Abstract
Bone marrow injury is a major adverse side effect of radiation and chemotherapy. Attempts to limit such damage are warranted, but their success requires a better understanding of how radiation and anticancer drugs harm the bone marrow. Here, we report one pivotal role of the BH3-only protein Puma in the radiosensitivity of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs). Puma deficiency in mice confers resistance to high-dose radiation in a hematopoietic cell–autonomous manner. Unexpectedly, loss of one Puma allele is sufficient to confer mice radioresistance. Interestingly, null mutation in Puma protects both primitive and differentiated hematopoietic cells from damage caused by low-dose radiation but selectively protects HSCs and HPCs against high-dose radiation, thereby accelerating hematopoietic regeneration. Consistent with these findings, Puma is required for radiation-induced apoptosis in HSCs and HPCs, and Puma is selectively induced by irradiation in primitive hematopoietic cells, and this induction is impaired in Puma-heterozygous cells. Together, our data indicate that selective targeting of p53 downstream apoptotic targets may represent a novel strategy to protecting HSCs and HPCs in patients undergoing intensive cancer radiotherapy and chemotherapy.
Introduction
Maintaining a steady supply of mature blood cells is essential for defense against infection, tumor surveillance, and maintenance of homeostasis. Normal hematopoiesis can be perturbed by DNA-damaging agents such as γ-radiation and chemotherapy drugs. Morbidity rates associated with cancer chemotherapy and radiotherapy limit the effectiveness of these treatments. Radiation and anticancer drugs cause apoptosis in both hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs), leading to severe anemia, bleeding, and infections.1,2
Bone marrow (BM) injury and gastrointestinal toxicity are the 2 most common limiting factors for cancer therapies of anticancer DNA-damaging agents, such as ionizing radiation and chemotherapeutical drugs. It was established that γ-irradiation (7-14 Gy) primarily causes damage to HSCs and HPCs in the BM. As a result, mice have severe BM failure and die between 20 and 30 days after radiation.3 In contrast, a radiation dose greater than 14 Gy in C57B6 wild-type mice caused lethality due to damage to the small intestine and gastrointestinal syndrome in addition to BM failure.3 The damage is not limited to radiation; some chemotherapeutic drugs may also have similar detrimental effects. Therefore, approaches for improving the safety of anticancer DNA-damaging agents must include measures to either increase tumor sensitivity or reduce toxicity to critical stem cells or progenitors, or both. The latter requires a careful analysis of DNA damage of signaling pathways in HSCs.
Mammalian cells evolved a network of interacting pathways that comprise the DNA damage response, leading to (1) cell-cycle arrest, (2) DNA damage repair, (3) senescence, or (4) apoptosis, depending on the cell type and extent of DNA damage. The specific conserved genes that participate in the DNA damage response are still being defined. In addition, the detailed pathways governing DNA damage response and outcomes, especially those within HSCs, are not completely understood. Therefore, efforts to enhance the effectiveness of intensive γ-radiation and chemotherapeutic cancer therapies have been limited. In vivo studies showed that inactivation of p53 increased radioresistance in HSCs and HPCs, thereby protecting mice against a lethal dose of radiation.4,5 It is thought that p53-mediated apoptosis is executed by several of proapoptotic target genes6 ; however, molecular mechanisms of the DNA damage response in HSCs and HPCs remain elusive.
We previously showed that a highly conserved antiapoptotic transcription factor, Slug, promoted the survival of HPCs by down-modulating radiation-induced up-regulation of Puma, thereby conferring radioresistance against 6.5-Gy total body irradiation (TBI), which is a sublethal dose of radiation for wild-type mice. Puma encodes a p53-responsive BH3-only proapoptotic factor. Deletion of Puma converted the radiosensitivity of the Slug-deficient mice to radioresistance to 6.5-Gy TBI.7 These results implicate a key role of Puma in radiation-induced apoptosis in these critical cells that are responsible for radioresistance. This finding prompted us to explore whether the deletion of Puma alone is sufficient to allow mice to withstand a higher or lethal dose of ionizing radiation by protecting HSCs and HPCs. Studies of the apoptotic pathways and molecules that selectively act in HSCs and HPCs will facilitate the possibility of finding small molecules that improve the therapeutic index of cancer radiotherapy or chemotherapy, or both.
In the present study, we demonstrate that the deletion of Puma allows mice to withstand lethal dose radiation in a hematopoietic cell–autonomous manner, and loss of one Puma allele renders mice radioresistance to 9-Gy TBI. Remarkably, deletion of Puma selectively protects primitive but not differentiated hematopoietic cells from lethal dose radiation, thereby accelerating hematopoietic regeneration. Consistently, Puma deficiency suppresses radiation-induced apoptosis in HSCs and HPCs. Interestingly, loss of one Puma allele impairs the full radiation induction of Puma in hematopoietic cells, and Puma is selectively induced in primitive hematopoietic cells. Thus, it suggests that radiosensitivity is determined by Puma gene dosage and radiation dosages. Collectively, our findings here provide the proof of principle that inhibition of Puma is probably a novel strategy for protecting HSCs and HPCs in patients undergoing intensive cancer radiotherapy and chemotherapy.
Methods
Animal studies
All animal studies were evaluated and approved by the Institutional Animal Care and Use Committee of Maine Medical Center. PUMA−/− mice were generated and used after 5 backcrosses to C57BL/6N mice. p53−/− mice were purchased from The Jackson Laboratory. C57BL/6N mice (Ly5.2) and B6.SJL-ptprcα Pep3b (B6.SJL) congenic mice (LY5.1) were purchased from Taconic. All mice were housed in the pathogen-free animal facility at the Maine Medical Center Research Institute. For TBI experiments, the mice were given acidified antibiotic water (1.1 g/L neomycin sulfate and 106 U/L polymyxin B sulfate) 3 days before γ-irradiation and then continuously for 30 days to reduce the chance of spontaneous infection. The mice were 4 to 14 weeks old, and γ-irradiation was performed in a 137Cs γ-ray Model 143-45 irradiator (J.L. Shepherd and Associates). The irradiated mice were inspected daily for up to 40 days for radiation-induced death.
BM histology
Puma+/+, Puma+/−, and Puma−/− mice (8 weeks old) were nonirradiated or γ-irradiated (9 Gy) and then killed at 10 days after irradiation. Femurs were collected and fixed in Bouin fixative (Labchem Inc) for an additional 8 days. Sections were made and stained with hematoxylin and eosin. The images were captured using Axiovision 4.8 image-acquisition software and an AxioCam MRM camera (Zeiss) on a Zeiss microscope (40×/0.75 NA objective).
BM transplantation
For BM transplantation experiments, mice (4-8 weeks old) were fed with acidified antibiotic water and then lethally irradiated with 9-Gy TBI or a total of 13 Gy, given in 2 fractions of 6.5 Gy 3 hours apart. BM cells were injected into the retroorbital venous sinus of irradiated recipients after depletion of red blood cells.
cDNA synthesis and real-time quantitative reverse transcription–polymerase chain reaction
RNA was extracted with Trizol (Invitrogen) according to the manufacturer's protocol. The cDNA was synthesized by a standard reverse transcription reaction with an oligo-dT primer. Real-time polymerase chain reaction (PCR) was performed on a Bio-Rad system with the use of SYBR Green-based assays with AmpliTaq Gold (Applied Biosystems). The Puma transcripts were analyzed by quantitative PCR (QPCR), as previously described.7 The gene expression values were normalized to the geometric mean of the expression values of the housekeeping gene Hprt to obtain relative expression levels. All reactions were performed in triplicate.
Western blot analysis of protein expression level
BM cells were harvested from Puma+/+ and Puma+/− mice at 0, 4, and 6 hours after irradiation (5 Gy) and lysed in sodium dodecylsulfate extraction buffer [6% sodium dodecylsulfate, 4% β-mercaptoethanol, 20% glycerol, Tris (tris(hydroxymethyl)aminomethane)–HCl, pH6.8]. The protein samples were boiled for 5 minutes before separation by polyacrylamide gel and blotted with anti-Puma polyclonal antibody (Abcam). β-actin was detected as a loading control.
Flow cytometric analysis of different lineages and apoptotic cells
The mice were killed at the indicated time points after TBI, and the BM cells were flushed from the femurs with phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS), filtered through a nylon mesh, and the red blood cells were depleted. BM cells (1 × 106) were blocked with the staining buffer (PBS plus 2% FBS) containing 5% rat serum and Fc-block for 10 minutes before antibody staining. For analysis of differentiated cells, the cells were stained with fluorescein isothiocyanate (FITC)–anti-CD11b (for myeloid cells), FITC–anti-CD3e (for T cells), or FITC–anti-B220 (for B cells) for 20 minutes on ice, followed by incubation with 7-amino-actinomycin D for 10 minutes on ice. The stained cells were washed with staining buffer and resuspended in 0.3 mL of staining buffer. For analysis of HPCs (Lin−Sca-1+), the cells were costained with FITC-labeled antibodies specific for lineage markers (CD3e, B220, TER119, CD11b, and Gr-1), phycoerythrin (PE)–anti-Scal-1, and 7-amino-actinomycin D. For analysis of HSCs, the cells were stained for 20 minutes on ice with FITC-labeled antibodies specific for lineage markers, washed with PBS containing 2% FBS, and then stained with a combinations of antibodies specific for c-Kit, Scal-1, Flk2, and CD150 to identify CD150+LSK (Lin−Sca1+c-kit+), Flk2lowLSK, and Flk2highLSK cells.
For analysis of apoptotic cells in LSK and differentiated cell populations,8 BM cells were harvested from nonirradiated and irradiated PUMA−/− and PUMA+/+ mice (9 Gy) and cultured in serum-free expansion medium (StemSpan; 10 ng/mL interleukin-3) for HSCs and HPCs (Stemcell Technologies). Six hours after irradiation, BM cells were stained with biotinylated antibodies specific for lineage markers and PE–anti-Scal-1 and Alexa Fluor 647–anti–c-Kit antibodies, followed by Avid-PE/cyanine 7 staining. The stained cells were further stained with annexin V according to the manufacturer's instruction (Biotium). Annexin V+ LSK cells were gated as apoptotic hematopoietic stem and progenitor cells. All of the stained cells were analyzed by the FACSCalibur instrument (Becton Dickinson). All antibodies were purchased from eBioscience and BioLegend.
Results
Radiosensitivity is determined by Puma gene dosage
Numerous studies implicate p53 as a pivotal factor that regulates apoptosis in hematopoietic cells exposed to DNA-damaging drugs or γ-radiation.6 Targets for p53 include a growing list of proapoptotic genes, including BAX, BAK, Puma, and NOXA.6 HSCs and HPCs are the most critical cells for survival of mammalian organisms after exposure to DNA-damaging agents9 ; however, it remains to be determined whether one or a combination of p53 targets mediate apoptosis in these key cells after a high dose of radiation. Our previous study indicated that Slug radioprotects HPCs through direct inhibition of Puma transcription in sublethally irradiated mice.7 Inhibition of Puma by Slug is not sufficient to protect mice against a higher than 8.5-Gy TBI, a lethal dose for wild-type mice.7
To test the effects of Puma on the BM failure and death of all wild-type mice (LD100) after exposure to a lethal dose of irradiation, we lethally irradiated Puma+/+, Puma+/−, and Puma−/− mice with 9-Gy TBI. As expected, all of the wild-type mice died within 20 days. Remarkably, deletion of Puma allowed 90% of the irradiated mice to survive more than 35 days after γ-irradiation (Figure 1A). Thus, mice lacking Puma survive an otherwise lethal dose of γ-irradiation, even though the mice are wild-type for p53 and Slug.
Deletion of Puma renders mice resistant to γ-irradiation. (A) Kaplan-Meier survival curves of mice exposed to 9 Gy TBI. Puma+/+ (n = 9), Puma+/− (n = 6), and Puma−/− (n = 8) mice were given a single dose of TBI (9 Gy) and were monitored for survival. The enhanced survival rate of Puma−/− and Puma+/− mice was significantly greater than Puma+/+ mice (P < .001 and P < .01, respectively), but the survival rate was not significantly different between Puma−/− mice versus Puma+/− mice (P > .05). (B) Kaplan-Meier survival curves of mice exposed to 9.5 Gy TBI. Puma+/+ (n = 6), Puma+/− (n = 9), and Puma−/− (n = 7) mice were then administrated a lethal TBI dose (9.5 Gy) and monitored daily for survival. The survival rate of Puma−/− and Puma+/− mice was significantly greater than Puma+/+ mice (Puma−/− mice vs Puma+/+ mice, P < .001; Puma+/− mice vs Puma+/+ mice, P < .01). (C) Diagram for generation of reconstituted mice. C57BL6/N recipients were lethally irradiated for a total of 13 Gy (6.5 Gy, 2 times, 3 hours apart) and received 1 × 107 total BM cells from Puma+/+ mice or Puma−/− mice (n = 6 mice/group). (D) Kaplan-Meier survival curves of reconstituted mice after 9 Gy TBI. After 8 weeks, the mice in panel C were given a second course of TBI (9 Gy) and monitored for survival. The survival rate of mice reconstituted with Puma−/− BM cells was significantly higher than in mice reconstituted with Puma+/+ BM cells (P < .001).
Deletion of Puma renders mice resistant to γ-irradiation. (A) Kaplan-Meier survival curves of mice exposed to 9 Gy TBI. Puma+/+ (n = 9), Puma+/− (n = 6), and Puma−/− (n = 8) mice were given a single dose of TBI (9 Gy) and were monitored for survival. The enhanced survival rate of Puma−/− and Puma+/− mice was significantly greater than Puma+/+ mice (P < .001 and P < .01, respectively), but the survival rate was not significantly different between Puma−/− mice versus Puma+/− mice (P > .05). (B) Kaplan-Meier survival curves of mice exposed to 9.5 Gy TBI. Puma+/+ (n = 6), Puma+/− (n = 9), and Puma−/− (n = 7) mice were then administrated a lethal TBI dose (9.5 Gy) and monitored daily for survival. The survival rate of Puma−/− and Puma+/− mice was significantly greater than Puma+/+ mice (Puma−/− mice vs Puma+/+ mice, P < .001; Puma+/− mice vs Puma+/+ mice, P < .01). (C) Diagram for generation of reconstituted mice. C57BL6/N recipients were lethally irradiated for a total of 13 Gy (6.5 Gy, 2 times, 3 hours apart) and received 1 × 107 total BM cells from Puma+/+ mice or Puma−/− mice (n = 6 mice/group). (D) Kaplan-Meier survival curves of reconstituted mice after 9 Gy TBI. After 8 weeks, the mice in panel C were given a second course of TBI (9 Gy) and monitored for survival. The survival rate of mice reconstituted with Puma−/− BM cells was significantly higher than in mice reconstituted with Puma+/+ BM cells (P < .001).
We also found that 70% of Puma+/− mice (with one Puma copy) withstood 9-Gy TBI (Figure 1A). When the radiation dose was increased from 9 Gy to 9.5 Gy, 20% of Puma+/− mice survived for more than 40 days after γ-irradiation (Figure 1B). In comparison to Puma+/− mice, the deletion of Puma (Puma−/−) rendered most (70%) of the lethally irradiated mice to survive beyond 40 days after irradiation (Figure 1B). These results show that Puma−/− mice phenocopy p53−/− mice in terms of their radioresistance.10 Taken together, these data indicate that Puma gene dosage is a critical determinant of radiosensitivity, implying that Puma serves as a convergence point downstream of p53 for the DNA damage-induced signaling pathway in HSCs and HPCs.
Radioresistance conferred by Puma deficiency is intrinsic to the transplanted BM cells
To investigate whether Puma deficiency confers resistance to lethal doses of radiation in a hematopoietic cell–autonomous manner, we reconstituted 2 groups of lethally γ-irradiated wild-type mice with Puma+/+ or Puma−/− BM cells as donor cells with the use of our previously published approach.7 Thus, these reconstituted mice had the same microenvironment of BM, but they had a different hematopoietic compartment (Figure 1C). As expected, these mice had an equivalent level of white blood cell counts (data not shown). After a second course of 9-Gy TBI given at 2 months after reconstitution, all of the mice reconstituted with Puma+/+ BM cells died within 20 days, as expected (Figure 1D). By contrast, 90% of the chimeric mice reconstituted with Puma−/− BM cells survived more than 30 days after γ-irradiation (Figure 1D). These results show that Puma deficiency confers radioresistance to lethal dose of radiation in a transplanted hematopoietic cell–autonomous manner, and the effect does not depend on the BM marrow microenvironment.
Puma deficiency selectively protects HSCs and HPCs against lethal dose radiation
HPCs and HSCs have predominant roles in radioprotection, and they are required for short-term and long-term radioprotection, respectively.9 We and others showed that Puma has an important role in apoptosis in myeloid progenitor cells7 and lymphocytes11-13 after exposure to a sublethal dose of irradiation. In addition, Puma deficiency also afforded radioresistance to intestinal progenitor cells.14 Therefore, we decided to determine whether the radioresistance of Puma−/− mice against lethal dose radiation (Figure 1) is because of increased survival of in vivo HSCs and HPCs. To address this possibility, we determined survival of hematopoietic primitive cells versus differentiated cells (T, B, and myeloid cells) in Puma+/+, Puma+/−, and Puma−/− mice before and after a sublethal dose of TBI (5 Gy). We found that the number of LSK cells (Figure 2A), HPCs (Figure 2B), T cells (Figure 2C), B cells (Figure 2D), and myeloid cells (Figure 2E) in nonirradiated mice was equivalent, regardless of Puma genotypes. Thus, under physiologic conditions, loss of Puma does not lead to abnormal hematopoiesis.
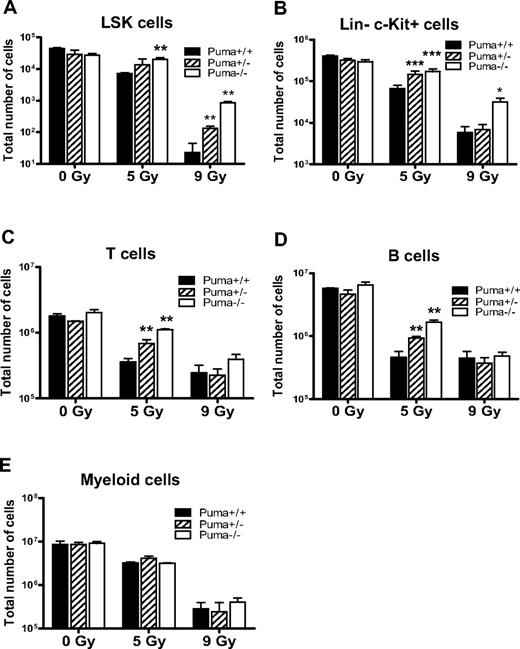
Radiosensitivity of hematopoietic cells is determined by Puma gene dosages and radiation dosages. (A) Total number of LSK cells in mice (n = 3 per group). The number of surviving LSK cells in 1 pair of femurs of Puma−/− mice was comparable with that from Puma+/+ mice before irradiation, but this number was significantly higher in Puma−/− than in Puma+/+ mice at 3 days after 5 Gy TBI and was significantly higher in Puma−/− and Puma+/− mice than in Puma+/+ mice at 3 days after 9 Gy TBI. **P < .01. (B) Total number of HPCs in 1 pair of mouse femurs before and after radiation. The number of HPCs (Lin−c-Kit+) in Puma−/−, Puma+/−, Puma+/+ mice was similar before irradiation but is significantly higher in Puma−/− and Puma+/− mice than in Puma+/+ mice at 3 days after 5 Gy TBI. There was a significantly higher number of Lin−c-Kit+ cells in Puma−/− mice than in Puma+/− and Puma+/+ mice at 3 days after 9 Gy TBI (n = 3 mice per group). *P < .05, ***P < .001. (C-D) Total number of T and B lymphocytes in 1 pair of femurs of mice. There was no significant difference in cell numbers of differentiated cells in nonirradiated mice, regardless of Puma genotype. The number of surviving differentiated T cells (CD3e+) and B cells (B220+) in Puma−/− and Puma+/− mice was significantly greater than those in Puma+/+ mice at 3 days after 5 Gy TBI. Three days after 9-Gy TBI, there was no difference in cell numbers of T and B cells in these mice (n = 3 per group). **P < .01. (E) Total number of myeloid cells in 1 pair of femurs of mice before and after exposure to radiation. There was no significant difference in myeloid cell numbers between Puma−/− or Puma+/− mice or Puma+/+ mice (n = 3) before and after radiation (5 or 9 Gy TBI). All data are shown as mean ± SD.
Radiosensitivity of hematopoietic cells is determined by Puma gene dosages and radiation dosages. (A) Total number of LSK cells in mice (n = 3 per group). The number of surviving LSK cells in 1 pair of femurs of Puma−/− mice was comparable with that from Puma+/+ mice before irradiation, but this number was significantly higher in Puma−/− than in Puma+/+ mice at 3 days after 5 Gy TBI and was significantly higher in Puma−/− and Puma+/− mice than in Puma+/+ mice at 3 days after 9 Gy TBI. **P < .01. (B) Total number of HPCs in 1 pair of mouse femurs before and after radiation. The number of HPCs (Lin−c-Kit+) in Puma−/−, Puma+/−, Puma+/+ mice was similar before irradiation but is significantly higher in Puma−/− and Puma+/− mice than in Puma+/+ mice at 3 days after 5 Gy TBI. There was a significantly higher number of Lin−c-Kit+ cells in Puma−/− mice than in Puma+/− and Puma+/+ mice at 3 days after 9 Gy TBI (n = 3 mice per group). *P < .05, ***P < .001. (C-D) Total number of T and B lymphocytes in 1 pair of femurs of mice. There was no significant difference in cell numbers of differentiated cells in nonirradiated mice, regardless of Puma genotype. The number of surviving differentiated T cells (CD3e+) and B cells (B220+) in Puma−/− and Puma+/− mice was significantly greater than those in Puma+/+ mice at 3 days after 5 Gy TBI. Three days after 9-Gy TBI, there was no difference in cell numbers of T and B cells in these mice (n = 3 per group). **P < .01. (E) Total number of myeloid cells in 1 pair of femurs of mice before and after exposure to radiation. There was no significant difference in myeloid cell numbers between Puma−/− or Puma+/− mice or Puma+/+ mice (n = 3) before and after radiation (5 or 9 Gy TBI). All data are shown as mean ± SD.
Subsequently, we irradiated Puma+/+, Puma+/−, and Puma−/− sex-matched littermates with a sublethal dose of TBI (5 Gy) and used flow cytometry to quantify each surviving hematopoietic lineages in the BM of these animals at 3 days after irradiation. According to the report from Wang et al,15 LSK cells or BM cells decreased to their lowest numbers at 3 days after irradiation. As expected, the number of surviving LSK cells (Figure 2A), progenitors (Figure 2B), T and B lymphocytes (Figure 2C-D), and myeloid cells (Figure 2E) in sublethally irradiated (5 Gy) mice were fewer than those in nonirradiated animals. Specially, we observed that Puma−/− mice retained 3 times more LSK cells, 2.5 times more HPCs (Lin−c-Kit+), at least 4 times more T cells (CD3e+), and 3 times more B cells (B220+) cells in their femurs, respectively, than did their Puma+/+ littermates after 5-Gy TBI (Figure 2A-D). Interestingly, the total number of myeloid cells is nearly equal in irradiated mice, regardless of Puma genotype (Figure 2E). The behavior of T and B cells after 5-Gy TBI is in agreement with the previous report.12
Next, we irradiated mice with a high dose of TBI (9 Gy), a lethal dose of irradiation for wild-type mice. The number of surviving LSK cells (Figure 2A) and HPCs (Figure 2B) in the femurs of Puma−/− mice was at least 36-fold and 6-fold higher, respectively, than in Puma+/+ counterparts who received 9-Gy TBI. Surprisingly, we found that the number of surviving T and B lymphocytes and myeloid cells in Puma+/+, Puma+/−, and Puma−/− mice was essentially the same after 9-Gy TBI, regardless of their genotypes (Figure 2C-E). These results suggest that the absence of Puma cannot protect these differentiated hematopoietic cells (T, B, and myeloid cells) against 9-Gy TBI.
Together, these results showed that the deletion of Puma selectively protected HSCs (LSK cells) and HPCs in mice exposed to a lethal dose of TBI (9 Gy) but not to a sublethal dose of TBI (5 Gy). Notably, loss of one Puma allele (Puma+/−) was sufficient to partially protect LSK cells against 9-Gy TBI (Figure 2A) and HPCs from 5-Gy TBI (Figure 2B), which may explain why Puma+/− mice were partially resistant to radiation (Figure 1A-B). In addition, heterozygosity of Puma radioprotected T and B cells against a sublethal dose but not a lethal dose of TBI (Figure 2C-D).
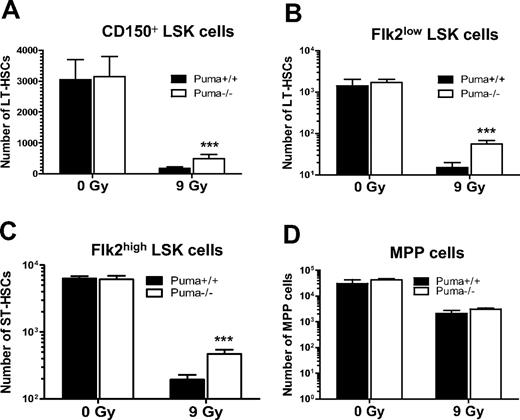
Although LSK cells enrich HSCs, LSK markers do not mark HSCs with high specificity. To further confirm that deletion of Puma indeed radioprotects HSCs, we used different combinations of surface markers to analyze long-term HSCs in Puma+/+ and Puma−/− mice before and after exposure to 9-Gy TBI. Our analysis indicated that Puma−/− mice have a similar number of long-term (LT) HSCs marked by CD150+LSK cells16 or Flk2lowLSK cells, compared with Puma+/+ mice, but had 3 times more CD150+LSK cells and 4 time more Flk2lowLSK cells at 3 days after 9-Gy TBI (Figure 3A-B). Similarly, the total number of short-term (ST) HSCs (Flkhigh LSK cells) was nearly the same in nonirradiated mice but was 2.5 times higher in Puma−/− mice than in Puma+/+ mice (Figure 3C). In contrast, these animals had similar numbers of multipotent progenitors marked as CD150−CD48−CD244+,16 regardless of Puma genotype (Figure 3D). Thus, these data indicated that Puma is a critical mediator of radiation-induced apoptosis in LT-HSCs and ST-HSCs, but not in multipotent progenitors. Taken together, our data indicate that the deletion of Puma shielded both differentiated cells (lymphocytes and myeloid cells) and undifferentiated cells (HSCs and HPCs) from sublethal doses of radiation but selectively protected HSCs and HPCs in mice exposed to lethal doses of radiation.
Ablation of Puma confers protection of primitive hematopoietic cells against a single dose of γ-irradiation. (A-D) Total number of surviving CD150+ LSK cells (A), Flk2low LSK cells (B), Flk2high LSK cells (C), and multipotent progenitor (MPP; CD150−CD48−CD244+) cells (D) in mice before and after 9 Gy TBI. The total number of CD150+ LSK cells, Flk2low LSK cells, and Flk2highLSK cells in 1 pair of femurs of Puma−/− mice (n = 6) was comparable with those in Puma+/+ mice (n = 5) under normal conditions but was significantly higher in Puma−/− mice than in Puma+/+ mice at 3 days after 9 Gy TBI. In contrast, the number of MPP cells (CD150−CD48−CD244+) was similar in both Puma−/− mice and Puma+/+ mice before and 3 days after 9 Gy TBI, respectively; ***P < .001. All data are shown as mean ± SD.
Ablation of Puma confers protection of primitive hematopoietic cells against a single dose of γ-irradiation. (A-D) Total number of surviving CD150+ LSK cells (A), Flk2low LSK cells (B), Flk2high LSK cells (C), and multipotent progenitor (MPP; CD150−CD48−CD244+) cells (D) in mice before and after 9 Gy TBI. The total number of CD150+ LSK cells, Flk2low LSK cells, and Flk2highLSK cells in 1 pair of femurs of Puma−/− mice (n = 6) was comparable with those in Puma+/+ mice (n = 5) under normal conditions but was significantly higher in Puma−/− mice than in Puma+/+ mice at 3 days after 9 Gy TBI. In contrast, the number of MPP cells (CD150−CD48−CD244+) was similar in both Puma−/− mice and Puma+/+ mice before and 3 days after 9 Gy TBI, respectively; ***P < .001. All data are shown as mean ± SD.
Puma deficiency accelerates regeneration of hematopoiesis in BM after irradiation
Deletion of Puma renders HSCs and HPCs radioresistant, based on their phenotype analysis. To ensure that phenotype of these spared HSCs correlate with their function in vivo, we designed an assay in which the repopulation ability of Puma−/− HSCs and Puma+/+ HSCs after radiation can be compared in each mouse. To this end, we generated reconstituted mice by transplanting a mixture of Puma−/− (Ly5.2+) and wild-type (Ly5.1+/5.2+) BM cells into lethally irradiated recipient mice (Ly5.1+) (Figure 4A). At 2 months after BM transplantation, the ratio of Puma−/− donor-derived cells was between 40% and 55% in peripheral blood of these reconstituted mice but increased to 85% at 2 months after a second course of 9-Gy TBI. This data further support the notion that Puma deficiency protects HSCs against γ-irradiation.
Deletion of Puma enhances repopulation of hematopoiesis after a single lethal dose of radiation. (A) Diagram for in vivo HSC competitive repopulating assay. PUMA−/− BM cells (Ly5.2+) were mixed with an equal number (1 × 106) of helper BM cells (Ly5.1+/Ly5.2+), and then transplanted into lethally irradiated recipients (Ly5.1+). The percentage of donor-derived cells (Ly5.2+) among hematopoietic cells derived from transplanted BM cells (Ly5.1+/Ly5.2+ and Ly5.2+) was determined by flow cytometric analysis in each recipient at 2 months after radiation. Reconstituted mice were then given a second course of γ-irradiation (9 Gy), and the percentage of donor-derived cells (Ly5.2+) in each recipient was determined by flow cytometric analysis at 2 months after the second radiation. (B) The percentage of donor-derived cells (Ly5.2+) in reconstituted mice in panel A. The percentage of donor-derived cells (Ly5.2+) in each of the reconstituted mice was significantly increased after a second course of γ-irradiation (9 Gy). The data shown are the means ± SD (n = 4 mice/group). P < .001. (C) Hematoxylin and eosin staining of the BM cavities of femurs from mice after TBI (9 Gy) or without treatment. Ten days after irradiation, hematopoietic cell clusters were evident in the BM of Puma−/− mice but not those of Puma+/+ mice.
Deletion of Puma enhances repopulation of hematopoiesis after a single lethal dose of radiation. (A) Diagram for in vivo HSC competitive repopulating assay. PUMA−/− BM cells (Ly5.2+) were mixed with an equal number (1 × 106) of helper BM cells (Ly5.1+/Ly5.2+), and then transplanted into lethally irradiated recipients (Ly5.1+). The percentage of donor-derived cells (Ly5.2+) among hematopoietic cells derived from transplanted BM cells (Ly5.1+/Ly5.2+ and Ly5.2+) was determined by flow cytometric analysis in each recipient at 2 months after radiation. Reconstituted mice were then given a second course of γ-irradiation (9 Gy), and the percentage of donor-derived cells (Ly5.2+) in each recipient was determined by flow cytometric analysis at 2 months after the second radiation. (B) The percentage of donor-derived cells (Ly5.2+) in reconstituted mice in panel A. The percentage of donor-derived cells (Ly5.2+) in each of the reconstituted mice was significantly increased after a second course of γ-irradiation (9 Gy). The data shown are the means ± SD (n = 4 mice/group). P < .001. (C) Hematoxylin and eosin staining of the BM cavities of femurs from mice after TBI (9 Gy) or without treatment. Ten days after irradiation, hematopoietic cell clusters were evident in the BM of Puma−/− mice but not those of Puma+/+ mice.
Furthermore, we directly examined the regeneration of hematopoiesis in the BM sections from lethally irradiated Puma+/+, Puma+/−, and Puma−/− mice. As expected, there was no noticeable difference in normal hematopoiesis, regardless of genotypes (Figure 4C upper panel). However, at day 10 after 9-Gy TBI, there were few organized hematopoietic cell clusters in Puma+/+ mice but significantly more hematopoietic cell clusters evident in the Puma−/− mice (Figure 4C lower panel). Interestingly, the number of repopulated hematopoietic cells in Puma+/− mice varied between Puma+/+ and Puma−/− mice, suggesting a Puma gene dosage effect. Collectively, our data indicated that loss of Puma accelerates the normal regeneration of hematopoietic compartments after γ-irradiation, suggesting that limiting apoptosis in HSCs is a primary mechanism for restoration of hematopoiesis in Puma−/− mice after irradiation.
Ablation of Puma limits apoptosis in primitive hematopoietic cells
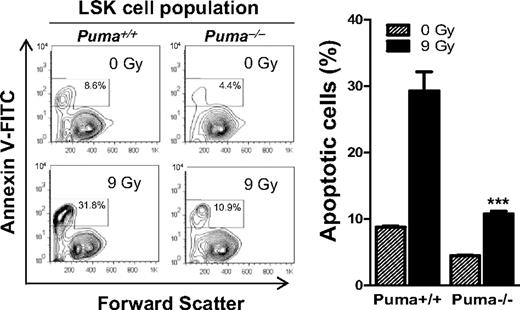
In mice, BM is the primary tissue damaged by γ-irradiation at doses up to 14 Gy,3 and HSCs and HPCs are critical for LT and ST radioprotection, respectively.9 Being a BH3-only protein, Puma's proapoptotic function has been extensively documented.11,17-19 Thus, we considered that Puma deficiency might render HSCs and HPCs more resistant to apoptosis after lethal dose radiation, which should accelerate recovery and regeneration of hematopoietic cells in the BM. To test this hypothesis, we irradiated Puma+/+ and Puma−/− mice with 9 Gy, the same dose for the prior survival experiments (Figures 1,Figure 2,Figure 3–4). We harvested BM cells from irradiated and nonirradiated mice and cultured them in the StemSpan serum-free expansion medium (10 ng/mL interleukin-3 for suppressing spontaneous apoptosis). We analyzed LSK cells enriched in HSCs and HPCs to quantify apoptosis cells with the use of annexin V staining and found that the percentage of apoptotic LSK cells is 2-fold lower in Puma−/− versus Puma+/+ mice before radiation and 3 times lower in Puma−/− versus Puma+/+ mice at 6 hours after 9-Gy TBI (Figure 5). Notably, the percentage of apoptotic LSK cells (10.9%) is only slightly higher in irradiated Puma−/− mice than in nonirradiated Puma+/+ mice. Thus, deletion of Puma renders LSK cells resistant to a higher dose of irradiation (9 Gy).
Puma deficiency diminishes radiation-induced apoptosis in primitive hematopoietic cells. Analysis of apoptotic rate in LSK cell population in BM with annexin V–based apoptosis assay. BM cells were harvested from nonirradiated and irradiated (9 Gy) Puma−/− and Puma+/+ mice and cultured in the StemSpan serum-free expansion medium supplemented with 10 ng/mL interleukin-3 (for suppressing spontaneous apoptosis). Six hours later, BM cells were stained for LSK markers, and the apoptotic cells were identified by a fluorescence-labeled annexin V. Apoptotic LSK cells (annexin V+ LSK) were analyzed by flow cytometry. The percentage of annexin V+ LSK cells (the numbers in boxes in left panel) is significantly increased in BM cells from Puma+/+ mice than those from Puma−/− mice before and after radiation. The data shown are representative of flow cytometric analysis (left) and the mean of the triplicate (right). ***P < .001 (Puma−/− vs Puma+/+ after radiation). FACS indicates fluorescence-activated cell sorting. Bar graph data are mean ± SD.
Puma deficiency diminishes radiation-induced apoptosis in primitive hematopoietic cells. Analysis of apoptotic rate in LSK cell population in BM with annexin V–based apoptosis assay. BM cells were harvested from nonirradiated and irradiated (9 Gy) Puma−/− and Puma+/+ mice and cultured in the StemSpan serum-free expansion medium supplemented with 10 ng/mL interleukin-3 (for suppressing spontaneous apoptosis). Six hours later, BM cells were stained for LSK markers, and the apoptotic cells were identified by a fluorescence-labeled annexin V. Apoptotic LSK cells (annexin V+ LSK) were analyzed by flow cytometry. The percentage of annexin V+ LSK cells (the numbers in boxes in left panel) is significantly increased in BM cells from Puma+/+ mice than those from Puma−/− mice before and after radiation. The data shown are representative of flow cytometric analysis (left) and the mean of the triplicate (right). ***P < .001 (Puma−/− vs Puma+/+ after radiation). FACS indicates fluorescence-activated cell sorting. Bar graph data are mean ± SD.
Puma is selectively up-regulated in primitive hematopoietic cells and its induction by radiation is in impaired in Puma-heterozygous hematopoietic cells
Our results indicated that deletion of one Puma allele is sufficient to protect animals and their LSK cells and HPCs from lethality caused by both sublethal and lethal-dose TBI (Figures 1–2). In addition, Puma expression is known to be up-regulated by γ-irradiation in a p53-dependent manner.7,11 Thus, we investigated whether loss of one Puma allele resulted in impairment of Puma induction by p53 after γ-irradiation. In agreement with the previous reports,7,11 Puma was indeed induced by γ-irradiation in Puma+/+ hematopoietic cells, and its expression increased up to 2-fold at 1.5 hours and 5-fold at 3 hours after γ-irradiation (Figure 6A). In contrast, Puma expression in Puma+/− hematopoietic cells only increased up to 1.5-fold at 1.5 hours and 3.5-fold at 3 hours after γ-irradiation (Figure 6A). Puma mRNA level in Puma+/− hematopoietic cells was 1.5-fold lower compared with Puma+/+ BM cells before γ-irradiation, 2-fold lower at 1.5 hours, and 2.5-fold lower at 3 hours after irradiation. Consistent with these results, Puma protein levels in Puma+/− BM cells were lower at each designated time points than in Puma+/+ BM cells (Figure 6B). As expected, expression of NOXA, another p53-responsive gene, in these samples was not altered by Puma gene dosage (data not shown). These results, together with the other data (Figures 1,Figure 2–3), indicated that deletion of one Puma allele impaired full induction of Puma expression, which resulted in increased survival of LSK cells and HPCs of Puma+/− mice, rendering these animals resistant to lethal doses of radiation.
Puma is up-regulated by γ-irradiation in a gene dose-dependent manner and is selectively expressed in LSK cells. (A) QPCR analysis of Puma mRNA expression levels in primary hematopoietic cells from Puma+/+ and Puma+/− mice at 1.5 and 3 hours after irradiation (5 Gy) or no treatment. Puma gene expression levels were normalized, based on the levels of the housekeeping gene HPRT. The cross comparison between Puma+/+ and Puma+/− mice at each time point was highly significant (2-tailed Student t test). *P < .05, ***P < .001 (Puma+/+ vs Puma+/− at each time point). (B) Western blot analysis of Puma expression levels. Lysates extracted from BM of Puma+/+ and Puma+/− mice at the indicated time points after irradiation (5 Gy) were probed with an anti-Puma and an anti–β-actin antibody (loading control). (C) QPCR analysis of PUMA induction by irradiation in LSK cells. LSK and Lin+ cells were sorted, respectively, from wild-type mice and cultured in the StemSpan serum-free expansion medium (10 ng/mL interleukin-3). The cells were untreated or treated with γ-irradiation (7 Gy) and then subjected to total RNA extraction at 3 hours after irradiation. QPCR analysis was used to qualify Puma mRNA level in nonirradiated and irradiated cells and normalized to HPRT expression level. ***P < .001 and *P < .05 (LSK vs Lin+ cells). Data in panels A and C are mean ± SD.
Puma is up-regulated by γ-irradiation in a gene dose-dependent manner and is selectively expressed in LSK cells. (A) QPCR analysis of Puma mRNA expression levels in primary hematopoietic cells from Puma+/+ and Puma+/− mice at 1.5 and 3 hours after irradiation (5 Gy) or no treatment. Puma gene expression levels were normalized, based on the levels of the housekeeping gene HPRT. The cross comparison between Puma+/+ and Puma+/− mice at each time point was highly significant (2-tailed Student t test). *P < .05, ***P < .001 (Puma+/+ vs Puma+/− at each time point). (B) Western blot analysis of Puma expression levels. Lysates extracted from BM of Puma+/+ and Puma+/− mice at the indicated time points after irradiation (5 Gy) were probed with an anti-Puma and an anti–β-actin antibody (loading control). (C) QPCR analysis of PUMA induction by irradiation in LSK cells. LSK and Lin+ cells were sorted, respectively, from wild-type mice and cultured in the StemSpan serum-free expansion medium (10 ng/mL interleukin-3). The cells were untreated or treated with γ-irradiation (7 Gy) and then subjected to total RNA extraction at 3 hours after irradiation. QPCR analysis was used to qualify Puma mRNA level in nonirradiated and irradiated cells and normalized to HPRT expression level. ***P < .001 and *P < .05 (LSK vs Lin+ cells). Data in panels A and C are mean ± SD.
These studies could not answer the critical question: why does deletion of PUMA result in selective radioprotection of primitive hematopoietic cells but not differentiated cells? To address this question, we examined the expression level of Puma before and after irradiation in LSK cell versus Lin+ cell populations. The QPCR analysis indicated that expression level of endogenous Puma is 3-times higher in LSK cells than in Lin+ cells, and that a 2.5-fold increase of Puma transcripts was shown in LSK cells versus Lin+ cells after irradiation (Figure 6C). Thus, Puma is endogenously expressed at a higher level and prominently induced by irradiation in primitive hematopoietic cells versus differentiated cells. Our data indicate that Puma is selectively expressed in primitive hematopoietic cells versus differentiated cells.
Discussion
Maintaining the integrity of hematopoiesis is essential for survival of mammalian organisms during lethal intrinsic and extrinsic insults. To accomplish this function, the hematopoietic system replenishes mature blood cells daily and maintains sufficient numbers of stem and progenitor cells to regenerate the hematopoietic system quickly. Therefore, apoptotic pathway(s) should operate differently in mature cells and in primitive hematopoietic cells when exposed uniformly to the same genotoxic agent. Although p53-deficient mice survive a lethal radiation dose due to increased resistance of HSCs and HPCs in the BM,4,5 it remains to be determined whether a common key factor downstream of p53 mediates radiation-induced apoptosis in these key cells.6
Our results indicate that deletion of Puma protects mice from lethal doses of irradiation in a cell-autonomous manner. Interestingly, deletion of one Puma allele (Puma+/−) rendered the mice resistant to 9-Gy TBI, but radioresistance declined when the γ-irradiation dose increased to 9.5 Gy, which is similar to the results noted with the p53+/− mice.10 In addition, our data showed that both Puma mRNA and protein levels are significantly lower in Puma+/− BM cells than in wild-type (Puma+/+) BM cells at specific time points after γ-irradiation, implying that the Puma mRNA level in Puma+/− mice is affected by its gene dosage. Consistent with these new findings, our previous studies indicate that Slug, a transcriptional repressor, protects HPCs in vivo by down-modulating transcriptional up-regulation of Puma in response to ionizing radiation.7 Taken together, these data clearly indicate that Puma is an essential target of p53 in HSCs and HPCs, and it mediates p53-induced apoptosis in these key cells.
In vivo proapoptotic functions of Puma in radiation-induced signaling were previously shown in lymphocytes and other nonhematopoietic cells.11-14 To our surprise, in contrast to a sublethal dose (5 Gy) effect, deletion of Puma selectively protected HSCs and HPCs from a lethal radiation dose (9 Gy). In addition, we demonstrated that surviving HSCs identified by their phenotypes in irradiated Puma−/− mice retained repopulating potential.
How does deletion of Puma protect HSCs and HPCs but not mature hematopoietic cells from lethal dose radiation? p53, as a principle transcriptional factor, responds to γ-irradiation and up-regulates several target genes, including Puma, NOXA, BAX, DR5, and others.17,18,20-23 The timing for p53 to induce its target genes is determined by both radiation dose and cell type.24-26 It is conceivable that Puma is primarily induced in primitive cells and thus is a rate-limiting factor for lethal-dose radiation–induced apoptosis in these cells. Indeed, our QPCR data indicated that Puma is selectively expressed in primitive versus differentiated hematopoietic cells before and after irradiation. The molecular mechanisms underlying lineage-specific Puma transcription regulation should be extensively studied in the future.
The role of Puma in determining the fate of HSCs and HPCs is relevant to cancer therapy because therapeutic doses of radiation and chemotherapy are primarily limited by the sensitivity of these key cells to genotoxic agents. Interestingly, a small molecule inhibitor of p53, which was recently identified by high-throughput screening, protects mice from γ-irradiation. There are some safety concerns with this approach due to the critical roles of p53 in tumor suppression and DNA damage repair.27
It has been widely assumed that the inability of p53 to induce apoptosis would enable irradiated cells to propagate any chromosomal aberrations sustained from γ-irradiation exposure, thereby increasing the likelihood of generating new malignant cells. However, recent work from Christophorou et al28 and Efeyan et al29 surprisingly showed that, in mouse models, the inability of p53 to induce apoptosis did not increase the onset of tumor formation and also that oncogenes, rather than p53-mediated apoptosis, provided the key signal to p53 to suppress tumorigenesis.
Consistent with previous reports from others,11,30-32 mice deficient in Puma are not inherently prone to form tumors. Interestingly, Puma deficiency accelerates Myc-induced lymphomagenesis,32,33 implying that Puma may function differently in tumors induced by different oncogenic signals. Thus, it is necessary to further test whether Puma inhibition will promote tumor progression or drug resistance in preexisting tumors. Nevertheless, according to our findings, inhibition of Puma rather than p53 represents a safer approach to protecting HSCs and HPCs from the detrimental effects of radiation and possibly chemotherapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Gerard P. Zambetti for Puma−/− mice. We thank our colleagues at Maine Medical Center Research Institute (MMCRI) for their critical review. We also thank the Cell Separation and Analysis Core for the cell analysis and the Histopathology Core at MMCRI for the bone marrow sections.
This work was supported in part by National Institutes of Health from the National Center for Research Resources (grant P20 RR018789). W.S.W was supported by a K01 award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01DK078180).
National Institutes of Health
Authorship
Contribution: L.S., Y.S., and Z.Z. designed and performed research and analyzed data; W.F. and Y.G. performed the research; Z.C. and Z.Z.W. contributed to research design; A.T.L. provided materials; W.-S.W. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wen-Shu Wu, Maine Medical Center Research Institute, 81 Research Dr, Scarborough, ME 04074; e-mail: wuw@mmc.org.
References
Author notes
L.S., Y.S., and Z.Z. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal