Abstract
The protein C (PC) pathway is an important anticoagulant mechanism that prevents thrombosis during the systemic inflammatory response. Thrombomodulin (TM), an endothelial cell membrane receptor, accelerates the conversion of PC to activated protein C (APC), which leads to the down-regulation of thrombin production and fibrin formation. Induction of acute endotoxemia in young and aged mice with a low dose of bacterial endotoxin lipopolysaccharide (LPS, 2.5 mg/kg) caused a high mortality rate in aged (80%) but not young (0%) mice. After injection with this dose of LPS, fibrin formation was significantly elevated only in aged mice, plasma APC levels were increased only in young mice, and TM expression was profoundly depressed in the aged. The increased thrombosis, suppressed APC level, and decreased TM expression were not observed in young mice receiving a higher dose of LPS (20 mg/kg), which resulted in a mortality rate (78%) equivalent to that seen in aged mice with the low-dose LPS. Mutant mice with reduced TM showed significantly less plasma APC and increased fibrin formation compared with wild-type mice after LPS. These results demonstrate that PC pathway activation is suppressed with aging and is partly responsible for age-associated thrombosis and high mortality during endotoxemia.
Introduction
Systemic inflammatory response syndrome (SIRS) occurs after severe infection, trauma, and burn or during pancreatitis. It is a clinical condition characterized by hyperthermia or hypothermia, increased heart and respiratory rate, and abnormally high or low white blood cell number.1-3 SIRS is a particularly serious problem in the geriatric population, as elderly patients with systemic inflammatory stress have much higher morbidity and mortality than younger patients.4-7 SIRS, along with its infectious counterpart, sepsis, affects nearly 700 000 people annually and has been the 10th leading cause of death in patients older than 65 years in the United States since 2001.4,8 Although incidence rates and deaths for sepsis in humans are known to increase with age, the exact mechanisms for this age-associated vulnerability remain largely unknown. Susceptibility to SIRS has been documented as a function of age in animal models; thus, the use of aged animals is particularly important as investigating such processes in the young may result in a different outcome.
SIRS is accompanied by increased production of inflammatory cytokines, and such responses are closely linked to the initiation of thrombosis.9 Widespread microvascular thrombosis, or disseminated intravascular coagulation (DIC), is a characteristic late complication of SIRS, which often leads to tissue ischemia, multiple organ failure, and death.10 It is estimated that 30% to 50% of patients with the most severe clinical manifestations of SIRS/sepsis experience DIC.11 As microvascular dysfunction is recognized as a major contributor to organ failure and death,12 it is particularly important to understand the mechanisms behind such complications.
During SIRS, the coagulation cascade is activated when coagulation factor VIIa (FVIIa) enters the tissue from the circulation and contacts tissue factor. The tissue factor-FVIIa complex catalyzes the conversion of FX to activated factor FXa, which along with its cofactor FVa produces thrombin through posttranslational cleavage of prothrombin. Thrombin converts fibrinogen to fibrin, which is then crosslinked by FXIIIa to form a clot. Thrombin production is accelerated by a positive feedback mechanism, in which FVIII and FXI (upstream of FX activation) and FV are activated by thrombin and contribute to the conversion of prothrombin to thrombin.9
The protein C (PC) pathway is a negative feedback mechanism that regulates thrombin production and subsequent fibrin formation.13,14 PC is converted to activated protein C (APC) by thrombin; this activation is augmented more than 1000-fold when thrombin binds to its receptor, thrombomodulin (TM), at the vascular endothelial cell surface.15 PC activation is further enhanced (20-fold) by its binding to endothelial protein C receptor.16 APC down-regulates thrombin production and fibrin formation by inactivating FVa and FVIIIa. TM is a 74-kDa glycoprotein that is most abundantly expressed in the lung17 and is known to be down-regulated during endotoxemia.18,19 TM acts as an anticoagulant not only by enhancing PC activation but also by capturing and inactivating circulating thrombin.20 In addition, TM serves as a negative regulator of complement activation21 ; thus, down-regulation of TM results in increased coagulation by both impairing the negative regulation of coagulation and by facilitating complement-mediated vascular injury. Accordingly, both TM and APC have important roles for preventing blood coagulation during SIRS. However, very little is known about whether TM or APC levels are altered with age.
Previously, we and others demonstrated that aged mice, compared with young mice, are significantly more susceptible to death during systemic inflammation caused by experimental acute endotoxemia.22-24 Although age-associated increases in production of inflammatory cytokines were well documented in these studies, age-associated alterations in the blood coagulation cascade have not been investigated. In the present study, we demonstrate an age-dependent decrease of TM and altered plasma APC levels in mice during endotoxemia. These findings provide an important foundation for explaining the origin of increased DIC and mortality observed in the elderly during SIRS.
Methods
Animals
Young (4-month-old) and aged (24-month-old) male C57BL/6 mice were obtained from a colony at the National Institute on Aging. Mice were acclimated for at least 14 days in a 12:12-hour light-dark cycle with free access to water and regular chow diet (LabDiet) before experiments began. Transgenic (TMpro) mice with a single amino acid homozygous mutation (Glu404Pro; GAA → CCA) in the TM gene25 were transferred from the Blood Research Institute at the Blood Center of Wisconsin to the University of Texas Medical Branch, where breeding and experiments were performed. The mutation in TMpro mice results in reduced TM expression,26 deficient PC activation,27 and increased mortality to endotoxemia.28 In experiments using these mutant mice, age- and sex-matched C57BL/6 mice (designated as wild-type) are used as controls. To elicit systemic inflammation, acute endotoxemia was induced by intraperitoneal injection with bacterial endotoxin lipopolysaccharide (LPS, derived from Pseudomonas aeruginosa, L8643, lot no. 045K4057; Sigma-Aldrich). For survival studies, mortality of each mouse was monitored for 7 days after LPS injection. Body temperature, as a parameter of severity of systemic inflammation,24 was assessed by rectal temperature probe with a digital thermometer (Precision Thermometer 4600, YSI Inc). Major organs from dead mice were examined, and animals with any evident signs of tumor were excluded from the study. For tissue sample collection, mice were intraperitoneally injected with 50 units of heparin sodium (Pharmacia) 5 minutes before death. Mice were then anesthetized with isoflurane inhalation, the inferior vena cava was cut, and the entire vasculature was perfused with physiologic saline through the cardiac ventricles as previously described.29 Immediately after dissection, tissues were frozen in liquid nitrogen and stored at −80°C. Noninjected mice were used as a control (designated 0 hours). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch and the University of Kentucky.
Western blot analysis
Protein was extracted from each individual frozen tissue, and Western blot analysis was performed as previously described.29 In brief, pulverized frozen tissues were homogenized in ice-cold lysis buffer with protease inhibitors and phosphatase inhibitors. Equal amounts of proteins were resolved on Nu-PAGE Bis-Tris SDS-polyacrylamide gels (Invitrogen) and electrophoretically transferred to polyvinylidene difluoride membranes (Bio-Rad). The membranes were incubated with antifibrin monoclonal antibody 59D830 or anti-TM polyclonal antibody M-17 (Santa Cruz Biotechnology). The membranes were washed and further incubated with horseradish peroxidase–conjugated anti-IgG secondary antibodies (Millipore). Chemiluminescent detection was performed using enhanced chemiluminescence detection reagents (GE Healthcare). Intensity of each protein was determined by densitometric analysis using Kodak one-dimensional software, Version 3.6. All membranes were reprobed with anti–β-actin antibody (no. 4967; Cell Signaling) for normalization.
APC assay
Blood samples were taken from the inferior vena cava and mixed with 0.1M sodium citrate and 0.3M benzamidine-HCl (Sigma-Aldrich) at a ratio of 1:10 and centrifuged at 2000g for 10 minutes at 4°C. The concentration of plasma APC was determined using an immunocapture assay as previously described,31 except for the substitution of chromogenic substrate S2366 (0.46mM in Tris-buffered saline, pH 7.5; DiaPharma) for Spectrozyme PCA. In each set of experiments, APC levels of all plasma samples were determined within a single 96-well plate, using a standard curve made from a set of positive control reactions in the same plate.
Immunohistochemistry
Lung tissues were fixed with 10% neutral-buffered formalin and embedded in paraffin. Sections (4 μm) were prepared on glass slides, and immunohistochemistry was performed with diluted (1:50) anti-TM antibody (Santa Cruz Biotechnology) using Dako Cytomation EnVision+ System-HRP Kit (Dako Denmark). Photomicrographs were taken using Nikon DXM-1200 digital camera and Nikon Microphot-FXA microscope with ACT-1 acquisition software (40× objective, total magnification 400×, aperture 0.95).
Northern blot analysis
Total RNA was isolated from tissues using guanidine/phenol solution as described previously.29 The RNA samples (20 μg each) were subjected to Northern blot analysis as recently described.32 To prepare probe DNA, a TM cDNA fragment (499-bp) was amplified by reverse transcriptase-polymerase chain reaction from mouse lung total RNA. The oligo-DNA primers used for the reverse transcriptase-polymerase chain reaction were 5′-GGAGAATGGTGGCTGTGAGT-3′ (sense), 5′-GCACGAAGTTTCATTGCAGA-3′ (TM antisense).
Statistical analysis
Results from the mortality experiment were analyzed using a log-rank test from StatView software (SAS Institute). All other data were analyzed with Student t test or analysis of variance using SigmaStat Statistical Software, Version 2.0 (Systat Software). A P value less than .05 was considered statistically significant.
Results
Age-associated mortality from endotoxemia
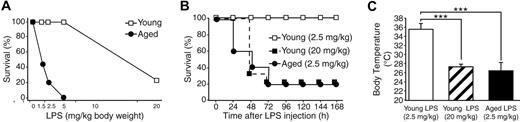
A survival study was performed with various doses of bacterial endotoxin to determine the difference in dose-response of young (4-month-old) and aged (24-month-old) C57BL/6 mice to LPS. Mice were intraperitoneally injected with 4 different doses of LPS (1.5, 2.5, 5, and 20 mg/kg body weight), and survival was monitored for 7 days. As shown in Figure 1A, the survival rate of young mice was 100% with LPS doses of 5 mg/kg or less and decreased to 22% with 20 mg/kg LPS. In contrast, aged mice displayed increased vulnerability to lower doses of LPS. After injection with 1.5, 2.5, and 5 mg/kg LPS, their survival rates were 42%, 20%, and 0%, respectively. As shown in Figure 1B, survival patterns of aged mice with 2.5 mg/kg LPS and young mice with 20 mg/kg were similar. The survival rates of these 2 groups became 40% and 33%, respectively, by 48 hours after LPS injection and 20% and 22%, respectively, by 72 hours and thereafter; there was no significant difference between the groups (P = .52). Compared with the 100% survival rate of young mice with 2.5 mg/kg LPS, the decrease in survival rates of aged mice with 2.5 mg/kg LPS and young mice with 20 mg/kg LPS was statistically significant (P < .001). As shown in Figure 1C, young mice with 2.5 mg/kg exhibited mild hypothermia (35.5 ± 1.3°C) 12 hours after LPS injection. In contrast, young mice receiving 20 mg/kg LPS and aged mice receiving 2.5 mg/kg LPS exhibited more profound hypothermia to a similar degree (27.4 ± 0.6°C and 26.5 ± 1.7°C, respectively); there was no statistical difference between these 2 groups (P = .54). The equivalent mortality rate and degree of hypothermia between young mice with the high dose of LPS (20 mg/kg) and aged mice with the low dose of LPS (2.5 mg/kg) suggest that both groups developed endotoxemia of similar severity.
Age-dependent mortality during endotoxemia. (A) A survival study demonstrating age-dependent mortality during endotoxemia. Young (4 months) and aged (24 months) male C57BL/6 mice received an intraperitoneal injection with LPS at various doses, and survival was monitored for 7 days. For each dose point, 8 to 14 mice were studied. No mortality was found in control mice with saline injection (not shown here). (B) Seven-day survival curves from the same experiment comparing mortality rates of young mice (LPS 2.5 mg/kg or 20 mg/kg) and aged mice (LPS 2.5 mg/kg). There was no statistical difference between the survival curve patterns of aged mice with 2.5 mg/kg LPS and young mice with 20 mg/kg LPS (P = .52). (C) Degree of hypothermia 12 hours after LPS injection was compared among young mice (LPS 2.5 mg/kg or 20 mg/kg) and aged mice (LPS 2.5 mg/kg). ***Statistical significance (P < .001). There was no statistical difference between body temperatures of aged mice with 2.5 mg/kg LPS and young mice with 20 mg/kg LPS (P = .54).
Age-dependent mortality during endotoxemia. (A) A survival study demonstrating age-dependent mortality during endotoxemia. Young (4 months) and aged (24 months) male C57BL/6 mice received an intraperitoneal injection with LPS at various doses, and survival was monitored for 7 days. For each dose point, 8 to 14 mice were studied. No mortality was found in control mice with saline injection (not shown here). (B) Seven-day survival curves from the same experiment comparing mortality rates of young mice (LPS 2.5 mg/kg or 20 mg/kg) and aged mice (LPS 2.5 mg/kg). There was no statistical difference between the survival curve patterns of aged mice with 2.5 mg/kg LPS and young mice with 20 mg/kg LPS (P = .52). (C) Degree of hypothermia 12 hours after LPS injection was compared among young mice (LPS 2.5 mg/kg or 20 mg/kg) and aged mice (LPS 2.5 mg/kg). ***Statistical significance (P < .001). There was no statistical difference between body temperatures of aged mice with 2.5 mg/kg LPS and young mice with 20 mg/kg LPS (P = .54).
Age-dependent coagulation during endotoxemia
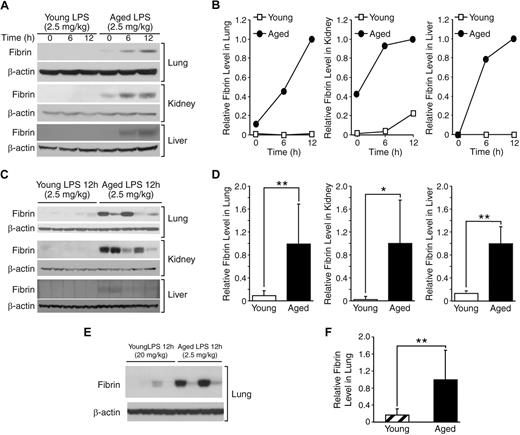
Fibrin formation in mouse tissues was assessed to examine age-associated changes in microvascular coagulation during systemic inflammation. Protein was extracted from tissues of young (4-month) and aged (24-month) mice that were sacrificed 0, 6, or 12 hours after injection with a dose of LPS (2.5 mg/kg), which was shown to cause 0% and 78% mortality in young and aged mice, respectively. Western blot analysis was performed to detect fibrin formation using a fibrin-specific monoclonal antibody 59D8 that does not react with fibrinogen, a precursor of fibrin. Fibrin formation in the lung, kidney, and liver clearly increased in aged mice 6 and 12 hours after LPS injection (Figure 2A-B). In contrast, fibrin levels were minimally increased in young mice treated with the same dose of LPS. Analysis of protein samples from individual mice showed that the age-associated increase in fibrin formation is statistically significant in all 3 tissues 12 hours after LPS injection (lung, P = .008; kidney, P = .016; liver, P = .001; Figure 2C-D); fibrin formation in the lung and kidney 6 hours after LPS injection also showed a statistically significant age-associated increase (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These results confirm that aged mice are significantly more prone to endotoxemia-induced coagulation than young mice.
Age-dependent coagulation during endotoxemia. (A) Western blot analysis of a time course experiment examining fibrin formation in lung, kidney, and liver of young (4 months) and aged (24 months) mice that were sacrificed 6 and 12 hours after LPS injection (2.5 mg/kg, intraperitoneal). The control mice without LPS injection are represented by 0 h. Each lane contained 40 μg of protein derived equally from 5 individual mice. Each membrane was reprobed with anti–β-actin antibody to assure equal protein loading. (B) Densitometric analysis of panel A. (C) Fibrin formation 12 hours after LPS injection was further compared among individual samples, which were pooled in panel A. Each lane contained 40 μg of protein from an individual mouse. (D) Densitometric analysis of panel C. (E) Western blot analysis comparing thrombosis in lungs from young mice with a high dose of LPS (20 mg/kg) and aged mice with a low dose of LPS (2.5 mg/kg; n = 4 in each group). Each lane represents a protein sample from an individual mouse. (F) Densitometric analysis of panel E. (D-F) Data are mean ± SD. *Statistical significance (P < .05). **Statistical significance (P < .01).
Age-dependent coagulation during endotoxemia. (A) Western blot analysis of a time course experiment examining fibrin formation in lung, kidney, and liver of young (4 months) and aged (24 months) mice that were sacrificed 6 and 12 hours after LPS injection (2.5 mg/kg, intraperitoneal). The control mice without LPS injection are represented by 0 h. Each lane contained 40 μg of protein derived equally from 5 individual mice. Each membrane was reprobed with anti–β-actin antibody to assure equal protein loading. (B) Densitometric analysis of panel A. (C) Fibrin formation 12 hours after LPS injection was further compared among individual samples, which were pooled in panel A. Each lane contained 40 μg of protein from an individual mouse. (D) Densitometric analysis of panel C. (E) Western blot analysis comparing thrombosis in lungs from young mice with a high dose of LPS (20 mg/kg) and aged mice with a low dose of LPS (2.5 mg/kg; n = 4 in each group). Each lane represents a protein sample from an individual mouse. (F) Densitometric analysis of panel E. (D-F) Data are mean ± SD. *Statistical significance (P < .05). **Statistical significance (P < .01).
To further examine the age-associated increase in coagulation during endotoxemia, we compared LPS-induced fibrin formation in lungs from young mice with a high dose of LPS (20 mg/kg) versus aged mice with a low dose of LPS (2.5 mg/kg; n = 4 in each group). As described in “Age-associated mortality from endotoxemia,” these 2 doses of LPS resulted in similar severity of endotoxemia in young and aged mice as measured by mortality rates (Figure 1B) and hypothermia (Figure 1C). Fibrin formation in lungs from aged mice with the low dose of LPS was significantly higher than that from young mice with the high dose of LPS (P = .007; Figure 2E-F). Absence of high levels of fibrin formation in young mice with a lethal dose of LPS (20 mg/kg) indicates that the strong fibrin formation seen in aged mice is not a result of increased severity of disease but an age-dependent mechanism that leads to increased susceptibility.
Age-associated changes in plasma APC levels during endotoxemia
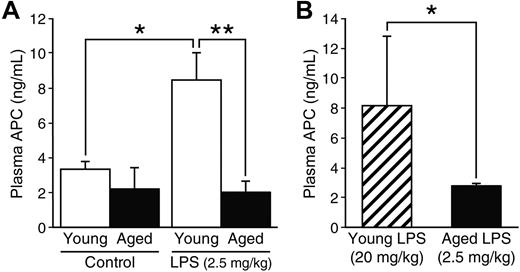
To establish whether APC levels are altered by age during endotoxemia, we measured the level of APC in plasma samples obtained from young and aged mice that were sacrificed without LPS injection or 12 hours after LPS injection (2.5 mg/kg). Plasma APC levels of young and aged mice without endotoxemia were 3.3 plus or minus 0.5 ng/mL and 2.2 plus or minus 1.2 ng/mL, respectively; there was no statistical difference between the age groups at basal levels (P = .62, Figure 3A). Plasma APC levels increased significantly in young mice during endotoxemia (8.5 ± 1.6 ng/mL, P = .023 compared with young mice without LPS), whereas this elevation was not noted in aged mice where the APC levels remained low (2.0 ± 0.6 ng/mL). Thus, the level of APC in the plasma during endotoxemia was 4.2-fold higher in young mice than in aged mice (P = .007).
Age-associated difference in plasma APC levels during endotoxemia. (A) Young (4 months, n = 6) and aged (24 months, n = 4) C57BL/6 mice were sacrificed 12 hours after LPS injection (2.5 mg/kg, intraperitoneal). Plasma APC levels were determined by immunocapture assay. For controls, plasma samples from young and aged mice (n = 4, each group) without LPS injection were assayed for APC. (B) Plasma APC levels were compared 12 hours after LPS injection in young mice with a high dose (20 mg/kg) versus aged mice with a low dose (2.5 mg/kg; n = 4 in each group). Data are mean ± SD. *Statistical significance (P < .05). **Statistical significance (P < .01).
Age-associated difference in plasma APC levels during endotoxemia. (A) Young (4 months, n = 6) and aged (24 months, n = 4) C57BL/6 mice were sacrificed 12 hours after LPS injection (2.5 mg/kg, intraperitoneal). Plasma APC levels were determined by immunocapture assay. For controls, plasma samples from young and aged mice (n = 4, each group) without LPS injection were assayed for APC. (B) Plasma APC levels were compared 12 hours after LPS injection in young mice with a high dose (20 mg/kg) versus aged mice with a low dose (2.5 mg/kg; n = 4 in each group). Data are mean ± SD. *Statistical significance (P < .05). **Statistical significance (P < .01).
Next, we compared plasma APC levels in young mice with the high dose of LPS (20 mg/kg) versus aged mice with the low dose of LPS (2.5 mg/kg; Figure 3B, n = 4 in each group). As mentioned previously, these 2 doses resulted in similar severity of endotoxemia in young and aged mice. Whereas plasma APC levels in aged mice remained low (2.7 ± 0.2 ng/mL), the levels in young mice with the lethal dose of LPS increased (8.1 ± 4.7 ng/mL). From these results, we conclude that low levels of plasma APC are an age-dependent phenomenon. Increased PC activation during endotoxemia in young mice with the lethal dose of LPS (20 mg/kg) indicates that the suppressed APC production seen in aged mice is not a result of severity of disease.
Age-dependent loss of TM during endotoxemia
The significantly low plasma APC levels in aged mice during endotoxemia (Figure 3A-B) prompted us to examine the expression of TM, a major factor that promotes PC activation. Western blot analysis showed that, in young mice, the level of TM decreased to 64% of its initial level by 6 hours but started to recover by 12 hours after injection with LPS (2.5 mg/kg). However, in aged mice, the level of TM decreased to 44% by 6 hours and further decreased to a level that was barely detectable by 12 hours after injection with the same dose of LPS (Figure 4A-B). There appeared to be no age-associated difference in basal TM levels (0 hours). Further analysis of individual protein samples from mice sacrificed 12 hours after LPS injection showed that pulmonary TM levels at this time point were significantly lower in aged mice than young mice (P = .002) with a magnitude of approximately 6-fold (Figure 4C-D). TM expression is mainly limited to lung, and we did not detect TM in kidney and liver (data not shown). We also compared TM expression in young mice with the high dose of LPS (20 mg/kg) versus aged mice with the low dose of LPS (2.5 mg/kg; n = 4 in each group). TM levels in aged mice were approximately 2-fold lower than the levels in young mice with the lethal dose of LPS (Figure 4E-F).
Age-dependent loss of TM in lungs during endotoxemia. (A) Western blot analysis of a time course experiment assessing TM levels in lungs of young (4 months) and aged (24 months) mice that were sacrificed 6 and 12 hours after LPS injection (2.5 mg/kg, intraperitoneal). The control mice without LPS injection are represented by 0 h. Each lane contained 40 μg of protein derived equally from 5 individual mice. The membrane was reprobed with anti–β-actin antibody to assure equal protein loading. (B) Densitometric analysis of panel A. (C) TM levels at 12 hours after LPS injection were further compared among individual samples, which were pooled in panel A. Each lane contained 40 μg of protein from an individual mouse. (D) Densitometric analysis of panel C. (E) TM levels in lungs were compared in young mice with a high dose of LPS (20 mg/kg) versus aged mice with a low dose of LPS (2.5 mg/kg) 12 hours after injection (n = 4 in each group). (F) Densitometric analysis of panel E. (D-F) Data are mean ± SD. *Statistical significance (P < .05). **Statistical significance (P < .01).
Age-dependent loss of TM in lungs during endotoxemia. (A) Western blot analysis of a time course experiment assessing TM levels in lungs of young (4 months) and aged (24 months) mice that were sacrificed 6 and 12 hours after LPS injection (2.5 mg/kg, intraperitoneal). The control mice without LPS injection are represented by 0 h. Each lane contained 40 μg of protein derived equally from 5 individual mice. The membrane was reprobed with anti–β-actin antibody to assure equal protein loading. (B) Densitometric analysis of panel A. (C) TM levels at 12 hours after LPS injection were further compared among individual samples, which were pooled in panel A. Each lane contained 40 μg of protein from an individual mouse. (D) Densitometric analysis of panel C. (E) TM levels in lungs were compared in young mice with a high dose of LPS (20 mg/kg) versus aged mice with a low dose of LPS (2.5 mg/kg) 12 hours after injection (n = 4 in each group). (F) Densitometric analysis of panel E. (D-F) Data are mean ± SD. *Statistical significance (P < .05). **Statistical significance (P < .01).
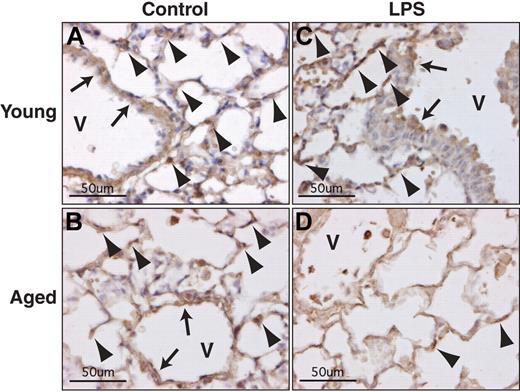
Immunohistochemical analysis demonstrated positive staining in the capillaries and large vessels of the lung from young and aged mice without LPS injection (Figure 5A-B). In the sections from mice with endotoxemia, TM staining was still detectable in young but not in aged mice (Figure 5C-D), confirming the results from our Western blot analyses.
Age-dependent loss of TM in pulmonary vascular cells during endotoxemia. Immunohistochemical analysis of TM was performed on lung sections from young (4 months) and aged (24 months) mice that were sacrificed 12 hours after LPS injection (2.5 mg/kg, intraperitoneal). Strong positive immunostaining for TM is seen in young control (A), aged control (B), and young mice with LPS (C), but not in aged mice with LPS (D). Control mice received no LPS. TM-positive cells in capillaries and large vessels (V) are indicated by arrowheads and arrows, respectively (original magnification ×400).
Age-dependent loss of TM in pulmonary vascular cells during endotoxemia. Immunohistochemical analysis of TM was performed on lung sections from young (4 months) and aged (24 months) mice that were sacrificed 12 hours after LPS injection (2.5 mg/kg, intraperitoneal). Strong positive immunostaining for TM is seen in young control (A), aged control (B), and young mice with LPS (C), but not in aged mice with LPS (D). Control mice received no LPS. TM-positive cells in capillaries and large vessels (V) are indicated by arrowheads and arrows, respectively (original magnification ×400).
In addition, we examined TM mRNA levels in the lungs from young and aged mice (supplemental Figure 2). The average TM mRNA levels in both young and aged mice decreased by 6 hours after LPS injection; however, the levels in young mice started to return to normal by 12 hours, whereas the levels in aged mice continued to decrease. TM mRNA levels at 12 hours after LPS injection were approximately 5-fold lower in aged mice. The pattern of change in TM mRNA levels in young and aged mice strongly resemble those of TM protein levels (Figure 4A).
From these results, we conclude that, although pulmonary TM levels decrease in both young and aged mice after LPS injection, the reduction of TM in the aged mouse lung is more profound and prolonged.
Augmented coagulation in TMpro mice during endotoxemia
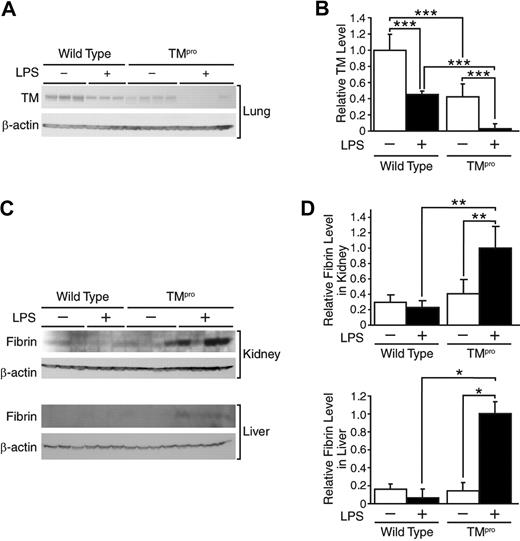
We used TMpro mice (mutant mice with reduced TM expression) to examine whether the reduced TM levels in aged mice during endotoxemia are causally linked to low APC levels and increased fibrin formation. TMpro mutant mice and wild-type control mice were sacrificed 6 hours after LPS injection, and APC, fibrin formation, and TM levels were assessed. As expected and previously observed,25 the TM levels in mice without LPS injection were significantly lower in TMpro mice than wild-type control mice. Although both groups showed decreased TM levels after LPS injection, the TM levels in TMpro mice were almost undetectable, whereas the TM levels in wild-type mice remained at nearly half the initial level (Figure 6A-B). LPS-mediated fibrin formation was very low or undetectable in the kidney and liver of wild-type mice but significantly increased in the same tissues from TMpro mice (Figure 6C-D).
Augmented coagulation in TMpro mice during endotoxemia. (A) Western blot analysis confirming TM levels in lung protein samples from TMpro mice and wild-type mice (both 4-5 months old) that were sacrificed 6 hours after LPS injection (5 mg/kg, intraperitoneal). Control mice received no LPS (indicated as LPS−). Each lane contained 40 μg of protein from an individual mouse. (B) Desitometric analysis of panel A. ***Statistical significance (P < .001). (C) Western blot analysis demonstrating fibrin formation in kidney and liver from the same animals. Each lane contained 40 μg of protein from an individual mouse. (D) Densitometric analysis of panel C. Data are mean ± SD. *Statistical significance (P < .05). **Statistical significance (P < .01). This experiment was repeated once with similar results.
Augmented coagulation in TMpro mice during endotoxemia. (A) Western blot analysis confirming TM levels in lung protein samples from TMpro mice and wild-type mice (both 4-5 months old) that were sacrificed 6 hours after LPS injection (5 mg/kg, intraperitoneal). Control mice received no LPS (indicated as LPS−). Each lane contained 40 μg of protein from an individual mouse. (B) Desitometric analysis of panel A. ***Statistical significance (P < .001). (C) Western blot analysis demonstrating fibrin formation in kidney and liver from the same animals. Each lane contained 40 μg of protein from an individual mouse. (D) Densitometric analysis of panel C. Data are mean ± SD. *Statistical significance (P < .05). **Statistical significance (P < .01). This experiment was repeated once with similar results.
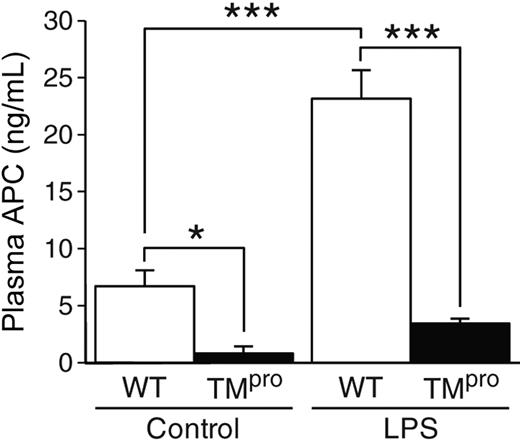
Plasma APC levels were significantly lower in TMpro mice compared with wild-type mice (Figure 7). Without LPS, the APC levels in TMpro mice were 8.4-fold lower than that in wild-type mice (0.8 ± 0.6 ng/mL vs 6.7 ± 1.4 ng/mL, P = .021). After LPS injection, the APC levels increased significantly in wild-type mice (23.1 ± 2.5 ng/mL; P < .001), whereas the APC levels in TMpro mice only increased to 3.6 plus or minus 0.3 ng/mL (P = .22). The average plasma APC level in TMpro mice during endotoxemia was 6.4-fold lower than the APC level in wild-type mice (P < .001). From these data, we conclude that TM reduction results in abnormally low protein C activation and leads to enhanced coagulation during endotoxemia.
Low plasma APC levels in TMpro mice during endotoxemia. Both TMpro mice (n = 4) and wild-type (WT, n = 5) mice (6 months old) were sacrificed 6 hours after LPS injection (5 mg/kg, intraperitoneal). Control mice received no injection (n = 4 in each group). The plasma APC levels were determined by immunocapture assay. Data are mean ± SD. *Statistical significance (P < .05). ***Statistical significance (P < .001).
Low plasma APC levels in TMpro mice during endotoxemia. Both TMpro mice (n = 4) and wild-type (WT, n = 5) mice (6 months old) were sacrificed 6 hours after LPS injection (5 mg/kg, intraperitoneal). Control mice received no injection (n = 4 in each group). The plasma APC levels were determined by immunocapture assay. Data are mean ± SD. *Statistical significance (P < .05). ***Statistical significance (P < .001).
Discussion
In the present study, we demonstrate, for the first time, that aging is accompanied by suppressed PC pathway activation during endotoxemia. When young and aged mice were given an equal dose of LPS (2.5 mg/kg), young mice showed no mortality (Figure 1), no significant fibrin formation (Figure 2A-D), but increased plasma APC levels (Figure 3A) and a modest temporal down-regulation of TM expression (Figures 4A-D, 5). In contrast, aged mice showed a high mortality rate (80%, Figure 1), significantly increased fibrin formation (Figure 2), no increase in plasma APC levels (Figure 3), and profound and sustained down-regulation of TM expression (Figures 4–5). We also compared young mice given a higher dose of LPS (20 mg/kg) with aged mice given the low dose of LPS (2.5 mg/kg). We did this comparison because these 2 groups showed very similar mortality rates (78% and 80%, Figure 1B) and degrees of hypothermia (27.4 ± 0.6°C and 26.5 ± 1.7°C, Figure 1C). Young mice with the high dose of LPS did not show significant fibrin formation (Figure 2E-F) but showed increased plasma APC levels (Figure 3B) and TM expression, which was significantly higher than that in aged mice with the low dose of LPS (Figure 4E-F). The significantly increased coagulation and suppressed PC pathway activation seen in aged mice were not observed in either young mouse group regardless of the severity of endotoxemia, indicating that such intense coagulation and suppression of PC pathway activation are an age-dependent phenomenon; the high levels of plasma APC in young mice with a lethal dose of LPS excludes the possibility that suppressed PC pathway activation in aged mice is a result of lethality.
In Figure 2, we show that aged animals are extremely more prone to coagulation during systemic inflammation than young animals. Thrombosis does not appear to be the cause of death in young mice with the lethal high dose of LPS (20 mg/kg), as demonstrated by lack of significant fibrin formation. In contrast, it is highly probable that the majority of aged mice died with thrombosis after injection with the lower dose of LPS (2.5 mg/kg).
Indeed, 40% of aged mice but no young mice died within 24 hours of LPS injection, although they showed equivalent mortality rates after 7 days (Figure 1B). The early death of those aged mice reflects acute coagulation found only in the aged mouse group 6 to 12 hours after LPS injection. The difference in intensity of fibrin formation seen in aged mice (Figure 2C-D) may indicate a variable outcome with respect to mortality. Although there is no direct evidence that increased thrombosis in aged mice is the main cause of mortality, Ely et al reported that recombinant APC administration to patients with sepsis was more effective in terms of short- and long-term survival for elderly patients than for young patients,33 consistent with the premise that thrombosis during systemic inflammation is a major contributing factor for mortality in the aged.
We also demonstrate that plasma APC levels in young mice were significantly increased after LPS injection, whereas those of aged mice remained low. TM was decreased in both young and aged animals; however, the decrease was more profound and prolonged in the aged. These findings indicate that (1) in the young, protein C is activated by the thrombin/TM interaction and facilitates inactivation of the coagulation cascade preventing microvascular coagulation; and (2) in the aged, protein C is not activated, at least in part, because of the down-regulation of TM; thus, thrombin is available to carry on the cascade, resulting in more fibrin deposition, which leads to DIC and death. Clinically, patients who are septic often exhibit elevated plasma APC levels; low APC levels are associated with a poor outcome.34 This trend is similar to the outcome noted in our current study. Young animals with elevated APC levels do not develop DIC and recover quickly from endotoxemia. In contrast, aged animals are associated with a poor prognosis; they develop severe coagulation, APC levels are not elevated, and they exhibit a higher mortality rate than young animals. The conundrum of having increased APC during endotoxemia, although TM is decreased, is probably because thrombin levels are elevated in endotoxemia and the amount of APC generated in healthy animals is roughly proportional to the thrombin levels.35
TMpro mice have a reduced capacity to activate exogenously injected human PC,25,36 and these mice have higher mortality rates after LPS injection than wild-type mice.28 In the present study, we further demonstrate that the TMpro mutation is associated with low endogenous plasma APC levels and significantly elevated fibrin formation during endotoxemia. Taken together, these results further support our conclusion that age-associated suppression of TM during endotoxemia contributes to loss of APC production, increased thrombosis, and high mortality in the aged. In addition, Li et al demonstrated that mutant mice overexpressing endothelial protein C receptor produced significantly higher levels of APC upon thrombin infusion and were more resistant to endotoxemia than control mice.31 The protective role of the APC during endotoxemia also supports our conclusion that age-associated suppression of PC pathway activation causes increased coagulation and mortality in the aged.
TM is down-regulated in young animals after treatment with LPS.37,38 The proinflammatory cytokine tumor necrosis factor α (TNF-α) appears to be a mediator of this down-regulation39-41 ; however, it is not clear at present why LPS-mediated down-regulation of TM becomes more profound and prolonged with aging. One possible explanation is that LPS-mediated production of TNF-α tends to be elevated with aging. Several studies have demonstrated that aged mice show approximately 2-fold higher levels of circulating TNF-α than young mice 1.5 to 2 hours after intraperitoneal injection with LPS.22,23,42 These studies also showed that TNF-α induction was not sustained and returned to basal levels within 3 hours of LPS injection. Thus, it is yet to be confirmed whether the modest age-associated difference in TNF-α levels occurring within a very short time period during the early phase of endotoxemia can produce a profound difference in TM expression at a much later phase. We and others have shown that several inflammatory factors, in addition to TNF-α, exhibit age-related changes in their pattern of induction during endotoxemia. For example, LPS-mediated induction of interleukin-1 (IL-1) and IL-6 is significantly increased with age,22-24,32,43 whereas interferon-γ induction is suppressed with age.22 Thus, it is probable that inflammatory mediators other than TNF-α also contribute to age-associated suppression of TM during endotoxemia.
We recently reported that pulmonary expression of extracellular superoxide dismutase (EC-SOD or SOD3), a type of SOD that is mainly expressed in the lungs, is down-regulated during endotoxemia.29 These findings led us to hypothesize that there is a common mechanism for the down-regulation of EC-SOD and TM in the lungs. Gut-enriched Kruppel-like factor (GKLF or KLF4) was identified as a transcriptional repressor of EC-SOD.44 Kruppel-like factors 2 and 4 (KLF2, KLF4), zinc finger transcription factors, were recently identified as potent inducers of TM.45,46 Overexpression of KLF2 in vitro resulted in an approximate 8-fold increase in TM activity as measured by the production of APC.46 We found that TM mRNA levels correspond to down-regulated protein levels, implying that the age-associated loss of TM is an effect of transcriptional regulation. Future studies will investigate the age-associated differences in KLF-2 and KLF-4 regulation.
Yamamoto et al42 previously reported that LPS-induced expression of plasminogen activator inhibitor-1, a major inhibitor of fibrinolysis, is augmented by aging and that this age-associated change suppresses fibrinolysis and increases DIC in aged mice during endotoxemia. This study and our present study point out that 2 major anticoagulant mechanisms, the PC pathway and fibrinolysis, are suppressed by aging, which accelerates endotoxin-induced coagulation. APC is known to possess both anticoagulant and anti-inflammatory functions.47-49 We and others have previously shown that age-associated high mortality in mice to endotoxemia is accompanied by elevated expression of proinflammatory factors, such as TNF-α, IL-6, and plasminogen activator inhibitor-1.22,24,42 Thus, the age-associated decline of APC levels during endotoxemia may be causally related to increased production of proinflammatory cytokines, which would also contribute to elevated DIC and mortality in aged animals. In addition, Xu et al recently reported that histones released during the inflammatory response contribute to endothelial dysfunction and APC cleaves these circulating histones, reducing their cytotoxicity.50 This protective mechanism may be absent in aged animals with suppressed PC pathway activation; such a novel mechanism may also partly contribute to the increased mortality observed in aged animals during endotoxemia.
In conclusion, endotoxemia-mediated down-regulation of TM expression is significantly more profound with aging. This age-associated loss of TM results in failure of APC production, which is partly responsible for the increased DIC and mortality observed in the aged during systemic inflammation. Although the mechanisms for age-associated suppression of PC pathway activation are still unclear, the novel findings presented here have potential clinical significance if confirmed in human patients with systemic inflammation. Therapeutic efforts should then be taken to reactivate the PC pathway, particularly in elderly patients, which would lead to reduced DIC and mortality from SIRS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Arnold J. Stromberg, Professor of the Department of Statistics at the University of Kentucky, for his advice on our statistical analyses, Dr Marshall Runge at the University of North Carolina for generously providing the monoclonal antibody 59D8, and Ms Karen K. Martin for manuscript preparation.
This work was supported by the National Institutes of Health (grant R01-AG025908) and the University of Texas Medical Branch, Claude D. Pepper Older American Independence Center, National Institutes of Health (P30AG024832).
National Institutes of Health
Authorship
Contribution: M.E.S. performed experiments, analyzed data, and wrote the paper; J.U. and H.T. performed experiments and analyzed data; H.W. provided transgenic animals and reviewed results; C.T.E. provided vital reagents and reviewed results; B.M.E. reviewed results; and H.S. designed research, performed experiments, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: C.T.E. is a consultant for Artisan Portola, Bayer, Vasculox, and Cardiome. The remaining authors declare no competing financial interests.
Correspondence: Hiroshi Saito, Department of Surgery, University of Kentucky, MS-476 Medical Science Bldg, 800 Rose St, Lexington, KY 40536-0298; e-mail: hiroshi.saito@uky.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal