LCH is a rare disorder affecting patients of all ages that is characterized by the pathologic accumulation of immature LC and other inflammatory cells in organs such as skin, bone, liver, lungs, bone marrow, and brain. Although first described over a century ago, the etiology of LCH has remained elusive. In this issue of Blood, Badalian-Very and colleagues provide exciting new insights into LCH by demonstrating that a subset of cases exhibit somatic activating mutations in the proto-oncogene BRAF.1
The clinical manifestations of Langerhans cell (LC) histiocytosis (LCH) are remarkably variable with some patients exhibiting localized involvement of specific sites and others developing a disseminated form of disease that mirrors acute leukemia. Although the majority of the patients with localized LCH may be cured of their disease, the outcome for those with systemic involvement is suboptimal with 20% to 50% of patients dying despite the use of intensive multiagent chemotherapy and/or stem cell transplantation. To improve the outcome for patients with LCH, particularly those with high-risk disease, understanding the pathogenesis of this puzzling disorder is imperative.
Researchers have long debated whether LCH represents a true malignancy or a reactive immune condition. Resolving this issue is important as the answers are likely to have consequences in terms of diagnostic testing, outcome prediction, therapy, and patient counseling. Studies in favor of the notion that LCH is a malignancy include the demonstration that LC from nonpulmonary lesions are monoclonal based on a pattern of highly skewed X-chromosome inactivation.2,3 Other supportive findings include the immature appearance of lesional LC, the existence of rare familial clusters, evidence of cell-cycle dysregulation within lesions, and the presence of significant telomere shortening of LCH cells compared with LC from other inflammatory lesions. Epidemiologic data also suggest a close association between LCH and cancer, particularly lymphoma and acute lymphoblastic leukemia. In support of a potential clonal relationship among the disorders are reports documenting identical molecular changes in the lymphoma or leukemia and the LCH.4-6 In contrast, supporters of the opinion that LCH is a reactive process emphasize that clonal cell populations are commonly present within the immune system and that phenotypically immature LC often accumulate in areas of chronic inflammation (eg, dermatopathic lymphadenitis). The lesional expression of inflammatory chemokines and cytokines (most recently, interleukin 17, a key cytokine in several autoimmune disorders) has been reported, although conflicting data exist.7,8 Finally, although high levels of p53 protein have been described in LCH, no mutations in the TP53 gene have been reported, nor have recurrent chromosomal translocations or other genomic abnormalities been consistently described.9
In this study, Badalian-Very and colleagues demonstrate that 35 (57%) of 61 LCH specimens exhibit mutations in BRAF that encode a known oncogenic V600E form of the BRAF protein. In contrast, the related disorders dermatopathic lymphadenopathy and Rosai-Dorfman disease did not exhibit BRAF gene mutations. Although the BRAFV600E mutation was present more often in younger LCH patients, it was not associated with site or stage of disease.
BRAF is a member of the RAF family of serine threonine kinases, which are components of the RAS-RAF-MAPK signaling pathway (see figure). In normal cells, the activity of this pathway is controlled by mitogens, including growth factors, cytokines, and hormones that bind to cell-surface receptors. Ligand-bound receptors then activate RAS, which in turn binds and activates RAF and its downstream substrates, thereby enhancing cell survival, proliferation, motility, and differentiation. Somatic-activating BRAF gene mutations are observed in premalignant cutaneous nevi and a range of cancers, including melanoma, papillary thyroid cancer, colorectal and lung cancer, low-grade ovarian carcinoma, and pediatric low-grade glioma.10 Among the mutations identified, V600E is by far the most common. This mutant version of BRAF is constitutively and highly active; it is proposed that unabated activation of the MEK-ERK pathway contributes to dysregulated cell proliferation, survival, and ultimate malignant progression.
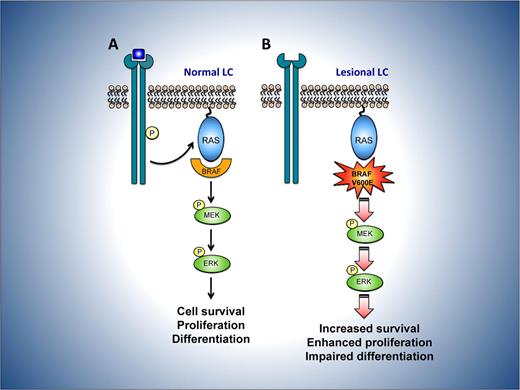
Potential consequences of mutant BRAF V600E in LCH. (A) In normal LC, mitogens such as growth factors bind to and activate cell-surface receptors, which signal through a complex consisting of adaptor proteins and exchange factors (not shown) to activate the small G-protein RAS on the inner surface of the plasma membrane. Once active, RAS binds to and activates the RAF family of proteins, comprising BRAF, ARAF, and CRAF. RAF then phosphorylates and activates MEK, which subsequently phosphorylates and activates ERK. ERK phosphorylates numerous substrates within the cytoplasm and nucleus, promoting cell division and enhancing survival, movement, and differentiation. (B) In LC from LCH lesions, constitutive activity of the mutant BRAF V600E protein is predicted to bypass the requirement for mitogen induced activation of RAF by RAS. This may lead to dysregulated signaling through the MEK-ERK pathway and thereby favor the survival and proliferation of lesional LCH cells while perturbing their differentiation.
Potential consequences of mutant BRAF V600E in LCH. (A) In normal LC, mitogens such as growth factors bind to and activate cell-surface receptors, which signal through a complex consisting of adaptor proteins and exchange factors (not shown) to activate the small G-protein RAS on the inner surface of the plasma membrane. Once active, RAS binds to and activates the RAF family of proteins, comprising BRAF, ARAF, and CRAF. RAF then phosphorylates and activates MEK, which subsequently phosphorylates and activates ERK. ERK phosphorylates numerous substrates within the cytoplasm and nucleus, promoting cell division and enhancing survival, movement, and differentiation. (B) In LC from LCH lesions, constitutive activity of the mutant BRAF V600E protein is predicted to bypass the requirement for mitogen induced activation of RAF by RAS. This may lead to dysregulated signaling through the MEK-ERK pathway and thereby favor the survival and proliferation of lesional LCH cells while perturbing their differentiation.
The study by Badalian-Very and colleagues provides the first molecular insights into the pathogenesis of LCH and is important for several reasons. First, the identification of activating BRAF gene mutations strongly supports the hypothesis that LCH is a neoplastic process, at least in some cases. This observation has significant clinical implications as it suggests that alternative therapeutic approaches aimed at targeting active BRAF should be tested in LCH, particularly in patients with the systemic and aggressive form of disease. Furthermore, this mutation should provide a means to assess minimal residual disease status in a subset of LCH patients. Second, the authors observe that lesional LC stain strongly for phospho-MEK and -ERK, regardless of BRAF mutational status. This finding suggests that RAF-MEK-ERK pathway activation may be a general feature of LCH. Thus, mutations in other genes that regulate this pathway should be sought in LCH cases with normal BRAF alleles. Third, it should now be possible to generate mouse models for LCH by activating the RAS-RAF-MEK signaling pathway specifically in LC. Such models will provide insight into mechanisms of disease initiation and progression and facilitate examination of the effects of BRAF-directed therapies. Elucidation of the effects of oncogenic BRAF and the consequences of its inhibition are critical to optimize therapeutic efficacy and minimize adverse effects resulting from manipulation of the RAS-RAF-ERK pathway.
The question of whether LCH is a neoplasm or an immune dysregulation has stimulated many important investigations into this enigmatic disorder. Although the current work by Badalian-Very and colleagues has not solved the puzzle of LCH, it has provided us with critical information that moves us in the right direction. For example, the findings of this study suggest several possible and exciting areas of investigation, including the initiation of multicenter clinical trials examining BRAF as a therapeutic target and further assessing the association between BRAF gene mutations with disease site, stage, clinical response, and outcome. In addition, the current work opens the door to future basic research studies examining whether mutations in other members of the RAS-RAF-MEK pathway participate in disease initiation and identifying the additional molecular events that cooperate with mutant BRAF to promote disease progression.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
Acknowledgments
We thank Mitchell Weiss for his critical reading of this commentary. We also thank Paul, Elizabeth, and Nikolas Kontoyannis for their continued support of the Nikolas Symposia annual “think tanks” on LCH, and their unending dedication and commitment to solving the mystery of LCH.