Abstract
Expression of the heparan sulfate proteoglycan syndecan-1 is a hallmark of both normal and multiple myeloma (MM) plasma cells. Syndecan-1 could affect plasma cell fate by strengthening integrin-mediated adhesion via its core protein and/or by accommodating and presenting soluble factors via its HS side chains. Here, we show that inducible RNAi-mediated knockdown of syndecan-1 in human MM cells leads to reduced growth rates and a strong increase of apoptosis. Importantly, knockdown of EXT1, a copolymerase critical for HS chain biosynthesis, had similar effects. Using an innovative myeloma xenotransplantation model in Rag-2−/−γc−/− mice, we demonstrate that induction of EXT1 knockdown in vivo dramatically suppresses the growth of bone marrow localized myeloma. Our findings provide direct evidence that the HS chains of syndecan-1 are crucial for the growth and survival of MM cells within the bone marrow environment, and indicate the HS biosynthesis machinery as a potential treatment target in MM.
Introduction
Within the lymphoid system, expression of the heparan sulfate proteoglycan (HSPG) syndecan-1 is characteristic for terminally differentiated B cells, that is, plasma cells, and their malignant counterpart multiple myeloma (MM),1 a plasma cell neoplasm, which expands in the bone marrow (BM).2,3 HSPGs are proteins with covalently attached HS chains, which consist of alternating N-acetylated glucosamine and D-glucuronic acid units. To exert their function, the HS chains undergo a complex series of processing reactions involving deacetylation, epimerization, and (de)sulfation.4,5 This endows HS chains with highly modified domains that provide specific docking sites for many bioactive molecules. Binding of these ligands serves a variety of functions, ranging from immobilization and concentration to distinct modulation of biologic functions. HSPGs are widely expressed in mammalian tissues as extracellular matrix components or cell-membrane-bound proteins. These membrane-localized HSPGs can also function independent of their HS side chains, for example, by the ability of their core protein to interact with signaling molecules or cytoskeletal proteins.6-8 Thus, HSPGs act as multifunctional scaffolds regulating important biologic processes, including cell adhesion and migration, tissue morphogenesis, and angiogenesis.4,6,7,9
Studies from several laboratories, including our own, point to a versatile role of the transmembrane HSPG syndecan-1 in the interaction of MM plasma cells with the BM microenvironment.10-13 Various growth factors have been implicated in controlling MM survival and growth, including hepatocyte growth factor, epidermal growth factor-family members, and WNTs.10-12,14-16 Most of these factors can bind to the HS chains of syndecan-1,11,12,17 which promotes their ability to stimulate MM cell growth and survival in vitro.11,12 Independent of HS chains, the syndecan-1 core protein can interact with, and regulate the activity of, integrin adhesion molecules.7,18 Thus, both the HS chains and the syndecan-1 core protein could contribute to the dynamic interaction of tumor with the BM milieu and influence tumor behavior.13,19 This notion prompted us to specifically study the impact of targeting of EXT1, an enzyme indispensable for HS-chain synthesis,20,21 on MM growth, both in vitro and in vivo.
Methods
Cell lines and culture
The human MM cell lines RPMI-8226 and L363 were cultured as described.22
Generation of inducible cell lines
Doxycycline-inducible cell lines were generated using the T-REx System (Invitrogen). L363 and RPMI-8226 were transfected by electroporation (Gene Pulser Apparatus; Bio-Rad) with the pcDNA6/TR construct alone (TetR), or in combination with a construct (pTER) containing an shRNA directed against either syndecan-1 (shSYN1) or EXT1 (shEXT1a, b, or c). shRNA sequences are given in the supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article), and the position of the target sites is shown in supplemental Figure 1A. To induce shRNA expression, 1 μg/mL doxycycline (Sigma-Aldrich) was used.
In vitro growth and apoptosis measurements
Cells were plated (104) in 96-well plates. Cells were quantified by fluorescence-activated cell sorter (FACS; BD Biosciences), using TO-PRO-3-′iodide to exclude dead cells. Apoptotic cells were identified by annexin V and TO-PRO-3-′iodide.
Transplantation of MM cells in mice
Rag-2−/−γc−/− mice (9-14 weeks) were bred and housed as described.22 Transplantation of GFP-luciferase transduced L363 or RPMI-8226 cells into Rag-2−/−γc−/− mice was performed essentially as described,22 except that 1 × 106 MM cells were injected intracardially. During the experiment, the mice were supplied with water ad libitum, containing 5% sucrose with or without 1 mg/mL doxycycline (Sigma-Aldrich). In addition, mice receiving doxycycline were injected intraperitoneally, twice a week, with 125 μg doxycycline to sustain adequate serum levels.
Bioluminescent imaging
Mice were anesthetized by isoflurane inhalation before they received an intraperitoneal injection of 100 μL of 7.5mM D-luciferine (Synchem Chemie). Bioluminescence images were acquired using a third generation cooled GaAs intensified charge-coupled device camera, controlled by the Photo Vision software and analyzed with M3Vision software (all from Photon Imager; Biospace Laboratory).
Statistical analysis
The unpaired 2-tailed Student t test was used to determine the significance of differences between means, unless stated otherwise.
Results and discussion
In this study, we have directly explored the impact of HS modification of syndecan-1 on MM growth in vivo, using a recently developed, innovative, xenotransplantation model.22 Key features of this model are that it uses immunodeficient Rag-2−/−γc−/− mice as recipients of human MM cells transduced with GFP-luciferase to allow noninvasive real-time monitoring of MM cell growth in vivo. Rag-2−/−γc−/− mice completely lack B, T, and NK cells and therefore permit highly reproducible engraftment of human lymphocytes.23-25 As we recently demonstrated, these Rag-2−/−γc−/− mice are also highly permissive to grafting of human MM cell lines, several of which displayed a strikingly selective tumor outgrowth in recipient's BM compartment on intravenous injection.22 For our current studies, 2 of these MM-cell lines (L363 and RPMI-8226) were selected and stably transfected with doxycycline-inducible shRNAs against either the syndecan-1 core protein or the HS copolymerase EXT1 (shSYN1 and shEXT1, respectively). To rule out off-target effects of the shRNAs, 3 different L363-shEXT1 lines, each containing a different nonoverlapping targeting sequence for EXT1, were generated (shEXT1a, b, and c; supplemental Methods; supplemental Figure 1A-B).
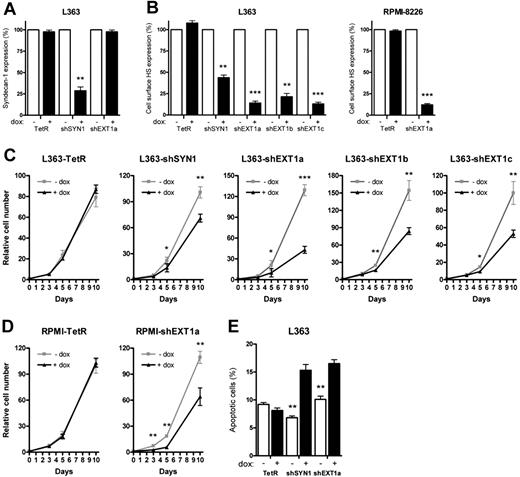
Doxycycline treatment of L363-shSYN1 cells led to an approximately 75% reduction of syndecan-1 expression (Figure 1A), whereas treatment of L363-shEXT1a, b, or c or RPMI-shEXT1a cells was even more effective and reduced cell-surface HS expression by approximately 90% (Figure 1B). In control (TetR) cells, doxycycline did not affect syndecan-1 or HS levels (Figure 1A-B). Interestingly, the doxycycline-induced knockdown of either syndecan-1 or EXT1 led to a marked reduction of the in vitro growth rate of the myeloma cells (Figure 1C-D), which was accompanied by a prominent increase in apoptosis (Figure 1E), as well as a minor increase in the percentage of cells in phase G1/G0 of the cell cycle (data not shown). Importantly, the expression of the syndecan-1 core protein was unaffected by EXT1 knockdown (Figure 1A). Therefore, our results underscore the importance of HS side chains, rather than the proteoglycan core protein per se, in the growth and survival of MM cells in vitro.
Loss of syndecan-1 and cell-surface HS reduces the in vitro growth of MM resulting from increased apoptosis. L363 and RPMI-8226 MM control cells (TetR) and L363 and RPMI-8226 MM cells containing inducible shRNA, targeting either syndecan-1 (shSYN1) or EXT1 (shEXT1a, b, and c), were incubated with (+dox) or without doxycycline (−dox) for 5 days before each experiment to allow for optimal knockdown of the target genes. (A-B) Expression of syndecan-1 (A) or cell-surface HS (B) measured by FACS using antibodies BB4 (Serotec) and 10E4 (Seikagaku America), respectively. The expression level of the untreated samples was normalized to 100%. Bars represent the mean ± SD of at least 5 independent experiments. (C-D) The growth rate of L363-TetR, -shSYN1, and -shEXT1a, b, and c MM cells (C) or RPMI-TetR and -shEXT1a MM cells (D) was analyzed over a 10-day culture period in the presence of 10% FCS. The growth curves represent the mean ± SD of 3 to 5 independent experiments (in triplicate). (E) The percentages of apoptotic cells were measured for the different L363 MM cell lines after 3 days of culture. Apoptotic cells were determined as annexin V+/TO-PRO−. Necrotic, TO-PRO single-positive cells, were excluded. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Loss of syndecan-1 and cell-surface HS reduces the in vitro growth of MM resulting from increased apoptosis. L363 and RPMI-8226 MM control cells (TetR) and L363 and RPMI-8226 MM cells containing inducible shRNA, targeting either syndecan-1 (shSYN1) or EXT1 (shEXT1a, b, and c), were incubated with (+dox) or without doxycycline (−dox) for 5 days before each experiment to allow for optimal knockdown of the target genes. (A-B) Expression of syndecan-1 (A) or cell-surface HS (B) measured by FACS using antibodies BB4 (Serotec) and 10E4 (Seikagaku America), respectively. The expression level of the untreated samples was normalized to 100%. Bars represent the mean ± SD of at least 5 independent experiments. (C-D) The growth rate of L363-TetR, -shSYN1, and -shEXT1a, b, and c MM cells (C) or RPMI-TetR and -shEXT1a MM cells (D) was analyzed over a 10-day culture period in the presence of 10% FCS. The growth curves represent the mean ± SD of 3 to 5 independent experiments (in triplicate). (E) The percentages of apoptotic cells were measured for the different L363 MM cell lines after 3 days of culture. Apoptotic cells were determined as annexin V+/TO-PRO−. Necrotic, TO-PRO single-positive cells, were excluded. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
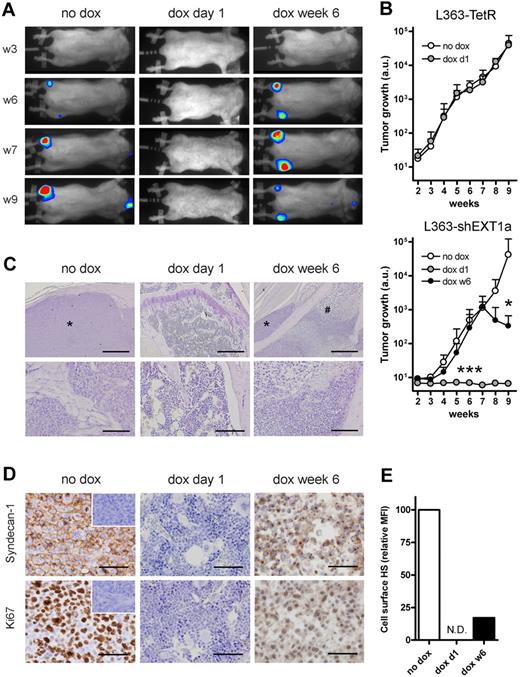
To directly assess the role of HS on tumor growth in vivo, the L363-shEXT1a and RPMI-shEXT1a MM cells were retrovirally transduced to express a GFP-luciferase fusion protein and injected intracardially into irradiated Rag-2−/−γc−/− mice. Consistent with our previous observations using untransfected L363 cells,22 mice that did not receive doxycycline displayed a rapid exponential tumor growth (Figure 2A-B; supplemental Figure 3A), localized in various parts of the skeleton, preferentially in the large bones, including the femurs (Figure 2A) and the lower spinal cord (supplemental Figure 2). Immunohistochemical analysis of the isolated bones confirmed the presence of highly proliferative tumor foci (Figure 2C-D). In striking contrast, mice injected with L363-shEXT1a that received doxycycline from the first day remained completely tumor free throughout the entire experiment (Figure 2A-D). Interestingly, in mice in which these tumors were allowed to form before doxycycline treatment, doxycycline administration from week 6 onward led to either growth arrest or even a reduction in tumor size. Histologic studies revealed the presence of areas of extensive necrosis within these tumors, which were found to be devoid of HS expression (Figure 2E), confirming EXT1 knockdown in vivo. In line with these findings, knockdown of EXT1 in RPMI-8226 cells also markedly inhibited the in vivo MM tumor growth, resulting in a significantly extended survival (supplemental Figure 3A-B). Importantly, doxycycline did not influence the tumor growth of L363-TetR or RPMI-TetR control cells in vivo (Figure 2B; supplemental Figure 3A).
In vivo growth of MM requires EXT1. L363-TetR or L363-shEXT1a MM cells, transduced to express a GFP-luciferase fusion protein, were injected intracardially into Rag-2−/−γc−/− mice. Subsequently, the mice were divided into 2 (L363-TetR) or 3 (L363-shEXT1a) groups; the first group did not receive doxycycline (no dox), the second group was treated with doxycycline throughout the entire experiment (dox day 1), and the third group (L363-shEXT1a only) received doxycycline after 6 weeks (dox week 6). Bones were collected after 9 weeks, fixed in normal buffered formalin, decalcified, and embedded in paraffin. (A) Bioluminescence images of the ventral side of mice injected with L363-shEXT1a MM cells, taken at week 3 (w3), w6, w7, and w9. Representative mice are shown for each group. (B) Tumor growth in mice injected with L363-TetR or L363-shEXT1a MM cells, as determined by the average photon emission intensity (arbitrary units) measured for the total body. Data are mean ± SD (n = 5 per group). A Mann-Whitney test (2-tailed) was applied to determine significance: *P < .05; **P ≤ .01; ***P ≤ .001. (C) Hematoxylin-stained femur sections of representative mice. Within the femurs of the no dox and dox week 6 mice, myeloma tumors (indicated with asterisks) were found. Bars represent 500 μm and 100 μm for top and bottom panels, respectively. #Necrotic MM cells. *MM cells. (D) To visualize the tumors and their proliferation, anti–human syndecan-1 (clone MI-15, Dako Denmark) and anti–human Ki67 (M7240, Dako Denmark) immunostainings were performed. The no dox and dox week 6 mice had tumors with approximately 87% and approximately 34% Ki67+ MM cells, respectively (data not shown). In the dox week 6 mice, areas of necrosis with weak and diffuse syndecan-1 and Ki67 staining were found. Representative areas are shown. Bars represents 50 μm. (Insets) Isotype controls. IC micrographs were obtained with an Olympus BX51 light microscope, using a 10×/0.30 Plan FI, 20×/0.50 Plan FI, or 40×/0.85 PlanApo objective, connected to an Olympus DP70 camera. Images were captured with Olympus DPController software (Version 1.2.1.108) and further processed with Adobe Photoshop (Version 7.0). (E) Cell-surface HS expression of GFP+ L363-shEXT1a MM cells harvested from the bones of no dox and dox week 6 mice, as determined by FACS. The mean fluorescence intensity (MFI) of the untreated mice was normalized to 100%. N.D. indicates not determined (no tumor).
In vivo growth of MM requires EXT1. L363-TetR or L363-shEXT1a MM cells, transduced to express a GFP-luciferase fusion protein, were injected intracardially into Rag-2−/−γc−/− mice. Subsequently, the mice were divided into 2 (L363-TetR) or 3 (L363-shEXT1a) groups; the first group did not receive doxycycline (no dox), the second group was treated with doxycycline throughout the entire experiment (dox day 1), and the third group (L363-shEXT1a only) received doxycycline after 6 weeks (dox week 6). Bones were collected after 9 weeks, fixed in normal buffered formalin, decalcified, and embedded in paraffin. (A) Bioluminescence images of the ventral side of mice injected with L363-shEXT1a MM cells, taken at week 3 (w3), w6, w7, and w9. Representative mice are shown for each group. (B) Tumor growth in mice injected with L363-TetR or L363-shEXT1a MM cells, as determined by the average photon emission intensity (arbitrary units) measured for the total body. Data are mean ± SD (n = 5 per group). A Mann-Whitney test (2-tailed) was applied to determine significance: *P < .05; **P ≤ .01; ***P ≤ .001. (C) Hematoxylin-stained femur sections of representative mice. Within the femurs of the no dox and dox week 6 mice, myeloma tumors (indicated with asterisks) were found. Bars represent 500 μm and 100 μm for top and bottom panels, respectively. #Necrotic MM cells. *MM cells. (D) To visualize the tumors and their proliferation, anti–human syndecan-1 (clone MI-15, Dako Denmark) and anti–human Ki67 (M7240, Dako Denmark) immunostainings were performed. The no dox and dox week 6 mice had tumors with approximately 87% and approximately 34% Ki67+ MM cells, respectively (data not shown). In the dox week 6 mice, areas of necrosis with weak and diffuse syndecan-1 and Ki67 staining were found. Representative areas are shown. Bars represents 50 μm. (Insets) Isotype controls. IC micrographs were obtained with an Olympus BX51 light microscope, using a 10×/0.30 Plan FI, 20×/0.50 Plan FI, or 40×/0.85 PlanApo objective, connected to an Olympus DP70 camera. Images were captured with Olympus DPController software (Version 1.2.1.108) and further processed with Adobe Photoshop (Version 7.0). (E) Cell-surface HS expression of GFP+ L363-shEXT1a MM cells harvested from the bones of no dox and dox week 6 mice, as determined by FACS. The mean fluorescence intensity (MFI) of the untreated mice was normalized to 100%. N.D. indicates not determined (no tumor).
In conclusion, although mutations in essential growth control genes underlie MM development, signals from the BM microenvironment are also essential for driving tumor growth.2,3 As targets for intervention, these signals may be equally important as mutated oncogene products. Our current study demonstrates that the HS chains decorating syndecan-1 are crucial for the growth and survival of MM cells within the BM environment and indicates these HS chains and their biosynthesis machinery5 as potential treatment targets in MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Gerard Geelen and Kiki Hesp for excellent animal care in the Central Animal Facility of the University of Utrecht, Berend Hooibrink for FACS sorting, Dr Guido David for kindly providing the 10E4 antibody, and Dr Hans Clevers for the generous gift of the Tet repressor expression plasmid.
This work was supported by a grant from the Dutch Cancer Society (M.S., S.T.P.) and the Netherlands Organization for Health Research and Development (TOP grant, ZonMw/NWO; M.S., S.T.P.).
Authorship
Contribution: R.M.R. and R.W.J.G. equally performed research, analyzed data, and wrote the paper; H.R and A.C.M.M. provided the xenotransplant human MM mouse model, provided technical assistance, and analyzed data; A.K., A.d.H.-K., and T.C. performed research; and M.S. and S.T.P. equally designed and supervised the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven T. Pals, Department of Pathology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: s.t.pals@amc.uva.nl.
References
Author notes
R.M.R. and R.W.J.G. contributed equally to this study.
M.S. and S.T.P. contributed equally to this study, sharing last authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal