Abstract
Myeloablative conditioning before bone marrow transplantation (BMT) results in thymic epithelial cell (TEC) injury, T-cell immune deficiency, and susceptibility to opportunistic infections. Conditioning regimen–induced TEC damage directly contributes to slow thymopoietic recovery after BMT. Keratinocyte growth factor (KGF) is a TEC mitogen that stimulates proliferation and, when given before conditioning, reduces TEC injury. Some TEC subsets are refractory to KGF and functional T-cell responses are not fully restored in KGF-treated BM transplant recipients. Therefore, we investigated whether the addition of a pharmacologic inhibitor, PFT-β, to transiently inhibit p53 during radiotherapy could spare TECs from radiation-induced damage in congenic and allogeneic BMTs. Combined before BMT KGF + PFT-β administration additively restored numbers of cortical and medullary TECs and improved thymic function after BMT, resulting in higher numbers of donor-derived, naive peripheral CD4+ and CD8+ T cells. Radiation conditioning caused a loss of T-cell zone fibroblastic reticular cells (FRCs) and CCL21 expression in lymphoid stroma. KGF + PFT-β treatment restored both FRC and CCL21 expression, findings that correlated with improved T-cell reconstitution and an enhanced immune response against Listeria monocytogenes infection. Thus, transient p53 inhibition combined with KGF represents a novel and potentially translatable approach to promote rapid and durable thymic and peripheral T-cell recovery after BMT.
Introduction
Allogeneic bone marrow transplantation (BMT) is used to treat malignant and nonmalignant disorders.1,2 Chemoradiotherapy conditioning preceding donor graft infusion damages thymic stroma, severely delaying peripheral CD4+ and CD8+ T-cell reconstitution.2-6 Thus, BM transplant recipients are at increased risk of opportunistic fungal and viral infections.7 Thymic stroma is composed of a 3-dimensional matrix of thymic epithelial cells (TECs), fibroblasts, macrophages, dendritic cells (DCs), and mesenchymal cells.8 Critical signals are supplied by TECs to developing thymocytes directing their thymic ingress,9,10 survival,11,12 trafficking,11 selection,13 and export.11 Conditioning depletes TECs, impairing T-cell production for prolonged periods of time after BMT.14-17 Restoring thymic function would speed peripheral T-cell recovery after BMT.
Developing thymocytes can be distinguished by their CD4 and CD8 cell surface expression. CD4−CD8− (double-negative, DN) thymocytes mature to become CD4+CD8+ (double-positive, DP) thymocytes and undergo positive selection on cortical TECs (cTECs). DP thymocytes continue their maturation into CD4+ or CD8+ (single-positive, SP) thymocytes and migrate into the thymic medulla where negative selection is mediated by medullary TECs (mTECs) and medullary DCs.8 SP thymocytes complete their maturation in the medulla and are exported into the periphery as mature T cells.13
Keratinocyte growth factor (KGF) is a fibroblast growth factor family member that controls cell migration, proliferation, and differentiation in epithelial tissues.18 Endogenous KGF is up-regulated in mucosal tissues after injury, and exogenous KGF enhances protection/repair of epithelial cells in models of chemoradiotherapy-induced injury.18 KGF is approved by the Food and Drug Administration for prevention of oral mucositis associated with high-dose chemoradiotherapy and hematopoietic stem cell transplantation.19-21 KGF is produced by thymic mesenchymal cells and binds exclusively to FGFR2-IIIb expressed by TECs.15,22 KGF is a potent mitogen for TECs.23 KGF pretreatment prevents thymic injury and prolonged T-cell deficiency in murine BMT models.15,23-27 KGF facilitates alloengraftment and abrogates graft-versus-host disease (GVHD)–induced lethality in murine BM transplant recipients.28 Although KGF can ameliorate many BMT-related side effects, KGF fails to completely protect mTECs and to restore CD8+ T-cell numbers and function after BMT.29
The tumor suppressor protein, p53, is activated to induce apoptosis and/or growth arrest after genotoxic stress, eliminating damaged cells from the organism.30 The massive cellular loss after chemoradiotherapy in epithelial tissues is partly determined by p53-mediated apoptosis.30 Transient pharmacologic p53 inhibition has been proposed as a therapy to minimize damage of epithelial tissues caused by radiation conditioning.31,32 A small molecule (pifithrin-β; PFT-β) was isolated for its ability to reversibly block p53 function in vitro and in vivo.32 PFT-β abrogated apoptosis in epithelial tissues and protected mice from lethality after supranormal doses of total body irradiation (TBI).31 Inhibition of p53 through PFT-β was transient, and p53 function returned to normal within 12 hours.32
We hypothesized that PFT-β could protect TECs from radiation injury and could speed thymopoietic recovery after BMT and that KGF + PFT-β could work in combination to restore thymic function after BMT. This study focuses on one agent (recombinant human KGF; Kepivance; Biovitrum) currently approved by the Food and Drug Administration and another agent (PFT-β) that will be the focus of upcoming clinical trials for ameliorating the general side effects of chemoradiotherapy. KGF + PFT-β additively restored numbers of cTECs and mTECs and improved thymic output, resulting in enhanced reconstitution of donor-derived, naive CD4+ and CD8+ T cells. This increase in T cells correlated with recovery of fibroblastic reticular cells (FRCs) and CCL21 expression in lymph nodes (LNs) and a robust immune response against Listeria monocytogenes (Lm) in vivo. These findings suggest that KGF + PFT-β may represent a novel, clinically translatable approach to accelerate thymic and T-cell recovery and function after BMT.
Methods
Animals
C57BL/6 (H-2b; termed B6) and [C57BL/6×Balb/c]F1 (H-2d/b; termed CB6F1) female mice were purchased from The Jackson Laboratory and BALB/c (H-2d) or C57BL/6.Ly5.1 mice were purchased from the National Cancer Institute. Mice were used at approximately 8 weeks of age. Bim−/− mice, backcrossed more than 10 generations onto B6 background, were provided by Dr P. Bouillet (The Walter and Eliza Hall Institute for Medical Research) and maintained in house. Mice were housed in specific pathogen-free facilities, and protocols were approved by the institutional care and use committee at the University of Minnesota.
KGF and PFT-β administration
Recombinant human KGF (Amgen) was administered subcutaneously for 3 consecutive days (5 mg/kg/d) before radiation as previously reported.15 PFT-β is a cyclic derivative of the small molecule inhibitor, pifithrin-alpha (PFT-α), that is significantly less toxic and possesses longer pharmacodynamic effects than does PFT-α.33 PFT-β was administered intraperitoneally (25 mg/kg) in phosphate-buffered saline (PBS) 30 to 45 minutes before radiation. The dose/timing of PFT-β administration provides optimal inhibition of p53 function with minimal toxicity32 (data not shown).
BMT
Single-cell suspensions of BM cells from B6.Ly5.1 (congenic) or BALB/c (allogeneic) donors were depleted of T cells to greater than 98% purity as described.29 CD4/8-depleted BM cells (107 [allogeneic] or 5 × 106 [congenic]) were administered intravenously to recipients that had received 9 Gy (C57BL/6) or 10 Gy (CB6F1) TBI from an x-ray source 24 hours before.
Lymphocyte analysis by fluorescence-activated cell sorting
Single-cell suspensions of thymocytes, splenocytes, and LNs were prepared by gentle dissociation, washed, filtered, resuspended in 2% fetal calf serum/PBS, and incubated with fluorochrome-conjugated monoclonal antibodies for 30 minutes at 4°C. Antibodies used were directed against CD4, CD8, CD3, T-cell receptor β), CD11c, B220, CD45.1, CD62L, and CD44 (eBioscience). Live events (≥ 105) were acquired on a BD FACSCanto and analyzed with FlowJo software (TreeStar).
TEC analysis by fluorescence-activated cell sorting
TECs were isolated as described with minor modifications.34,35 Individual thymi were incubated at 37°C twice in collagenase-D/DNase-I and twice in collagenase/dispase/DNase-I (Roche). Pooled digestions were stained with anti–CD45-PerCp-Cy5.5, anti–EpCAM-PE, anti–Ly51/CDR1-biotin plus streptavidin-conjugated PE/Cy7, anti–MHC-II-Pacific Blue (eBioscience), and FITC-conjugated Ulex-europaeus-agglutinin-1 (UEA-1; Vector Laboratories). Mouse AIRE-specific rat mAb (5H12) was detected with mouse anti–rat IgG2c-Cy5. A total of 3 × 106 live events were acquired per sample.
Recent thymic emigrant detection
Anesthetized mice were injected in one thymic lobe with 50 μg sulfo-NHS-biotin (Pierce) in 10 μL PBS. After 24 hours, thymus and spleen were stained with streptavidin-conjugated PE/Cy7, CD4, CD8, CD3, CD45.1, CD44, and CD62L and were analyzed by flow cytometry as described.36
Immunofluorescence microscopy
Tissues were embedded in OCT, snap-frozen in liquid nitrogen. Acetone-fixed 8-μm thymic sections were blocked with 10% normal horse serum/PBS and stained with Ly51/CDR1-FITC and polyclonal rabbit anti–mouse CK5 (Covance Research Products) plus Cy5-conjugated goat anti–rabbit immunoglobulin G (IgG; Invitrogen). For LN/spleen analysis, 6-μm cryosections were acetone-fixed and stained for glycoprotein-38 (gp38; purified clone 8.1.1; ATCC) or CCL21 (R&D Systems) along with B220-FITC (clone RA3-6B2; BD) for 3 hours at room temperature. CCL21 and gp38 signals were amplified with Tyramide Signal Amplification kit according to the manufacturer's instructions (Invitrogen). Slides were mounted with VECTASHIELD (Vector Laboratories) and images were acquired through a 10×/0.40 Olympus UPlanApo or 40×/0.80 Olympus UPlanApo Oil lens and an Olympus FV500 camera, compiled with Fluoview software (v.4.3), then analyzed and cropped in Adobe Photoshop CS2.
Lm infection and determination of CFU in organs
Recombinant Lm strains Lm-OVA and ΔactA-Lm-OVA37 (attenuated) expressing full-length chicken ovalbumin (OVA) were provided by Dr S. S. Way (University of Minnesota). Strain 2C38 was provided by Drs Jonathan Hardy and Christopher Contag (Stanford University). Mice were inoculated with early logarithmic–phase bacteria grown in brain heart infusion (BHI) broth at 37°C. Congenic BM transplant recipients were infected with 106 colony-forming units (CFUs) of ΔactA-Lm-OVA and rechallenged with 105 CFU of Lm-OVA. For allogeneic BMT studies, mice were intravenously immunized with 5 × 104 CFU and rechallenged with 2 × 106 CFU of Lm 2C. Three days after secondary infection, livers/spleens were homogenized in 0.05% Triton X-100/PBS, plated onto BHI plates, and Lm colonies were enumerated after 24 hours at 37°C.
Quantification of Lm-OVA–specific CD8 T cells
MHC-I-DimerX:mouse-Ig-PE (BD PharMingen) and purified OVA257-64 (SIINFEKL) peptide (Anaspec) were mixed to form MHC-I-DimerX:mouse-Ig:OVA257-64-PE conjugates according to the manufacturer's instructions and incubated with red blood cell–lysed peripheral blood for 1 hour at 4°C, washed, and then incubated with antibodies against surface markers. More than 104 donor CD8 T cells were collected per sample.
Statistical analysis
Differences between treated and untreated BMT groups were analyzed by a 2-tailed, unpaired Student t test with unequal distribution.
Results
Pretreatment with KGF + PFT-β additively restores TEC subsets by 4 weeks after BMT
When KGF, PFT-β, or KGF + PFT-β was administered to healthy animals, thymocyte cellularity increased in KGF-treated mice, whereas PFT-β treatment did not alter thymocyte numbers at 2 weeks after administration (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). KGF + PFT-β combined treatment resulted in a significant increase of DN and CD4 SP thymocytes compared with individual treatments (supplemental Figure 1B-E). CD4 and CD8 T-cell numbers in LNs were unaffected by any treatment (supplemental Figure 1F-G).
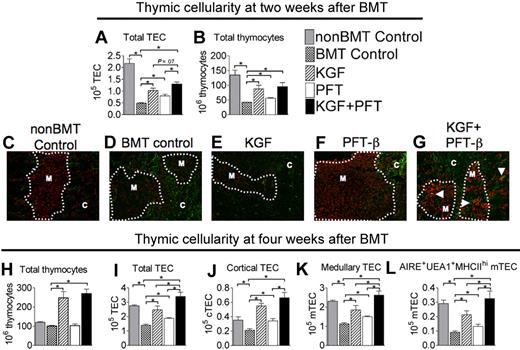
To determine whether KGF and p53 inhibition would augment thymopoiesis and TEC recovery after lethal TBI and BMT, congenic recipients of T cell–depleted BM were left untreated (BM transplant control) or pretreated with KGF, PFT-β, or KGF + PFT-β. Two weeks after BMT, TECs (CD45−EpCAM+MHC-II+) were dramatically depleted (∼ 75%) in untreated BM transplant recipients compared with non-BM transplant controls (Figure 1A). BM transplant recipients treated with KGF or PFT-β partially abrogated this reduction, maintaining significantly higher numbers of TECs than did the untreated BM transplant controls. KGF + PFT-β treatment resulted in a further increase in total TEC cellularity, albeit not to significant levels above KGF alone (P = .06; Figure 1A). Thymocyte cellularity was significantly reduced (> 75%) in untreated BM transplant recipients at 2 weeks after BMT compared with non-BM transplant controls, but it was significantly increased in KGF-, PFT-β–, and KGF + PFT-β–treated BM transplant recipients (Figure 1B). Thymocyte subset distribution (DN, DP, CD4 SP, and CD8 SP) was undisturbed by any treatment, and the levels of donor chimerism were equal (70%-80%) in all BMT groups at this early time after BMT (data not shown).
Combined pretreatment with KGF + PFT-β additively restores all TEC subsets by 4 weeks after congenic BMT. Lethally irradiated B6 recipients of congenic (B6 Ly5.1+) BM were left untreated (BMT Control) or pretreated with KGF, PFT-β, or KGF + PFT-β and analyzed for absolute numbers of total (A) TECs (CD45−EpCAM+MHC-II+) and (B) thymocytes (SSClowCD45+EpCAM−MHC-II−) at 2 weeks after BMT. (C-G) Immunofluorescence staining of thymic sections at 2 weeks after BMT for the cytokeratin-5, K5 (red) and Ly51 (green) identified mature cortical TECs (Ly51+K5−) and medullary TECs (Ly51−K5+). These methods were used to assess recovery of distinct cortical and medullary TEC populations. Images were acquired on an Olympus FV500 confocal microscope with the use of a 10×/0.40 objective lens with associated Olympus Software. (H) Total thymocytes, (I) total TECs, (J) cTECs (CD45− EpCAM+ MHC-II+Ly51+), (K) mTECs (CD45−EpCAM+MHC-II+Ly51−), and (L) AIRE+ mTEChi (CD45−EpCAM+UEA-1highMHC-IIhighLy51− AIRE+) were quantified in thymi from BM transplant recipients and non-BM transplant controls (nonBMT Control) at 4 weeks after BMT. For FACS analysis, data shown are the mean numbers of cells ± SEMs and are representative of 3 experiments of 4 mice per group; *P < .05. For immunofluorescence microscopy, data are representative of 2 experiments, each with 3 mice per group.
Combined pretreatment with KGF + PFT-β additively restores all TEC subsets by 4 weeks after congenic BMT. Lethally irradiated B6 recipients of congenic (B6 Ly5.1+) BM were left untreated (BMT Control) or pretreated with KGF, PFT-β, or KGF + PFT-β and analyzed for absolute numbers of total (A) TECs (CD45−EpCAM+MHC-II+) and (B) thymocytes (SSClowCD45+EpCAM−MHC-II−) at 2 weeks after BMT. (C-G) Immunofluorescence staining of thymic sections at 2 weeks after BMT for the cytokeratin-5, K5 (red) and Ly51 (green) identified mature cortical TECs (Ly51+K5−) and medullary TECs (Ly51−K5+). These methods were used to assess recovery of distinct cortical and medullary TEC populations. Images were acquired on an Olympus FV500 confocal microscope with the use of a 10×/0.40 objective lens with associated Olympus Software. (H) Total thymocytes, (I) total TECs, (J) cTECs (CD45− EpCAM+ MHC-II+Ly51+), (K) mTECs (CD45−EpCAM+MHC-II+Ly51−), and (L) AIRE+ mTEChi (CD45−EpCAM+UEA-1highMHC-IIhighLy51− AIRE+) were quantified in thymi from BM transplant recipients and non-BM transplant controls (nonBMT Control) at 4 weeks after BMT. For FACS analysis, data shown are the mean numbers of cells ± SEMs and are representative of 3 experiments of 4 mice per group; *P < .05. For immunofluorescence microscopy, data are representative of 2 experiments, each with 3 mice per group.
Immunofluorescence staining of thymic sections at 2 weeks after BMT indicated that untreated BM transplant recipients showed a dramatic loss in the detection of mTECs compared with non-BM transplant controls, whereas mTECs (Ly51−K5+) were protected in PFT-β–treated BM transplant recipients (Figure 1C vs D,F). At this early time after BMT, KGF treatment appeared to have minimal effect on mTECs (Figure 1D vs E). Interestingly, thymi from KGF + PFT-β–treated BM transplant recipients contained higher numbers of TECs coexpressing markers for cTECs (Ly51) and mTECs (K5), thought to represent bipotent TECs that can give rise to mature cTECs or mTECs (Figure 1G white arrowheads).
Untreated BM transplant recipients had restored thymocyte numbers by 4 weeks after BMT, in agreement with expected kinetics of the congenic BMT model. The effect of PFT-β treatment alone on thymocyte cellularity was no longer evident; however, thymocyte cellularity in KGF- and KG + PFT-β–treated BM transplant recipients remained significantly greater (2- to 3-fold) than all other BM transplant groups and non-BM transplant controls (Figure 1H). At 4 weeks after BMT, numbers of cTECs (defined as CD45−EpCAM+Ly51+UEA-1−MHC-II+) and mTECs (defined as CD45−EpCAM+Ly51−UEA-1+/−MHC-II+) were significantly (∼ 75% and ∼ 50%, respectively) lower in thymi from untreated BM transplant recipients than from non-BM transplant controls. Pretreatment with KGF or PFT-β alone had significantly restored both the cTEC and mTEC compartments (Figure 1J-K). Thymi from KGF + PFT-β–treated BM transplant recipients contained additively higher numbers of cTECs and mTECs (Figure 1J-K) with a complete restoration of the mTEC compartment (Figure 1J). Numbers of UEA-1+MHC-IIhiAIRE+ mTECs, responsible for promiscuous gene expression and hence for the deletion of autoreactive SP cells contained within the medulla, were completely restored in KGF + PFT-β–treated BM transplant recipients (Figure 1L). These data suggest that KGF and PFT-β improve early thymocyte recovery and that combined pretreatment with KGF + PFT-β maximally improves TEC recovery after BMT.
Pretreatment with KGF and PFT-β enhances thymic output at 5 weeks after BMT
We next investigated whether improved TEC regeneration permitted higher thymic export of SP thymocytes. Thymic export was quantified at 5 weeks after BMT by detection of splenic T cells that were intrathymically labeled with biotin.29,36 Numbers of labeled CD4+ and CD8+ cells (ie, recent thymic emigrants, RTEs) were markedly reduced in untreated BM transplant recipients (5-fold and 8-fold, respectively) compared with non-BM transplant controls (supplemental Figure 2A-B). Export of donor-derived CD4+ RTEs was significantly improved in BM transplant recipients treated with PFT-β or KGF + PFT-β but not with KGF alone (supplemental Figure 2A). Thymic export of donor-derived CD8+ RTEs was significantly improved in KGF-, PFT-β–, or KGF+PFT-β–treated BM transplant recipients (supplemental Figure 2B). These findings suggest that thymic function (culminating in RTE export) is deficient in untreated BM transplant recipients and that TEC protection consequent to KGF and/or PFT-β treatment partially corrects this deficiency.
Pretreatment with KGF + PFT-β additively enhances peripheral T-cell reconstitution after BMT
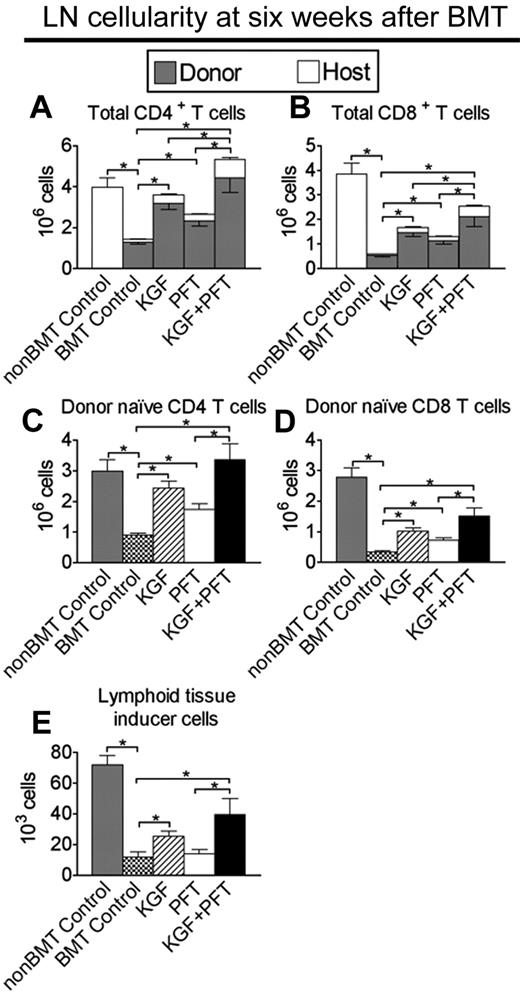
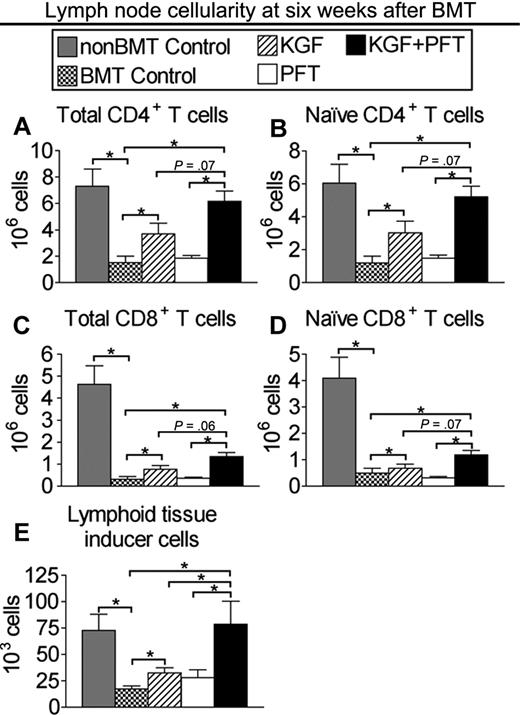
To determine whether improved TEC recovery and thymic export resulted in enhanced peripheral T-cell reconstitution, CD4+ and CD8+ T cells were quantified in LNs of congenic BM transplant recipients at 6 weeks after BMT. CD4+ and CD8+ T-cell numbers in untreated BM transplant recipients were dramatically reduced (75% and 90%, respectively) compared with non-BM transplant controls (Figure 2A-B). CD4+ T-cell numbers were significantly greater in LNs of BM transplant recipients pretreated with KGF (3-fold) or PFT-β (2-fold) alone and were additively increased (5-fold) and completely restored in BM transplant recipients pretreated with KGF + PFT-β compared with non-BM transplant controls (Figure 2A). KGF or PFT-β treatment alone resulted in a significant (2-fold) increase in CD8+ T-cell numbers, whereas combined KGF + PFT-β provided a further increase (5-fold) in CD8+ T cellularity (Figure 2B). Donor-derived, naive CD4 and CD8 T cells were quantified to assess the contribution of thymus-derived T cells to peripheral T-cell reconstitution. A similar trend in donor-derived, naive CD4+ and CD8+ T cells was observed in LNs of BM transplant recipients treated with KGF + PFT-β (Figure 2C-D). Donor-derived DC numbers were additively increased in KGF + PFT-β–treated BM transplant recipients, and similar effects for all lymphoid subsets were observed in the spleen (data not shown; supplemental Figure 3).
Combined pretreatment with KGF + PFT-β additively restores numbers of total and donor-derived, naive CD4+ and CD8+ T cells in LNs by 6 weeks after congenic BMT. Lethally irradiated B6 recipients of congenic (B6 Ly5.1+) BM were left untreated (BMT Control) or pretreated with KGF, PFT-β, or KGF + PFT-β and analyzed for the presence of T cells and Lti cells in the LNs at 6 weeks after BMT alongside unmanipulated age-/sex-matched B6 controls (non-BMT Control). Mean absolute numbers ± SEMs of (A) total CD4+CD3+ T cells; (B) total CD8+CD3+ T cells; (C) donor-derived, naive (CD62LhighCD44low) CD4+CD3+ T cells; (D) donor-derived, naive (CD62LhighCD44low) CD8+CD3+ T cells; and (E) donor-derived, CD4+CD3−CD11c−B220− Lti cells in the LNs are shown. LN node cells were pooled from inguinal, axillary, and mesenteric LNs. Data are representative of 3 experiments, each with 4 mice per group; *P < .05.
Combined pretreatment with KGF + PFT-β additively restores numbers of total and donor-derived, naive CD4+ and CD8+ T cells in LNs by 6 weeks after congenic BMT. Lethally irradiated B6 recipients of congenic (B6 Ly5.1+) BM were left untreated (BMT Control) or pretreated with KGF, PFT-β, or KGF + PFT-β and analyzed for the presence of T cells and Lti cells in the LNs at 6 weeks after BMT alongside unmanipulated age-/sex-matched B6 controls (non-BMT Control). Mean absolute numbers ± SEMs of (A) total CD4+CD3+ T cells; (B) total CD8+CD3+ T cells; (C) donor-derived, naive (CD62LhighCD44low) CD4+CD3+ T cells; (D) donor-derived, naive (CD62LhighCD44low) CD8+CD3+ T cells; and (E) donor-derived, CD4+CD3−CD11c−B220− Lti cells in the LNs are shown. LN node cells were pooled from inguinal, axillary, and mesenteric LNs. Data are representative of 3 experiments, each with 4 mice per group; *P < .05.
Accumulation of CD4+CD3−CD11c−B220− lymphoid tissue-inducer (Lti) cells in secondary lymphoid organs correlates with preferential restoration of the lymphoid stromal compartment after injury (eg, viral infection).39,40 We consistently observed a significant and additive increase in the numbers of donor-derived (> 90%) CD4+CD3−CD11c−B220− Lti cells in LNs nodes of KGF + PFT-β–treated BM transplant recipients at 6 weeks after BMT (Figure 2E). Sorted CD4+CD3−CD11c−B220− cells from the LN/spleen of B6 mice expressed RORγt, confirming their identity as Lti cells, but did not express FGFR2-IIIb (supplemental Figure 4). These data show that combined KGF + PFT-β pretreatment before BMT markedly improved the reconstitution of the peripheral T-cell and Lti compartment after BMT.
Enhanced T-cell reconstitution correlates with improved recovery of T-cell zone FRCs
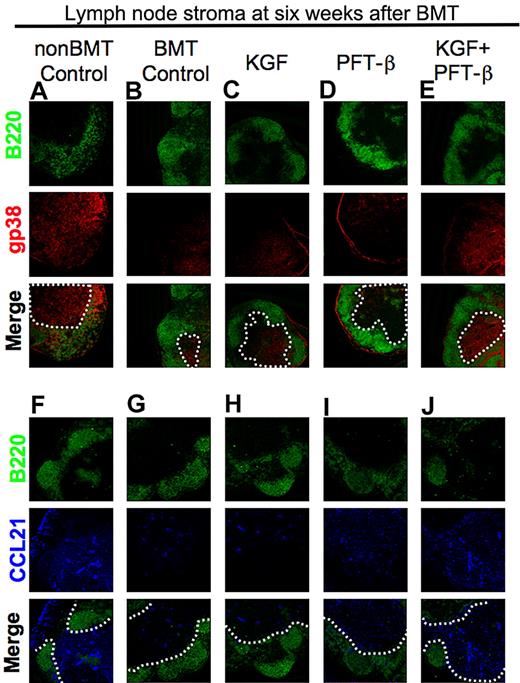
We hypothesized that radiation conditioning before BMT injures the lymphoid stromal compartment, including T-cell zone FRCs that support naive T-cell ingress and survival in secondary lymphoid organs through provision of critical cytokines (eg, interleukin-7 [IL-7]) and chemokines (eg, CCL21).39,41,42 If true, then KGF and/or PFT-β treatment could augment the recovery of these critical components of lymphoid stromal “health.” Such a finding would agree with the high numbers of Lti cells (which can drive recovery of FRCs after viral infection) in LNs in KGF + PFT-β–treated BM transplant recipients. We first examined LN sections with the use of immunofluorescence microscopy for the expression of gp38 to identify FRCs39,41 along with a B-cell marker (B220) for an architectural frame of reference. Compared with non-BM transplant controls, LNs of untreated BM transplant recipients consistently exhibited a loss of reticular gp38 staining in the T-cell zones (ie, B220-negative areas; Figure 3A vs B inside white dotted line). Pretreatment with KGF partially restored gp38-staining density, in contrast to PFT-β alone (Figure 3B vs C-D). However, gp38 staining in LNs isolated from KGF + PFT-β–treated BM transplant recipients was strikingly greater than all other treatment groups but comparable with that of non-BM transplant controls (Figure 3A vs E). Consistent with the FRC staining, LNs from KGF- or PFT-β–treated BM transplant recipients contained intermediately higher CCL21 expression levels than did untreated BM transplant recipients (Figure 3E vs F-G). CCL21 staining was most robust in LN T-cell zones of KGF + PFT-β–treated BM transplant recipients to levels approaching that of non-BM transplant controls (Figure 3H-I). Technical limitations precluded costaining for gp38 and CCL21 on the same section. Together, these findings provide qualitative evidence to support the idea that preferential recovery of the lymphoid stromal compartment in KGF + PFT-β–treated BM transplant recipients may augment the homing and survival of naive CD4+ and CD8+ T cells in LNs.
Combined pretreatment with KGF + PFT-β augments T-cell zone FRC and CCL21 expression after congenic BMT. (A-E) Immunofluorescence staining of peripheral LN cryosections for B220+ B cells (green) and gp38+ FRCs (red) was used to assess the relative abundance of gp38+ FRCs in T-cell zones of (A) unmanipulated, age-/sex-matched B6 control (non-BMT Controls) or BM transplant recipients that were (B) left untreated or treated with (C) KGF, (D) PFT-β, or (E) KGF + PFT-β. (F-J) Immunofluorescence staining of LNs for B220 (green) and CCL21 (blue) in (F) unmanipulated, age-/sex-matched B6 control (non-BMT Controls) or BM transplant recipients that were (G) left untreated or treated with (H) KGF, (I) PFT-β, or (J) KGF + PFT-β. In all merged images, a white, dashed line encircles the B220− T-cell zones. Data are representative of 2 experiments, each with 3 mice per group; *P < .05.
Combined pretreatment with KGF + PFT-β augments T-cell zone FRC and CCL21 expression after congenic BMT. (A-E) Immunofluorescence staining of peripheral LN cryosections for B220+ B cells (green) and gp38+ FRCs (red) was used to assess the relative abundance of gp38+ FRCs in T-cell zones of (A) unmanipulated, age-/sex-matched B6 control (non-BMT Controls) or BM transplant recipients that were (B) left untreated or treated with (C) KGF, (D) PFT-β, or (E) KGF + PFT-β. (F-J) Immunofluorescence staining of LNs for B220 (green) and CCL21 (blue) in (F) unmanipulated, age-/sex-matched B6 control (non-BMT Controls) or BM transplant recipients that were (G) left untreated or treated with (H) KGF, (I) PFT-β, or (J) KGF + PFT-β. In all merged images, a white, dashed line encircles the B220− T-cell zones. Data are representative of 2 experiments, each with 3 mice per group; *P < .05.
Pretreatment with PFT-β or KGF + PFT-β enhances immune response against Lm after BMT
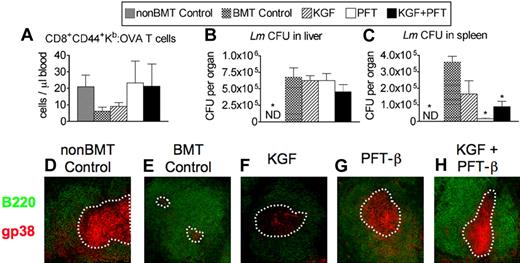
To determine whether improved T-cell reconstitution induced by treatment with KGF/PFT-β before BMT would permit a functional immune response to challenge with a live intracellular pathogen, mice were immunized with 106 CFU of an attenuated strain of Lm engineered to express chicken ovalbumin (ΔactA-Lm-OVA). Activated Lm-specific CD8 T cells were quantified in peripheral blood by MHC class-I:OVA257-264 tetramer binding to CD44+ CD8 T cells 8 days after primary infection (ie, peak of response). KGF-treated BM transplant recipients showed a modest increase above untreated BM transplant recipients, which contained approximately 4-fold fewer numbers of responding OVA-specific CD44+ CD8+ T cells than did non-BM transplant controls (Figure 4A). PFT-β or KGF + PFT-β treatment before BMT resulted in higher numbers of OVA-specific CD44+ CD8+ T cells that were comparable with non-BM transplant controls (Figure 4A).
Pretreatment with PFT-β or KGF + PFT-β significantly improves primary and secondary immune responses against Lm after congenic BMT. Lethally irradiated B6 recipients of congenic (B6 Ly5.1+) BM were left untreated (BMT Control) or pretreated with KGF, PFT-β, or KGF + PFT-β and immunized at 4 weeks after BMT alongside unmanipulated age-/sex-matched B6 controls (non-BMT control). For primary immunization, 106 CFU of an attenuated strain of Lm that express recombinant full-length chicken ovalbumin (ΔactA-Lm-OVA) was intravenously injected. (A) Absolute numbers of CD44+CD8+ Kb-OVA257-64–specific T cells were quantified in peripheral blood of infected animals by FACS 8 days after primary infection. (B) Immunized mice were then rechallenged with 105 CFU of the virulent parent strain, Lm-OVA, 5 weeks after primary infection. After 3 days, bacterial CFUs in (B) liver and (C) spleen were determined by plating of serial dilutions of organ homogenates onto BHI agar. (D-H) Immunofluorescence staining of spleen cryosections for B220+ B cells (green) and gp38+ FRCs (red) was used to assess the relative abundance of gp38+ FRCs in T-cell zones of (A) unmanipulated, age-/sex-matched B6 control (non-BMT Controls) or BM transplant recipients that were (B) left untreated (BMT control) or treated with (C) KGF, (D) PFT-β, or (E) KGF + PFT-β. A white, dashed line encircles the B220− T-cell zones. Data are representative of 2 experiments, each with 4 mice per group; *P < .05 compared with BMT controls.
Pretreatment with PFT-β or KGF + PFT-β significantly improves primary and secondary immune responses against Lm after congenic BMT. Lethally irradiated B6 recipients of congenic (B6 Ly5.1+) BM were left untreated (BMT Control) or pretreated with KGF, PFT-β, or KGF + PFT-β and immunized at 4 weeks after BMT alongside unmanipulated age-/sex-matched B6 controls (non-BMT control). For primary immunization, 106 CFU of an attenuated strain of Lm that express recombinant full-length chicken ovalbumin (ΔactA-Lm-OVA) was intravenously injected. (A) Absolute numbers of CD44+CD8+ Kb-OVA257-64–specific T cells were quantified in peripheral blood of infected animals by FACS 8 days after primary infection. (B) Immunized mice were then rechallenged with 105 CFU of the virulent parent strain, Lm-OVA, 5 weeks after primary infection. After 3 days, bacterial CFUs in (B) liver and (C) spleen were determined by plating of serial dilutions of organ homogenates onto BHI agar. (D-H) Immunofluorescence staining of spleen cryosections for B220+ B cells (green) and gp38+ FRCs (red) was used to assess the relative abundance of gp38+ FRCs in T-cell zones of (A) unmanipulated, age-/sex-matched B6 control (non-BMT Controls) or BM transplant recipients that were (B) left untreated (BMT control) or treated with (C) KGF, (D) PFT-β, or (E) KGF + PFT-β. A white, dashed line encircles the B220− T-cell zones. Data are representative of 2 experiments, each with 4 mice per group; *P < .05 compared with BMT controls.
To investigate the effects of KGF/PFT-β on a secondary immune response against Lm, immunized animals were rechallenged, 5 weeks after primary infection, with 105 CFU of the virulent parent strain, Lm-OVA. Three days later, we assessed mice for clearance of Lm as measured by CFU determination in livers and spleens of infected animals. Although non-BM transplant controls had cleared all traces of infection from both liver and spleen, untreated BM transplant recipients contained a significantly higher bacterial burden in liver (≥ 6-fold) and spleen (≥ 4-fold; Figure 4B-C). BM transplant recipients treated with KGF or PFT-β contained similar bacterial burdens in the liver compared with untreated BM transplant recipients. whereas KGF + PFT-β–treated BM transplant recipients showed a nearly significant decrease in bacterial burden (P = .06; Figure 4B). KGF-treated BM transplant recipients showed modestly improved clearance from the spleen compared with untreated BM transplant recipients, whereas PFT-β and KGF + PFT-β–treated BM transplant recipients had significantly reduced the bacterial burden in the spleen (Figure 4C).
In addition to supporting T-cell homeostasis, FRCs provide critical signals that allow for a robust immune response against viral pathogens.39,42 To qualitatively correlate clearance of Lm from spleen to gp38+ FRC numbers, we analyzed spleen sections by immunofluorescence microscopy and observed the highest intensity of reticular gp38 staining in T-cell zones of PFT-β– and KGF + PFT-β–treated BM transplant recipients (Figure 4G-H). These data indicate that improved immune response against Lm observed in PFT-β– and KGF + PFT-β–treated BM transplant recipients correlates with higher numbers of responding CD8+ T cells and FRC recovery after BMT.
Pretreatment with KGF + PFT-β additively restores thymocyte cellularity after allogeneic BMT
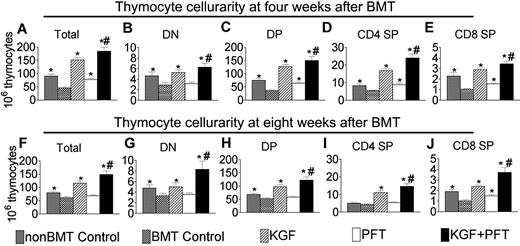
With the insights into the general biology of PFT-β and KGF effects on promoting thymic and peripheral T-cell recovery after congenic BMT, we investigated whether these findings could be extended to a clinically relevant and more challenging allogeneic BMT model. Allogeneic murine BM transplant recipients of rigorously T cell–depleted BM cells were left untreated or pretreated with KGF, PFT-β, or KGF + PFT-β. At 4 weeks after BMT, total thymocytes were approximately 50% reduced in untreated BM transplant recipients compared with non-BM transplant controls (Figure 5A). Mice treated with KGF or PFT-β completely (KGF) or partially (PFT-β) abrogated this reduction in thymocytes, whereas KGF + PFT-β resulted in additive increases in thymocyte numbers that were significantly greater than untreated, KGF-, and PFT-β–treated BM transplant recipients as well as non-BM transplant controls (Figure 5A). The relative distribution of thymocyte subsets was not affected by treatment with KGF and/or PFT-β; therefore, concomitant increases in the DN, DP, CD4+ SP, and CD8+ SP subsets were observed (Figure 5B-E). The additive effects of KGF + PFT-β were also observed at 8 and 12 weeks after BMT, showing the durability of these effects in an allogeneic BMT setting (Figure 5F-J; data not shown). By 4 weeks after BMT, more than 90% of thymocytes were of donor origin, increasing to more than 99% by 8 weeks (data not shown). Therefore, increased thymic cellularity was due to increased numbers of donor-derived thymocytes and not due to the preservation of host-derived thymocytes.
Combined pretreatment with KGF + PFT-β additively restores thymocyte cellularity after allogeneic BMT. Lethally irradiated B6 recipients of allogeneic (balb/c) BM were left untreated (BMT control) or pretreated with KGF, PFT-β, or KGF + PFT-β and analyzed for thymocyte cellularity at (A-E) 4 and (F-J) 8 weeks after BMT alongside age-/sex-matched, unmanipulated B6 controls (non-BMT control). Data shown are mean absolute numbers ± SEMs of total thymocytes. The data are pooled from 5 independent experiments with 4 to 5 mice per group; *P < .05 compared with BMT controls; #P < .05 compared with KGF- and PFT-β–treated BM transplant recipients.
Combined pretreatment with KGF + PFT-β additively restores thymocyte cellularity after allogeneic BMT. Lethally irradiated B6 recipients of allogeneic (balb/c) BM were left untreated (BMT control) or pretreated with KGF, PFT-β, or KGF + PFT-β and analyzed for thymocyte cellularity at (A-E) 4 and (F-J) 8 weeks after BMT alongside age-/sex-matched, unmanipulated B6 controls (non-BMT control). Data shown are mean absolute numbers ± SEMs of total thymocytes. The data are pooled from 5 independent experiments with 4 to 5 mice per group; *P < .05 compared with BMT controls; #P < .05 compared with KGF- and PFT-β–treated BM transplant recipients.
Pretreatment with KGF + PFT-β additively improves T-cell reconstitution after allogeneic BMT
To determine whether improved thymopoiesis induced by KGF/PFT-β resulted in higher numbers of peripheral T cells, LNs of BM transplant recipients were analyzed for CD4+ and CD8+ T cells at 6 weeks after allo-BMT. CD4+ and CD8+ T-cell numbers in untreated BM transplant recipients were reduced approximately 75% and approximately 90%, respectively (Figure 6A,C). KGF pretreatment resulted in partial but significant restoration of total CD4+ (2-fold) and CD8+ (2-fold) T cells compared with untreated BM transplant recipients. In contrast, PFT-β pretreatment alone did not confer benefit for restoring CD4+ or CD8+ T-cell numbers. However, the combined pretreatment with KGF + PFT-β improved reconstitution of CD4+ (3-fold) and CD8+ (4-fold) T cells to levels above those of KGF-treated BM transplant recipients, with a trend toward significance (Figure 6A,C). In fact, the CD4+ T-cell compartment was nearly restored in KGF + PFT-β–treated BM transplant recipients (Figure 6A). Reconstitution of naive CD4+ and CD8+ T cells was also further improved with combined KGF + PFT-β pretreatment, albeit not significantly above KGF alone (Figure 6B,D). Numbers of Lti cells were significantly and additively increased in LNs of KGF + PFT-β–treated BM transplant recipients (Figure 6E). Similar observations for all lymphoid subsets were observed in the splenic T-cell compartment (data not shown). Therefore, pretreatment with KGF + PFT-β maximally enhances the recovery of peripheral CD4+ and CD8+ T cells after allo-BMT to a greater degree than either KGF or PFT-β alone.
Combined pretreatment with KGF + PFT-β additively restores numbers of total and naive CD4+ and CD8+ T cells in LN by 6 weeks after allogeneic BMT. Lethally irradiated CB6F1 recipients of allogeneic (BALB/c) BM were left untreated (BMT Control) or pretreated with KGF, PFT-β, or KGF + PFT-β and analyzed for the presence of T cells in the LNs at 6 weeks after BMT alongside unmanipulated age-/sex-matched CB6F1 controls (non-BMT Control). Mean absolute numbers ± SEMs of (A) total CD4+CD3+ T cells, (B) naive (CD62LhighCD44low) CD4+ T cells, (C) total CD8+CD3+ T cells, (D) naive (CD62LhighCD44low) CD8+ T cells, and (E) CD4+CD3−CD11c−B220− Lti cells in the LNs are shown. LN cells were pooled from inguinal, axillary, and mesenteric LNs. Data are representative of 2 experiments, each with 4 mice per group; *P < .05.
Combined pretreatment with KGF + PFT-β additively restores numbers of total and naive CD4+ and CD8+ T cells in LN by 6 weeks after allogeneic BMT. Lethally irradiated CB6F1 recipients of allogeneic (BALB/c) BM were left untreated (BMT Control) or pretreated with KGF, PFT-β, or KGF + PFT-β and analyzed for the presence of T cells in the LNs at 6 weeks after BMT alongside unmanipulated age-/sex-matched CB6F1 controls (non-BMT Control). Mean absolute numbers ± SEMs of (A) total CD4+CD3+ T cells, (B) naive (CD62LhighCD44low) CD4+ T cells, (C) total CD8+CD3+ T cells, (D) naive (CD62LhighCD44low) CD8+ T cells, and (E) CD4+CD3−CD11c−B220− Lti cells in the LNs are shown. LN cells were pooled from inguinal, axillary, and mesenteric LNs. Data are representative of 2 experiments, each with 4 mice per group; *P < .05.
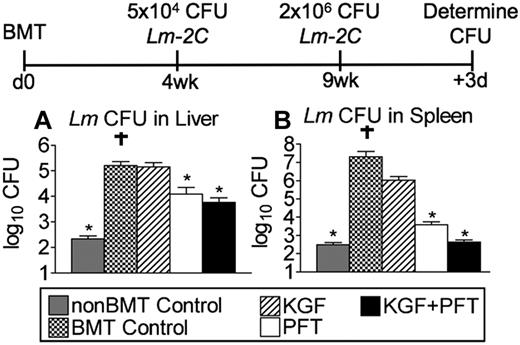
Pretreatment with KGF and PFT-β enhances functional immune response against Lm after allogeneic BMT
To investigate the effects of KGF and PFT-β on a secondary immune response against Lm in the allogeneic BMT setting, untreated, KGF-, PFT-β, or KGF + PFT-β–treated allogeneic BM transplant recipients were immunized with 5 × 104 CFU of Lm 2C at 4 weeks after BMT and rechallenged with 2 × 106 CFU of Lm 2C 5 weeks later. After 3 days, we assessed for clearance of Lm as measured by CFU determination in livers and spleens of infected animals. Although non-BM transplant controls had nearly completely cleared the infection from liver and spleen, untreated and KGF-treated BM transplant recipients contained significantly higher (3-log) bacterial burdens in both organs compared with non-BM transplant controls (Figure 7A-B). BM transplant recipients treated with PFT-β alone or with combined KGF + PFT-β had significantly reduced the bacterial burden in both liver and spleen, with the latter group clearing Lm from spleen as effectively as non-BM transplant controls (Figure 7A-B). These findings indicate that allogeneic BM transplant recipients pretreated with PFT-β or KGF + PFT-β are able to mount a superior in vivo immune response to secondary infection with an intracellular pathogen.
Pretreatment with KGF and PFT-β significantly improves immune clearance of Lm after allogeneic BMT. Lethally irradiated CB6F1 recipients of allogeneic (balb/c) T cell–depleted BM were left untreated (BMT Control) or pretreated with KGF, PFT-β, or KGF + PFT-β and immunized at 4 weeks after BMT alongside unmanipulated age-/sex-matched CB6F1 controls (non-BMT Control). For primary immunization, 5 × 104 CFU of Lm (strain 2C) was injected intravenously. Immunized mice were rechallenged with 2 × 106 CFU of Lm-2C, 5 weeks after primary infection. Then after 3 days, bacterial CFUs in liver (A) and spleen (B) were determined by plating of serial dilutions of organ homogenates onto BHI agar. Data are representative of 2 experiments, each with 4 mice per group. † indicates mouse succumbed to infection; *P < .05 compared with BMT controls.
Pretreatment with KGF and PFT-β significantly improves immune clearance of Lm after allogeneic BMT. Lethally irradiated CB6F1 recipients of allogeneic (balb/c) T cell–depleted BM were left untreated (BMT Control) or pretreated with KGF, PFT-β, or KGF + PFT-β and immunized at 4 weeks after BMT alongside unmanipulated age-/sex-matched CB6F1 controls (non-BMT Control). For primary immunization, 5 × 104 CFU of Lm (strain 2C) was injected intravenously. Immunized mice were rechallenged with 2 × 106 CFU of Lm-2C, 5 weeks after primary infection. Then after 3 days, bacterial CFUs in liver (A) and spleen (B) were determined by plating of serial dilutions of organ homogenates onto BHI agar. Data are representative of 2 experiments, each with 4 mice per group. † indicates mouse succumbed to infection; *P < .05 compared with BMT controls.
PFT-β works independently of the Bim proapoptotic pathway in the thymus
The proapoptotic BH3 family of proteins are key effectors of p53-mediated apoptosis after genotoxic stress.43 Bcl-2 interacting mediator of cell death (Bim) is a prominent member of the BH3 family that binds with high affinity to all antiapoptotic Bcl-2 proteins and is expressed in the thymus.44,45 Therefore, we next investigated whether the effects of PFT-β on thymocyte recovery were dependent on or complemented Bim function. Untreated or PFT-β–treated, lethally irradiated Bim+/− and Bim−/− mice received a transplant with T cell–depleted allogeneic BM. Age-/sex-matched littermates were included as non-BM transplant controls. At 4 weeks after BMT, thymi from Bim+/− BM transplant recipients contained significantly fewer thymocytes than did non-BM transplant controls, but were significantly restored with PFT-β pretreatment (supplemental Figure 5A). Bim−/− thymocytes were partially refractory to radiation-induced depletion, which is in agreement with previous reports46 ; nevertheless, PFT-β pretreatment resulted in significantly higher numbers of thymocytes compared with untreated Bim−/− BM transplant recipients (supplemental Figure 5A).
To more directly study whether PFT-β operates independently of the Bim pathway in thymic stroma, Bim+/− and Bim−/− mice first received a transplant with wild-type congenic BM to create chimeric mice in which loss of Bim function was restricted to thymic stroma. Complete donor chimerism (> 95% donor) was confirmed after 12 weeks by fluorescence-activated cell sorting (FACS) analysis of peripheral blood (data not shown). Chimeric mice were left untreated or pretreated with PFT-β before radiation and congenic BMT. Cohorts of age-/sex-matched chimeric mice were included as non-BM transplant controls. Thymocyte cellularity at 4 weeks after BMT was significantly reduced (3-fold) in untreated BM transplant recipients of both chimeric groups compared with non-BM transplant controls, suggesting that the loss of proapoptotic Bim in TECs does not protect from overall thymocyte loss after BMT (supplemental Figure 5B). Interestingly, PFT-β treatment significantly restored thymocyte cellularity in both chimeric groups, indicating that the PFT-β–mediated benefit is independent of the Bim pathway in thymic stroma (supplemental Figure 5B).
Discussion
Current treatment options for speeding T-cell recovery after BMT generally require repeated infusion of proteins or donor lymphocytes, which can be cumbersome, costly, and plagued by negative side effects. The most promising approach to durably restore T cells after BMT is through restoring thymic function. This report describes a novel approach to restore thymic function and peripheral T-cell numbers after BMT by combining transient inhibition of p53 with the potent epithelial mitogen, KGF. Pretreatment with KGF + PFT-β before BMT additively restored the TEC compartment and improved thymopoietic recovery. Combined pretreatment also resulted in significant increases in donor-derived, naive CD4+ and CD8+ T cells in the periphery and enhanced immune response against Lm. Our data also suggest that radiation/BMT injures the lymphoid stromal compartment and suggest a novel link between recovery of lymphoid stroma (eg, FRCs) and T-cell reconstitution after BMT.
KGF pretreatment before BMT has been shown to improve thymopoietic recovery after murine and nonhuman primate models of BMT in an IL-7–dependent manner.15,24,29 KGF administration to healthy (non-BMT) animals expands FGFR2-IIIb+ TECs and boosts thymopoiesis.23 In the present study, pretreatment with KGF or PFT-β significantly enhanced cTEC and mTEC regeneration after BMT, whereas combined KGF + PFT-β additively restored cTEC and mTEC numbers. Because thymocyte development is tightly controlled by the size of the thymic stromal niche, improved TEC regeneration may increase the stromal scaffold available to support development and export of newly derived T cells.10,11,29,47
Data from several reports support the notion that thymocytes supply “crosstalk” signals to support TEC development in both mice and humans.48-53 This idea may also hold true for TEC regeneration after depletive regimens. Additive restoration of TECs by 4 weeks after BMT observed in KGF + PFT-β–treated BM transplant recipients may be due to PFT-β–mediated protection combined with TEC expansion driven by mature SP thymocyte-derived crosstalk. However, TEC recovery in BM transplant recipients treated with PFT-β alone was not as robust because this group lacks the early and late crosstalk signals provided by KGF-expanded thymocytes. Although the precise mechanism(s) for TEC protection/repair remain unknown, we have excluded the contribution of Bim, a member of the proapoptotic BH3 family of key effectors of p53-mediated apoptosis after genotoxic stress.43,44
AIRE+ mTECs mediate negative selection of SP thymocytes on tissue-restricted antigens in the thymus and thus maintain central tolerance. AIRE+ mTECs remained severely diminished in untreated BM transplant recipients, but they were completely restored in KGF + PFT-β–treated BM transplant recipients by 4 weeks after BMT. Restoration of AIRE+ mTECs increases the probability that SP thymocytes encounter tissue-restricted antigens for their negative selection.36 This importance is underscored by the findings that thymus-derived donor T cells cause chronic GVHD (an autoimmune-like condition) and highlight the importance of restoring central tolerance after BMT.54,55
Reconstitution of a polyclonal population of donor-derived, naive T cells is particularly important for BM transplant recipients to ward off opportunistic infections and leukemia relapse.5 Although thymic output was not additively increased in KGF + PFT-β–treated BM transplant recipients above KGF or PFT-β alone, numbers of peripheral T cells were additively greater by 6 weeks after BMT. We found that FRC numbers and CCL21 expression levels, which both provide key homeostatic signals to naive T cells, were depleted after BMT and that BM transplant recipients pretreated with KGF + PFT-β had preferentially restored these in T-cell zones of the LNs. FGFR2-IIIb transcripts were undetectable by reverse transcription–polymerase chain reaction in RORγt+ Lti cells isolated from LNs/spleens of wild-type mice; thus, it is unlikely that the observed accumulation of donor-derived Lti cells is due to direct effects of KGF on Lti cells. Recent data suggest that systemic availability of IL-7 controls the size of the adult Lti pool.56 We and others have shown that KGF administration restores IL-7 expression, at least in the thymus, in murine models of aging and after BMT.15,23,26 IL-7 restoration may have contributed to the higher Lti numbers in BM transplant recipients treated with KGF and KGF + PFT-β. Consequently, higher numbers of Lti cells may support faster FRC recovery, in line with previous studies showing the role of Lti cells in promoting FRC genesis during lymphoid development and recovery after viral infection.39,40,57 Our findings provide novel links between improved recovery of secondary lymphoid stromal “health” and promotion T-cell reconstitution after BMT.
Low numbers of peripheral CD4+ and CD8+ T cells and general T-cell dysfunctions after BMT increase the susceptibility to opportunistic infections and negatively correlate with survival after BMT.4,58-62 In the present study and in our previous work, untreated BM transplant recipients were unable to mount an effective immune response against Lm as evidenced by a lack of responding CD8+ T cells and inadequate clearance of the pathogen from liver or spleen.29 Pretreatment with KGF significantly improved T-cell reconstitution after congenic and allogeneic BMTs, but it did not augment an immune response against Lm.29 KGF has been shown to skew toward a T helper type 2 anti-inflammatory environment, which may consequently inhibit responses against certain pathogens, including Lm, which require robust T helper type 1 responses.28,63-66 However, KGF reduces incidence and severity of acute GVHD through its T helper type 2–skewing and epithelial protective effects.25,28,65,66 Epithelial protection mediated by KGF and augmented by PFT-β may reduce general damage and thus hold promise for speeding T-cell recovery in the setting of GVHD.
Reversible p53 pharmacologic suppression with PFT-β has been used to prevent certain conditioning-related side effects without higher incidence of tumor development.31,32,67 Nevertheless, the high incidence of cancer observed in p53-deficient mice and humans have raised concerns about the safety of p53 inhibition for clinical use.31 Collective preclinical data argue that temporary and reversible inhibition of p53 with pharmacologic inhibitors such as PFT-β does not predispose recipients to develop tumors.31-33,67-70 In fact, accumulating evidence indicates that transient inhibition of p53 during radiation conditioning regimens may actually promote eradication of solid tumors through destruction of stromal support and/or the tumor itself and increase efficacy of chemoradiotherapy conditioning.33
Ongoing efforts are aimed at identifying pharmacologic modulators of p53 to inhibit singular functions of p53. One candidate molecule (termed PFT-μ) reversibly inhibits p53 binding to mitochondria, but, unlike PFT-β, it does not affect p53 transactivation functions.70 Preliminary data indicate that treatment with PFT-μ does not enhance restoration of thymic function after allogeneic BMT (R.M.K. and B.R.B., unpublished observations, January 2007), suggesting that temporary blockade of p53 transactivation functions is critical for the radioprotective effect observed in the TEC compartment.
In summary, reversible and temporary blockade of p53 in combination with KGF is highly effective in restoring thymic function after BMT. KGF + PFT-β maximally enhanced TEC and thymocyte recovery, leading to a more rapid and durable restoration of peripheral CD4 and CD8 T cells. Improved T-cell reconstitution correlated with superior immune responses against Lm infection. Collectively, the data presented here suggest the possibility for clinical trials to investigate the safety and therapeutic value of KGF + PFT-β on immune recovery after BMT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Philippe Bouillet and Andreas Strasser for providing Bim−/− mice, Dr Sing Sing Way (University of Minnesota) for providing Lm-OVA, Drs Chris Contag and Jonathan Hardy (Stanford University) for providing Lm-2C, and Ann Bohac (Oligonucleotide & Peptide Synthesis Facility, BioMedical Genomics Center, University of Minnesota) for help with primer design and for rapid and reliable primer synthesis. We also thank Steven Highfill for critical reading of the manuscript.
This work was supported by the National Institutes of Health (grants R01-HL073794, R01-HL55209, R01-A1057477-01, R01-A1057477-01, R01-CA75179, P01CA067493), Swiss National Science Foundation (SNF; grant 3100-68310.02), the Children's Cancer Research Fund (CCRF), and a grant from the European Community 6th Framework Program Eurothymaid Integrated Project. We acknowledge the use of the confocal microscope made available through a National Center for Research Resources (NCRR) Shared Instrumentation Grant (no. 1 S10 RR16851).
National Institutes of Health
Authorship
Contribution: R.M.K. designed and performed research, analyzed and interpreted data, made the figures, and wrote the paper; E.M.G. and M.J.O. performed research; P.A.T., S.N.M., and G.A.H. advised on experimental design and edited the paper; H.E.S. performed research and edited the paper; H.S.S. provided AIRE staining reagents and edited the paper; E.A.K. established optimal conditions for PFT-β administration; A.V.G. advised on experimental design and provided PFT-β compound; and B.R.B. designed research, advised on experimental design, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, University of Minnesota, Rm 460F Cancer Center Research Bldg, 425 E River Rd, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.