Abstract
Elevated cytokines in bone marrow (BM) micro-environment (interleukin-6 [IL-6], transforming growth factor-beta [TGF-β], and IL-1β) may play an important role in observed immune dysfunction in multiple myeloma (MM). As IL-6 and TGF-β are important for the generation of T-helper 17 (TH17) cells, we evaluated and observed a significantly elevated baseline and induced frequency of Th17 cells in peripheral blood mononuclear cells (PBMCs) and BM mononuclear cells (BMMCs) from MM patients compared with healthy donors. We observed significant increase in levels of serum IL-17, IL-21, IL-22, and IL-23 in blood and BM in MM compared with healthy donors. We also observed that myeloma PBMCs after TH17 polarization significantly induced IL-1α, IL-13, IL-17, and IL-23 production compared with healthy donor PBMCs. We next observed that IL-17 promotes myeloma cell growth and colony formation via IL-17 receptor, adhesion to bone marrow stromal cells (BMSCs) as well as increased growth in vivo in murine xenograft model of human MM. Additionally, we have observed that combination of IL-17 and IL-22 significantly inhibited the production of TH1-mediated cytokines, including interferon-γ (IFN-γ), by healthy donor PBMCs. In conclusion, IL-17–producing Th17 cells play an important role in MM pathobiology and may be an important therapeutic target for anti-MM activity and to improve immune function.
Introduction
A significant impairment of T-cell function is observed in patients with monoclonal gammopathy of undetermined significance and multiple myeloma (MM).1 Both phenotypic and functional aberrations in CD4 and CD8 cells have been described.2 Although recent studies show that T cells from MM patients contain defective T-cell receptor variable repertoire3 and impaired viral-specific cytotoxic T lymphocyte, particularly against influenza and Epstein-Barr virus,4 presence of clonal CD4 and CD8 cells has been observed in MM.5,6 The significance of the presence of these clonally expanded T cells in MM patients is not well understood; however, this phenomenon is associated with better prognosis. It is presumed that these expanded T cells could play a role in controlling tumor cell growth and survival.7 Increased hyper-reactive T cells are observed in myeloma that is associated with impaired TCR signaling and increased sensitivity to costimulatory signals.8
CD4 helper T lymphocytes are important in both cell-mediated and antibody-mediated immune responses.9 T-helper 17 (TH17) cells, a new CD4 subset, are differentiated in the presence of, interleukin-6 (IL-6), IL-1β, IL-21, and IL-23 with or without transforming growth factor-beta (TGF-β) and produce IL-17 and IL-22. These cytokines protect against fungal and parasitic infections; and participates in inflammatory reactions and autoimmunity.10-18 Activated TH17 cells produce most of the IL-17 but CD8 cells, γδ T cells, natural killer cells, natural killer T cells, and neutrophils also produce variable amounts of IL-17.19,20 IL-17 is a structural homologue of cystine knot family of proteins with intrachain disulfide bonds.21 It is closely related to TGF-β, nerve growth factor, bone morphogenetic protein and platelet-derived growth factor with similar structural motifs. IL-17 induces expression of a number of chemokines and cytokines22-27 including IL-6, TGF-β, granulocyte-colony stimulating factor or granulocyte, macrophage-colony stimulating factor; matrix metalloproteinase and intercellular adhesion molecule-1 in a variety of cell types, including the BM stromal cells. A number of molecules influence IL-17 production including, prostaglandin E2,28 granulocyte, macrophage-colony stimulating factor,29 and aryl hydrocarbon receptor.13 In addition to c-Jun-N-terminal kinase, and MAP kinase kinase, a number of additional signaling molecules play a role in IL-17 production including, caspase recruitment domain-containing-9,30 nucleotide-binding oligomerization domain-containing-2,31 interferon regulatory factor and 4.32 Even though Janus kinase/signal transducer and activator of transcription (STAT) pathway is critical in most of TH17-related cytokine-mediated effects, STAT-3 mutations are shown to be detrimental for IL-17 production.33 suppressor of cytokine signaling-3 inhibits TH17 cell differentiation by inhibiting IL-23–mediated signaling.34
A significant body of information has emerged supporting a critical role for immune cells and associated cytokines in MM pathobiology as well as observed immune dysregulation in MM. IL-6, TGF-β, and IL-1β have been implicated in this process.35 IL-6 and TGF-β both have been reported to enhance the generation of TH17 cells, and are differentiated by number of inflammatory cytokines including, IL-21, IL-22, IL-23, and IL-27. In addition, there is also epidemiologic data that suggest several fold higher incidence of myeloma in patients with autoimmune diseases including ulcerative colitis as well as rheumatoid arthritis.36 Therefore, we have here evaluated the role of TH17 cells and associated pro-inflammatory cytokines in myeloma. We demonstrate that TH17 cells and IL-17 are elevated in myeloma; and IL-17 it promotes myeloma cell growth both in vitro and in vivo via IL-17 receptors (IL17R), and inhibits TH1 responses.
Methods
Patient samples
Both peripheral blood and BM samples were collected from newly diagnosed myeloma patients, and from patients without treatment for at least 3 months, after informed consent in accordance with the Declaration of Helsinki and approval by the institutional review board (IRB) at Dana-Farber Cancer Institute. Healthy donor samples were obtained from the blood donor center at Children's Hospital, Boston, MA. Normal donor bone marrow samples were obtained from AllCells.
Intracellular IL-17–producing TH17 cell analysis by flow cytometry
To evaluate baseline frequency of TH17 cells, PBMCs and BMMCs isolated from healthy donors and myeloma patients were stimulated with phorbol 12-myristate13-acetate (PMA) and ionomycin for 6 hours in the presence of Golgi stop (eBioscience) as described previously.14,15 After washing, cells were fixed with fix/perm buffer (Beckman Coulter) and stained for CD4 (eBioscience). Intracellular staining for interferon-γ (IFN-γ; BD Biosciences) and IL-17 (eBioscience) were performed using conjugated anti–IFN-γ (BD Biosciences) and anti–IL-17 (eBioscience) antibodies and analyzed using Canto II flow cytometer (BD Biosciences). To evaluate induced TH17 cells, naive CD4 cells purified from negatively selected CD4 population using CD45RA micro beads (Miltenyi Biotec) were polarized with TH17-polarizing cocktail consisting of IL-1 (10 ng/mL), IL-6 (20 ng/mL), and IL-23 (10 ng/mL) in addition to anti–IFN-γ (10 μg/mL), anti–IL-4 (10 μg/mL) TGF-β (1 ng/mL; R&D Systems) and anti-CD3 and anti-CD28 antibodies for 6 days in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). Cells were further expanded with IL-2 (20 U/mL; R&D Systems) for additional 6 days prior to restimulation with PMA and ionomycin and staining with anti–IL-17 and anti–IFN-γ antibodies described above in this paragraph. The purified naive CD4 cells were positive for CD45RA in more than 95% cells by flow cytometry.
To evaluate induced TH1 cells, healthy donor PBMCs were polarized with TH1-polarizing cocktail consisting of IL-12 (10 ng/mL; R&D Systems) and anti-CD3 antibodies for 6 days in RPMI 1640 supplemented with 10% FBS. Cells further expanded with IL-2 (20 U/mL; R&D Systems) for additional 6 days prior to restimulation with PMA and ionomycin and staining with anti–IFN-γ antibodies described in the previous paragraph. In these experiments activated cells were stained with CD4 and CD69 prior to intracellular staining of IFN-γ.
Quantitative PCR for IL-17 and IL-17R expression
After polarization, RNA from CD4 cells in case of IL-17 and from primary myeloma cells purified with CD138 micro-beads (Miltenyi Biotec) in case of IL-17R was isolated using the RNeasy kit according to the manufacturer's instructions (QIAGEN). Reverse transcription of RNA to cDNA was prepared using the RETROscript kit (Ambion) according to the manufacturer's protocol. Quantitative real-time reverse-transcriptase polymerase chain reaction (qRT-PCR) was performed using the Applied Biosystems 7500 apparatus with the SYBR Green PCR master mix (Applied Biosystems) according to manufacturer's suggestions. Primer pairs were used as previously described.15
Myeloma cell-proliferation assays
Seven different myeloma cell lines (RPMI 8226, KMS-12BM, OPM-1, OPM-2, INA-6, U226, and H929) were cultured in RPMI 1640 supplemented with 10% FBS for 3 days in the presence or absence of IL-17. Proliferation was assessed by 3h-thymidine incorporation over 6 hours. Colony forming assay was performed using MethoCult agar media (StemCell Technologies) in the presence or absence of IL-17 for 3 weeks. Primary MM cells were purified from bone marrow mononuclear cells by positive selection with CD138 micro-beads (Miltenyi Biotec), according to the manufacturer's instructions. BMMCs were isolated using Ficoll-Hypaque density gradient sedimentation from BM aspirates from MM patients after informed consent and IRB (Dana-Farber Cancer Institute) approval. BMMCs were cultured in RPMI 1640 supplemented with 20% FBS (4-8 weeks) to establish bone marrow stromal cells. MM cell lines were cultured in RPMI 1640 supplemented with 10% FBS. After overnight culturing of BMMCs, MM cell lines were cocultured in the presence or absence of IL-17. These experiments were also performed with or without anti–IL-17 receptor antibody.
Adhesion assay
Cell adhesion assay was done as previously described.37 In brief, serum-starved MM cells (5 × 106/mL) were labeled with calcein (Molecular Probes) for 30 minutes at 37°C, washed, and resuspended in culture medium. Cells were added to bone marrow stromal cell (BMSC)–coated 96-well plates, treated with or without IL-17, and incubated at 37°C for 4 hours. Unbound cells were removed by 4 washes with RPMI 1640 complete medium. The absorbance of each well was measured using 492/520 nm filter set with a fluorescence plate reader (Wallac VICTOR2; Perkin-Elmer).
Myeloma murine xenograft model
Six- to 8-week-old male CB-17 severe combined immunodeficient (SCID) mice (Taconic) were housed and monitored in the VA Boston Healthcare System Animal Research Facility. All experimental procedures and protocols had been approved by the Institutional Animal Care and Use Committee. Five million OPM-1 MM cells treated with or without IL-17 (R&D Systems) at 100 ng/mL concentration were injected subcutaneously into SCID mice and tumor volumes were measured at 3 weeks as described previously.38
Cytokine measurements using ELISA
Serum samples were collected from blood and bone marrow after informed consent approved by the IRB from Dana-Farber Cancer Institute and stored at −80°C until used. TH17-associated cytokines were measured using multiplex luminex assay (Luminex Corp) for IL-17 and standard enzyme-linked immunosorbent assay (ELISA) kits for IL-22 (R&D Systems) and IL-21 and IL-23 (eBiosciences). Supernatants collected after TH17 polarization were analyzed using Quansys multiplex ELISA assay. Measurements for IFN-γ were performed using standard commercial ELISA kits (R&D Systems).
Western blotting
Myeloma cell lines were grown in RPMI-1640 supplemented with 10% FCS (FBS; Sigma Chemical), 2μM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO). Cells were pelleted at 500g for 5 minutes at 4°C, washed with ice-cold phosphate-buffered saline twice, and resuspended in 50 μl CelLytic M (Sigma-Aldrich) supplemented with complete mini ethylenediaminetetraacetic acid-free protease inhibitor cocktail (Roche Diagnostics) and 25mM N-Ethylmaleimide (Sigma-Aldrich). Cells were pipetted up and down thrice, rotated for 15 minutes at 4°C, centrifuged at 12 000g for 15 minutes and supernatant was then removed and used as total cell lysate.
Samples were separated by electrophoresis on Supersep Ace 5%-20% gradient gels (Wako). Gels were transferred to nitrocellulose membranes (Invitrogen), blocked with 5% carnation nonfat dry milk (Nestle) in tri-buffered saline supplemented with 0.5% Tween 20 (Sigma-Aldrich) for 1 hour and then incubated with anti–IL-17 receptor antibody used at final concentration of 1:1000 and anti-glyceraldehydes 3-phosphate dehydrogenase antibody used at final concentration of 1:5000, (Santa Cruz Biotechnology) for overnight at 4°C. Secondary antibodies were goat anti–rabbit IgG-horseradish peroxidase and goat anti–mouse IgG-horseradish peroxidase (Santa Cruz Biotechnology) were used at a final concentration of 1:2000 for 1 hour at room temperature. Immunoblots were developed using the enhanced chemiluminescence (ECL) reagent system from GE Healthcare Bio-Sciences and Kodak BioMax MR Film.
Immunohistochemistry
Immunohistochemistry was performed using 5m-thick zenker-fixed, paraffin-embedded tissue sections. Briefly, slides were soaked in xylene, passed through graded alcohols, and put in distilled water. Slides were then pretreated with 10-mM citrate, pH 6.0 (Zymed) in a steam pressure cooker (Decloaking Chamber; BioCare Medical) per manufacturer's instructions, followed by washing in distilled water. All further steps were performed at room temperature in a hydrated chamber. Slides were pretreated with Peroxidase Block (DAKO USA) for 5 minutes to quench endogenous peroxidase activity. Primary rabbit anti–IL-17R antibody (Santa Cruz Biotechnology) was applied at a 1:50 dilution in antibody diluent (DAKO) for 1 hour. Slides were washed in 50mM Tris-Cl, pH 7.4, and anti–rabbit horseradish peroxidase–conjugated antibody (Envision detection kit; DAKO) was applied for 30 minutes. After further washing, immunoperoxidase staining was developed using a DAB chromogen kit (DAKO) per the manufacturer and counterstained with Harris hematoxylin. Sections were observed and photographed with an Olympus BX41 microscope with 40× objective lens and 0.75 numeric aperture at room temperature (total magnification 200×) and an Olympus-QColor 5 camera, and analyzed with acquisition software QCapture (QImaging) and Adobe Photoshop (Adobe).
Confocal microscopy
Myeloma cell lines were stained with anti–IL-17 receptor antibodies in addition to isotype antibody controls (phycoerythrin) and then analyzed using multiphoton microscopy at room temperature (BioRad MRC 1024ES multiphoton system; Bio-Rad). A Zeiss Axiovert S 100 inverted microscope equipped with a high-quality water-immersion 40×/1.2 numeric aperture C-Apochromat objective was used to obtain images (total magnification, 640×). Images were reconstructed using the Bio-Rad LaserSharp and/or MetaMorph software (MetaMorph Imaging Series; Universal Imaging).
Statistical analysis
Statistical analyses were performed by Student t test as well as Mann-Whitney test. P values less than .05 were considered statistically significant.
Results
Presence of elevated TH17 cells in freshly isolated mononuclear cells from myeloma
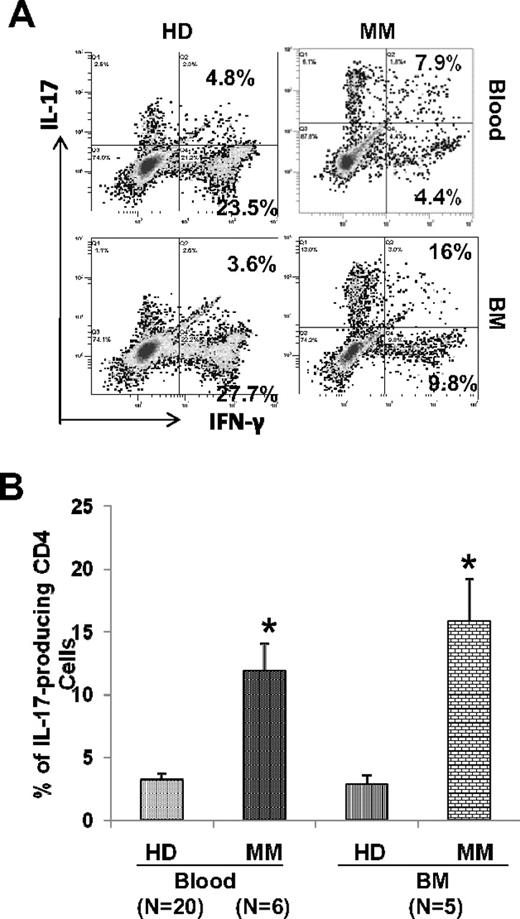
Significant dysregulation in T-helper cell subsets, TH1, TH2, and T regulatory cells, have been reported in myeloma. Some of these effects are mediated by the cytokines produced by myeloma cells and/or bone marrow stromal cells. Because these cytokines, especially IL-6, TGF-β, and IL-1 also impact generation of TH17 cells, we first evaluated the baseline frequency of TH17 cells in freshly isolated PBMCs and BMMCs from myeloma patients and compared it with its frequency in healthy donor PBMCs and BMMCs. Freshly isolated PBMCs and BMMCs were stimulated for 6 hours with PMA and ionomycin and stained for intracellular IL-17 and IFN-γ and analyzed by flow cytometry. The mean frequency of TH17 cells was 4.49% (± 0.78%) in MM PBMCs compared with 2.05% (± 0.3%) in healthy donor (HD) PBMCs (P < .05) and 2.85% (± 0.78%) in MM BMMCs compared with 1.67% (± 0.29%) in HD BMMCs (Figure 1B). As seen in Figure 1A and B, significantly increased frequency of TH17 cells was observed in PBMCs (N = 11) from MM patients compared with HD PBMCs (N = 12); however, the increase of TH17 cells seen in BMMCs (N = 4) from MM patients compared with HD BMMCs (N = 3), although higher, is not statistically significant. Furthermore, we observed that both IL-17+/IFN-γ+ as well as IL-17+/IFN-γ− cell ratios are significantly increased in MM PBMCs compared with healthy donor PBMCs (data not shown). We have also confirmed the increased frequency of IL-17–producing cells in CD4 cells from MM compared with HD by qPCR (Figure 1C).
Increased frequency of Th17 cells in freshly isolated mononuclear cells in myeloma. (A) Mononuclear cells were isolated from multiple myeloma (MM) patients (blood, N = 11, bone marrow [BM], N = 4) and from healthy donors (HDs; blood, N = 12; BM, N = 3) and stimulated for 6 hours with PMA and ionomycin and stained for intracellular interleukin-17 (IL-17) and interferon-γ (IFN-γ). Proportion of IL-17–producing Th17 cells was determined in CD4 population by flow cytometry. A representative dot plot analysis showing percentage of cells that are positive for intracellular IL-17 and IFN-γ within gated CD4 population using matching peripheral blood and BM samples from MM and HDs is shown. (B) Composite results presented as mean value with SEM for Th17 with in CD4 population. (C) RNA was isolated from CD4 cells from MM patients (N = 3) and healthy donors (N = 3) using QIAGEN kits and quantitative PCR was performed. *P < .05.
Increased frequency of Th17 cells in freshly isolated mononuclear cells in myeloma. (A) Mononuclear cells were isolated from multiple myeloma (MM) patients (blood, N = 11, bone marrow [BM], N = 4) and from healthy donors (HDs; blood, N = 12; BM, N = 3) and stimulated for 6 hours with PMA and ionomycin and stained for intracellular interleukin-17 (IL-17) and interferon-γ (IFN-γ). Proportion of IL-17–producing Th17 cells was determined in CD4 population by flow cytometry. A representative dot plot analysis showing percentage of cells that are positive for intracellular IL-17 and IFN-γ within gated CD4 population using matching peripheral blood and BM samples from MM and HDs is shown. (B) Composite results presented as mean value with SEM for Th17 with in CD4 population. (C) RNA was isolated from CD4 cells from MM patients (N = 3) and healthy donors (N = 3) using QIAGEN kits and quantitative PCR was performed. *P < .05.
Increased number of induced-TH17 cells in myeloma
Next, we evaluated the frequency of induced TH17 cells under TH17 polarizing conditions. Cultures in polarizing condition determine the potential for further increase in TH17 cells. To determine the frequency of induced TH17 cells, we stimulated naive CD4 cells with anti-CD3 and anti-CD28 antibodies, in the presence of IL-6, IL-1, IL-23, and TGF-β for 6 days. Cells were further expanded with IL-2 for 6 additional days, restimulated with PMA and Ionomycin, and analyzed by flow cytometry for percentage of cells expressing intracellular IL-17 in the CD4 population. As seen in Figure 2, there was significant increase in induced TH17 cells after culture in polarizing condition from MM compared with healthy donor PBMCs (11.9% ± 2.2% and 3.2% ± 0.5%, respectively; P < .05, N = 6) and BMMCs (15.8% ± 3.4% and 2.9% ± 0.7%, respectively; P < .05, N = 5). The starting frequency of TH17 cells in purified naive CD45RA positive cells is very low and similar in all groups of samples.
Increased frequency of induced Th17 cells in myeloma. Purified naive CD4 cells from peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs) were polarized with Th17 cocktail for 12 days. After restimulation with PMA and ionomycin, IL-17–expressing cells were measured by intracellular IL-17 staining using flow cytometry. (A) A representative dot plot analysis shows IL-17– and IFN-γ–expressing cells as percentage of CD4 cells in peripheral blood and BM samples from HD and myeloma (MM) patients. (B) Composite results presented as mean values with SEM for IL-17–expressing cells within CD4 population. *P < .05.
Increased frequency of induced Th17 cells in myeloma. Purified naive CD4 cells from peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs) were polarized with Th17 cocktail for 12 days. After restimulation with PMA and ionomycin, IL-17–expressing cells were measured by intracellular IL-17 staining using flow cytometry. (A) A representative dot plot analysis shows IL-17– and IFN-γ–expressing cells as percentage of CD4 cells in peripheral blood and BM samples from HD and myeloma (MM) patients. (B) Composite results presented as mean values with SEM for IL-17–expressing cells within CD4 population. *P < .05.
Elevated TH17-associated cytokines in myeloma microenvironment
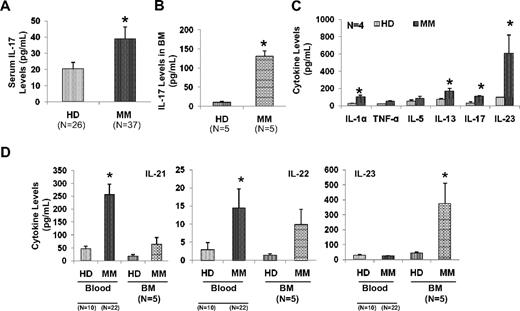
We next analyzed the pro-inflammatory cytokine network supporting the generation of TH17 cells in myeloma. We observed significantly elevated levels of serum IL-17 in myeloma patients (N = 37) compared with healthy donor sera (N = 26; 39 + 7 vs 20.5 ± 3.9, respectively; P < .05) as measured by multiplex luminex assay (Figure 3A). We also observed significant elevation of IL-17 in sera from BM from MM compared with healthy donors (Figure 3B). Furthermore, we evaluated the supernatants from PBMCs cultured in presence of Th17 polarizing conditions from myeloma patients and healthy donors (N = 4) using multiplex ELISA assay (Quansys). As shown in Figure 3C, IL-1α, IL-13, IL-17, and IL-23 were significantly elevated in supernatants from PBMCs from myeloma patients compared with healthy donor samples. In addition, we analyzed the levels of other pro-inflammatory cytokines that are important in relationship to TH17 cells in sera of myeloma patients. We observed significant elevation of IL-21, IL-22, and IL-23 in sera from both blood and BM from MM compared with healthy donors. These results suggest that Th17-associated pro-inflammatory cytokines may be present in the myeloma BM microenvironment and may modulate MM cell growth as well as immune responses.
Elevated levels of Th17-related cytokines in myeloma. Sera samples from myeloma patients (MM) and from healthy donors (HD) were analyzed by ELISA for the presence of IL-17 in (A) peripheral blood, (B) IL-17 in BM, and (D) IL-21, IL-22, and IL-23 in blood and BM by ELISA. (C) PBMCs isolated from myeloma patients (N = 4) and healthy donors (N = 4) were stimulated with anti-CD3 antibody in the presence of IL-6 and TGF-β for 6 days and cell supernatant was evaluated for various cytokines by multiplex ELISA assay. All the values presented in the bar graphs consist of mean plus or minus SEM. *P < .05.
Elevated levels of Th17-related cytokines in myeloma. Sera samples from myeloma patients (MM) and from healthy donors (HD) were analyzed by ELISA for the presence of IL-17 in (A) peripheral blood, (B) IL-17 in BM, and (D) IL-21, IL-22, and IL-23 in blood and BM by ELISA. (C) PBMCs isolated from myeloma patients (N = 4) and healthy donors (N = 4) were stimulated with anti-CD3 antibody in the presence of IL-6 and TGF-β for 6 days and cell supernatant was evaluated for various cytokines by multiplex ELISA assay. All the values presented in the bar graphs consist of mean plus or minus SEM. *P < .05.
IL-17 promotes myeloma cell growth both in vitro and in vivo
We next evaluated effect of IL-17 on myeloma cell growth and survival in vitro. MM cell lines (N = 7) were incubated in the presence or absence of IL-17 and cell proliferation was measured by 3H-thymidine incorporation. As seen in Figure 4A, IL-17 significantly induced proliferation of all myeloma cell lines tested (30.7% ± 2.8%; Figure 4A). In addition, we also observed that in both myeloma cell lines and primary patient MM cells, IL-17 significantly induced colony size and number as observed by methoCult colony assay (Figure 4B and C, respectively). We also observed increased number of MM cells in the S phase and reduced number of cells in G1 phase (data not shown). As seen in Figure 4D, we showed the induction of proliferation in 3 myeloma cell lines by IL-17 even in presence of BMSCs, as measured by 3H-thymidine incorporation (range, 32.6% to 48.6%). We also observed that IL-17 significantly increased adhesion of myeloma cells to BMSCs (Figure 4E). We next evaluated whether IL-17 promotes myeloma cell growth in vivo in a murine xenograft model of MM. We subcutaneously injected OPM-1 myeloma cells with and without IL-17 pretreatment and evaluated the tumor growth after 3 weeks after MM cell injection. As seen in Figure 4F, IL-17 pretreatment led to development of significantly larger tumors compared with control (P < .05).
IL-17 promotes myeloma cell growth in vitro and in vivo. (A) Myeloma cell lines (N = 7) were incubated with or without IL-17 and proliferation was measured by 3H-thymidine incorporation after 3 days. Data are presented as percentage increase in proliferation in presence of IL-17 compared with control and showed as mean plus or minus SEM. (B) Myeloma cell lines (OPM-1 and U266) were cultured in methocult agar plates in the presence or absence of IL-17. Representative photomicrograph is presented. Photographs were obtained using a Nikon TE200 microscope (40× objective) with attached camera (Nikon) at room temperature (total magnification 200×) and analyzed with Metafluor software (Molecular Devices). (C) Primary MM cells (N = 3) were cultured in methocult agar plates in the presence or absence of IL-17 and number of colonies was counted in unit area and presented as mean plus or minus SEM. (D) MM cell lines were cultured with or without BMSCs in the presence or absence of IL-17 and proliferation was increased as measured by 3H-thymidine incorporation after 3 days and presented as percentage of proliferation of control. (E) Serum-starved MM cells were labeled with calcein, washed, and added to BMSC-coated plates for 4 hours and nonadherent cells were removed by washing. Adhesion was measured by measuring the absorbance using 492/520 nm filter set with a fluorescence plate reader. Results represent mean plus or minus SEM of 4 independent experiments performed in triplicate. (F) Myeloma cells suspended in medium with or without IL-17 were injected subcutaneously in SCID mice (3 mice per group), and tumor size was measured after 3 weeks after MM cell injection. *P < .05.
IL-17 promotes myeloma cell growth in vitro and in vivo. (A) Myeloma cell lines (N = 7) were incubated with or without IL-17 and proliferation was measured by 3H-thymidine incorporation after 3 days. Data are presented as percentage increase in proliferation in presence of IL-17 compared with control and showed as mean plus or minus SEM. (B) Myeloma cell lines (OPM-1 and U266) were cultured in methocult agar plates in the presence or absence of IL-17. Representative photomicrograph is presented. Photographs were obtained using a Nikon TE200 microscope (40× objective) with attached camera (Nikon) at room temperature (total magnification 200×) and analyzed with Metafluor software (Molecular Devices). (C) Primary MM cells (N = 3) were cultured in methocult agar plates in the presence or absence of IL-17 and number of colonies was counted in unit area and presented as mean plus or minus SEM. (D) MM cell lines were cultured with or without BMSCs in the presence or absence of IL-17 and proliferation was increased as measured by 3H-thymidine incorporation after 3 days and presented as percentage of proliferation of control. (E) Serum-starved MM cells were labeled with calcein, washed, and added to BMSC-coated plates for 4 hours and nonadherent cells were removed by washing. Adhesion was measured by measuring the absorbance using 492/520 nm filter set with a fluorescence plate reader. Results represent mean plus or minus SEM of 4 independent experiments performed in triplicate. (F) Myeloma cells suspended in medium with or without IL-17 were injected subcutaneously in SCID mice (3 mice per group), and tumor size was measured after 3 weeks after MM cell injection. *P < .05.
Significant expression of IL-17 receptor in myeloma cells
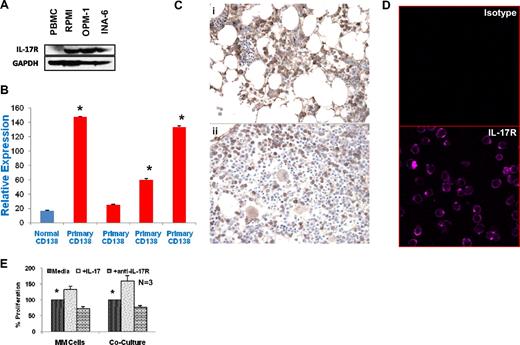
We have observed significant up-regulation of myeloma cell growth by IL-17, both in vitro and in vivo. Therefore, we further investigated whether MM cells expressed IL-17R. As seen in Figure 5A, we observed by Western blot analysis the expression of IL-17R in all 3 myeloma cell lines tested. We confirmed this observation in primary myeloma cells by quantitative PCR as shown in Figure 5B. Three of 4 primary CD138+ myeloma cells showed significantly higher expression of IL-17R than normal plasma cells. We have further validated IL-17R expression on primary MM cells by evaluating the paraffin-embedded bone marrow biopsy sections from myeloma patients with immune-histochemistry using anti–IL-17R antibody. As seen in Figure 5C, a majority of myeloma cells express IL-17R in 7 of 10 patient samples tested; while 2 patients showed less frequent staining of plasma cells and 1 sample was negative for IL-17R. This finding is further confirmed with confocal microscopy using myeloma cell lines as shown in Figure 5D. We have further evaluated the role of IL-17R in IL-17-mediated MM cell proliferation using anti–IL-17R antibody. As seen in Figure 5E, presence of anti–IL-17R antibody significantly suppresses MM cell proliferation in presence of IL-17. Similarly, anti–IL-17R antibody was also able to significantly block MM cell growth in the presence of BMSCs and IL-17.
Significant expression of IL-17 receptor in myeloma cells. (A) Total cell lysates were prepared from MM cells, and separated by electrophoresis on 5% to 20% polyacrylamide gradient gels. Samples were probed with antisera to IL-17 receptor and GAPDH as indicated. (B) RNA was isolated from purified primary MM cells and qPCR was performed as described in “Quantitative PCR for IL-17 and IL-17R expression.” Results are presented as relative expression value. *P < .05. (C) Paraffin-embedded tissue sections from MM patients (N = 10) were stained using anti–IL-17R antibody as described in “Immunohistochemistry” and evaluated using a Nikon transmitted light microscope. The majority of the MM cells are positively stained with IL-17R receptor antibody in 7 of 10 patients. Two representative stained sections are shown at 200× magnification. (D) Myeloma cell lines were stained with isotype control antibody (top panel) or anti–IL-17R antibody (bottom panel) and analyzed by confocal microscopy. One representative cell line of 4 experiments is shown at 640× magnification. (E) MM cell lines (N = 3) were cultured alone or cocultured with BMSCs with or without IL-17 in the presence or absence of anti–IL-17R antibody. Proliferation was measured by 3H-thymidine incorporation after 3 days. Data are presented as percentage proliferation in the presence of IL-17 or IL-17R antibody compared with control and shown as mean plus or minus SEM. *P < .05.
Significant expression of IL-17 receptor in myeloma cells. (A) Total cell lysates were prepared from MM cells, and separated by electrophoresis on 5% to 20% polyacrylamide gradient gels. Samples were probed with antisera to IL-17 receptor and GAPDH as indicated. (B) RNA was isolated from purified primary MM cells and qPCR was performed as described in “Quantitative PCR for IL-17 and IL-17R expression.” Results are presented as relative expression value. *P < .05. (C) Paraffin-embedded tissue sections from MM patients (N = 10) were stained using anti–IL-17R antibody as described in “Immunohistochemistry” and evaluated using a Nikon transmitted light microscope. The majority of the MM cells are positively stained with IL-17R receptor antibody in 7 of 10 patients. Two representative stained sections are shown at 200× magnification. (D) Myeloma cell lines were stained with isotype control antibody (top panel) or anti–IL-17R antibody (bottom panel) and analyzed by confocal microscopy. One representative cell line of 4 experiments is shown at 640× magnification. (E) MM cell lines (N = 3) were cultured alone or cocultured with BMSCs with or without IL-17 in the presence or absence of anti–IL-17R antibody. Proliferation was measured by 3H-thymidine incorporation after 3 days. Data are presented as percentage proliferation in the presence of IL-17 or IL-17R antibody compared with control and shown as mean plus or minus SEM. *P < .05.
Down-regulation of TH1 cell responses by Th17-secreted cytokines in myeloma
To determine the effect of cytokines produced by Th17 cells (IL-17 and IL-22) on Th1 cells, we incubated PBMCs isolated from healthy donors under Th1 polarizing conditions for 12 days in the presence or absence of IL-17 and/or IL-22. Cells were then restimulated with PMA and ionomycin for 6 hours and intracellular IFN-γ was measured in the CD4 population. Although IL-17 and IL-22 by themselves had little effect on IFN-γ–producing cells (data not shown), significant inhibition of IFN-γ–producing cells (Figure 6A-B), as well as IFN-γ production (Figure 6C), was observed in the presence of combination of IL-17 and IL-22.
Down-regulation of protective TH1 response by TH17-related cytokines. (A) Healthy donor PBMCs were activated with TH1 polarizing cytokines as described in “Intracellular IL-17–producing Th17 cell analysis by flow cytometry” in presence or absence of IL-17 and IL-22 for 12 days. Cells were treated with PMA and ionomycin, stained for intracellular IFN-γ, and evaluated by flow cytometry. IFN-γ–producing cell number was evaluated in CD69+ cell population in CD4 gated cells. A representative dot plot analysis showing percentage of cells that are positive for intracellular IFN-γ within gated CD4 population. (B) Composite results of 9 experiments presented in a bar graph. Results are mean plus or minus SEM in healthy donors. (C) Healthy donor PBMCs were activated with TH1 polarizing cytokines in the presence or absence of IL-17 and IL-22 for 6 days, and supernatants were analyzed for IFN-γ by ELISA. *P < .05.
Down-regulation of protective TH1 response by TH17-related cytokines. (A) Healthy donor PBMCs were activated with TH1 polarizing cytokines as described in “Intracellular IL-17–producing Th17 cell analysis by flow cytometry” in presence or absence of IL-17 and IL-22 for 12 days. Cells were treated with PMA and ionomycin, stained for intracellular IFN-γ, and evaluated by flow cytometry. IFN-γ–producing cell number was evaluated in CD69+ cell population in CD4 gated cells. A representative dot plot analysis showing percentage of cells that are positive for intracellular IFN-γ within gated CD4 population. (B) Composite results of 9 experiments presented in a bar graph. Results are mean plus or minus SEM in healthy donors. (C) Healthy donor PBMCs were activated with TH1 polarizing cytokines in the presence or absence of IL-17 and IL-22 for 6 days, and supernatants were analyzed for IFN-γ by ELISA. *P < .05.
Discussion
TH17 cells induced by IL-6, IL-1β, IL-21, and IL-23 participate in protection against fungal and parasitic infections and their levels are elevated in a number of inflammatory and autoimmune diseases.39,40 We have shown here that Th17 cells are significantly elevated in peripheral blood and bone marrow of myeloma patients compared with healthy donors. Interestingly, interactions between MM cells and the BM microenvironment lead to production of a number of cytokines and chemokines35,41 with immuo-modulatory activity that may skew TH subsets toward TH17 cells. The interplay of TGF-β and IL-6, which are both expressed at high levels in MM bone marrow,35,41 may affect generation of TH17 cells both directly and via other pro-inflammatory cytokines and thereby modulate antitumor immune responses. TH17 cells and the ratios of IL-17+/IFN-γ+ cells were significantly higher in MM than in healthy donor samples. These results suggest increased frequency of TH17 cells present in the total CD4 cell population consisting of both naive and memory cell pools. Furthermore, when purified naive CD4 cells were cultured under Th17 polarizing conditions, Th17 cells were induced in significantly higher numbers in myeloma compared with healthy donors. These results are consistent with a recent report showing increased TH17 cells polarized by DCs in BM compared with peripheral blood in MM patients.42 Increased frequency of Th17 cells is also observed in tumor microenvironment in a number of human tumors, including ovarian, prostate, renal, and pancreatic carcinomas.43,44 Some animal studies have shown that TH17 cells are important in antitumor activity.45 Interestingly, a human study has also shown that IL-17–producing TH17 cells facilitate antitumor activity via enhancing the presence of TH1 effectors in the tumor microenvironment of ovarian cancer patients.46 Elevated levels of TH17 cells and associated cytokines have also been documented in rheumatoid arthritis, and these pro-inflammatory cytokines also participate in bone damage observed in this disease. Interestingly, there have been reports of increased incidence of myeloma in patients with autoimmune disorders,36 raising question regarding relation between the TH17 cells and pro-inflammatory cytokines and the development of MM in patients with autoimmune disorders.
We have evaluated the serum levels of Th17-associated cytokines in peripheral blood and the bone marrow of healthy donors and MM patients. Our results demonstrate that a number of Th17-associated cytokines, including IL-17, are significantly elevated in myeloma compared with healthy donors. Significantly elevated IL-17 levels have been previously reported in stage II and III MM.47 Interestingly, reduced levels of serum IL-17 were reported after bis-phophonate therapy.48 Serum levels of IL-23, a Th17-associated cytokine, observed to be elevated in MM bone marrow, is also elevated in colon, ovarian, head/neck, lung, breast, and stomach cancer as well as melanoma. This has been associated with reduced CD8 T-cell infiltration into the tumor micro-environment.49 Consistent with these observations, we report that combination of these Th17-associated pro-inflammatory cytokines suppresses T-cell responses. In addition, we have shown that a number of other Th17-associated pro-inflammatory cytokines, including IL-1, IL-13, IL-17, and IL-23, are elevated after Th17 polarization in myeloma compared with healthy donors. Of course, these results will now excite us to conduct larger studies to understand their role in the progression of myeloma and the relationship between disease stage and response to therapy as well as survival.
We have further demonstrated by both 3H-thymidine incorporation and clonogenic assay that IL-17 increases myeloma cell proliferation. In addition, we have observed that IL-17 promotes myeloma tumor cell growth in SCID mouse model. We have further shown, using various techniques, that MM cell lines and primary cells express IL-17 receptor, providing the biologic mechanism for IL-17 effects on MM cells. As predicted, blocking IL-17R by antibody abolishes IL-17 effects on MM cells. This provides a rationale for IL-17 and IL-17R blockade as a potential therapy in MM. We are in the process of evaluating IL-17 blockade experiments in vivo. A recent study50 shows that IL-21, a Th17-associated pro-inflammatory, is also capable of inducing STAT-3–mediated myeloma growth-promoting effects in synergism with IGF-1. The biologic basis for this growth-stimulating effect remains unclear. However, based on observation in rheumatoid arthritis, we postulate that IL-17 may induce production of IL-6 in the bone marrow milieu. It will be important to evaluate the effect of these cytokines not only on MM cell growth but also on BMSCs, as well as on production of other growth-promoting cytokines and chemokines in MM. In addition, we also report that IFN-γ–producing cells are reduced when PBMCs from healthy donors are polarized in the presence of IL-17 and IL-22, suggesting immune suppressive activity of these cytokines. Because most Th17 cells produce IL-17 and IL-22, we believe that the observed immune-suppression in MM may be partly induced by this pathway.
In conclusion, we observed significantly increased numbers of Th17 cells in MM along with increased levels of IL-17 and other pro-inflammatory cytokines supporting MM cell growth as well as suppressing immune responses. These results suggest Th17 cells and IL-17 as an important therapeutic target in MM for both anti-MM responses as well as to improve immune function.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Department of Veterans Affairs Merit Review Award (N.C.M.) and National Institutes of Health grants RO1-124929 (N.C.M.), P50-100707, and PO1-78 378 (N.C.M., K.C.A.). This work was also supported by Multiple Myeloma Research Foundation awards to R.H.P.
National Institutes of Health
Authorship
Contribution: R.H.P. conceived and developed the experimental plan, performed experiments, analyzed the data, and prepared the manuscript; D.P., M.F., H.K.P., P.N., W.S., C.P., S.A., J.F.D., and J.L.K. assisted in experiments; Y.-T.T., P.G.R., I.M.G., and S.P.T. provided patient samples; K.C.A. helped in data analysis and patient samples; N.C.M. participated in study design and coordination, data analysis, patient samples, and manuscript preparation; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nikhil C. Munshi, MD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: nikhil_munshi@dfci.harvard.edu; or Rao H. Prabhala, PhD, VA Boston Healthcare System/Harvard Medical School, 1400 VFW Pkwy, West Roxbury, MA 02132, e-mail: rao_prabhala@dfci.harvard.edu.

![Figure 1. Increased frequency of Th17 cells in freshly isolated mononuclear cells in myeloma. (A) Mononuclear cells were isolated from multiple myeloma (MM) patients (blood, N = 11, bone marrow [BM], N = 4) and from healthy donors (HDs; blood, N = 12; BM, N = 3) and stimulated for 6 hours with PMA and ionomycin and stained for intracellular interleukin-17 (IL-17) and interferon-γ (IFN-γ). Proportion of IL-17–producing Th17 cells was determined in CD4 population by flow cytometry. A representative dot plot analysis showing percentage of cells that are positive for intracellular IL-17 and IFN-γ within gated CD4 population using matching peripheral blood and BM samples from MM and HDs is shown. (B) Composite results presented as mean value with SEM for Th17 with in CD4 population. (C) RNA was isolated from CD4 cells from MM patients (N = 3) and healthy donors (N = 3) using QIAGEN kits and quantitative PCR was performed. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/26/10.1182_blood-2009-10-246660/4/m_zh89991054430001.jpeg?Expires=1767785572&Signature=DS0uFmB9bRkH1it5oPHYnM220FngwBDtkJFlzWb8iEL-jC3GcUwDRviswz9kQuxDcZReRUSUPkGX3SfeMPIjf6T3IkUDr8lRG-K7mAzrDRnQtUlFE32~iySUPgPNnb6M-qoLApfgBaH1vOe~yKidd62ysJxgk0W2XUcTlRPu~a1dDr~JU8T04F1vdwAT7kY1OfQU31u8gMRjXX7Vz4TpVuBbcW8BW5wXl5qOA~CxSMCzpHElKjreCv8MY8qLfU2vWFRm0ENycYWLvOlYmmNuBcImUXec-VKJ3LkimNuJanvMh~jJa8fkW8cvbFkcBALhOSPapBQTqfSve~Zby0r8-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal