were smaller (Figure 1A-B), and approximately 55% died during the first postnatal week. Importantly, however, approximately 20% survived (Figure 1B), had normal weights and life spans, and were fertile.
Macroscopic phenotype of podoplanin−/− mice in postnatal and embryonic development. (A-B) Podoplanin−/− mice in postnatal development are significantly smaller (right mouse) than podoplanin+/+ littermates (left mouse) or heterozygotes. Podoplanin−/− mice also show ectatic vessels and some bleeding in their skin. Approximately 20% of podoplanin−/− mice reach fertility age. (C) No significant differences between podoplanin−/− and podoplanin+/+ mice are detected in their activated partial thromboplastin time (aPTT), but a lower hematocrit is found in P5 podoplanin−/− mice. Values in panels B and C are means ± SD. (D-G) Blood-filled capillary network (black arrows) that increases in size with age, and blood extravasations (white arrows) are found in podoplanin−/−, but not in podoplanin+/+ embryos (H); Olympus SZ zoom stereo microscope, Sony DSC W200 camera with adapter VAE-WD. Immunofluorescence staining (I) identifies the dermal blood-filled vessels of podoplanin−/− embryos as Lyve-1+ lymphatics (green) that contain Ter119+ erythrocytes (red). Cell nuclei are stained with DAPI (blue); Olympus AX70, 20 ×/0.7 UPlanApo air objective. Scale bar in panel I equals 50 μm.
Macroscopic phenotype of podoplanin−/− mice in postnatal and embryonic development. (A-B) Podoplanin−/− mice in postnatal development are significantly smaller (right mouse) than podoplanin+/+ littermates (left mouse) or heterozygotes. Podoplanin−/− mice also show ectatic vessels and some bleeding in their skin. Approximately 20% of podoplanin−/− mice reach fertility age. (C) No significant differences between podoplanin−/− and podoplanin+/+ mice are detected in their activated partial thromboplastin time (aPTT), but a lower hematocrit is found in P5 podoplanin−/− mice. Values in panels B and C are means ± SD. (D-G) Blood-filled capillary network (black arrows) that increases in size with age, and blood extravasations (white arrows) are found in podoplanin−/−, but not in podoplanin+/+ embryos (H); Olympus SZ zoom stereo microscope, Sony DSC W200 camera with adapter VAE-WD. Immunofluorescence staining (I) identifies the dermal blood-filled vessels of podoplanin−/− embryos as Lyve-1+ lymphatics (green) that contain Ter119+ erythrocytes (red). Cell nuclei are stained with DAPI (blue); Olympus AX70, 20 ×/0.7 UPlanApo air objective. Scale bar in panel I equals 50 μm.
Development of vascular abnormalities in podoplanin−/− mice
In podoplanin−/− embryos of the mixed as well as the C57BL/6 background, tortuous cutaneous blood-perfused vessels (Figure 1D; black arrows) were detected as early as at E13.5 in the jugular and axillary areas, extending toward the hind limb and the abdominal region (Figure 1E), and at E15 and E16.5 also toward most of the lateral aspects (Figure 1F-G). These vessels were Lyve-1+ and contained erythrocytes (Figure 1I), indicating their lymphatic origin and a connection to blood vessels. Cutaneous bleedings (white arrows) were found mainly in the flank regions (Figure 1E-G). Microvascular abnormalities disappeared postnatally after 10 to 14 days, and were absent from mice surviving beyond this point. The cutaneous vasculature of podoplanin+/+ and podoplanin+/− littermates was devoid of aberrant vessels during the entire developmental period (Figure 1H). Postnatally, some podoplanin−/− animals developed chylothorax or chylous ascites and exhibited portolymphatic shunts (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The thromboplastin time was similar between wild-type and podoplanin−/− littermates, whereas the hematocrit was significantly reduced in podoplanin−/− mice (Figure 1C), presumably due to bleeding into tissues.
Lymphatic vessels of podoplanin−/− embryos exhibit a “nonseparation” phenotype
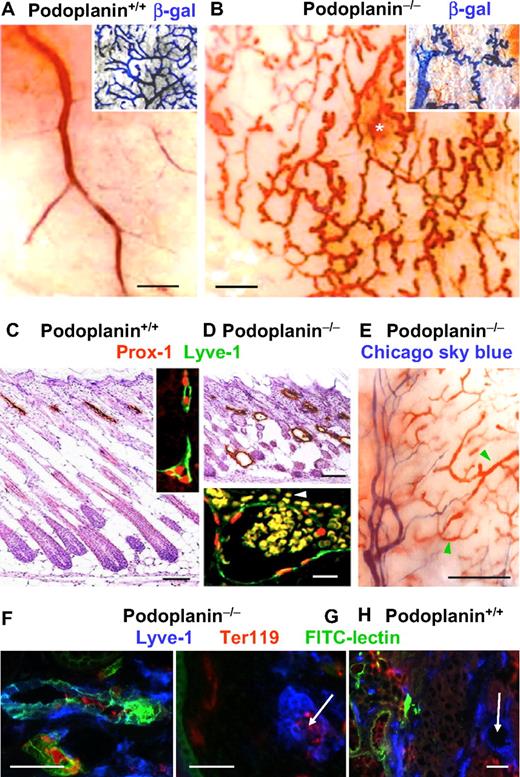
The macroscopic dermal aspect of the abdominal skin of podoplanin+/+ P3 mice showed blood-filled veins and venules (Figure 2A). The dermal lymphatics identified by double fluorescence for Prox-1 and Lyve-1 did not contain blood (Figure 2C). In contrast, tortuous blood-filled blind-ending cutaneous vessels were encountered in podoplanin−/− mice (Figure 2B). They were identified as lymphatics, because their endothelial cells expressed Lyve-1, Prox-1 (Figure 2D), and β-galactosidase when intercrossed with a mouse strain that expresses this reporter under the VEGFR-3 promoter and thus, exclusively in lymphatic endothelial cells33 (Figure 2A-B insets). The erythrocyte-containing lymphatic vessels were indicative of a “nonseparation” phenotype of the lymphatic and blood vasculatures. Tortuous blind-ending and blood-filled lymphatic vessels were also found in the intestine and in the thoracic walls (supplemental Figure 1).
Malformations and blood perfusion (the “nonseparation” phenotype) of cutaneous lymphatic vessels in podoplanin−/− mice. (A) In a wild-type P3, mouse the en face view of the dermal side of the skin of the flanks and abdomen shows blood-filled veins of different calibers. The lymphatic system is visualized by VEGFR-3–driven, lymphatic endothelium–specific expression of β-galactosidase (inset). (B) By contrast, in P3 podoplanin−/− mice, a fractal pattern of blood-filled, tortuous, blind-ending vessels are found, besides occasional areas of bleeding (white asterisk). These vessels are of lymphatic origin because they express VEGFR-3–driven β-galatosidase (inset); Olympus SZ zoom stereo microscope, Sony DSC W200 camera with adapter VAE-WD. (C) Sections through the flank skin of P3 podoplanin+/+ mice show Lyve-1–expressing lymphatics (brown) in the upper dermis. These vessels also express Prox-1 (red) on top of Lyve-1 (green) by double immunofluorescence (inset). (D) Skin of age-matched podoplanin−/− mice contains extended, randomly distributed lymphatic vessels. Their endothelial cells express Prox-1 (red) and Lyve-1 (green), and their dilated lumen contains erythrocytes (yellow) that also have leaked into the perivascular space (white arrowhead; inset). (E) In a P3 podoplanin−/− mouse, intravenously injected Chicago sky blue fills dermal veins and arteries (“purple vessel” on the left side of the image; Olympus SZ40); however, the blood-filled lymphatics (green arrowheads) are not entered by the tracer, indicating that this compartment is already sequestered from the circulation in this P3 mouse. Identification of perfused lymphatic vessels (F) by intravenous injection of FITC-lectin (green). Lyve-1 is blue and Ter119 is red. FITC-lectin is not detectable in the small erythrocyte-filled lymphatic capillaries (arrow in panel G). (H) Control injection of FITC-lectin into wild-type mice. FITC-lectin is confined to blood vessels and not detectable in large (arrow) or small lymphatic vessels (the latter are not shown in this picture). Scale bars in panels A, B, and E equal 300 μm; in panels C and D, 100 μm; in panel D inset, 15 μm; and in panels F through H, 50 μm.
Malformations and blood perfusion (the “nonseparation” phenotype) of cutaneous lymphatic vessels in podoplanin−/− mice. (A) In a wild-type P3, mouse the en face view of the dermal side of the skin of the flanks and abdomen shows blood-filled veins of different calibers. The lymphatic system is visualized by VEGFR-3–driven, lymphatic endothelium–specific expression of β-galactosidase (inset). (B) By contrast, in P3 podoplanin−/− mice, a fractal pattern of blood-filled, tortuous, blind-ending vessels are found, besides occasional areas of bleeding (white asterisk). These vessels are of lymphatic origin because they express VEGFR-3–driven β-galatosidase (inset); Olympus SZ zoom stereo microscope, Sony DSC W200 camera with adapter VAE-WD. (C) Sections through the flank skin of P3 podoplanin+/+ mice show Lyve-1–expressing lymphatics (brown) in the upper dermis. These vessels also express Prox-1 (red) on top of Lyve-1 (green) by double immunofluorescence (inset). (D) Skin of age-matched podoplanin−/− mice contains extended, randomly distributed lymphatic vessels. Their endothelial cells express Prox-1 (red) and Lyve-1 (green), and their dilated lumen contains erythrocytes (yellow) that also have leaked into the perivascular space (white arrowhead; inset). (E) In a P3 podoplanin−/− mouse, intravenously injected Chicago sky blue fills dermal veins and arteries (“purple vessel” on the left side of the image; Olympus SZ40); however, the blood-filled lymphatics (green arrowheads) are not entered by the tracer, indicating that this compartment is already sequestered from the circulation in this P3 mouse. Identification of perfused lymphatic vessels (F) by intravenous injection of FITC-lectin (green). Lyve-1 is blue and Ter119 is red. FITC-lectin is not detectable in the small erythrocyte-filled lymphatic capillaries (arrow in panel G). (H) Control injection of FITC-lectin into wild-type mice. FITC-lectin is confined to blood vessels and not detectable in large (arrow) or small lymphatic vessels (the latter are not shown in this picture). Scale bars in panels A, B, and E equal 300 μm; in panels C and D, 100 μm; in panel D inset, 15 μm; and in panels F through H, 50 μm.
Abnormal communications between the blood and the lymphatic circulatory systems were visualized upon injection of Chicago sky blue intravenously into the periorbital sinus. In podoplanin+/+ mice, the tracer appeared in the large subcutaneous blood vessels, but not in lymphatic vessels, whereas in podoplanin−/− mice, both vessel types were filled with the tracer (supplemental Figure 2). Interestingly, the tracer was absent from the subcutaneous tortuous blood-filled initial “lymphatic” microvasculature of P3 podoplanin−/− mice, indicating that at this point the microvessels had become disconnected from the circulation (Figure 2E). These findings were confirmed by intravenous injection of FITC-lectin into P3 podoplanin−/− mice, where the FITC signal was detected primarily in large lymphatic vessels that were Lyve-1+ and contained Ter119+ erythrocytes (Figure 2F). Such FITC-lectin signal was absent from most of the capillaries positive for Lyve-1 and Ter119 (Figure 2G). In contrast, in podoplanin+/+ mice, the FITC signal was restricted to blood vessels and absent from Lyve-1+ vessels (Figure 2H). The communication of large lymphatic vessels and veins persisted in adult podoplanin−/− mice (supplemental Figure 2).
Podoplanin induces platelet aggregation under flow conditions in vitro
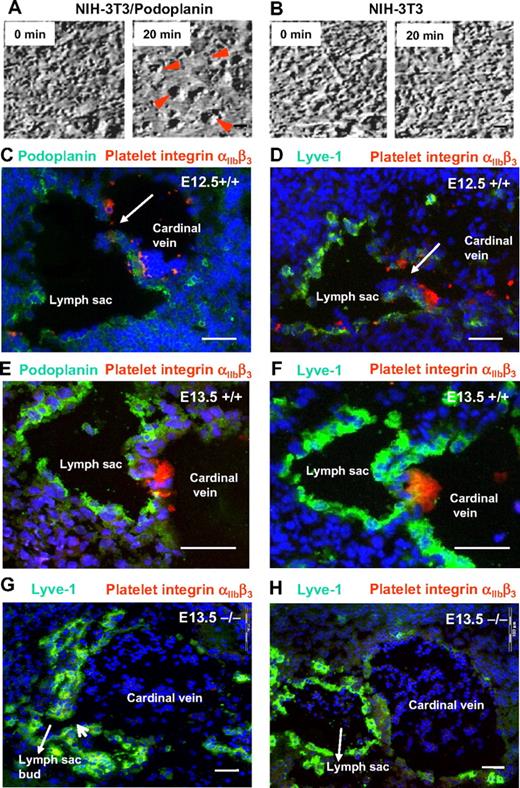
It has been reported previously that podoplanin can induce platelet aggregation in vitro in a static assay system.37 To demonstrate that podoplanin-expressing cells induce platelet aggregation under flow conditions that resemble the in vivo situation, we expressed podoplanin in NIH-3T3 fibroblasts at levels comparable with dermal lymphatic endothelial cells (data not shown). Human or mouse platelet suspensions were then continuously superfused onto the surface of fibroblast or endothelial monolayers to produce permanent laminar flow at speeds similar to that found in veins and presumably also in the patent lymph sacs. In these conditions, podoplanin-transfected NIH-3T3 fibroblasts triggered the formation of platelet aggregates already in 5 minutes, and aggregation was complete in 20 minutes (Figure 3A), regardless of whether the platelets were from wild-type or podoplanin-deficient mice. In sharp contrast, neither wild-type nor podoplanin−/− platelets from mice (Figure 3B) or humans (data not shown) formed aggregates on untransfected or mock-transfected NIH-3T3 fibroblasts. Furthermore, incubation of platelets with podoplanin+/+-transfected fibroblasts in the presence of a blocking rabbit anti-podoplanin IgG36 inhibited the formation of platelet aggregates. Finally, platelet aggregation could also be induced on cultured lymphatic, but not blood vascular endothelial cells (supplemental Table 1).
Platelet aggregation is driven by podoplanin and linked to separation of the lymph sacs from the cardinal veins. (A-B) Still frames of movies showing coincubation under flow conditions of isolated normal mouse platelets with monolayers of NIH-3T3 cells that transgenetically express podoplanin (A) or NIH-3T3 cells that were transfected with an empty vector (B). While large platelet aggregates form on the surfaces of podoplanin-expressing NIH-3T3 cells (red arrowheads), NIH-3T3 cells that lack podoplanin fail to interact with platelets even after 20 minutes. Imaged on an Olympus IX50 using a 40×/0.65 air LCAch (objective lens and a FViewII) camera (Olympus). (C-F) Direct demonstration in E12.5 and E13.5 podoplanin+/+ embryos of platelet thrombi (labeled for integrin αIIbβ3 in red) at the junction of cardinal veins and lymphatic sacs that are marked by podoplanin (green; C,E) or Lyve-1 (green; D,F). By contrast, platelets are absent from the lymph sac's orifice in E13.5 podoplanin−/− embryos (G-H) as seen on serial sections (220 μm apart) stained for Lyve-1 (green) and the platelet integrin αIIbβ3 (red). Note the still persisting connection (G) between lymph sac and vein. In the section 220 μm apart (H), the connection between lymph sac and cardinal vein is no longer visible; in panels G and H, erythrocytes/reticulocytes are present in the lymph sacs (Ter119 staining not shown). Cell nuclei are stained with DAPI (blue); Olympus AX70, 10×/0.40 air UPlanApo. Scale bars in panels A and B equal 10 μm; in panels C through H, 50 μm.
Platelet aggregation is driven by podoplanin and linked to separation of the lymph sacs from the cardinal veins. (A-B) Still frames of movies showing coincubation under flow conditions of isolated normal mouse platelets with monolayers of NIH-3T3 cells that transgenetically express podoplanin (A) or NIH-3T3 cells that were transfected with an empty vector (B). While large platelet aggregates form on the surfaces of podoplanin-expressing NIH-3T3 cells (red arrowheads), NIH-3T3 cells that lack podoplanin fail to interact with platelets even after 20 minutes. Imaged on an Olympus IX50 using a 40×/0.65 air LCAch (objective lens and a FViewII) camera (Olympus). (C-F) Direct demonstration in E12.5 and E13.5 podoplanin+/+ embryos of platelet thrombi (labeled for integrin αIIbβ3 in red) at the junction of cardinal veins and lymphatic sacs that are marked by podoplanin (green; C,E) or Lyve-1 (green; D,F). By contrast, platelets are absent from the lymph sac's orifice in E13.5 podoplanin−/− embryos (G-H) as seen on serial sections (220 μm apart) stained for Lyve-1 (green) and the platelet integrin αIIbβ3 (red). Note the still persisting connection (G) between lymph sac and vein. In the section 220 μm apart (H), the connection between lymph sac and cardinal vein is no longer visible; in panels G and H, erythrocytes/reticulocytes are present in the lymph sacs (Ter119 staining not shown). Cell nuclei are stained with DAPI (blue); Olympus AX70, 10×/0.40 air UPlanApo. Scale bars in panels A and B equal 10 μm; in panels C through H, 50 μm.
Platelet thrombi form at the orifices of embryonic lymph sacs
In E12.5 and E13.5 wild-type embryos, both podoplanin (Figure 3C,E) and Lyve-1 (Figure 3D,F) were expressed in large amounts in the endothelial cells of forming lymph sacs. Aggregates of platelets with activated αIIbβ3 integrins were encountered at the separation zone of lymph sacs and the cardinal veins (Figure 3C-F), but were absent from podoplanin−/− embryos (Figure 3G-H). This finding strongly suggests that podoplanin induces platelet aggregation also in vivo, which likely terminates the connection between the lymph sacs and the blood vasculature. In podoplanin−/− embryos, we detected no platelet aggregates at the “neck” region (Figure 3G), but we found persisting connections between the lymph sac and vein, and in erythrocytes in the lymph sac up to 220 μm away from the persisting connection (Figure 3H).
Interference with platelet aggregation/activation in vivo produces a transient “nonseparation” phenotype
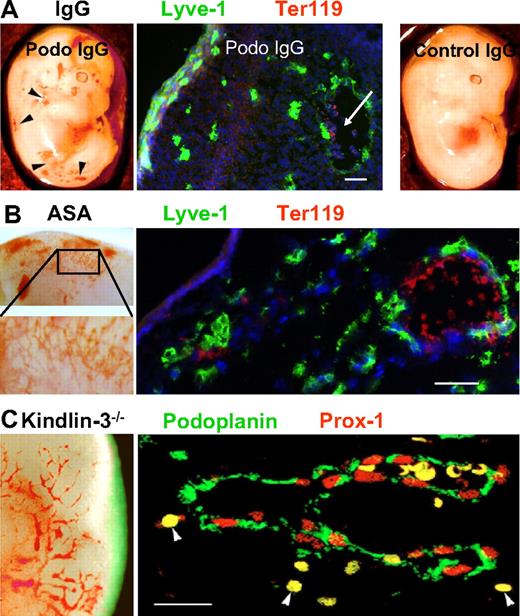
To further corroborate our hypothesis that podoplanin-induced platelet aggregation is critical for the separation of lymph sacs from the blood vasculature, we tested to see if different means of blocking platelet aggregation in vivo would also interfere with the separation process. A “nonseparation” phenotype was produced in E13.5 embryos by injecting blocking anti-podoplanin into pregnant mice, whereas control IgG gave no phenotype (Figure 4A). The blood-filled vessels generated upon anti-podoplanin antibody treatment were Lyve-1+, confirming their lymphatic identity (Figure 4A middle panel).
Inhibition of platelet aggregation causes a “nonseparation” phenotype. (A) E13.5 podoplanin+/+ embryo whose mother was injected with rabbit anti-podoplanin IgG that blocks podoplanin-platelet interaction (left panel). Subtle vascular malformations (arrowheads) similar to those in podoplanin−/− mice are locally observed. Immunofluorescence staining (middle panel) demonstrates dermal Lyve-1+ lymphatic vessels (green) containing erythrocytes (red; white arrow). Cell nuclei are stained with DAPI (blue). Right panel shows macroscopically normal vasculature in podoplanin+/+ embryo whose mother was injected with irrelevant rabbit IgG. (B) E14.5 podoplanin+/+ embryo whose mother was treated with acetyl salicylic acid (ASA; 25 mg/kg/d) to inhibit platelet aggregation. Vascular lesions reminiscent of those found in podoplanin−/− mice are locally observed (left panel; inset shows higher magnification). Immunofluorescence staining (right panel) shows that the dermal vessels are Lyve-1+ lymphatics (green) and contain erythrocytes (Ter119 in red). Cell nuclei are stained with DAPI (blue). (C) Kindlin-3−/− E14.0 embryo that shows blood-containing vascular malformations in the skin (left panel). Double immunofluorescence of dermal vessel (right panel); endothelium is positive for Prox1 (red nuclei) and podoplanin (green), and the vessel is filled with erythrocytes (yellow). Arrowheads mark extravasated erythrocytes (fluorescent images were taken on an Olympus AX70 with a 20× objective lens; all other images with the Olympus SZ40). Scale bars in panels A and B equal 50 μm; in panel C, 20 μm.
Inhibition of platelet aggregation causes a “nonseparation” phenotype. (A) E13.5 podoplanin+/+ embryo whose mother was injected with rabbit anti-podoplanin IgG that blocks podoplanin-platelet interaction (left panel). Subtle vascular malformations (arrowheads) similar to those in podoplanin−/− mice are locally observed. Immunofluorescence staining (middle panel) demonstrates dermal Lyve-1+ lymphatic vessels (green) containing erythrocytes (red; white arrow). Cell nuclei are stained with DAPI (blue). Right panel shows macroscopically normal vasculature in podoplanin+/+ embryo whose mother was injected with irrelevant rabbit IgG. (B) E14.5 podoplanin+/+ embryo whose mother was treated with acetyl salicylic acid (ASA; 25 mg/kg/d) to inhibit platelet aggregation. Vascular lesions reminiscent of those found in podoplanin−/− mice are locally observed (left panel; inset shows higher magnification). Immunofluorescence staining (right panel) shows that the dermal vessels are Lyve-1+ lymphatics (green) and contain erythrocytes (Ter119 in red). Cell nuclei are stained with DAPI (blue). (C) Kindlin-3−/− E14.0 embryo that shows blood-containing vascular malformations in the skin (left panel). Double immunofluorescence of dermal vessel (right panel); endothelium is positive for Prox1 (red nuclei) and podoplanin (green), and the vessel is filled with erythrocytes (yellow). Arrowheads mark extravasated erythrocytes (fluorescent images were taken on an Olympus AX70 with a 20× objective lens; all other images with the Olympus SZ40). Scale bars in panels A and B equal 50 μm; in panel C, 20 μm.
Similarly, inhibition of platelet aggregation by treatment of pregnant podoplanin+/+ mice with acetyl salicylic acid at approximately 50 mg/kg per day or approximately 25 mg/kg per day from approximately E8.5 until E16.5 resulted in 50% (5 of 10) and approximately 45% (13 of 28), respectively, of embryos exhibiting LYVE-1+ vessels in the skin containing red blood cells (Figure 4B). The lower concentration of acetyl salicylic acid (7.5 mg/kg/d) did not induce such phenotype in any of the 14 embryos, and this phenotype was never observed in embryos from mothers treated with the vehicle alone.
Finally, we studied kindlin-3–deficient mouse strains, which display a prominent platelet aggregation defect due to an inability to activate platelet integrins.34 Importantly, the lymphatic vessels of kindlin-3–deficient embryos around E15 also contained blood like the podoplanin-deficient animals (Figure 4C). Altogether, these results demonstrate that platelet aggregation is required for efficient separation of blood and lymphatic vessels.
Discussion
Centrifugal lymphangiogenesis4 starts by focal expression of lymphatic markers, such as Prox-1 and podoplanin, by endothelial cells of cardinal veins, followed by sprouting and formation of lymph sacs from which the peripheral lymphatics are generated.8 A critical step in this development is the separation of the newly formed lymph sacs and the parental cardinal veins.
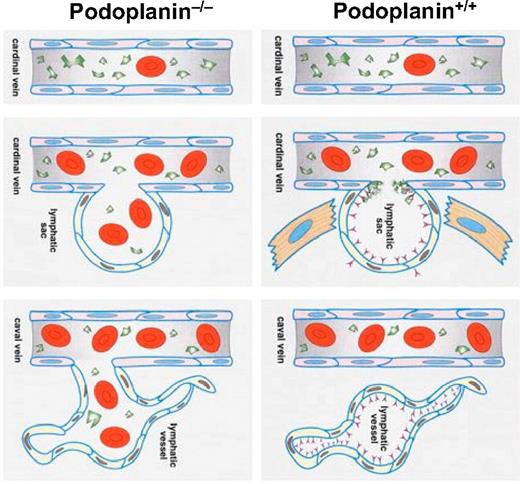
In this investigation, we have discovered the basic mechanism necessary for the lymphatic and blood vascular separation during embryonic development. Based on the vascular phenotype in podoplanin−/− mice generated in a mixed 129S/v × Swiss or C57Bl/6 background, we ascribe a decisive role in this process to the interaction of podoplanin that is expressed on the surface of nascent lymphatic endothelial cells, with circulating platelets that arrive from the blood stream of the cardinal vein (Figure 5).
Schematic depiction of the mechanisms involved in separation of lymph sacs from the cardinal veins, resulting in the “separation phenotype” and “nonseparation phenotype.” The developmental default “separation” phenotype in podoplanin+/+ mice (right column) starts with formation of lymph sacs from the cardinal vein around E12. Endothelial cells of the lymph sacs acquire the lymphatic phenotype and express podoplanin (depicted by red “Y”-shaped objects) on their surface that binds and activates circulating platelets (depicted in green). Platelet thrombi develop around the orifice of the lymph sacs and lead to separation of the lymphatic sac from the cardinal vein either directly by an occluding platelet aggregate or indirectly by release of vasoconstrictive substances or growth factors released from activated platelets recruiting mural cells. At the stage of E14 and later, the separated lymph sacs are fully sequestered from the cardinal veins and sprout centrifugally to form a separate lymphatic circulatory system. The “nonseparation” phenotype in podoplanin−/− mice (left column) is characterized by persisting patent connections between the venous and the lymphatic systems. This is due to the absence of podoplanin on the lymphatic sac's endothelial cells, and thus the lack of local platelet activation and aggregation at the lymph sac's orifices. This results in blood perfusion of the outgrowing lymphatic vessels.
Schematic depiction of the mechanisms involved in separation of lymph sacs from the cardinal veins, resulting in the “separation phenotype” and “nonseparation phenotype.” The developmental default “separation” phenotype in podoplanin+/+ mice (right column) starts with formation of lymph sacs from the cardinal vein around E12. Endothelial cells of the lymph sacs acquire the lymphatic phenotype and express podoplanin (depicted by red “Y”-shaped objects) on their surface that binds and activates circulating platelets (depicted in green). Platelet thrombi develop around the orifice of the lymph sacs and lead to separation of the lymphatic sac from the cardinal vein either directly by an occluding platelet aggregate or indirectly by release of vasoconstrictive substances or growth factors released from activated platelets recruiting mural cells. At the stage of E14 and later, the separated lymph sacs are fully sequestered from the cardinal veins and sprout centrifugally to form a separate lymphatic circulatory system. The “nonseparation” phenotype in podoplanin−/− mice (left column) is characterized by persisting patent connections between the venous and the lymphatic systems. This is due to the absence of podoplanin on the lymphatic sac's endothelial cells, and thus the lack of local platelet activation and aggregation at the lymph sac's orifices. This results in blood perfusion of the outgrowing lymphatic vessels.
Expression of genes related to the lymphatic phenotype of endothelial cells has provided crucial insights into the formation and outgrowth of lymph sacs from the cardinal vein, but—with the exception of podoplanin—no evidence for their involvement in the separation of the 2 circulatory systems was found. For example, disruption of the gene of the homeobox transcription factor Prox-1 indicated its role in the budding and migration of lymphatic endothelial cells from the cardinal vein, leading to a complete absence of the lymphatic vascular system.8,38 VEGF-C in turn was required for migration and survival of Prox-1–expressing endothelial cells and the formation of lymph sacs.14 Angiopoietin-2,13 ephrinB2,39 FoxC2,40 or α9 integrin11 play a role in lymphatic vessel differentiation and maturation, but not in the lymphatic sprouting and segregation of lymph sacs. Interestingly, in a report by Johnson et al on conditional down-regulation of Prox1, scattered blood-filled vessels were detected when Prox1 was excised in embryos from venous lymphatic endothelial cell precursors at early developmental stages. A much larger number of scattered, superficial, blood-filled vessels were observed in conditional mutant embryos in which Prox1 was excised from developing lymph sacs at later stages.41
A possible role for podoplanin in the separation of blood and lymphatic vasculature was suggested by the findings that endothelial cell O-glycan deficiency24 causes down-regulation of podoplanin expression and a “nonseparation” phenotype. Here, we confirm a central role of the expression of podoplanin by lymphatic endothelial cells26 for the separation of blood and lymphatic vasculature, specifically by its capacity to bind, activate, and aggregate platelets.31 Podoplanin is identical with aggrus that was previously discovered on the surface of murine colon carcinoma cells and found to induce platelet aggregation.31 Consistent with this observation, we demonstrate that aggregation of mouse and human platelets is induced in vitro under flow conditions by cells expressing podoplanin (supplemental Table 1).
Importantly, the in vivo significance of platelet-podoplanin interaction for the development of the “separation” phenotype in vivo is underscored by the direct immunohistologic demonstration of aggregated activated platelets at the orifices of sprouting lymphatic sacs on the side of the cardinal veins. The occlusion of this vascular communication is presumably due to the platelet aggregate itself serving as a plug, and/or to mediators released from aggregated platelets, such as vasoconstricting serotonin or PDGF and TGF-β, that attract mural cells.
Strong support for our “platelet hypothesis” of the separation of the lymphatic from the blood vasculature is further provided by experiments in which we interfere selectively with the function of platelets or with their interaction with podoplanin. All manipulations generate a “nonseparation” phenotype. Specifically, we have used approaches to inhibit in vivo podoplanin-platelet interaction by injecting blocking anti-podoplanin antibody, or treating pregnant wild-type mice with large dosages of acetyl salicylic acid. The effects of acetyl salicylic acid treatment in mice cannot be directly related to humans, because of their clear species-specific differences in sensitivity for the effects of acetyl salicylic acid.42,43 Most compelling were the results obtained with mice lacking the platelet integrin-binding protein kindlin-3. These mice suffer from a failure to aggregate platelets but display no alterations of endothelial cells.34 The nonseparation phenotype in these animals is transient, and is restricted to the time around E15. The further phenotypic development of the kindlin-3 knockout mice is, however, different from that of podoplanin knockouts, and is dominated by hemorrhages, anemia, and their clinical consequences.44 Taken together with the results obtained on podoplanin−/− mice, these observations strongly indicate that any interference with podoplanin-mediated platelet aggregation results in a “nonseparation” phenotype in embryos.
Failure to separate emerging lymphatic vessels from blood vessels similar to that observed in our podoplanin−/− mice was previously described in mice lacking the hematopoietic signaling molecules Syk19 or SLP-76;16,18 however, the underlying mechanisms for this defect were not elucidated, and the established roles of these proteins in lymphocyte differentiation did not offer an explanation either. It was speculated that vascular progenitor cells expressing these proteins differentiate into endothelial cells after their recruitment to the sites of separation, or that Syk or SLP-76 expressed by circulating hematopoietic cells convey signals critical for lymphatic or vascular endothelial cell homing and differentiation.16,45 Our findings offer an alternative simple explanation, as lack of Syk and/or SLP-76 strongly affect the capacity of platelets to activate integrins, and to undergo aggregation and activation.21-23,46 Therefore, formation of platelet plugs that separate the cardinal vein and the lymph sacs is likely compromised in embryos with deficiencies of these proteins. Further support for the validity of our “platelet hypothesis” of blood and lymphatic vascular separation is provided by the finding that ablation of other components of the intracellular Syk and SLP-76 pathways also results in a similar phenotype. This applies to phospholipase-Cγ2 that is phosphorylated by Syk and SLP-76,16,21,22 and most probably also to Rap1b, an abundant small GTPase in platelets acting downstream of phospholipase-Cγ2 and regulating the cross-talk between platelet integrin α2β1 and integrin αIIbβ3.47,48 The link between podoplanin and this described pathway of platelet aggregation was revealed recently, because Syk, SLP-76, and phospholipase-Cγ2 in platelets act downstream of CLEC-2,49 which was found to be the platelet receptor responsible for platelet aggregation induced by podoplanin.32
In our study, we also show that mice lacking the platelet integrin-binding protein kindlin-3 and displaying a prominent platelet aggregation defect due to an inability to activate platelet integrins34 exhibit a “nonseparation phenotype” similarly as the other measures interfering with platelet aggregation/activation described here do. Collectively, these findings provide evidence that expression of Syk and SLP-76 and downstream components of their intracellular pathways, as well as other components such as kindlin-3 that are essential for correct aggregation/activation of platelets, are required for proper separation of the blood and the lymphatic systems, and that defective podoplanin-driven platelet activation is the dominating common causative factor for a “nonseparation” phenotype.
An open question might be the lack of a “nonseparation” phenotype in NF-E2–deficient mice50 despite a 96% to 99% reduction in their platelet count during adulthood.51 A possible explanation could be that during the embryonic development, platelet counts in NF-E2–deficient embryos are not decreased to the same extent as that reported for the adult animals. In fact, the platelet counts in NF-E2–deficient embryos at E15.5 were estimated to be still as high as 70 000 to 80 000/μL, compared with approximately 650 000 platelets/μL in heterozygous mutant pups (Figure 6A in Levin et al 51 ), which may be sufficient to disconnect the lymphatic sac from the cardinal vein.
There are also indications that platelet-mediated separation also plays a role in human pathologies. We have recently found that in human inflammatory skin diseases, single endothelial cells of venules express lymphatic markers, similar to the initial events that precede lymph sac formation in cardinal veins.52 Moreover, preliminary results on the healing of incisional wounds in podoplanin−/− and wild-type mice provide evidence for transient, erroneous communications of newly formed lymphatic and blood microvessels, as aggregates of activated platelets are found in the lymphatics of wild-type mice, and erythrocytes are found in the lymphatics of podoplanin−/− mice (supplemental Figure 3A-B). In addition, the expression of podoplanin on the surface of angioma endothelial cells26 in partly blood-perfused vascular tumors causes thrombogenicity, and could provide a sink for platelets that causes thrombocytopenic bleeding disorders53 in some cases (supplemental Figure 3C-D).
Taken together, our results identify the interaction of platelets and podoplanin on the surface of lymphatic endothelial cells as a novel central event in the separation of lymphatic and blood vessels that integrates all currently known findings, including the vascular phenotype observed in Syk- or SLP-76–deficient animals. The relevance of this simple mechanism remains to be established for lymphangiogenesis in human pathology such as in inflammation52 or vascular tumors.26
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr E. Molinari (deceased), Technoclone Inc, Vienna, for the lipid analysis, Dr H. Kowalski (Pathology) for help in designing of the podoplanin knockout construct, Mario Hilpert (Department of Vascular Biology and Thrombosis Research) for excellent technical support, and T. Nardelli (Department of Vascular Biology and Thrombosis Research) and A. Jaeger (Pathology) for help with the artwork.
This work was supported by the Austrian Science Foundation program project grant F005 Microvascular injury and repair 5-03 to H.S., 5-07 to D.K., and 5-09 to B.R.B.; the European Union 6th framework program Integrated Projects Lymphangiogenomics (LSGH-CT-2004-503573 to D.K.) and Cancerdegradome (LSHC-CT-2003-503297 to B.R.B.); and was performed partially within the European Vascular Genomics Network of Excellence (EVGN), contract no. LSHM-CT-2003-503254 (B.R.B. laboratory).
Authorship
Contribution: P.U, J.Z., and P.C. generated the podoplanin−/− mice and analyzed the gross phenotype; J.M.B. and D.O. performed immunohistochemistry and performed platelet experiments; H.S. and E.F. provided the podoplanin-expressing NIH-3T3 fibroblasts; M.M. and R.F. provided the kindlin-3−/− mice; P.H. and K.A. produced data on the β-galactosidase–expressing mice under the VEGFR-3 promoter; B.R.B. designed the experiments and wrote the manuscript together with D.K., P.U., and J.M.B.; and D.K. provided lymphatic endothelial cells, data from the tufted angioma, and the podoplanin antibody. The podoplanin knockout mouse is a joint project of the Kerjaschki and Binder laboratories.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for P.C. is Animal Production Research Centre and SUA FBFS Nitra, Nitra, Slovak Republic.
Correspondence: Bernd R. Binder, Schwarzspanier Str 17, A-1090 Vienna, Austria; e-mail: bernd.binder@meduniwien.ac.at.
References
Author notes
P.U. and J.Z. contributed equally to this article.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal