Abstract
We prospectively studied the pharmacokinetics (PK) and clinical outcomes of intravenous busulfan (Bu) in 71 children with preexisting liver damage who underwent hematopoietic stem cell transplantation for thalassemia. Intravenous Bu was administered every 6 hours as part of a conditioning regimen with PK-based dose adjustment to target a conservative area under the concentration-versus-time curve (AUC) range (900-1350 μMol*min). The first-dose Bu clearance (CL) was significantly higher than the subsequent daily CL that remained unchanged in the ensuing days. One-third of patients required dose escalation based on dose 1 AUC, whereas dose reduction was needed in the subsequent days. At doses 5, 9, and 13, 78%, 81%, and 87% of patients, respectively, achieved the target range of AUC. A population PK analysis confirmed that the first-dose CL was 20% higher and that body weight was the most important covariate to explain PK variability. Patients with variant GSTA1*B had a 10% lower Bu CL than wild-type. These results suggest that the disease-specific behavior of intravenous Bu PK should be considered for PK-guided dose adjustment in patients with thalassemia, and the use of a conservative AUC range resulted in low toxicity, good engraftment, and good survival rate.
Introduction
Hematopoietic stem cell transplantation (HSCT) from a suitable related or unrelated matched donor provides the only cure for patients with thalassemia.1-3 High-dose busulfan (Bu) combined with cyclophosphamide (Cy) is the preferred preparatory regimen for patients with thalassemia and is a valid alternative to regimens that include total body irradiation. Although the BuCy regimen has been extensively used in patients with thalassemia few data are available on relationship of Bu pharmacokinetics (PK) to transplantation outcome with controversial results: one study found no effect of oral Bu exposure to transplantation outcome,4 whereas in 2 other studies a relationship between oral Bu PK and rejection, hepatic veno-occlusive disease (VOD),5 or mortality6 was observed. These discrepancies might be the result of the unreliability of oral Bu pharmacokinetic assessment because of erratic intestinal absorption and possible dose loss with emesis. Furthermore, children have a significantly higher total body clearance (CL) of Bu compared with adults, which results in underexposure or overexposure with standard oral doses.7,8 To optimize Bu treatment and overcome these complications therapeutic drug monitoring (TDM) of oral Bu with dose adjustment is widely practiced but with variable results.9-11 A recently developed intravenous formulation of Bu exhibits less interpatient as well as dose-to-dose variability, leading to more reliable and consistent PK estimations.12 Although in adult patients undergoing allogeneic transplantation intravenous Bu dosed at 0.8 mg/kg was found to have a dose equivalency to oral Bu at 1 mg/kg,12,13 there is no agreement in the literature regarding optimal pediatric dosing strategy of intravenous Bu. Some studies used age-based dosing of intravenous Bu with 1 mg/kg for patients 4 years of age or younger and 0.8 mg/kg for patients more than 4 years assuming that children's CL of Bu is higher in the former than in the latter age group.14-16 Other studies used 2 dosing levels (0.8 mg/kg for children > 12 kg and 1.1 mg/kg for those ≤ 12 kg) of intravenous Bu. Recently, it has been shown that intravenous Bu CL is better correlated to body weight as a nonlinear function than with age, which led to the development in children of a weight-based nomogram.17,18 This nomogram has been approved in the European Union. The conflicting PK data on intravenous Bu in pediatric patients owe to both small and heterogeneous patient populations studied. Indeed, studies have shown that oral Bu CL varies based on the underlying disease where children affected by immune deficiencies have the slowest CL, whereas children with hemoglobinopathies the most rapid CL.19 To date, no study has addressed this issue with intravenous Bu, especially in uniform disease populations, such as thalassemia.
Bu is metabolized mainly in the liver by glutathione S-transferase (GST) enzymes, primarily by GSTA1 form, with minor contributions from GSTM1 and GSTP1.20,21 Therefore, genetic polymorphisms of GST genes could in part explain high interpatient variability of Bu PK. However, there are conflicting data regarding the association of Bu CL with GST polymorphisms: some studies demonstrate an association with GSTA1*B and intravenous Bu CL,21 whereas others do not.22 In the present study, we hypothesized that intravenous Bu PK in children with thalassemia and preexisting liver damage because of iron overload and/or hepatitis could be different from other populations, and therefore the PK-guided dose adjustment after weight-based fix dosing could also require a disease-specific approach and optimization of the therapeutic window. We also studied whether the polymorphisms of the GST genes are associated with intravenous Bu PK. This is the first investigation reporting sequential intravenous Bu PK, and its relationship to clinical outcomes in a large group of children with thalassemia.
Methods
This study was a prospective, single-center investigation of the PK of intravenous Bu in children. The study included 71 consecutive patients with thalassemia (n = 68) or sickle cell anemia (n = 3) who underwent HSCT from a human leukocyte antigen (HLA)–identical family donor between June 2006 and March 2009. The Mediterranean Institute of Hematology Institutional Review Board approved the treatment protocol, and all parents or patients provided written informed consent, in accordance with the Declaration of Helsinki.
Treatment regimen
All patients were conditioned with intravenous Bu (Busilvex; Pierre Fabre Medicament) and Cy with or without thiotepa (Table 1). Patients who received stem cells from phenoidentical relatives or sickle cell anemia were also given thymoglobulin (Genzyme) to facilitate engraftment. Class 3 patients, before conditioning with intravenous Bu/Cy160 with or without TT10, were given preconditioning chemotherapy consisting of azathioprine, hydroxyurea, and fludarabine to reduce a large disease burden and increase immunosuppression to avoid peritransplantation drug toxicity.24 Bone marrow was used in 70 patients, with 1 patient receiving peripheral blood stem cells for second transplantation. Diagnosis and degree of acute and chronic graft-versus-host disease (GVHD) were assessed according to consensus criteria.25
Patient, transplantation, and graft characteristics
| Variable . | Patients (n = 71) . |
|---|---|
| Patient sex, male/female | 42/29 |
| Median patient age, y (range) | 9 (1.6-27) |
| Mean patient age, y (± SD) | 10.2 (± 5.2) |
| Patients older than 18 y, n | 4 |
| Mean patient weight ± SD, kg (range) | 26.4 ± 11.15 (10-60) |
| Median donor age, y (range) | 11 (1.3-52) |
| Diagnosis | |
| Thalassemia | 68 |
| Sickle cell anemia | 3 |
| Risk class (1/2/3/not applicable) | 6/23/39/3 |
| Median AST, IU/L (range) | 33 (13-216) |
| Median ALT, IU/L (range) | 38 (8-247) |
| Median bilirubin, mg/dL (range) | 0.9 (0.3-2.8) |
| Median serum ferritin, ng/mL (range) | 2126 (279-9458) |
| Serum ferritin > 2000 ng/mL, n | 38 |
| Median liver iron concentration, mg/g dw (range) | 17 (0.6-47.8) |
| Liver iron concentration > 7 mg/g dw, n | 55 |
| Median packed RBC units received before transplantation, n (range) | 95 (2-500) |
| Liver size ≤ 3 cm, n | 55 |
| Liver size > 3 cm, n | 16 |
| Splenectomy, yes/no | 17/54 |
| Liver fibrosis score (Ishak et al23 ; staging 0-6) | 1 (0-6) |
| Incomplete cirrhosis | 4 |
| Cirrhosis | 2 |
| Hepatitis C (HCV-RNA–positive) | 7 |
| Hepatitis B (HBs antigen–positive) | 1 |
| HLA-identical siblings | 62 |
| HLA-phenotypically identical parents or relatives | 9 |
| Donor/patient CMV serology | |
| Both positive | 60 |
| Any positive | 9 |
| Both negative | 2 |
| Conditioning regimen | |
| IV Bu/CY200 | 11 |
| IV Bu/TT10/CY200 | 14 |
| IV Bu/CY160 preceded by HU AZA FL20 | 15 |
| IV Bu/TT10/CY160 preceded by HU AZA FL30 | 15 |
| IV Bu/TT10/CY90 preceded by HU AZA FL30 | 3 |
| IV Bu/TT10/CY200/ATG10 preceded by HU AZA FL30 | 10 |
| IV Bu/CY200/ATG10 | 3 |
| GVHD prophylaxis | |
| CSA + methylprednisolone + short MTX 10 mg/m2 (d+1, d+3, d+6) | 39 |
| CSA + methylprednisolone + CY7.5 mg/kg (d+1) + MTX 10 mg/m2 (d+3, d+6) | 32 |
| Median nucleated cell dose, ×108/kg (range) | 4.4 (1.4-10.8) |
| Median CD34+ cell dose, ×106/kg (range) | 7.1 (1.4-26) |
| Variable . | Patients (n = 71) . |
|---|---|
| Patient sex, male/female | 42/29 |
| Median patient age, y (range) | 9 (1.6-27) |
| Mean patient age, y (± SD) | 10.2 (± 5.2) |
| Patients older than 18 y, n | 4 |
| Mean patient weight ± SD, kg (range) | 26.4 ± 11.15 (10-60) |
| Median donor age, y (range) | 11 (1.3-52) |
| Diagnosis | |
| Thalassemia | 68 |
| Sickle cell anemia | 3 |
| Risk class (1/2/3/not applicable) | 6/23/39/3 |
| Median AST, IU/L (range) | 33 (13-216) |
| Median ALT, IU/L (range) | 38 (8-247) |
| Median bilirubin, mg/dL (range) | 0.9 (0.3-2.8) |
| Median serum ferritin, ng/mL (range) | 2126 (279-9458) |
| Serum ferritin > 2000 ng/mL, n | 38 |
| Median liver iron concentration, mg/g dw (range) | 17 (0.6-47.8) |
| Liver iron concentration > 7 mg/g dw, n | 55 |
| Median packed RBC units received before transplantation, n (range) | 95 (2-500) |
| Liver size ≤ 3 cm, n | 55 |
| Liver size > 3 cm, n | 16 |
| Splenectomy, yes/no | 17/54 |
| Liver fibrosis score (Ishak et al23 ; staging 0-6) | 1 (0-6) |
| Incomplete cirrhosis | 4 |
| Cirrhosis | 2 |
| Hepatitis C (HCV-RNA–positive) | 7 |
| Hepatitis B (HBs antigen–positive) | 1 |
| HLA-identical siblings | 62 |
| HLA-phenotypically identical parents or relatives | 9 |
| Donor/patient CMV serology | |
| Both positive | 60 |
| Any positive | 9 |
| Both negative | 2 |
| Conditioning regimen | |
| IV Bu/CY200 | 11 |
| IV Bu/TT10/CY200 | 14 |
| IV Bu/CY160 preceded by HU AZA FL20 | 15 |
| IV Bu/TT10/CY160 preceded by HU AZA FL30 | 15 |
| IV Bu/TT10/CY90 preceded by HU AZA FL30 | 3 |
| IV Bu/TT10/CY200/ATG10 preceded by HU AZA FL30 | 10 |
| IV Bu/CY200/ATG10 | 3 |
| GVHD prophylaxis | |
| CSA + methylprednisolone + short MTX 10 mg/m2 (d+1, d+3, d+6) | 39 |
| CSA + methylprednisolone + CY7.5 mg/kg (d+1) + MTX 10 mg/m2 (d+3, d+6) | 32 |
| Median nucleated cell dose, ×108/kg (range) | 4.4 (1.4-10.8) |
| Median CD34+ cell dose, ×106/kg (range) | 7.1 (1.4-26) |
RBC indicates red blood cell; IV, intravenous; TT, thiotepa; HU, hydroxyurea; AZ, azathioprine; FLU, fludarabine; ATG, thymoglobulin; MTX, methotrexate; CSA, cyclosporine A; and dw, dry weight.
Intravenous Bu doses were based on actual patient body weight (9 to < 16 = 1.2 mg/kg/dose [n = 13]; 16 to < 23 = 1.1 mg/kg/dose [n = 17]; 23 to 34 = 0.95 mg/kg/dose [n = 26]; and > 34 = 0.8 mg/kg/dose [n = 15]) and was administered over 4 consecutive days in 4 divided doses as an intravenous infusion (concentration, 0.6 mg/mL) for 2 hours. No drugs that potentially could interfere with Bu metabolism were given during treatment. No hepatic VOD prophylaxis was given. All patients received valproic acid (Depakin; Sanofi-Aventis) at a dose of 30 mg/kg/day in 3 divided doses starting at 24 hours before the first Bu administration and continuing until 24 hours after the last Bu dose as an anticonvulsant prophylaxis. To control emesis, patients received granisetron at 40 μg/kg (intravenously) before the first dose of intravenous Bu each day. The interval between the last dose of Bu and the first dose of Cy was 14 hours in 30 patients, and it was 38 hours for the remaining 41 patients.
Bu assay
After sample purification,26 Bu assaying was performed using high- performance liquid chromatography coupled with a mass spectrometer operating in multiple reaction monitoring in positive mode.27 Bu concentration was determined using a linear 7-point calibration curve ranging from 39 to 2500 ng/mL. [2H8]-Bu (Eurisotop) was used as an internal standard. Quality control plasma samples at 3 concentration levels (250, 500, and 1000 ng/mL) were analyzed during each batch run. The recovery of the assay was between 87% and 91%. The linear regression constant (r2) was more than 0.99 in all cases. The intra- and interassay variability were less than 10% for all tested concentrations. The interassay SD was less than 10% for calibration standard and quality controls. The limit of detection was 2 ng/mL, and quantitation limit was 7 ng/mL.
Blood sampling and TDM
Blood samples were collected immediately before and 2, 4, and 6 hours after the 1st, 5th, 9th, and 13th doses of intravenous Bu as suggested by the limited sampling strategies.28,29 In 68 patients, samples were collected from a peripheral line, and in 3 very young children from a central line after withdrawal of 5 mL of blood (preceded by normal saline washing of the line) before taking each sample. The samples of 67 patients were assayed immediately, whereas the samples of the remaining 4 patients were centrifuged and the plasma was stored at −80°C until analysis the next day. The area under the curve (AUC) was calculated by the linear trapezoidal rule with a noncompartmental approach. The AUC at dose 1 was calculated by extrapolated area to time infinity after the last measurable plasma concentration (AUCinf). The average steady-state Bu plasma concentration (Css) was calculated as observed AUC (0-6 hours) divided by the dosing interval. In 67 patients, PK-guided dose adjustments were performed at dose 3, and in 4 patients at dose 7 if necessary, to target the AUC to a steady state of 1125 μMol*min (range, 900-1350 μMol*min). A total of 282 PK profiles and 1087 plasma samples were processed. For dose adjustment, the following formula was used: adjusted dose (mg) = actual dose (mg) × target dose AUC (μMol*min)/actual AUC (μMol*min), where the target dose was 1125 μMol*min.
Population PK analysis
The data were then retrospectively analyzed by population PK modeling using the NONMEM software (Version 6.2; ICON Dev). The population PK analysis was performed to search for relevant relationships between Bu total body CL and covariates. Studied covariates included demographic, biochemical, and other thalassemia-oriented characteristics, such as class of risk, serum ferritin concentrations, liver iron concentrations, liver fibrosis score, hepatomegaly, splenomegaly, number of red blood cells units, and severity of hepatitis. In addition, GST genotype data were tested as categorical covariates. As previously published,17,18 concentration-time data were fitted using a one-compartment PK model with first-order elimination from the central compartment. Both interpatient and intrapatient variabilities were described by exponential models assuming log-normal distribution of PK parameters, and residual variability was heteroscedastic. The first order with conditional estimation method was used throughout the modeling process. The log-likelihood ratio test evaluated the significance of covariates inclusion into the model; a difference in the objective function value higher than 3.8 (1 degree of freedom) was used for comparison between hierarchical models. A forward inclusion and backward deletion procedure was used to select the relevant covariates into the model. Once established, the final population PK model was used to provide empirical Bayesian estimates of individual PK parameters for subsequent evaluation of the relationships with transplantation outcomes.
GST genotype determination
Genomic DNA was purified from 200 μL of whole blood using the MagNA Pure LC DNA Isolation Kit I in the automated extractor MagNa Pure LC (Roche Diagnostics GmbH). Genotyping of GSTP1, GSTM1, and GSTT1 genes was performed as previously described.30 GSTA1 was sequenced as follows: 100 ng of genomic DNA was amplified in 20 μL containing polymerase buffer, 1.5mM MgCl2, 0.25mM deoxyribonucleoside triphosphates, 0.25μM primer sense (5′-CCCTACATGGTATAGGTGAAAT-3′) and antisense (5′-GTGCTAAGGACACATATTAGC-3′) and 1.5 U of Taq polymerase. Polymerase chain reaction (PCR) conditions were: 95°C for 10 minutes, (96°C for 30 seconds, 53°C for 30 seconds, 72°C for 1 minute 30 seconds) for 35 cycles, and lastly 72°C for 10 minutes; 20 ng of PCR products, purified with the Agencourt AMPure PCR Purification kit (Agencourt Bioscience Corporation), was sequenced in 10 μL of reaction solution containing sequencing buffer, 0.5 pmol of reverse primer, and ABI PRISM BigDye Terminator, Version 3.1 Ready Reaction Cycle Sequencing Kit (Applied Biosystems). Sequencing reaction conditions were 96°C for 1 minute, (96°C for 10 seconds, 50°C for 5 seconds, and 60°C for 4 minutes) for 40 cycles. Products were purified with the CleanSEQ dye terminal removal kit (Agencourt Bioscience Corporation) and analyzed by a 3730 DNA Analyzer Instrument (Applied Biosystems).
Regimen-related toxicity
Regimen-related toxicity was scored using the NCI Common Toxicity Criteria, Version 2.0. During the first 30 days after transplantation, oral mucositis, central nervous system, gastrointestinal, hepatic, pulmonary, cardiac, and renal toxicity were evaluated. Any positive blood culture for bacteria or fungal species was documented as bacteremia or fungemia, respectively. The Jones criteria were used for the diagnosis of VOD.31
Disease monitoring after transplantation
The first chimerism analysis was performed on bone marrow samples at 20 days after transplantation for the percentage of donor/recipient DNA using PCR-based analysis of short tandem repeats (Profiler Plus Applera). Subsequently, at 60, 90, 180, and 365 days after transplantation, lineage-specific chimerism was performed.
Definitions of outcomes
The day of neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count of 500 or higher. Platelet engraftment was defined as the first of 7 consecutive days with platelet counts higher than 20 000 without transfusion. Primary graft failure or rejection was defined by persistent pancytopenia with no evidence of hematologic recovery of donor cells beyond 28 days after transplantation, and secondary graft failure by a rapid decrease in neutrophil count after successful engraftment.
Supportive care
For infectious disease prophylaxis patients were given systemic antibacterial antibiotics and antifungal drugs (amphotericin B) until the neutrophil level exceeded 1.0 × 109/L. They also received acyclovir as herpes virus and trimethoprim/sulfamethoxazole for Pneumocystis jiroveci prophylaxis. Patients were monitored weekly for the Epstein-Barr virus, cytomegalovirus, adenovirus, and BK virus in the blood and/or urine using sensitive reverse transcription PCR starting before conditioning until at least 100 days after transplantation. Test for cytomegalovirus pp65 antigen was performed twice weekly at hematologic recovery.
Relationships between Bu PK parameters and transplantation outcomes
The relationships between PK parameters and transplantation outcomes were characterized using logistic regression analysis. When appropriate, Fisher exact test was used. Study endpoints included graft failure/rejection, survival, thalassemia-free survival, transplantation-related mortality, and acute GVHD. The relationship between PK parameters and survival and disease-free survival was evaluated using the Kaplan-Meier method,32 graft failure, GVHD, and transplantation-related mortality using cumulative incidence curves. For this purpose, the PK parameters were categorized into groups above and below the median. All P values were 2-tailed and considered significant when P was less than .05. All statistical analysis were performed with StatView 5 (SAS Institute) statistical software and R statistical program, Version 2.9.1 (R Project for Statistical Computing).33
Results
Patients' characteristics
Table 1 summarizes patients, disease, and transplantation characteristics. Patients with severe organ system dysfunction were not eligible for this study. The median age of patients was 9 years (range, 1.6-27 years), and the mean weight was 26.4 plus or minus 11.15 kg. Seven patients had hepatitis C and one chronic HBAg-positive hepatitis at the time of transplantation. Most patients had severe iron overload with serum ferritin more than 2000 ng/mL in 38 (54%), and liver iron concentration more than 7 mg/g dry weight (normal value < 1.6 mg/g dry weight) in 55 (78%) of 65 evaluable patients. Fifty-eight (82%) patients had stage 1-6 liver fibrosis on liver biopsies. Four of these patients had incomplete cirrhosis and 2 had cirrhosis. Most patients were in the advanced phase of disease (class 3 of risk).
Table 2 illustrates the frequencies of GST variants. Forty-two of the 61 patients studied were heterozygous (n = 31) or homozygous (n = 11) for GSTA1*B. The GSTM1*0 (null) and GSTT1*0 (null) homozygous genotypes were observed in 28 and 11 patients, respectively. Thirty-six patients were homozygous for GSTP1*A (n = 28) or GSTP1*B (n = 8).
Genotypes of GST (n = 61)
| Isoenzyme/genetic variant . | Frequency (%) . |
|---|---|
| GST A1 | |
| A/B | 31 (51) |
| A/A | 19 (31) |
| B/B | 11 (18) |
| GST M1 | |
| M1+ | 33 (54) |
| M1 null | 28 (46) |
| GST T1 | |
| T1+ | 50 (82) |
| T1 null | 11 (18) |
| GST P1 | |
| A/A | 28 (46) |
| A/B | 15 (25) |
| A/C | 7 (11) |
| B/B | 8 (13) |
| B/C | 3 (5) |
| Isoenzyme/genetic variant . | Frequency (%) . |
|---|---|
| GST A1 | |
| A/B | 31 (51) |
| A/A | 19 (31) |
| B/B | 11 (18) |
| GST M1 | |
| M1+ | 33 (54) |
| M1 null | 28 (46) |
| GST T1 | |
| T1+ | 50 (82) |
| T1 null | 11 (18) |
| GST P1 | |
| A/A | 28 (46) |
| A/B | 15 (25) |
| A/C | 7 (11) |
| B/B | 8 (13) |
| B/C | 3 (5) |
Bu PK
TDM results.
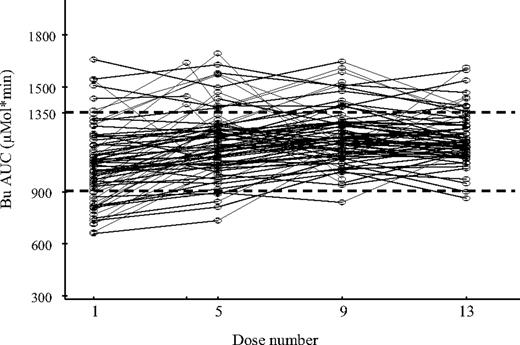
Table 3 presents noncompartmental PK parameters. Figure 1 shows individual patient AUC values at doses 1, 5, 9, and 13. All patients were available for all PK parameters at dose 1, 5, 9, and 13. Two patients' PK parameters were not evaluable at dose 13 due to incomplete sample collection. After the initial dose, the median AUCinf was 971 μMol*min (range, 630-1621 μMol*min), and the median CL normalized to body weight was 4.1 mL/min per kilogram (range, 2.38-6.19 mL/min per kilogram). The median volume of distribution (Vd) was 0.63 L/kg (range, 0.39-0.90 L/kg). Without dose adjustment, 42 patients (59%) had AUCinf within the target range (900-1350 μMol*min), 24 patients (34%) less than 900 μMol*min, and 5 patients (7%) more than 1350 μMol*min. On the basis of the first-dose PK analysis, 24 patients required dose escalation by a median of 29% (range, 10%-62%), whereas 5 patients required dose reduction by 29% (range, 9%-45%) to achieve the targeted range (Tables 4–5). For the subsequent dose adjustments, patients outside the target required mainly dose reduction. At days 2, 3, and 4, dose reduction was required in 18%, 10%, and 16% of patients, respectively, whereas dose increase was needed in 6%, 4%, and none of the patients. After dose adjustment, 78% of patients at dose 5, 81% at dose 9, and 87% at dose 13 maintained an AUC within the target range.
Intravenous busulfan pharmacokinetic data
| . | Day 1 . | Day 2 . | Day 3 . | Day 4 . |
|---|---|---|---|---|
| No. of evaluable patients | 71 | 71 | 71 | 69 |
| Bu AUC, μMol*/min | ||||
| Mean ± SD | 996 ± 213 | 1210 ± 201 | 1234 ± 176 | 1210 ± 138 |
| Median (range) | 971 (630-1621) | 1202 (746-1664) | 1219 (845-1700) | 1193 (855-1709) |
| Percentage coefficient of variation | 21.4 | 16.6 | 14.3 | 11.4 |
| Bu concentration (Css), ng/mL | ||||
| Mean ± SD | 682 ± 146 | 828 ± 137 | 844 ± 121 | 828 ± 94 |
| Median (range) | 664 (431-1109) | 822 (510-1138) | 830 (578-1163) | 816 (585-1169) |
| Percentage coefficient of variation | 21.4 | 16.6 | 14.3 | 11.4 |
| Bu clearance, mL/min per kilogram | ||||
| Mean ± SD | 4.15 ± 0.87 | 3.62 ± 0.80 | 3.71 ± 0.75 | 3.41 ± 0.78 |
| Median (range) | 4.10 (2.38-6.19) | 3.55 (2.08-5.75) | 3.30 (1.93-5.50) | 3.36 (1.8-5.58) |
| Percentage coefficient of variation | 30.0 | 22.1 | 20.2 | 22.8 |
| T1/2, hours | ||||
| Mean ± SD | 1.85 ± 0.23 | 2.13 ± 0.28 | 2.26 ± 0.30 | 2.27 ± 0.33 |
| Median (range) | 1.83 (1.2-2.48) | 2.1 (1.43-3.06) | 2.25 (1.33-3.08) | 2.24 (1.42-3.28) |
| Percentage coefficient of variation | 12.4 | 13.2 | 13.3 | 14.5 |
| . | Day 1 . | Day 2 . | Day 3 . | Day 4 . |
|---|---|---|---|---|
| No. of evaluable patients | 71 | 71 | 71 | 69 |
| Bu AUC, μMol*/min | ||||
| Mean ± SD | 996 ± 213 | 1210 ± 201 | 1234 ± 176 | 1210 ± 138 |
| Median (range) | 971 (630-1621) | 1202 (746-1664) | 1219 (845-1700) | 1193 (855-1709) |
| Percentage coefficient of variation | 21.4 | 16.6 | 14.3 | 11.4 |
| Bu concentration (Css), ng/mL | ||||
| Mean ± SD | 682 ± 146 | 828 ± 137 | 844 ± 121 | 828 ± 94 |
| Median (range) | 664 (431-1109) | 822 (510-1138) | 830 (578-1163) | 816 (585-1169) |
| Percentage coefficient of variation | 21.4 | 16.6 | 14.3 | 11.4 |
| Bu clearance, mL/min per kilogram | ||||
| Mean ± SD | 4.15 ± 0.87 | 3.62 ± 0.80 | 3.71 ± 0.75 | 3.41 ± 0.78 |
| Median (range) | 4.10 (2.38-6.19) | 3.55 (2.08-5.75) | 3.30 (1.93-5.50) | 3.36 (1.8-5.58) |
| Percentage coefficient of variation | 30.0 | 22.1 | 20.2 | 22.8 |
| T1/2, hours | ||||
| Mean ± SD | 1.85 ± 0.23 | 2.13 ± 0.28 | 2.26 ± 0.30 | 2.27 ± 0.33 |
| Median (range) | 1.83 (1.2-2.48) | 2.1 (1.43-3.06) | 2.25 (1.33-3.08) | 2.24 (1.42-3.28) |
| Percentage coefficient of variation | 12.4 | 13.2 | 13.3 | 14.5 |
Therapeutic dose strata of intravenous busulfan
| . | Busulfan dose, mg/kg . | |||
|---|---|---|---|---|
| 0.8 . | 0.95 . | 1.1 . | 1.2 . | |
| No. of patients | 15 | 26 | 17 | 13 |
| Total initial dose, mg/kg | 12.8 | 15.2 | 17.6 | 19.2 |
| Total final dose, mg/kg (mean ± SD) | 13.8 ± 2 | 16.4 ± 3 | 16.7 ± 1.7 | 18.6 ± 2.3 |
| Range | 10.7-17.1 | 13.6-24.3 | 13.6-21 | 13.9-21.8 |
| . | Busulfan dose, mg/kg . | |||
|---|---|---|---|---|
| 0.8 . | 0.95 . | 1.1 . | 1.2 . | |
| No. of patients | 15 | 26 | 17 | 13 |
| Total initial dose, mg/kg | 12.8 | 15.2 | 17.6 | 19.2 |
| Total final dose, mg/kg (mean ± SD) | 13.8 ± 2 | 16.4 ± 3 | 16.7 ± 1.7 | 18.6 ± 2.3 |
| Range | 10.7-17.1 | 13.6-24.3 | 13.6-21 | 13.9-21.8 |
Therapeutic dose adjustment of intravenous busulfan
| . | Day 1 . | Day 2 . | Day 3 . | Day 4 . |
|---|---|---|---|---|
| No. of evaluable patients | 71 | 71 | 71 | 69 |
| Mean dose elevation, percentage (± SD) | 31 (±15) | 18 (±11) | 24 (±12) | 0 |
| Median (range) | 29 (10-62) | 14 (9-33) | 18 (18-38) | — |
| Mean dose reduction, percentage (± SD) | 27 (±15) | 20 (±7) | 16 (±6) | 25 (±10) |
| Median (range) | 29 (9-45) | 20 (7-33) | 17 (7-25) | 23 (11-40) |
| Patients requiring dose elevation, n (%) | 24 (34) | 4 (6) | 3 (4) | 0 |
| Patients requiring dose reduction, n (%) | 5 (7) | 13 (18) | 7 (10) | 11 (16) |
| Patients not requiring dose adjustment, n (%) | 42 (59) | 54 (76) | 61 (86) | 58 (84) |
| . | Day 1 . | Day 2 . | Day 3 . | Day 4 . |
|---|---|---|---|---|
| No. of evaluable patients | 71 | 71 | 71 | 69 |
| Mean dose elevation, percentage (± SD) | 31 (±15) | 18 (±11) | 24 (±12) | 0 |
| Median (range) | 29 (10-62) | 14 (9-33) | 18 (18-38) | — |
| Mean dose reduction, percentage (± SD) | 27 (±15) | 20 (±7) | 16 (±6) | 25 (±10) |
| Median (range) | 29 (9-45) | 20 (7-33) | 17 (7-25) | 23 (11-40) |
| Patients requiring dose elevation, n (%) | 24 (34) | 4 (6) | 3 (4) | 0 |
| Patients requiring dose reduction, n (%) | 5 (7) | 13 (18) | 7 (10) | 11 (16) |
| Patients not requiring dose adjustment, n (%) | 42 (59) | 54 (76) | 61 (86) | 58 (84) |
— indicates not applicable.
The first dose intravenous Bu CL normalized to weight (median, 4.1 mL/min per kilogram; range, 2.38-6.19 mL/min per kilogram) was significantly higher compared with subsequent daily dose CL (P = .02, Student t test), whereas no change in CL from dose 5 to dose 13 was observed (Table 3). There was approximately 20% difference between CL at dose 1 and the subsequent doses. This finding has never been reported before. To investigate a possible impact of a short sampling interval (limited to 4 hours after end of infusion) on dose 1 AUC or CL calculation, the second dose of intravenous Bu was delayed to 12 hours in 7 patients enrolled in this study, and PK sampling was performed over 12 hours first-dose sampling at times: 0, 2, 4, 6, 9, and 12 hours. Comparing the 2 sampling intervals (0-6 vs 0-12 hours), there was no significant difference in the estimation of AUCinf or CL. The mean percentage deviation between the 2 calculations was less than 1%, showing that there is no impact of the limited sampling on AUC and CL calculations at dose 1.
Population PK analysis results.
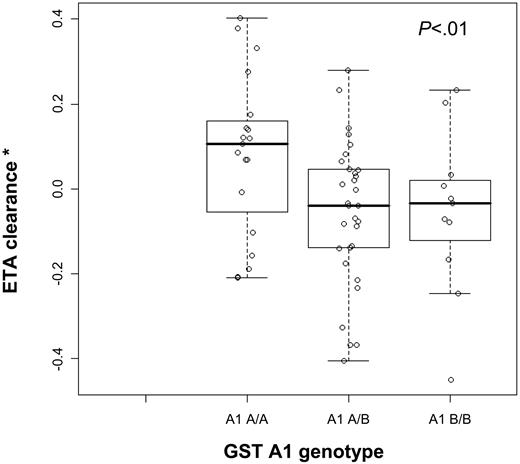
In the covariate-free model, interpatient variability in CL and Vd was 39% and 42%, respectively. Intrapatient (dose-to-dose) variability for CL was 11%. Based on the finding that the first-dose Bu CL was significantly higher than that of the subsequent doses, this difference in CL was taken into account in the model. A 20% difference was estimated by the population PK approach. This “dose 1 effect” significantly improved the fit of the data and was therefore retained in the next steps of the modeling process. We subsequently performed the covariate analysis. The model showed that weight was the most important covariate to explain variability in CL and in the Vd. Furthermore, it was seen that GST polymorphism had a statistically significant influence on CL with approximately 10% lower CL in GSTA1*B versus wild-type (Figure 2). Other GST polymorphisms had no influence on the PK parameters of intravenous Bu. All other covariates had no impact on CL. When the relevant covariates, such as weight, GSTA1 genotype, and “dose 1 effect,” were added to the model, the interpatient variability for CL and for Vd decreased from 39% (covariate-free) to 19%, and from 42% (covariate-free) to 16%, respectively. Intrapatient variability for CL was 6%. So covariates accounted for approximately 50% of the variabilities observed in these patients.
Bu clearance versus GST A1 polymorphism. ETA (Empirical Bayes Estimates) clearance is calculated as the difference between each individual value and the mean population model value given that the model included both body weight and “dose 1 effect” covariates. ETACL value represents the remaining unexplained variability in Bu clearance once body weight and “dose 1 effect” are accounted for.
Bu clearance versus GST A1 polymorphism. ETA (Empirical Bayes Estimates) clearance is calculated as the difference between each individual value and the mean population model value given that the model included both body weight and “dose 1 effect” covariates. ETACL value represents the remaining unexplained variability in Bu clearance once body weight and “dose 1 effect” are accounted for.
Clinical outcomes
Engraftment
Sixty-seven (94%) patients had sustained engraftment. Graft failure/rejection occurred in 4 thalassemia patients with a cumulative incidence of 5% (95% confidence interval [CI], 1%-12%). Two patients had primary and 2 secondary graft failure. Three of these 4 patients were in class 3 of risk. None of these 4 patients at the first dose had intravenous Bu AUC less than 900 μMol*min. The median time to ANC more than or equal to 500 × 109/L was 20 days (range, 14-30 days), and median time to a platelet count more than or equal to 20 000 × 109/L was 24 days (range, 13-56 days). At 2 months after transplantation, 61 (91%) of 67 evaluable patients had complete myeloid and lymphoid chimerism and 6 patients (9%) mixed donor chimerism between 85% and 97%. At 6 months after transplantation, 94% of patients had complete and 6% had mixed chimerism. The only patient who had 85% of donor chimerism at 2 months subsequently lost his graft. All the remaining 5 patients had stable mixed chimerism with donor cells between 93% and 97%.
GVHD
The cumulative incidence of grade II-IV and III-IV acute GVHD was 30% (95% CI, 19%-40%) and 6% (95% CI, 2%-14%), respectively. Eight patients experienced extensive chronic GVHD with a cumulative incidence of 12% (95% CI, 6%-21%).
Survival, disease-free survival, and transplantation-related mortality
Overall, within the first 100 days after transplantation, 69 patients were alive and 66 of them were disease-free. The probability of overall survival and disease-free survival at day 100 was 97% (95% CI, 90%-99%) and 92% (95% CI, 83%-96%), respectively. Death occurred within 100 days in 2 patients, and the cause was acute GVHD. There were an additional 4 deaths beyond 100 days resulting from infections (n = 2), chronic GVHD (n = 1), and posttransplantation splenectomy (n = 1), yielding 3-year overall survival and disease-free survival of 91% (95% CI, 82%-96%) and 87% (95% CI, 77%-93%), respectively, with a median follow-up of 31 months (range, 9-42 months) for surviving patients.
Regimen-related toxicities
Toxic complications observed in our patients are summarized in Table 6. No intravenous Bu infusion-related toxicity was observed. One patient (1.4%) developed moderate hepatic VOD, which resolved with supportive care within 7 days. One patient had grade 3 hyperbilirubinemia without other features of hepatic VOD, which resolved within 5 days. Both patients had plasma Bu AUCs greater than 1350 μMol*min during 2 or 3 plasma concentration assessments requiring dose adjustment. Most frequent grade 2 toxicity was aspartate aminotransferase (AST) and alanine aminotransferase (ALT) elevations (38%) followed by stomatitis (17%), diarrhea (9.8%), and early hemorrhagic cystitis (7%). Ten (14%) patients experienced grade 3 toxicity. None of the patients had grade 4 toxicity. After the 15th dose of intravenous Bu, one patient had grade 2 neurotoxicity with somnolence, confusion, and mental slowing, and this necessitated switching the last 16th dose of the drug. His mean AUC was 1145 μMol*min (range, 1031-1215 μMol*min). Blood concentration of valproic acid determined immediately at the onset of neurologic symptoms was within the normal range, and he continued drug administration for the next 3 days with complete resolution of neurotoxicity. This patient had sustained complete donor chimerism. There were no cases of convulsions. None of the patients developed interstitial pneumonia. Six patients developed Gram-negative, 2 patients Gram-positive bacteremia, and one patient lung aspergillosis during the aplastic phase. Cytomegalovirus reactivation occurred in 58% of patients.
Regimen-related toxicity according to NCI-CTC, Version 2.0
| . | Toxicity grade, no. (%) of patients . | ||||
|---|---|---|---|---|---|
| Grade 0 . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | |
| Heart | 71 | — | — | — | — |
| Bladder | 66 (93) | — | 5 (7) | — | — |
| Kidney | 71 | — | — | — | — |
| Lung | 71 | — | — | — | — |
| Liver | |||||
| AST and ALT | 26 (37) | 13 (18) | 27 (38) | 5 (7) | — |
| Bilirubin | 69 (97) | — | — | 2 (2.8) | — |
| Central nervous system | 70 | — | 1 (1.4) | — | — |
| Stomatitis | 40 (56) | 17 (24) | 12 (17) | 2 (2.8) | — |
| Gut | 59 (83) | 4 (5.6) | 7 (9.8) | 1 (1.4) | — |
| . | Toxicity grade, no. (%) of patients . | ||||
|---|---|---|---|---|---|
| Grade 0 . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | |
| Heart | 71 | — | — | — | — |
| Bladder | 66 (93) | — | 5 (7) | — | — |
| Kidney | 71 | — | — | — | — |
| Lung | 71 | — | — | — | — |
| Liver | |||||
| AST and ALT | 26 (37) | 13 (18) | 27 (38) | 5 (7) | — |
| Bilirubin | 69 (97) | — | — | 2 (2.8) | — |
| Central nervous system | 70 | — | 1 (1.4) | — | — |
| Stomatitis | 40 (56) | 17 (24) | 12 (17) | 2 (2.8) | — |
| Gut | 59 (83) | 4 (5.6) | 7 (9.8) | 1 (1.4) | — |
— indicates not applicable.
The relationship between Bu PK and transplantation outcomes
The effect of intravenous Bu PK parameters on various outcomes was examined by modeling PK parameters as a continuous variable and by dichotomizing variables as more than or equal to the median versus less than the median. There was no correlation between intravenous Bu PK parameters and toxicity, graft failure, mixed chimerism, hepatic VOD, acute GVHD, chronic GVHD, and transplantation-related mortality (data not shown).
Discussion
High Bu exposure (AUC > 1500 μMol*min) was associated with greater toxicity, whereas low exposure (AUC < 900 μMol*min) was associated with graft rejection or relapse.34,35 Consequently, targeting the therapeutic range of exposure may improve clinical outcomes. Therefore, better targeting strategies are needed to define optimal dosing and thus avoid subtherapeutic or supratherapeutic drug exposure. The choice of a therapeutic window from 900 to 1350 μMol*min in our patients was based on the underlying disease usually characterized by the presence of liver damage resulting from severe iron overload and/or hepatitis, which might increase the risk of drug-related toxicity. Therefore, we used a more conservative threshold for toxicity (upper limit of 1350 instead of 1500 μMol*min). This therapeutic window should minimize the failure to engraft and the incidence of serious toxicities.
Disease-specific variations of PK of Bu, although in a small series of children, have been suggested.15,19,36 To date, no study has investigated the PK of intravenous Bu in a large uniform cohort of children with thalassemia undergoing bone marrow transplantation. The main finding of our study was that Bu CL at the first dose of intravenous Bu was significantly higher compared with subsequent day doses. This finding has never been reported before. Interestingly, no significant difference was noted in CL across the following 3 days of intravenous Bu administration as it has been observed in patients with malignant diseases.8 Bertholle-Bonnet et al19 observed high Bu CL in 13 patients with hemoglobinopathies compared with patients with malignant diseases or other nonmalignant conditions who had been treated with oral Bu. However, in this study, Bu CL in subsequent doses was not evaluated. A methodologic issue related to the sampling time interval was hypothesized to explain the difference in CL between the first dose and subsequent doses. Indeed, the samples collection restricted over 6 hours on the first dosing might have underestimated the half-life and, as a result, underpredicted the AUCinf. To explore this hypothesis, the second dose was delayed 12 hours and the sample collection was extended over 12 hours in 7 patients. The data indicated that AUCinf was the same at sample intervals of 6 hours and 12 hours. Therefore, we ruled out a possible impact of the limited sampling design on the first-dose AUC calculation, which could have been responsible for the difference in CL.
To search for further explanations, a population PK modeling was performed enabling identification of influential patient's characteristics explaining the variability in Bu PK. A number of covariates reported in “Population PK analysis” were included in the analysis, and it was demonstrated that the dose 1 effect was still significant after including all other relevant covariates, such as body weight and the GST A1*B variant effect.
One explanation for the higher CL at the first dose of intravenous Bu could be the high hepatic GST activity and high plasma α-GST level in children with thalassemia compared with normal controls and age-matched leukemic patients.37 This probably led to increased Bu metabolism during its first administration in drug-naive patients. Consequently, depletion of excessive amounts of GST after the first-day doses led to a stabilization of CL in subsequent days. This finding shows that the AUC value at the first dose of intravenous Bu in patients with thalassemia could not reliably predict the subsequent days' AUC. Therefore, TDM in patients with thalassemia should consider disease-specific PK behavior of intravenous Bu.
In this study, we found that 59% of patients achieved the target range at the first dose of intravenous Bu. AUC targeting performance was 62% if a wider target window (900-1500 μMol*min) was considered. These data are different from that reported by Vassal et al18 who demonstrated the high targeting rate at first dose of intravenous Bu in children undergoing HSCT mostly for malignant diseases (76.4% of patients' AUC was within the range of 900-1350 μMol*min and 91% of patients AUC was within 900-1500 μMol*min). The lower performance achieved in our study could be explained by the faster CL and therefore the lower exposure observed on the first dose compared with subsequent doses. This specific PK behavior led to dose escalations in most of the patients (24 of 29) requiring dose adjustment. Consequently, because of a “normalization” of the CL (lower elimination capacity than at the first dose) on the subsequent administrations, the dose was increased too much, leading to higher AUC than expected in a substantial number of patients, which required further dose readjustment (mainly decreasing) to achieve the desired target. These fluctuations in drug exposure and doses adjustment followed the pattern of Bu CL. To avoid these large fluctuations in drug exposure and frequent dose readjustments, we suggest targeting the lower range of exposure for the dose adjustment based on dose 1 AUC, and the next dose should be calculated to achieve a median AUC of 900 μMol*min. This procedure should prevent unexpected high exposures at subsequent doses observed in this patient population. For the subsequent dose adjustment, the target dose is the conventional one (ie, 1125 μMol*min) because the CL remained unchanged after dose 1.
Little and conflicting data exist to associate graft rejection with oral Bu exposure. Slattery et al,38 in a heterogeneous patient population, found increased graft rejection with Bu Css levels less than 200 ng/mL in HLA-matched sibling and less than 600 ng/mL in those partially matched or unrelated donors who received BuCy conditioning regimen. Similar results were observed in children receiving transplantations from different donor sources for malignant and nonmalignant diseases.10,35 In contrast, no relationship between Bu Css and graft rejection was observed in children with thalassemia receiving bone marrow transplantation from an HLA-matched sibling, even though 6 of 64 patients had Css less than 200 ng/mL and 53 had Css less than 600 ng/mL.4 In all these studies, patients were treated with oral Bu and no study has evaluated the association of intravenous Bu and rejection in patients with thalassemia. The 4 patients who experienced rejection had received more than 50 packed red blood cells mainly without leukodepletion filters and therefore were immunized to HLA antigens, which might have contributed to their risk of graft failure. There was no correlation between any intravenous Bu PK parameters and rejection in our patients. None of 4 patients who rejected their grafts had Css less than 600 ng/mL. However, it must be acknowledged that the ability to identify a relationship between graft rejection and Bu Css may be blunted by (1) administration of other cytotoxic drugs, such as thiotepa and/or fludarabine with known myeloablative/immunosuppressive activity, and (2) low rejection number as a result of optimal drug exposure resulting from TDM.
One of the important findings of this study is that, despite preexisting disease and treatment-related liver damage, intravenous Bu was well tolerated with no significant toxicity. None of the patients experienced grade 4 toxicity. There are suggestions that intravenous Bu reduces the incidence of hepatic VOD39,40 compared with oral Bu; however, more studies are needed to confirm these results. The incidence of hepatic VOD in our study was lower (1.4%) compared with the reported incidence of VOD in children with thalassemia and treated with oral Bu, which varied between 4.5% and 45%.4-6 It has been suggested that dosing Bu close to the dose of Cy may increase hepatotoxicity that occurred more frequently with shorter intervals (7-15 hours) compared with longer intervals (24-48 hours) between the last dose of Bu and the first dose of Cy.41 In the present study, the interval between the last dose of Bu and the first dose of Cy was 14 hours in 42%, and it was 38 hours in the remaining 58% of patients. Furthermore, the latter group of patients received thiotepa between the last dose of Bu and the first dose of Cy, which might enhance hepatotoxicity of both drugs. However, treatment-related toxicity was similar between these 2 groups of patients. Another important observation that can be made from our experience is the low incidence of infections. The results of the present study suggest that optimal Bu delivery is important not only in relation to regimen-related toxicity, but also in the development of clinically significant acute GVHD and for survival. Indeed, in this high-risk patient population, the incidence of GVHD was low and survival rate was high. We conclude that the low toxicity profile observed in our study resulted from the use of intravenous Bu and the more conservative target range with TDM.
In conclusion, this study revealed a new pharmacokinetic behavior of intravenous Bu in children with thalassemia that is characterized by significantly higher CL after the first dose compared with subsequent day doses, leading to low AUC and dose adjustment in one third of patients. More importantly, this finding shows the necessity to consider disease-specific pattern of intravenous Bu CL for TDM in patients with thalassemia to avoid drug overexposure after dose adjustment. Another important consideration is the use of a more conservative AUC target (900-1350 μMol*min) for PK-guided intravenous Bu treatment in this patient population who usually have preexisting liver damage because of iron overload and/or hepatitis, thus avoiding excessive toxicity. Our data showed that this target range is safe and associated with low toxicity, a high engraftment rate, low severe acute or chronic GVHD, and an optimal survival rate in a group of patients known to be at high risk for high graft failure and toxicity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the nursing and the medical staff of the International Centre for Transplantation in Thalassemia and Sickle Cell Anemia of the Mediterranean Institute of Hematology, the technician staff of the Department of Internal Medicine and Laboratory of Molecular Biology of the University of Rome, Tor Vergata, and Luca Spitaleri, who died prematurely.
Authorship
Contribution: J.G. designed and performed research, analyzed data, performed statistical analysis, and wrote the manuscript; L.N. analyzed data, performed pharmacokinetic population analysis, and contributed to the writing of the manuscript; C.P. analyzed data, performed pharmacokinetic population analysis, and contributed to the writing of the manuscript; A.F.M., M.C., M.P.D., P.G., P.S., M.M., A.I., M.D.S., M.A., A.F., M.T., G.F., and S.B. performed research; and G.L. designed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Javid Gaziev, International Centre for Transplantation in Thalassemia and Sickle Cell Anemia, Mediterranean Institute of Hematology, Policlinico Tor Vergata, Viale Oxford 81, Rome 00133, Italy; e-mail: j.gaziev@fondazioneime.org.