Abstract
Transfusion of granulocytes from granulocyte-colony stimulating factor (G-CSF)/dexamethasone (dexa)–treated donors can be beneficial for neutropenic recipients that are refractory to antimicrobial therapy. G-CSF/dexa treatment not only increases the number of circulating neutrophils but also affects their gene expression. Because of the intended transfusion of these granulocytes into patients who are severely ill, it is of importance to establish to what extent mobilization affects the cellular behavior of neutrophils. Here, we studied the effects of mobilization on Toll-like receptor (TLR)–mediated responses. Mobilized granulocytes displayed increased gene and protein expression of TLR2, TLR4, TLR5, and TLR8. Although mobilized granulocytes displayed normal priming of nicotinamide adenine dinucleotide phosphate oxidase activity and a slight increase in adhesion in response to TLR stimulation, these cells produced massive amounts of interleukin-8 (IL-8), in particular to TLR2 and TLR8 stimulation. The increase in IL-8 release occurred despite reduced IL-8 mRNA levels in the donor granulocytes after in vivo G-CSF/dexa treatment, indicating that the enhanced TLR-induced IL-8 production was largely determined by posttranscriptional regulation. In summary, granulocytes mobilized for transfusion purposes show enhanced TLR responsiveness in cytokine production, which is anticipated to be beneficial for the function of these cells on transfusion into patients.
Introduction
Granulocytes are a critical constituent of the immune system. They act as a first line of defense against invading microbial pathogens, in particular bacteria and fungi. Patients with granulocyte deficiencies or prolonged periods of chemotherapy-induced neutropenia are extremely vulnerable to such infections. Despite antimicrobial drugs and the use of growth factors such as granulocyte-colony stimulating factor (G-CSF), to promote mobilization of cells from the bone marrow, some patients may remain refractory, and alternative approaches are warranted. Granulocyte transfusions (GTXs) constitute a promising adjuvant therapy in case of neutropenic and immunocompromised patients in which the exclusive use of modern antimicrobial drugs is ineffective.1,2
To acquire a sufficient number of cells for transfusion purposes the donor is treated with a combination of G-CSF and dexamethasone (dexa) before the collection of the neutrophils by leukapheresis.3,4 Clearly, for transfusions to be beneficial it is important that the donor granulocytes are functional. We and others have previously shown that most neutrophil functions are unaffected by the in vivo mobilization by G-CSF/dexa treatment, even though the expression of surface antigens as well as the resistance of the neutrophils to become apoptotic has changed considerably.5,6 We have also shown that granulocytes mobilized for transfusion present a strongly altered gene expression pattern compared with normal circulating neutrophils. Some of those changes were shown to contribute to their prolonged life span.7 The results also indicated that mobilized granulocytes have an increased mRNA expression of several Toll-like receptors (TLRs), whereas the level of other messengers, including those encoding several chemokines, was strongly diminished.7 However, the functional relevance of these changes was unclear and formed the focus of the present study.
TLRs are essential regulators of innate immunity, initiating a signaling cascade culminating in the activation of nuclear factor κB, mitogen-associated protein kinases (MAPKs), and interferon response factors.8 Neutrophils express all TLRs described, except TLR3 and TLR7. Activation of TLRs by their respective ligands stimulates neutrophil functions, including the respiratory burst, activation of adhesion molecules, induction of cytokine production,9,10 and prolongs their life span.11
Neutrophils, in addition to being professional phagocytes, produce a range of inflammatory mediators, including cytokines and chemokines that are involved in both innate and acquired immunity.12 One of the most prominent cytokines produced by neutrophils is interleukin-8 (IL-8; CXC chemokine ligand 8 [CXCL8]). IL-8 has chemotactic activity for all granulocytes and a subset of T lymphocytes.13 IL-8 is able to enhance the expression of integrins on the surface of neutrophils as well as to stimulate many neutrophil activities, including the respiratory burst, exocytosis of specific granules, and release of proteases.14 In addition, IL-8 causes monocytes to interact firmly with endothelial cells and may contribute to neovascularization.15,16
The study was performed to investigate whether the increased level of TLR transcripts in granulocytes used for transfusion, which we had noted previously,7 would correlate with a change in protein expression and contaminant change in functional behavior in response to TLR ligands. This could have a role in the outcome of GTX in the recipients who have indolent or overt infections at the time of infusion of such granulocyte concentrates.
Here, we show a significantly increased surface expression of several TLRs on mobilized neutrophils. Remarkably, the expression of mRNA for IL-8 is strongly diminished in mobilized neutrophils, but their stimulation with TLR ligands induces these neutrophils to produce massive amounts of this chemokine in comparison to control cells, as a consequence of posttranslational and improved secretory mechanisms. Our findings underscore that donor granulocytes obtained for transfusion purposes have undergone functional changes in neutrophil responsiveness, which may well contribute to their antimicrobial activity.
Methods
Granulocyte isolation and culture
Heparinized venous blood was collected from healthy granulocyte donors, with or without G-CSF/dexa treatment. Donors received G-CSF (600 μg subcutaneously) and dexa (8 mg orally), 16 to 20 hours before blood donation. The study was approved by the Sanquin Research Ethical Medical Committee and in accordance with the Declaration of Helsinki.
Granulocytes were isolated as described previously.17,18 In short, the granulocytes and erythrocytes were separated from the mononuclear leukocytes and platelets over isotonic Percoll with a specific density of 1.076 g/mL. Erythrocytes in the pellet were lysed in ice-cold medium containing 155mM NH4Cl, 10mM KHCO3 and 0.1mM EDTA (ethylenediaminetetraacetic acid), pH 7.4. Granulocytes were washed and resuspended in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered saline solution (132mM NaCl, 6.0mM KCl, 1.0mM CaCl2, 1.0 mM MgSO4, 1.2mM potassium phosphate, 20mM HEPES, 5.5mM glucose, and 0.5% [wt/vol] human serum albumin, pH 7.4). Purity and viability of granulocytes isolated with this method was always greater than 95%, based on positive staining for CD16 and negative for both CD36 (monocytes) and CD56 (natural killer cells), as measured by flow cytometry (see “Immunostaining and flow cytometric analysis”). Viability was similarly assessed by annexin V/propidium iodide staining and flow cytometry.
RNA isolation and reverse transcription
Total cellular RNA was extracted from a minimum of 20 × 106 cells with TRIzol reagent (Invitrogen) according to the protocol provided by the manufacturer, with the following minor modifications. An additional phenol-chloroform extraction was performed, and the isopropanol precipitation at −20°C was facilitated by the addition of 20 μg/mL glycogen (Roche). Purity and integrity of the RNA samples were confirmed on the Agilent 2100 bioanalyzer (Agilent Technologies Netherlands BV) by the RNA 6000 Nano LabChip kit.
Subsequently, first-strand complimentary DNA (cDNA) was synthesized with the Superscript III first-strand synthesis system for reverse transcription–polymerase chain reaction (RT-PCR; Invitrogen), as previously described.19
Primers
Intron-spanning primers were designed to specifically reduce the possibility of amplifying genomic DNA. In case of the TLR2 gene, which consists of only one exon, cDNA samples were tested for possible genomic DNA contamination, by performing quantitative RT-PCR with primers designed to recognize untranslated regions of genomic DNA. All primers were synthesized by Invitrogen. The primers used are listed in Table 1.
Primers used for quantitative PCR
| Human mRNA . | Forward primer . | Reverse primer . | Accession no. . |
|---|---|---|---|
| TLR2 | GAGCCACAAAACTGTCTTTGTTGC | AACCTAGGACTTTATCCCAGCTCTC | NM_003263 |
| TLR4 | ACTGCAGGTGCTAGATTTATCCAG | GTCCAATGGGGAAGTTGTCTAGAG | XM_057452 |
| TLR5 | TATCAGGACAGTCACAGCTTCATC | AATACAGCATCAGAGAGACCACAG | NM_003268 |
| TLR8 | CACTTCAGTGTTAGGGAACATCAG | TTTCTTCGGCGCATAACTCACAGG | NM_016610 |
| CXCL8 (IL-8) | CTCTTGGCAGCCTTCCTGATTTCT | CAAGGAAAACTGGGTGCAGAGGGT | NM_000584 |
| Human mRNA . | Forward primer . | Reverse primer . | Accession no. . |
|---|---|---|---|
| TLR2 | GAGCCACAAAACTGTCTTTGTTGC | AACCTAGGACTTTATCCCAGCTCTC | NM_003263 |
| TLR4 | ACTGCAGGTGCTAGATTTATCCAG | GTCCAATGGGGAAGTTGTCTAGAG | XM_057452 |
| TLR5 | TATCAGGACAGTCACAGCTTCATC | AATACAGCATCAGAGAGACCACAG | NM_003268 |
| TLR8 | CACTTCAGTGTTAGGGAACATCAG | TTTCTTCGGCGCATAACTCACAGG | NM_016610 |
| CXCL8 (IL-8) | CTCTTGGCAGCCTTCCTGATTTCT | CAAGGAAAACTGGGTGCAGAGGGT | NM_000584 |
Quantitative RT-PCR
PCR amplification was performed on a LightCycler instrument (Roche) and analyzed with software Version 3.5. The reaction was performed with Lightcycler FastStart DNA MasterPLUS SYBR Green I (Roche Diagnostics). The annealing temperature used for all primers was 65°C. The reaction mixture consisted of 4 μL of cDNA, 1 μL of relevant primer combination, and 4 μL of SYBR Green I mix in a total volume of 20 μL. All amplified cDNA was compared with the standard within the same run, and in every run the same standard was used, although there was very little variation in the standard between runs.
For amplification, the following LightCycler protocol was used. The chemical cleft of the Taq polymerase was removed by preincubation for 10 minutes at 95°C; the template was amplified for 40 cycles, with annealing of the primers at 65°C. The fluorescence was measured at the end of each cycle at 72°C. At the end of 40 cycles, a melting curve was generated to determine the unique features of the DNA amplified. The specific size of the product was determined on a 1% (wt/vol) agarose gel. Subsequently, the obtained band was purified by the GFX PCR DNA and Gel Band purification kit (Amersham Biosciences) according to the manufacturer's instructions to remove excess deoxynucleoside triphosphates and primers. The product was sequenced by Big-dye Terminator Sequencing and ABI Prism software (Applied Biosystems). The sequence was verified with BLAST to determine its specificity.20 All products obtained were unique and had no overlap with other isoforms. A standard curve was made and relative quantitation was performed as previously described.19
Immunostaining and flow cytometric analysis
After erythrocyte lysis from the blood cells, expression of surface-bound TLRs on granulocytes was assayed in total leukocyte samples by flow cytometry, with saturating concentrations of commercially available monoclonal antibodies, either directly labeled with fluorescein isothiocyanate, phycoerythrin, or allophycocyanin (APC) or indirectly labeled with Alexa 488–rabbit–anti–mouse immunoglobulin (Molecular Probes). Anti–TLR2-APC (clone T2.5) and anti–TLR4-APC (clone HTA125) were from eBioscience; anti–TLR5-fluorescein isothiocyanate (clone 85B152.5) was from Abcam.
Samples were analyzed on an LSRII flow cytometer equipped with FACSDiva software (BD Biosciences). Cells were gated based on their forward and side scatter, and 10 000 gated events were collected per sample (100% positive staining for CD16 and negative for both CD36 [monocytes] and CD56 [natural killer cells], confirming the purity of the analyzed neutrophil population).
Neutrophil stimulation
Neutrophils were isolated as described in “Granulocyte isolation and culture” and diluted to the desired concentration. TLR agonists were used at the following concentrations, unless otherwise noted: LPS/LPS-binding protein (LBP; 20 ng/mL/50 μg/mL; LPS isolated from Escherichia coli strain 055:B5 was obtained from Sigma-Aldrich; LBP was from R&D Systems), Pam3Cys-SK4 (20 μg/mL; EMC Microcollections), macrophage-activating lipoprotein 2 kDa (MALP-2; 1 μg/mL; EMC Microcollections), flagellin (100 ng/mL; InvivoGen), and resiquimod (50 μg/mL; Alexis Biochemicals). As non-TLR ligands, tumor necrosis factor α (TNF-α; 10 ng/mL; PeproTech EC), platelet activating factor (PAF; 1μM; Sigma-Aldrich), and phorbol myristate acetate (100 ng/mL; Sigma-Aldrich) were used.
Priming of NADPH oxidase
Neutrophils were preincubated with various TLR ligands for 30 minutes at 37°C on 96-well plates in the presence of 44a/IB4 antibody (directed against CD11b and CD18 chains of the major β2 integrin on neutrophils, respectively) to prevent “adhesion-induced” activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Afterward, NADPH oxidase activity in response to formyl-methionyl-leucyl-phenylalanine (fMLP) was assessed as hydrogen peroxide release determined by the Amplex Red kit (Molecular Probes). Neutrophils (0.25 × 106/mL) were stimulated with 1μM fMLP (Sigma-Aldrich) in the presence of Amplex Red (0.5μM) and horseradish peroxidase (1 U/mL). Fluorescence was measured at 30-second intervals for 20 minutes with the HTS7000+ plate reader (Tecan). Maximal slope of H2O2 release was assessed over a 2-minute interval.
Adhesion
Adhesion was determined in 96-well Maxisorb plates (Nunc). Calcein-labeled cells (100 μL; 2 × 106/mL) were pipetted into each well, and cells were stimulated with various TLR ligands. Plates were incubated for 30 minutes at 37°C. Thereafter, the plates were washed 3 times with phosphate-buffered saline at room temperature. Adherent cells were lysed with 125 μL of H2O, containing 0.5% (wt/vol) Triton X-100 (10 minutes, room temperature). Fluorescence was measured with the HTS7000+ plate reader at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. Adhesion was determined as a percentage of total cell input.
Cytokine production
Neutrophils (5 × 106/mL) were stimulated overnight with the indicated TLR ligands. Next, the cells were spun down for 5 minutes at 1200g, and the supernatants were collected and stored at −20°C before further use. The cell pellets were lysed by incubating them in phosphate-buffered saline supplemented with complete protease inhibitor cocktail mix (Roche Diagnostic), 1mM EDTA, and 0.5% Triton X-100 (vol/vol) for 30 minutes on ice. Afterward, the samples were spun down for 15 minutes at 12000g, and the supernatants containing cell-associated chemokines were collected and stored at −20°C until further use.
Production of IL-8 was measured in the supernatants and in the cell fraction with a commercially available Pelikine IL-8 enzyme-linked immunoabsorbent assay kit, according to the manufacturer's protocol (Sanquin Reagents). The lysis procedure did not interfere with the detection of IL-8, as shown by spiking a series of different neutrophil lysates with well-defined doses of the exogenous IL-8 from the calibration curve, resulting in additive results (ie, exogenous and endogenous background levels of IL-8).
Statistics
Graphs were drawn, and statistical analysis was performed with GraphPad Prism Version 5.00 for Windows (GraphPad Software). The results are presented as the mean plus or minus SEM, as indicated. Results were analyzed by independent analysis of variance test. Significance is mentioned when P values are less than .05, or P values less than .01 for normalized data.
Results
Mobilized granulocytes display changes in the expression of TLRs
TLRs play a central role in the activation by microbial stimuli of innate immune cell effector functions, including those of neutrophils. GTXs are being infused to patients with prolonged neutropenia to recover from persistent and life-threatening infections when clinically resistant to modern combinations of antimicrobials. Therefore, TLR responsiveness of granulocytes mobilized for transfusion is an important issue to address. In a previous study we performed a microarray analysis of the transcriptome of granulocytes mobilized for transfusion purposes.7 The analysis of the fluorescence intensity of different probes indicated that neutrophils express all TLR mRNAs, except for TLR3 and TLR7 (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), which is in agreement with previously published studies.10,21
Previously, we noted that 4 mRNAs, in particular those for TLR2, TLR4, TLR5, and TLR8, were significantly elevated in mobilized granulocytes compared with the respective levels on granulocytes isolated before G-CSF/dexa administration from the same donors (supplemental Table 1).
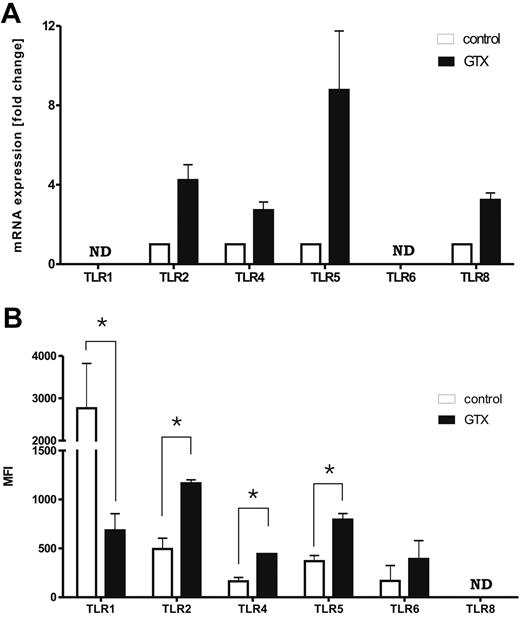
To verify the microarray data, we performed real-time PCR and confirmed the increased expression of all 4 TLR mRNAs (Figure 1A). Moreover, higher expression of surface-expressed TLR2, TLR4, and TLR5 was also detected on mobilized granulocytes by flow cytometry (Figure 1B; P < .05). The TLR2-partnering proteins, TLR1 and TLR6, were also determined. The mRNA and surface expression of TLR6 appeared unchanged. Notably, the surface expression of TLR1 on mobilized granulocytes was reduced (P < .05), whereas no difference was detected at the mRNA level. Because of the lack of a specific antibody, a similar quantitative analysis for TLR8 could not be performed. Taken together, these data suggest that the expression of at least TLR2, TLR4, and TLR5, and possibly TLR8, is enhanced on mobilized granulocytes. The microarray data have been deposited in National Center for Biotechnology Information's Gene Expression Omnibus and are accessible through GEO Series accession number GSE12841 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE12841).
Mobilization of neutrophils with G-CSF/dexa affects the expression of TLRs. (A) Expression of mRNA for various TLRs in control and G-CSF/dexa–mobilized neutrophils. (B) Surface expression of various TLRs on control and mobilized neutrophils, measured by flow cytometry. Results represent the data from 3 different experiments, with cells from 3 different donors (mean ± SEM). ND indicates not determined. Data were analyzed by an independent 2-way analysis of variance test; *P < .05 (significant difference).
Mobilization of neutrophils with G-CSF/dexa affects the expression of TLRs. (A) Expression of mRNA for various TLRs in control and G-CSF/dexa–mobilized neutrophils. (B) Surface expression of various TLRs on control and mobilized neutrophils, measured by flow cytometry. Results represent the data from 3 different experiments, with cells from 3 different donors (mean ± SEM). ND indicates not determined. Data were analyzed by an independent 2-way analysis of variance test; *P < .05 (significant difference).
Priming of NADPH-oxidase activity with TLR ligands
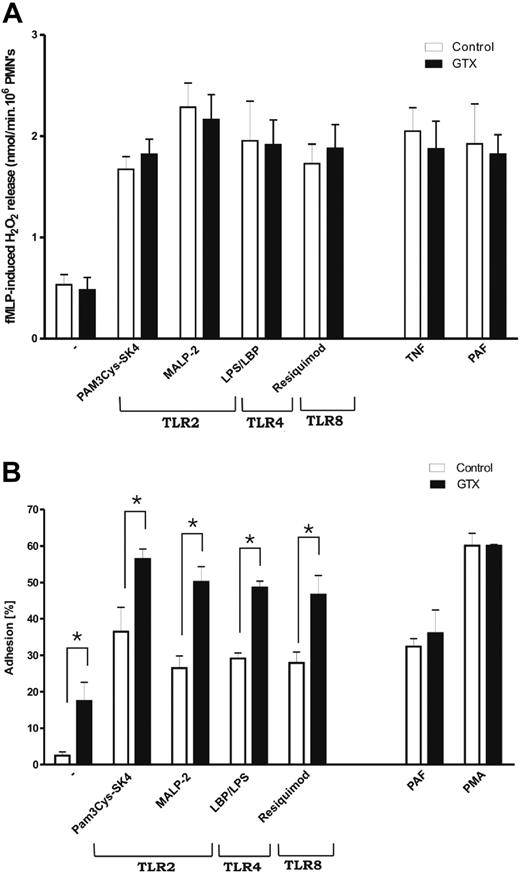
The generation of superoxide, which is induced rapidly after appropriate stimulation, is an important antimicrobial response of neutrophils. Stimulation of neutrophils with TLR ligands is known to generate rather modest levels of oxygen radicals. However, these ligands are potent priming agents for the fMLP-stimulated respiratory burst.22-24 To measure whether the increased expression of TLRs had any effect on their ability to prime the cells, neutrophils isolated from control donors or donors treated with G-CSF/dexa were incubated for 30 minutes with various TLR ligands or control non-TLR stimuli before subsequent fMLP stimulation. As shown in Figure 2A, GTX neutrophils primed with TLR ligands on fMLP stimulation produced essentially identical amounts of hydrogen peroxide (H2O2) as did the control cells, despite the increased expression levels of TLRs. There was also no difference after priming with PAF or TNF. Dose-titration curves of the TLR ligands to identify a change in the sensitivity for the respective ligands indicated a largely unaltered optimal priming concentration of the various ligands for the fMLP-induced respiratory burst (supplemental Figure 1). The increased expression of the other TLRs did not result in a lower optimal concentration of TLR ligands to activate mobilized neutrophils.
Functions of mobilized neutrophils in response to TLRs ligands. (A) Priming of NADPH oxidase activity with various TLR ligands. Neutrophils from control and GTX donors were incubated either for 30 minutes at 37°C with various TLR ligands or with TNF-α, or for 5 minutes with PAF. Afterward, fMLP-induced respiratory burst was measured by Amplex Red assay. (B) Adhesion of neutrophils to plastic surface in response to different TLR ligands. Significant difference, between control and GTX neutrophils, was observed after stimulation with all TLR ligands tested. However, this significance was absent when corrected for the basal level of adhesion, which was increased in the case of GTX neutrophils. Results represent the data from 6 different experiments (mean ± SEM). Data were analyzed by an independent 2-way analysis of variance test; *P < .05 (significant difference).
Functions of mobilized neutrophils in response to TLRs ligands. (A) Priming of NADPH oxidase activity with various TLR ligands. Neutrophils from control and GTX donors were incubated either for 30 minutes at 37°C with various TLR ligands or with TNF-α, or for 5 minutes with PAF. Afterward, fMLP-induced respiratory burst was measured by Amplex Red assay. (B) Adhesion of neutrophils to plastic surface in response to different TLR ligands. Significant difference, between control and GTX neutrophils, was observed after stimulation with all TLR ligands tested. However, this significance was absent when corrected for the basal level of adhesion, which was increased in the case of GTX neutrophils. Results represent the data from 6 different experiments (mean ± SEM). Data were analyzed by an independent 2-way analysis of variance test; *P < .05 (significant difference).
Adhesion of mobilized granulocytes in response to TLR stimulation
Stimulation of TLRs also has been shown to result in increased surface expression and activation of adhesion molecules such as CD11b at the neutrophil surface.25 Therefore, it has been investigated whether various TLR ligands can induce neutrophil adhesion to plastic, which completely depends on CD11b/CD18 (ie, blocking antibody against CD11b CD18 completely abolishes this type of adhesion; data not shown) and whether any differences were detectable between control and mobilized cells. Mobilized granulocytes showed slightly increased adhesion in the absence of any stimulus (Figure 2B; P < .056), although no increase in the basal expression of β2-integrins had been found previously.6 Stimulation of different TLRs resulted in increased adhesion of granulocytes compared with control cells (all P < .05), but when the numbers were corrected for the increased background, the elevation was not significant anymore. The PAF- and phorbol myristate acetate–induced adhesion was comparable between control and GTX neutrophils.
Chemokine expression in mobilized granulocytes
Neutrophils are known to produce various cytokines in response to stimulation with several ligands, including TLR ligands.10,26 Although neutrophils have been previously described to also secrete CCL2 (monocyte chemoattractant protein 1),27 CCL3 (macrophage inflammatory protein 1-α), CCL4 (macrophage inflammatory protein 1-β), CCL5 (regulated on activation normal T cell expressed and secreted), and TNF-α,26,28 depending on the (combination of) stimuli used, the most prominent cytokine generated by neutrophils is IL-8 (supplemental Table 2).
In the context of the enhanced expression of TLRs the analysis of cytokine production was of particular interest, because an increased expression of TLRs has previously been found to be associated with an increased cytokine secretion on cell stimulation.9,26
Once more we took advantage of the mRNA profile of control human neutrophils, previously obtained by expression analysis,7 and confirmed that unstimulated neutrophils express significant amounts of mRNA for various chemokines, including those already mentioned (supplemental Table 2), except for CCL2, as was also reported previously.27 Several chemokines were found to be strongly down-regulated in GTX neutrophils, with IL-8 being the most affected (Table 2).
Changes in expression level of various chemokines in mobilized granulocytes
| Accession number . | Gene symbol . | Protein encoded . | mRNA, fold change . |
|---|---|---|---|
| NM_001511 | CXCL1 | Chemokine (C-X-C motif) ligand 1; GROβ (melanoma growth stimulating activity, alpha) | −3.20 |
| NM_001001437 | CCL3L3 | Chemokine (C-C motif) ligand 3-like 3 | −5.98 |
| NM_002983 | CCL3 | Chemokine (C-C motif) ligand 3; MIP-1α | −6.35 |
| NM_002985 | CCL5 | Chemokine (C-C motif) ligand 5; RANTES | −7.71 |
| NM_002619 | CXCL4 | Chemokine (C-X-C motif) ligand 4; PF4 | −11.0 |
| NM_005064 | CCL23 | Chemokine (C-C motif) ligand 23; MPIF-1 | −15.06 |
| NM_002984 | CCL4 | Chemokine (C-C motif) ligand 4; MIP-1β | −15.45 |
| NM_002704 | CXCL7 | Chemokine (C-X-C motif) ligand 7; pro-platelet basic protein | −16.30 |
| NM_002001 | CXCL8 | Chemokine (C-X-C motif) ligand 8; interleukin-8 | −35.22 |
| Accession number . | Gene symbol . | Protein encoded . | mRNA, fold change . |
|---|---|---|---|
| NM_001511 | CXCL1 | Chemokine (C-X-C motif) ligand 1; GROβ (melanoma growth stimulating activity, alpha) | −3.20 |
| NM_001001437 | CCL3L3 | Chemokine (C-C motif) ligand 3-like 3 | −5.98 |
| NM_002983 | CCL3 | Chemokine (C-C motif) ligand 3; MIP-1α | −6.35 |
| NM_002985 | CCL5 | Chemokine (C-C motif) ligand 5; RANTES | −7.71 |
| NM_002619 | CXCL4 | Chemokine (C-X-C motif) ligand 4; PF4 | −11.0 |
| NM_005064 | CCL23 | Chemokine (C-C motif) ligand 23; MPIF-1 | −15.06 |
| NM_002984 | CCL4 | Chemokine (C-C motif) ligand 4; MIP-1β | −15.45 |
| NM_002704 | CXCL7 | Chemokine (C-X-C motif) ligand 7; pro-platelet basic protein | −16.30 |
| NM_002001 | CXCL8 | Chemokine (C-X-C motif) ligand 8; interleukin-8 | −35.22 |
Granulocytes were isolated from blood of donors before and after treatment with G-CSF/dexa. Changes in gene expression were evaluated by Agilent Whole Human Oligomicroarray, and shown in order of magnitude of changes in transcription. Significantly regulated genes were selected with Rosetta Resolver. Genes with a fold change of 3 or more together with a P-value cutoff of .01 or less (1-way ANOVA test with the Benjamini-Hochberg false-discovery rate correction) were considered significantly different.
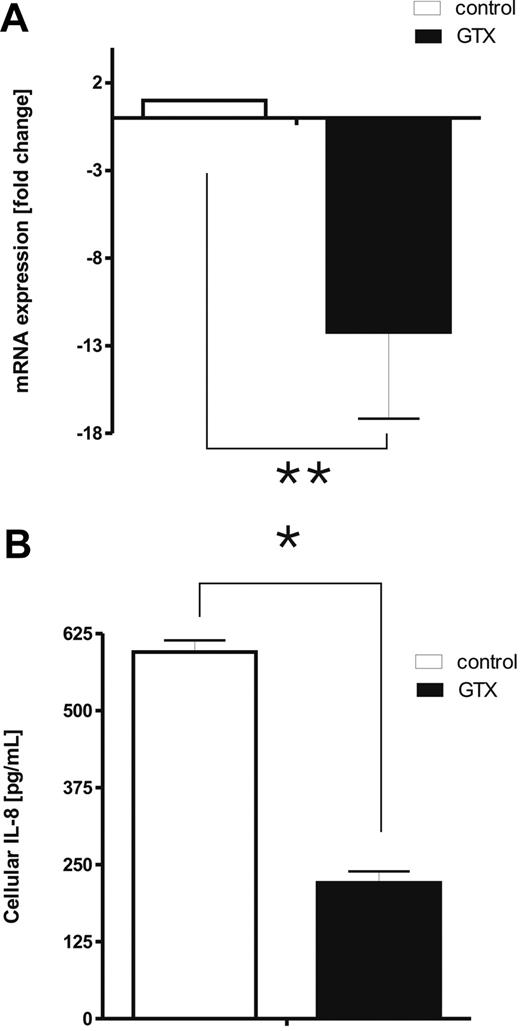
Quantitative RT-PCR analysis confirmed that mobilized granulocytes contain dramatically decreased levels of mRNA for IL-8, compared with the neutrophils obtained from the same donors before GTX/dexa mobilization (Figure 3A; P < .01). In addition, the amount of IL-8 in neutrophils derived from GTX donors was reduced accordingly (Figure 3B; P < .05).
IL-8 in mobilized neutrophils. (A) mRNA was isolated from fresh control and mobilized neutrophils. Relative expression of IL-8 was assessed by quantitative RT-PCR and compared with the expression of the housekeeping gene GUS. Data were analyzed by independent 2-way analysis of variance; **P < .01 (significant difference). (B) Freshly isolated neutrophils were lysed with Triton-100–based buffer, and the levels of cell-associated IL-8 were assessed with enzyme-linked immunoabsorbent assay. Results represent the data from 3 different experiments, with cells from 3 different donors (mean ± SEM). Data were analyzed by independent 2-way analysis of variance test; *P < .05 (significant difference).
IL-8 in mobilized neutrophils. (A) mRNA was isolated from fresh control and mobilized neutrophils. Relative expression of IL-8 was assessed by quantitative RT-PCR and compared with the expression of the housekeeping gene GUS. Data were analyzed by independent 2-way analysis of variance; **P < .01 (significant difference). (B) Freshly isolated neutrophils were lysed with Triton-100–based buffer, and the levels of cell-associated IL-8 were assessed with enzyme-linked immunoabsorbent assay. Results represent the data from 3 different experiments, with cells from 3 different donors (mean ± SEM). Data were analyzed by independent 2-way analysis of variance test; *P < .05 (significant difference).
Induction of IL-8 mRNA in mobilized granulocytes on TLR stimulation
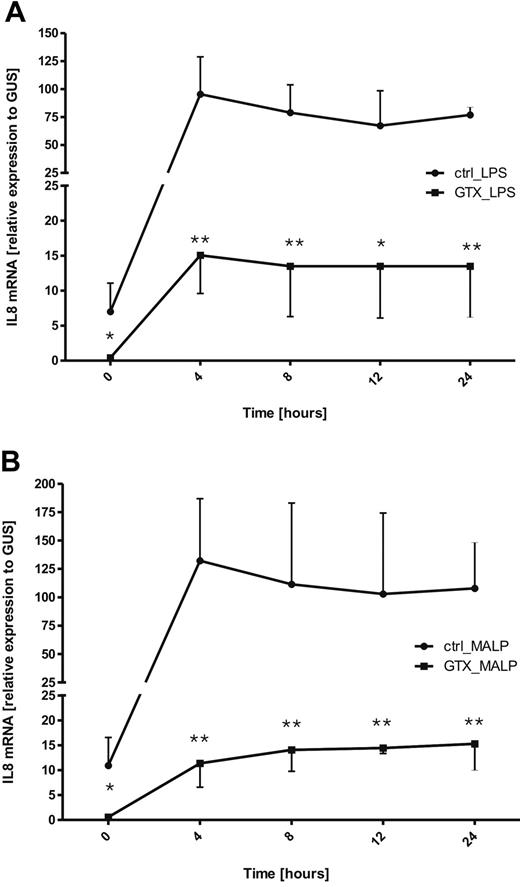
Subsequently, we studied whether the down-regulation of mRNA for IL-8 in mobilized neutrophils persisted on subsequent stimulation with TLR ligands. Neutrophils (control or after mobilization from the same donors) were stimulated, with either LPS/LBP (TLR4) or MALP-2 (TLR2/6) over various time periods (ranging from 0 to 24 hours). Afterward, the samples were collected, and the amounts of IL-8 mRNA were assessed with quantitative RT-PCR.
As observed before, freshly isolated GTX neutrophils displayed much lower levels of IL-8 mRNA than did control cells. On TLR triggering of control neutrophils, within 4 hours after stimulation the levels of IL-8 mRNA increased dramatically and thereafter started to decline slowly (Figure 4A-B). Stimulation of neutrophils derived from GTX donors also resulted in the increase of IL-8 mRNA within the first 4 hours, but the levels were still much lower than those in control cells. However, in contrast to control cells, the levels of IL-8 transcripts seemed to stay more constant over 24 hours in GTX neutrophils (Figure 4; significance per time point are indicated by asterisks).
Kinetic of changes in IL-8 mRNA expression by neutrophils, in response to TLR stimulation. Control and mobilized neutrophils were incubated over time with LPS/LBP (A) or with MALP-2 (B). At various time points samples were taken and lysed with TRIzol. The expression of mRNA for IL-8 was assessed by quantitative RT-PCR. Results represent the data from 3 different experiments, with cells from 3 different donors. Data were analyzed by independent 2-way analysis of variance test; *P < .05, **P < .01 (significant difference).
Kinetic of changes in IL-8 mRNA expression by neutrophils, in response to TLR stimulation. Control and mobilized neutrophils were incubated over time with LPS/LBP (A) or with MALP-2 (B). At various time points samples were taken and lysed with TRIzol. The expression of mRNA for IL-8 was assessed by quantitative RT-PCR. Results represent the data from 3 different experiments, with cells from 3 different donors. Data were analyzed by independent 2-way analysis of variance test; *P < .05, **P < .01 (significant difference).
TLR-induced IL-8 release
Increase in protein levels can be achieved by an increase in the total amount of messenger RNA (because of derepression of the promoter, activation of transcription, or stabilization of the mRNA), accelerated translation, or posttranslational stabilization of the protein.
Having investigated the levels of IL-8 mRNA on TLR stimulation in GTX and control neutrophils, the effect of prolonged mRNA transcriptional activity identified in the mobilized neutrophils was further studied. Neutrophils isolated from control and GTX donors were cultured in the presence or absence of various TLR ligands. After overnight incubation the cell-free supernatant was collected, and the levels of IL-8 were assessed by enzyme-linked immunoabsorbent assay.
In line with the mRNA data, unstimulated control neutrophils released small amounts of IL-8 when cultured overnight, as was observed before.29 There was even significantly less release of IL-8 from the mobilized neutrophils (Figure 5). Yet these mobilized neutrophils were able to respond with a massive IL-8 release reaction to stimulation with TLR ligands (P < .05).
Release of IL-8 in response to TLR stimulation. Isolated, control and mobilized neutrophils were cultured overnight in HEPES buffer with various TLR ligands. Afterward, the supernatants were collected, and the levels of released cytokine were measured by enzyme-linked immunoabsorbent assay. Results represent data from 6 independent experiments, with cells from 6 different donors (mean ± SEM). Data were analyzed by independent 2-way analysis of variance test; *P < .05 (significant difference).
Release of IL-8 in response to TLR stimulation. Isolated, control and mobilized neutrophils were cultured overnight in HEPES buffer with various TLR ligands. Afterward, the supernatants were collected, and the levels of released cytokine were measured by enzyme-linked immunoabsorbent assay. Results represent data from 6 independent experiments, with cells from 6 different donors (mean ± SEM). Data were analyzed by independent 2-way analysis of variance test; *P < .05 (significant difference).
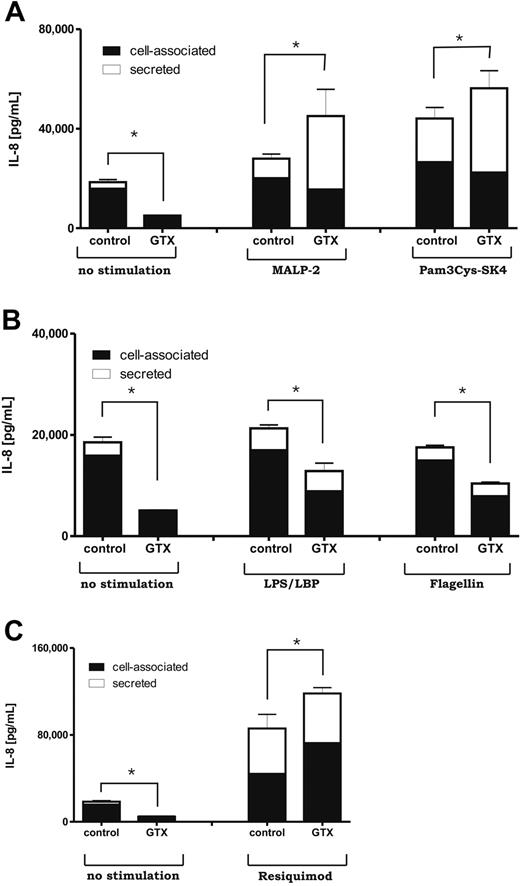
Although LPS/LBP-, flagellin-, and resiquimod-stimulated GTX cells secreted similar levels of IL-8 as did the control cells, Pam3Cys-SK4 (TLR2/TLR1) and MALP-2 (TLR2/TLR6) activation induced significantly higher amounts of IL-8 release in GTX than the control cells did (Figure 5). This difference appeared even more pronounced when we looked at the relative release of IL-8 for various TLR ligands (ie, after subtraction of background IL-8 levels on overnight culture in the absence of any activation; supplemental Figure 2A), including the IL-8 in response to flagellin (P < .05).
Cell-associated IL-8 versus released IL-8
According to the literature, only 30% of IL-8 produced by neutrophils in response to LPS activation is actually secreted into the culture media,30,31 whereas the rest remains cell associated as can be assayed on cell lysis. The mechanism(s) of regulation of IL-8 secretion has only been poorly defined. Because any differences between mobilized and control neutrophils may have been influenced by changes in secretion, we investigated both the secreted and the cell-associated pools of IL-8 on TLR stimulation of mobilized versus control neutrophils. We could confirm that isolated neutrophils produced significant amounts of IL-8 during overnight incubation with most remaining cell associated under all conditions (Figure 6). As expected, based on mRNA data, much (∼ 3-fold) less total IL-8 (ie, the sum of secreted plus cell-associated IL-8) was generated by GTX cells in the absence of stimulation (P < .05).
Total production of IL-8 by neutrophils in response to various TLR ligands. (A-C) Control and mobilized neutrophils were cultured overnight with various TLR ligands. Afterward, the supernatants were collected, and the pellets were incubated in Triton-100–based buffer for 30 minutes on ice. After lysis, samples were spun down at 12 000g for 15 minutes, and the supernatants, representing the cell-associated fraction, were collected. Secreted and cell-associated levels of chemokine were measured by enzyme-linked immunoabsorbent assay. Results represent data from 6 independent experiments, with cells from 6 different donors. Error bars represent SEM for the total production of IL-8 (sum of secreted and cell-associated protein). Data were analyzed by independent 2-way analysis of variance test; *P < .05 (significant difference).
Total production of IL-8 by neutrophils in response to various TLR ligands. (A-C) Control and mobilized neutrophils were cultured overnight with various TLR ligands. Afterward, the supernatants were collected, and the pellets were incubated in Triton-100–based buffer for 30 minutes on ice. After lysis, samples were spun down at 12 000g for 15 minutes, and the supernatants, representing the cell-associated fraction, were collected. Secreted and cell-associated levels of chemokine were measured by enzyme-linked immunoabsorbent assay. Results represent data from 6 independent experiments, with cells from 6 different donors. Error bars represent SEM for the total production of IL-8 (sum of secreted and cell-associated protein). Data were analyzed by independent 2-way analysis of variance test; *P < .05 (significant difference).
Again, this was compensated by a stronger response to all TLR ligands tested in the mobilized cells. This analysis showed that the TLR ligands also promoted the total IL-8 protein production. Interestingly, the GTX cells appeared again considerably more sensitive to the TLR-induced rise in relative IL-8 total production than the control cells (supplementary Figure 2B; P < .05 for TLR1/2, TLR2/6, and TLR8).
However, in case of TLR2 stimulation by either MALP-2 or Pam3Cys-SK4, the enhanced secretion mechanism seemed to contribute most to the regulation of massive IL-8 release.
Whether the observed results are caused by the increased expression of TLRs and therefore increased strength of a TLR-induced signal is not clear. However, incubation of whole blood for 16 to 20 hours with amounts of G-CSF/dexa comparable to those observed in the circulation of mobilized donors after drug administration did not increase the surface expression of TLRs on neutrophils, whereas there was a similar decrease in IL-8 mRNA levels as observed in mobilized cells (data not shown). After neutrophil purification and subsequent TLR activation overnight, stimulation of these in vitro “preactivated” cells with TLR ligands still induced IL-8 production, but the final levels remained much lower compared with the “nonactivated” control cells (supplemental Figure 3). These results clearly differed from the findings obtained with the in vivo mobilized neutrophils. Collectively, these results essentially show a higher TLR responsiveness in GTX cells compared with control cells. Furthermore, they suggest that TLRs signal for IL-8 production not only by promoting total IL-8 protein synthesis but also by stimulating more efficient IL-8 secretion.
Discussion
Administration of G-CSF and dexa to healthy volunteers is a well-established method to mobilize sufficient numbers of granulocytes for transfusion purposes.32 Neutrophils obtained in this fashion display a relatively normal functional behavior when tested in vitro, with only minor differences in their phenotype.6 However, extensive genetic analysis of their transcriptome showed considerable changes in relative gene expression caused by the mobilization procedure. For example, an increased expression of the calpain inhibitor calpastatin was observed previously in GTX neutrophils, and this was found to contribute to the prolonged life span of these cells.7
In the present report, we focused on the effects of GTX on genes involved in microbial recognition and host defense, in particular TLRs and cytokines. Such a study is relevant, because these granulocyte transfusions are administered to patients who are often severely immunocompromised, persistently infected, and refractory to extensive antimicrobial treatment.3,4 It has been previously reported that single administration of G-CSF in vivo augments neutrophil antibacterial and antifungal responses.33 However, Leavey et al34 reported that repeated stimulation of the donor with G-CSF for several consecutive days results in the mobilization of granulocytes with impaired killing activity against Staphylococcus aureus, especially at higher bacterial loads. Moreover, glucocorticoids such as dexa are well known for their potent anti-inflammatory activity.35
Our current findings showed prominent differences in the expression levels of TLRs. In particular, the expression of 4 of the 8 TLR mRNAs present in human neutrophils10,21 was increased in GTX cells. Concomitant increase was seen for these TLRs at the protein level. It has been previously reported that various cytokines, for example, G-CSF and granulocyte-macrophage CSF, can enhance TLR2 expression on neutrophils in vitro, which is reflected in their augmented response to TLR2-specific stimulation, including increased generation of superoxide and IL-8 production.9 We studied whether the in vivo increase in surface expression of TLRs on GTX neutrophils was associated with an enhanced neutrophil responsiveness to TLR stimulation. To this end, several TLR-regulated responses in neutrophils were evaluated that are relevant for host defense, including adhesion, respiratory burst, and the secretion of cytokines, in particular the chemokine IL-8. GTX neutrophils and control cells showed comparable priming of the fMLP-induced NADPH oxidase activity when treated with the various TLR ligands. Neutrophil adhesion, which is completely governed by β2-integrins, showed a greater increase on TLR stimulation in GTX than in control cells. However, also basal levels of adhesion without any further activation were already increased for GTX cells. Because the CR3 expression levels in GTX and control cells are comparable,6 the latter is most probably caused by an enhanced activation of the CD11b/CD18 complex. Therefore, the increased adhesion of GTX neutrophils caused by TLR stimulation cannot be simply interpreted as an effect caused by the increase in TLR or integrin expression.
On stimulation neutrophils generate superoxide within minutes. Adhesion in response to external ligands happens within the same time frame. In contrast, cytokine secretion does not reach detectable levels until hours after stimulation, indicating the involvement of different signaling pathway and perhaps secondary factors, which can be subject to regulation by the mobilization procedure. Various cytokines can be produced by neutrophils,10,28,36 among which is the chemokine IL-8 (CXCL8).37,38 Analysis of neutrophil gene expression profiles confirmed the presence of mRNA coding for various other chemokines, eg, CCL3, CCL4, and CCL5. Comparison of control and mobilized cells showed strong down-regulation of several chemokine transcripts in GTX neutrophils with IL-8 mRNA levels being most prominently affected (Table 2; Figure 3).
IL-8 transcription is believed to be induced by a combination of 3 different mechanisms: first, derepression of the gene promoter; second, transcriptional activation of the gene by nuclear factor κB and the Jun-N-terminal protein kinase pathway; and third, stabilization of the IL-8 mRNA by a p38 MAPK pathway.39 Although our results do not provide insight into the mechanism of GTX-mediated IL-8 mRNA repression, one obvious scenario is that dexa, applied in vivo as part in the mobilization procedure, could have caused this decrease. Glucocorticoids have already been described to down-regulate various cytokines, including IL-8,40 by decreasing mRNA stability or by repression of the promoter.41 Of note, this mRNA down-regulation was mirrored at the protein level, as the amount of cell-associated IL-8 obtained from freshly isolated cells or total IL-8 after overnight culture were both strongly decreased in GTX neutrophils.
Despite the lowered IL-8 mRNA, the mobilized cells secreted roughly equal or in case of TLR2 stimulation a massively increased amount of IL-8, suggesting that the GTX-mediated effect on IL-8 production may largely occur at the posttranscriptional level. Our results support a scenario in which the GTX treatment potentiates neutrophil IL-8 production at least at 2 levels: the first mechanism involves posttranscriptional enhancement in IL-8 protein production, and the second mechanism seems to promote its secretion, as illustrated in particular by the 2 different ligands for TLR2/1 and TLR2/6. Mobilized neutrophils showed increased TLR2 surface expression, whereas TLR6 remained unchanged, and TLR1 surface expression was even reduced. These data suggest that under these conditions the level of TLR-mediated IL-8 regulation is independent of the TLR surface expression level per se.
One of the posttranscriptional regulation mechanisms is governed by AU-rich elements (AREs), that were originally identified in the 3′-untranslated region of several cytokine genes.42,43 AREs control gene expression by accelerating the decay of their mRNAs. Another mechanism of action attributed to AREs is involvement in the translational control of their mRNAs.44,45 ARE sequences control mRNA degradation and translation by interactions with specific binding proteins. These proteins constitute the targets of the earlier mentioned signaling pathways regulating gene expression on cell activation, eg, p38 MAPK pathway. By increasing the mRNA stability and/or the translation rate, these pathways modulate the final protein output, thus providing an appropriate response to the specific activating stimulus received by the cells. IL-8 mRNA contains several AREs in its 3′-untranslated region which are involved in stabilization and destabilization induced by different signaling pathways.46,47 Our data indicate that the increased stability of mRNA, highly accelerated process of translation and efficient secretion, GTX neutrophils would be able to overcome the foreseen repression of the IL-8 gene and produce high amounts of this chemokine on TLR triggering. Translational control of existing mRNA allows for more rapid changes in cellular concentrations of the encoded proteins and adaptation to the local environmental cues, once transfused into the patient.
Collectively, the mobilized cells have an altered TLR-mediated cytokine responsiveness, which is expected to be beneficial once the cells have migrated to the site of infection in the neutropenic patient. Increased production and local release of IL-8 will contribute to the accumulation and activation of the transfused neutrophils for appropriate antimicrobial activity in the infected tissues of the patient.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof D. Roos for critical reading and discussion of the manuscript.
This work was supported by the Sanquin Foundation for Cellular Blood Product Development (grant no. PPO-C-03-011-2003).
Authorship
Contribution: A.D. designed and performed research, analyzed data, and wrote the paper; A.T.J.T and J.G. helped perform parts of the research; R.v.B. helped to analyze the data; and T.K.v.d.B. and T.W.K. supervised the project and reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Agata Drewniak, Department of Blood Cell Research, Phagocyte Laboratory, Sanquin Research and Landsteiner Laboratory, Plesmanlaan 125, 1066CX Amsterdam, The Netherlands; e-mail: a.drewniak@sanquin.nl.