Abstract
Dendritic cells (DCs) play an important role in viral infections both as initiators of immunity and as viral targets. Interaction between DCs and the innate-like CD1d-restricted natural killer T (NKT) cells results in the mutual activation of both cells and the subsequent initiation of cellular immune responses. Here, we show that HIV-1 inhibits the surface expression of CD1d in productively infected DCs and identify this as a novel activity of the HIV-1 vpu gene product. Interestingly, the viral protein U (Vpu) does not enhance constitutive CD1d endocytosis or induce rapid CD1d degradation. Instead, the Vpu protein interacts with CD1d and suppresses its recycling from endosomal compartments to the cell surface by retaining CD1d in early endosomes. This interference with the CD1d antigen presentation pathway strongly inhibits the ability of infected DCs to activate CD1d-restricted NKT cells. Given that the interaction with CD1d-expressing DCs is central to the ability of NKT cells to regulate immunity, these data suggest that interference with the CD1d antigen presentation pathway represents an HIV-1 strategy to evade innate cellular immune responses and imply a role for the innate-like CD1d-restricted NKT cells in the host defense against HIV-1.
Introduction
CD1d molecules present lipid antigens to CD1d-restricted natural killer T (NKT) cells expressing an invariant αβ T-cell receptor.1 Activation of NKT cells can occur by recognition of an exogenous pathogen-derived lipid antigen or by recognition of an endogenous lipid antigen in combination with a cytokine stimulus provided by professional antigen presenting cells (APCs) after pathogen encounter.2 They rapidly secrete T helper type 1 and 2 cytokines to activate and regulate a variety of other cell types, including dendritic cells (DCs), NK cells, B cells, and conventional T cells.3 Indeed, during microbial infections NKT cells may respond early and act as a bridge between innate and adaptive immunity. The quality of the NKT-cell cytokine response is determined by several factors, including the type of antigen recognized, the type and activation status of the APC, and the cytokine milieu provided by the APC.4,5
Most HIV-1 transmissions occur at mucosal surfaces in the genital and intestinal tracts where the virus, after crossing the epithelial barrier, will meet susceptible target cells and encounter both innate and adaptive immune cells.6 Some of the cells targeted by the virus are professional APCs, such as monocytes, macrophages, and DCs, which constitutively express CD1d.7,8 CD1d is also expressed in intestinal epithelial cells and epidermal keratinocytes,9,10 as well as in vaginal, ectocervical, and penile urethral epithelia,11 and a role in the defense against microbial invasion at the mucosal barrier has been suggested.8 Human NKT cells are distributed in blood, liver, and the intestinal mucosa.12-15 Furthermore, NKT cells have been detected in lung biopsies of patients with chronic asthma,16 and in the skin of patients with allergic contact dermatitis.17 CD1d and CD1d-restricted NKT cells are thus present at relevant entry sites for pathogens into the human body, supporting a role for the CD1d system in pathogen recognition and immune responses early after pathogen encounter.
The viral protein U (Vpu) is an accessory protein that is unique to HIV-1 and a subset of related simian immunodeficiency viruses (SIVs).18,19 Vpu is an oligomeric type I integral membrane protein fulfilling at least 2 functions in the viral life cycle; it mediates proteasomal degradation of CD420 and enhances the release of progeny virions from infected cells. The cellular protein CD317 (tetherin/BST-2) acts to retain virions on the cell surface, and Vpu is able to antagonize this host cell restriction factor.21,22 It remains controversial if Vpu is a major virulence factor, but several lines of evidence indicate a role of Vpu in HIV-1 pathogenesis. In macaque models, SIV/HIV chimeric viruses with a mutation in the vpu initiation codon revert rapidly, and reversion correlates with a phase of profound loss of CD4 T cells.23,24 If reversion is prevented by introducing larger deletions in the vpu gene, infected animals do not show significant CD4 T cell loss, indicating nonprogressive infection.25 Finally, naturally occurring viruses that lack expression of a functional Vpu protein, such as HIV-2 and most SIV isolates, show slower disease progression and cause less severe disease, implicating Vpu in pathogenesis.26
Considering its importance in early innate immune responses and presence at major HIV-1 transmission sites, the CD1d system for lipid antigen presentation may be a target for HIV-1 immune evasion. In this study, we identify the HIV-1 protein Vpu as a factor promoting evasion from CD1d-restricted immunity. We show that HIV-1 interferes with the surface expression of CD1d in productively infected DCs and demonstrate that this is a novel activity of the viral protein Vpu. Mechanistically, Vpu inhibits the efficient recycling of CD1d and retains the protein in early endosomal compartments, thereby inhibiting cell surface expression of CD1d and consequently the activation of NKT cells.
Methods
Cell culture and viruses
293T cells were cultured in RPMI 1640 medium (GIBCO Invitrogen Corporation) with 10% fetal calf serum, 2mM l-glutamine, and antibiotics. Cells were transiently transfected with DNA constructs with the use of Lipofectamine 2000 (Invitrogen) or ExGen 500 (Fermentas) according to the manufacturer's protocols. 293T cell lines stably expressing CD1d or CD1d mutants were selected in 50 μg/mL Zeocin (Invitrogen). The HIV-1 isolate BaL was grown on stimulated peripheral blood mononuclear cell cultures as described.27 81A-EGFP (enhanced green fluorescent protein) was generated by recombinant polymerase chain reaction technology inserting a cassette consisting of the EGFP gene followed by an internal ribosomal entry site between the env and nef genes into the 81A backbone. The gene cassette was derived from the plasmid pEGFP-IRES2-nef-ΔXbaI.28 To generate the mutants 81AΔvpu and 81AΔnefΔvpu, a 49 nucleotide deletion was introduced in the vpu gene of the viral plasmids 81A and 81AΔnef, respectively. Virus stocks of 81A and 81A mutants were generated by transfecting proviral DNA into 293T cells with the use of Lipofectamine 2000. After a 2-day culture, supernatants were harvested, debris was removed by centrifugation and filtration, and virus stocks were frozen. For some experiments proviral DNA was cotransfected with the plasmid pVPack VSV-G (Stratagene) to obtain VSV-G pseudotyped virions with enhanced infectivity.
Plasmid constructs
The CD1d gene was cloned into the vector pcDNA3.1/Zeo(+). The vpu gene was amplified from HIV-1 81A, cloned into pEGFP-N3 (BD Biosciences Clontech) and expressed as an EFGP fusion protein. CD1d and Vpu mutants were generated by polymerase chain reaction–based standard methods.
Flow cytometry and surface protein down-regulation
Cell surface and intracellular flow cytometry was performed as described.29 Antibody to HIV-1 p24 was from Beckman Coulter; antibodies to CD1d, CD59, CD71, human leukocyte antigen A2, and goat anti–mouse immunoglobulin were from BD Biosciences. Percentage of protein down-regulation from the cell surface was calculated by comparing mean fluorescence intensity (MFI) of antibody staining in EPGF/p24− cells (MFI−) versus EGFP/p24+ cells (MFI+) in the respective experiments: (MFI− − MFI+) × 100/MFI−. Data were acquired on a FACSCalibur (BD Biosciences) instrument and analyzed with FlowJo Version 8.8.3 software (TreeStar).
HIV-1 infection of DCs
DCs were generated and infected as described.27,29 Briefly, buffy coats were obtained from healthy blood donors as approved by the ethics committee at Karolinska Institutet. After enrichment with RosetteSep human monocyte enrichment cocktail (StemCell Technologies), monocytes were cultured for 6 days in RPMI 1640 medium supplemented with 5% human serum (Cambrex), 6.5 ng/mL recombinant human interleukin-4 (R&D Systems) and 250 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (PeproTech Inc) to obtain immature DCs. DCs were infected with viral stocks in the presence of cytokines and serum, cultured for up to 7 days, and analyzed for the expression of HIV-1 p24 and surface proteins by flow cytometry.
Activation of CD1d-restricted NKT cells
CD1d-restricted NKT-cell lines were established as described.29 To show immunologic synapse formation between NKT cells and DCs, DCs were infected with VSV-G pseudotyped virus mutant 81A-EGFP or MOCK-infected for 6 days, loaded with αGalCer (KRN7000; Biomol International), and coincubated with NKT cells at a 1:2 ratio for 30 minutes. After paraformaldehyde fixation, complexes were permeabilized with saponin and stained with anti–α-tubulin monoclonal antibody (mAb; Invitrogen). Synapse formation was quantified microscopically by evaluating centrosome polarization in NKT cells. To assess interferon-γ (IFN-γ) production by NKT cells, cocultures were run for 2 hours in the presence of brefeldin A (GolgiPLUG; 2 mg/mL; BD Biosciences), stained with anti–IFN-γ mAb (R&D Systems) after permeabilization with saponin, and microscopically analyzed.
Internalization assay
Internalization of CD1d was measured with an acid stripping technique.30 293T-CD1d cells were transfected with Vpu-EGFP or EGPF plasmids. After 48 hours, cells were incubated for 30 minutes at 4°C with purified anti-CD1d mAb followed by incubation with allophycocyanin-conjugated goat anti–mouse mAb. After washing, aliquots of cells were treated with ice-cold phosphate-buffered saline or acid buffer (pH 2), respectively, to measure total bound antibody (t0) and background signal (t0′). Cells were then incubated at 37°C for various times to allow internalization. Subsequently, all samples were divided into 2, washed with either phosphate-buffered saline (tn) or acid buffer (tn′), and analyzed by flow cytometry. The percentage of CD1d internalized at a given time point was calculated by the CD1d MFI in the EGFP+ population with the use of the following formula: (MFI tn′ − MFI t0′) × 100/(MFI tn − MFI t0′).
Recycling assay
CD1d recycling was assessed by flow cytometry as described.31 293T-CD1d cells transiently expressing Vpu-EGFP or EGPF were incubated with saturating amounts of purified anti-CD1d mAb (1 μg/106 cells) for 60 minutes at 4°C to block surface CD1d. After washes, cells were incubated at 37°C for 0 to 30 minutes and collected on ice. The surface arrival of unblocked internal CD1d was detected by staining with a phycoerythrin-conjugated anti-CD1d antibody. Recycling of CD1d was measured as an increase in cell-surface CD1d MFI and analyzed separately for EGFP− and EGFP+ cells in the same culture.
Immunoprecipitation and Western blot
At 24 hours after transfection, 293T cells cotransfected with CD1d and Vpu-EGFP or EGFP plasmids were lysed in buffer containing 1% Igepal (Sigma-Aldrich). Lysates were magnetically labeled with anti-GFP microbeads (Miltenyi Biotec), and proteins were isolated according to the manufacturer's instructions. Samples were run on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel, transferred onto a nitrocellulose membrane, and probed with anti-CD1d mAb (clone NOR3.2; Serotec). Membranes were subsequently stripped and reprobed with anti–GFP-horseradish peroxidase (Miltenyi Biotec).
Immunofluorescence
293T cells were grown on glass coverslips and cotransfected with CD1d and Vpu-EGFP plasmids. Twenty-four hours after transfection, cells were fixed at room temperature for 15 minutes with 1% paraformaldehyde, left intact or permeabilized with 0.1% saponin, and incubated with unconjugated primary mAbs directed against CD1d (BD PharMingen), lysosomal-associated membrane protein 1 (LAMP-1; Abcam), and early endosomal antigen 1 (EEA-1; Santa Cruz Biotechnology). Fluorochrome-conjugated secondary mAbs were from Molecular Probes. Images were obtained on a Leica TCS SP2 confocal system with a 63×/1.3 glycerol objective using Leica Confocal Software (Version 2.61). Slides were covered with 90% glycerol during image acquisition.
Statistical analysis
Data were analyzed with Student t test for paired or unpaired samples or with nonparametric Mann-Whitney or Wilcoxon signed rank test, as appropriate, with the use of using SigmaStat software (SPSS Inc). P values less than .05 were considered statistically significant.
Results
HIV-1 down-regulates surface expression of CD1d in productively infected DCs
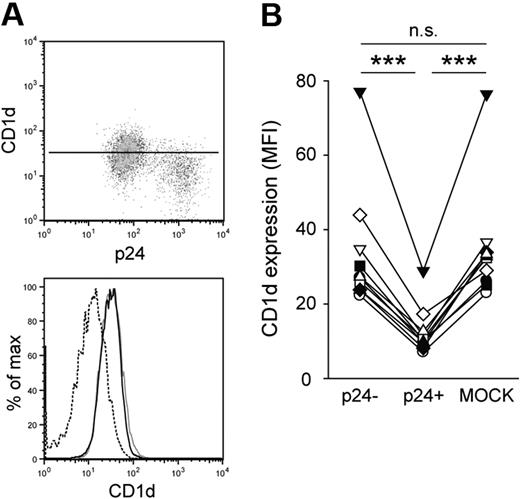
To investigate the effect of HIV-1 infection on CD1d expression, we infected monocyte-derived DCs with the CCR5-tropic HIV-1 strain BaL.27 DCs were generated in the presence of human serum to obtain cells expressing high levels of CD1d.29 Seven days after infection, cells were stained for CD1d surface expression followed by intracellular staining for the HIV-1 capsid protein p24 to identify productive infection. The p24-positive fraction of DCs had significantly lower CD1d surface expression compared with p24-negative cells in the same culture (P < .001; Figure 1A-B). The reduction of CD1d surface expression was 63% on average, comparable with that seen for CD4 and human leukocyte antigen A2 (data not shown). Virus-exposed but p24-negative cells in the cultures had CD1d surface expression levels similar to those seen in MOCK-infected cells (Figure 1B), indicating that viral gene expression was required for CD1d down-regulation. Inhibition of CD1d expression was not restricted to infection with the BaL strain but was also observed to a similar extent after infection with the HIV-1 strain 81A (data not shown), which is a derivative of the NL4-3 virus carrying the V3 loop of BaL. To exclude that reduced CD1d surface expression was an effect of infection-induced DC maturation (indicated by major histocompatibility complex class II up-regulation; data not shown), we treated immature DCs with lipopolysaccharide or polyinosine-polycytidylic acid. Neither treatment resulted in a significant change of CD1d surface expression, indicating that CD1d down-regulation was not a consequence of DC maturation (data not shown). These data collectively suggest that a viral factor is directly involved in the down-regulation of CD1d surface expression in DCs.
HIV-1 inhibits CD1d surface expression in DCs. (A-B) Human immature DCs were infected with HIV-1 BaL, stained for CD1d surface expression and intracellular expression of p24 at day 7 after infection, and analyzed by flow cytometry. CD1d MFI was calculated for infected (p24+; dashed black line) and uninfected (p24−; solid black line) cells in the culture, and for MOCK-infected cells (gray solid line). The vertical line in the dot plot indicates CD1d MFI of the p24− DC population. Data in panel B show 10 experiments with DCs from different donors. ***P < .001.
HIV-1 inhibits CD1d surface expression in DCs. (A-B) Human immature DCs were infected with HIV-1 BaL, stained for CD1d surface expression and intracellular expression of p24 at day 7 after infection, and analyzed by flow cytometry. CD1d MFI was calculated for infected (p24+; dashed black line) and uninfected (p24−; solid black line) cells in the culture, and for MOCK-infected cells (gray solid line). The vertical line in the dot plot indicates CD1d MFI of the p24− DC population. Data in panel B show 10 experiments with DCs from different donors. ***P < .001.
Interference with CD1d surface expression is a novel activity of HIV-1 Vpu
HIV-1 encodes several regulatory and accessory proteins that influence and regulate the expression of both viral and host cell proteins.32 To test the possibility that the accessory protein Vpu plays a role in HIV-1 interference with CD1d surface expression, we generated a plasmid encoding Vpu derived from the viral strain 81A as a fusion protein with EGFP. When Vpu-EGFP was transfected into a 293T-cell line stably expressing CD1d, we observed a significant reduction in CD1d surface expression (Figure 2A-B). The average reduction of CD1d surface expression upon Vpu expression was around 60%, comparable with the extent of down-regulation observed in infected DCs. To exclude any cell clone–specific effects, 3 independently generated 293T-CD1d clones were tested, all showing similar degrees of down-regulation upon expression of Vpu (data not shown). Vpu-mediated inhibition of CD1d cell surface expression was confirmed by confocal microscopy (Figure 2C). To investigate whether the effects were specific for CD1d, we assessed the surface expression of other glycoproteins in Vpu-expressing cells. CD59 is a glycosylphosphatidylinositol–anchored surface glycoprotein known to colocalize with HIV-1 Gag in lipid rafts. CD71, the transferrin receptor, is a type II surface glycoprotein containing a tyrosine-based trafficking motif, similar to that of CD1d, regulating endocytosis and recycling. In contrast to CD1d, surface expression of both CD59 and CD71 was largely resistant to Vpu-mediated down-regulation in 293T-CD1d cells (Figure 2D). Taken together, these data show that CD1d is down-regulated by HIV-1 Vpu and identify interference with CD1d surface expression as a novel activity of this viral protein.
CD1d is down-regulated from the cell surface by HIV-1 Vpu. (A-B) 293T cells stably expressing CD1d were transiently transfected with EGFP or Vpu-EGFP and examined for CD1d cell surface expression 48 hours after transfection. Dashed black lines indicate EGFP+ cells; solid black lines, EGFP− cells in the same culture; gray solid lines, isotype control stainings. CD1d down-regulation was calculated as described in “Flow cytometry and surface protein down-regulation.” Data are representative of 8 independent experiments. Error bars represent SD; ***P < .001. (C) Surface immunofluorescence microscopy of 293T cells 48 hours after cotransfection with CD1d and Vpu-EGFP. Arrowheads indicate representative EGFP+ cells; asterisks, representative EGFP− cells. Original magnification of the images, ×630; scale bar, 15 μm. (D) Forty-eight hours after transfection, 293T-CD1d cells transfected with Vpu-EGFP were stained for surface expression of CD59 or CD71. Dashed black lines indicate EGFP+ cells; solid black lines, EGFP− cells; gray solid lines, isotype controls. Representative data from 1 of 3 experiments are shown.
CD1d is down-regulated from the cell surface by HIV-1 Vpu. (A-B) 293T cells stably expressing CD1d were transiently transfected with EGFP or Vpu-EGFP and examined for CD1d cell surface expression 48 hours after transfection. Dashed black lines indicate EGFP+ cells; solid black lines, EGFP− cells in the same culture; gray solid lines, isotype control stainings. CD1d down-regulation was calculated as described in “Flow cytometry and surface protein down-regulation.” Data are representative of 8 independent experiments. Error bars represent SD; ***P < .001. (C) Surface immunofluorescence microscopy of 293T cells 48 hours after cotransfection with CD1d and Vpu-EGFP. Arrowheads indicate representative EGFP+ cells; asterisks, representative EGFP− cells. Original magnification of the images, ×630; scale bar, 15 μm. (D) Forty-eight hours after transfection, 293T-CD1d cells transfected with Vpu-EGFP were stained for surface expression of CD59 or CD71. Dashed black lines indicate EGFP+ cells; solid black lines, EGFP− cells; gray solid lines, isotype controls. Representative data from 1 of 3 experiments are shown.
CD1d down-regulation inhibits activation of CD1d-restricted NKT cells
The formation of an immunologic synapse between APCs and T cells requires interaction of antigen-presenting molecules and the T-cell receptor and goes along with several defined cellular events, such as centrosome polarization, leading to T-cell activation.33 To study the effect of CD1d down-regulation on the efficiency of synapse formation between DCs and CD1d-restricted NKT cells, we loaded HIV-1 or MOCK-infected DCs with the model lipid-antigen αGalCer and performed cocultures with NKT-cell lines. DCs were infected with a virus mutant (81A-EGFP) containing an open reading frame for the EGFP gene to identify productively infected DCs. Immunologic synapse formation was visualized by antitubulin staining and quantified by evaluating polarization of the centrosome in NKT cells. Whereas coculture with MOCK-infected DCs loaded with αGalCer clearly induced centrosome polarization toward the side of DC-NKT contact, such relocalization could not be observed in NKT cells forming complexes with 81A-EGFP–infected DCs (Figure 3A). Quantification showed a significant shift in centrosome localization with an almost random distribution in NKT cells in contact with HIV-1 infected DCs (P = .001; Figure 3B). Similarly, Vpu-transfected 293T-CD1d cells had a significantly reduced capacity to induce centrosome polarization in NKT cells, compared with 293T-CD1d cells not expressing Vpu (P < .05; Figure 3C). We next wanted to determine whether the observed effect of CD1d down-regulation on synapse formation also results in reduced cytokine production by NKT cells. In a similar microscopic approach as above, the percentage of IFN-γ+ cells of NKT cells in contact with a DCs clearly depended on the infection status of the DCs (Figure 3D). Whereas αGalCer-loaded HIV-1− DCs induced production of IFN-γ in more than 50% of the NKT cells, HIV-1+ DCs could only activate 20% of NKT cells (P < .05; Figure 3E). These data show that HIV-1–mediated CD1d down-regulation impairs both early and late events in NKT-cell activation.
CD1d down-regulation inhibits activation of CD1d-restricted NKTcells. (A) NKT cells were coincubated with αGalCer-loaded MOCK- or 81A-EGFP (green) infected DCs. Cell complexes were fixed, permeabilized, and stained with anti–α-tubulin mAb (red) and DAPI (4,6 diamidino-2-phenylindole; blue). White arrowheads indicate centrosomes; white lines show division of NKT cells for quantification of centrosome polarization. (B) Percentage of centrosomes located proximal, middle, or distal to the site of NKT-DC contact. At least 35 complexes in 2 experiments were analyzed; **P = .001. (C) As in panels A and B, but NKT cells were coincubated with αGalCer-loaded MOCK- or Vpu-transfected 293T-CD1d cells. At least 70 complexes in 2 experiments were analyzed; *P < .05. (D) Staining with anti–IFN-γ mAb (red) was performed after coincubation of NKT cells with αGalCer-loaded 81A-EGFP (green) infected DCs for 2 hours in the presences of brefeldin A. Minuses indicate IFN-γ negative, asterisks IFN-γ positive NKT cells in contact with DCs. (E) Quantification of IFN-γ production in 5 independent experiments with DCs from different donors. **P < .01. Scale bars, 15 μm.
CD1d down-regulation inhibits activation of CD1d-restricted NKTcells. (A) NKT cells were coincubated with αGalCer-loaded MOCK- or 81A-EGFP (green) infected DCs. Cell complexes were fixed, permeabilized, and stained with anti–α-tubulin mAb (red) and DAPI (4,6 diamidino-2-phenylindole; blue). White arrowheads indicate centrosomes; white lines show division of NKT cells for quantification of centrosome polarization. (B) Percentage of centrosomes located proximal, middle, or distal to the site of NKT-DC contact. At least 35 complexes in 2 experiments were analyzed; **P = .001. (C) As in panels A and B, but NKT cells were coincubated with αGalCer-loaded MOCK- or Vpu-transfected 293T-CD1d cells. At least 70 complexes in 2 experiments were analyzed; *P < .05. (D) Staining with anti–IFN-γ mAb (red) was performed after coincubation of NKT cells with αGalCer-loaded 81A-EGFP (green) infected DCs for 2 hours in the presences of brefeldin A. Minuses indicate IFN-γ negative, asterisks IFN-γ positive NKT cells in contact with DCs. (E) Quantification of IFN-γ production in 5 independent experiments with DCs from different donors. **P < .01. Scale bars, 15 μm.
HIV-1 Vpu does not affect the rate of CD1d endocytosis
After initial trafficking to the cell surface, CD1d molecules constitutively recycle between the cell surface and endosomal compartments.34 Internalization by clathrin-mediated endocytosis depends on a tyrosine-based targeting motif in the cytoplasmic tail of CD1d. Removal of this motif results in retention of CD1d on the cell surface and loss of antigen-presentation function.35 To determine whether down-regulation of CD1d by Vpu requires an intact targeting motif, we constructed a mutant in which the cytoplasmic tyrosine was replaced by alanine (CD1dY331A). 293T cells stably expressing either wild-type (wt) or mutant CD1d were transfected with Vpu-EGFP and analyzed for CD1d cell surface expression. Exchange of tyrosine 331 resulted in a marked reduction in Vpu-mediated CD1d down-regulation (Figure 4A). This suggests that intact intracellular trafficking of CD1d is a prerequisite for efficient down-regulation and implies that Vpu interferes with the rate of internalization and/or recycling of CD1d. In addition,Y331 may be part of a sequence motif that is required for a direct or indirect interaction with Vpu. A CD1d mutant lacking the last 8 cytoplasmic amino acids (QTSYQGVL; CD1dΔtail) was down-regulated to a similar degree as CD1dY331A, showing that the cytoplasmic tail does not contain further motifs relevant for Vpu interference with CD1d surface expression (Figure 4A).
Vpu impairs recycling of CD1d to the cell surface. (A) 293T cells stably expressing wt CD1d or mutants CD1dY331A or CD1dΔtail, respectively, were transiently transfected with Vpu-EGFP. CD1d down-regulation was calculated 48 hours after transfection. Data are representative of 5 independent experiments. Error bars represent SD; ***P < .001. (B) The rate of CD1d internalization in 293T-CD1d cells expressing Vpu-EGFP or EGFP alone was measured 48 hours after transfection with the use of a flow cytometry–based assay as described in “Recycling assay.” Data are representative of 6 independent experiments. Error bars represent SD. (C) For flow cytometric analysis of CD1d recycling, 293T-CD1d cells transfected with Vpu-EGFP were pretreated with purified anti-CD1d mAb to block surface CD1d. After incubation at 37°C for different times, the surface arrival of unblocked internal CD1d was detected by staining with a phycoerythrin-conjugated CD1d antibody. Recycling of CD1d was measured as an increase in cell surface CD1d MFI and analyzed separately for Vpu-EGFP+ (closed circles) and Vpu-EGFP− (open circles) cells in the same culture. Data from 1 representative of 4 independent experiments are shown. All experiments were performed in duplicates. (D) Quantification of CD1d recycling rates in Vpu-EGFP+ and Vpu-EGFP− cells. The rate of recycling is expressed as an increase in CD1d MFI per minute and corresponds to the slope of the regression line in panel C. Data from 4 independent experiments performed in duplicates are shown. **P < .01.
Vpu impairs recycling of CD1d to the cell surface. (A) 293T cells stably expressing wt CD1d or mutants CD1dY331A or CD1dΔtail, respectively, were transiently transfected with Vpu-EGFP. CD1d down-regulation was calculated 48 hours after transfection. Data are representative of 5 independent experiments. Error bars represent SD; ***P < .001. (B) The rate of CD1d internalization in 293T-CD1d cells expressing Vpu-EGFP or EGFP alone was measured 48 hours after transfection with the use of a flow cytometry–based assay as described in “Recycling assay.” Data are representative of 6 independent experiments. Error bars represent SD. (C) For flow cytometric analysis of CD1d recycling, 293T-CD1d cells transfected with Vpu-EGFP were pretreated with purified anti-CD1d mAb to block surface CD1d. After incubation at 37°C for different times, the surface arrival of unblocked internal CD1d was detected by staining with a phycoerythrin-conjugated CD1d antibody. Recycling of CD1d was measured as an increase in cell surface CD1d MFI and analyzed separately for Vpu-EGFP+ (closed circles) and Vpu-EGFP− (open circles) cells in the same culture. Data from 1 representative of 4 independent experiments are shown. All experiments were performed in duplicates. (D) Quantification of CD1d recycling rates in Vpu-EGFP+ and Vpu-EGFP− cells. The rate of recycling is expressed as an increase in CD1d MFI per minute and corresponds to the slope of the regression line in panel C. Data from 4 independent experiments performed in duplicates are shown. **P < .01.
To determine whether the reduced CD1d surface expression in Vpu-positive cells was caused by an accelerated rate of CD1d internalization from the cell surface, we compared the rate of CD1d endocytosis in 293T-CD1d cells expressing Vpu-EGFP or EGFP alone. The cells were antibody-labeled for CD1d and incubated at 37°C for different times. At each time point, an aliquot of the cells was treated with an acidic buffer (pH 2) to remove surface-bound noninternalized antibody. Finally, cells were subjected to flow cytometric analysis, and the percentage of internalized CD1d was calculated. The rate of CD1d endocytosis was similar in all cells regardless of the expression of Vpu-EGFP or EGFP with a maximum of around 70% of the labeled CD1d internalized after 30 minutes (Figure 4B). Hence, Vpu does not alter the rate of CD1d endocytosis, excluding this as a possible mechanism behind the reduced CD1d cell surface expression seen in Vpu-expressing cells.
HIV-1 Vpu inhibits CD1d recycling
We next investigated the effect of Vpu expression on the recycling of CD1d from endosomal compartments to the cell surface. 293T-CD1d cells transfected with Vpu-EGFP were incubated at 4°C with saturating amounts of unconjugated anti-CD1d mAb to block surface CD1d. Subsequently, recycling was analyzed by incubating the cells at 37°C for different times and detecting the surface arrival of unblocked intracellular CD1d with phycoerythrin-conjugated anti-CD1d. The arrival of CD1d at the cell surface was clearly slower in cells expressing Vpu than in Vpu-negative cells in the same culture (Figure 4C-D). Noteworthy, the CD1d recycling rate was unaffected by expression of EGFP alone (data not shown). To exclude that the result of this assay was skewed by potential Vpu interference with the transport of newly synthesized CD1d to the cell surface, we performed the same experiment in the presence of cycloheximide, a drug that inhibits protein synthesis. Here, we also observed a reduced rate of CD1d surface arrival (data not shown), further supporting the conclusion that Vpu interferes with CD1d recycling. These results indicate that HIV-1 Vpu interferes with CD1d cell surface expression by inhibiting recycling of CD1d from endosomal compartments back to the cell surface.
Colocalization and interaction of CD1d and Vpu in early endosomal compartments
The ability of HIV-1 Vpu to specifically interfere with the recycling step in the life cycle of CD1d suggests its physical presence in CD1d-containing endosomal/lysosomal compartments. Therefore, we studied the potential interaction of CD1d and Vpu and the subcellular distribution of both proteins. First, we examined the distribution of CD1d transiently expressed in 293T cells in the absence and presence of Vpu. We again observed a strongly reduced expression of CD1d on the surface of cells coexpressing Vpu. Moreover, we detected a pronounced vesicular distribution of CD1d when Vpu was present (Figure 5A). Together with the finding that Vpu interferes with CD1d recycling, this may indicate that Vpu retains CD1d in intracellular vesicular compartments. Interestingly, confocal microscopy showed that CD1d and Vpu colocalized to a significant extent in these structures, suggesting physical interaction of the 2 proteins (Figure 5B). To test this possibility, 293T cells coexpressing CD1d and Vpu-EGFP were lysed in a mild buffer to maintain protein-protein interactions. Immunoprecipitation was performed with anti-GFP microbeads and was followed by Western blotting with anti-CD1d antibody. With the use of this approach, we could precipitate CD1d from the samples containing Vpu-EGFP but not from control samples containing only the EGFP protein (Figure 5C top panel). Interestingly, Vpu seems to predominantly interact with the fully processed high-molecular-weight form of CD1d, supporting that Vpu targets CD1d primarily after surface arrival and internalization. Expression and precipitation of Vpu-EGFP and EGFP was confirmed by reprobing with anti-GFP antibody (Figure 5C bottom panel). This shows physical interaction and complex formation between CD1d and Vpu.
Complex formation of CD1d and Vpu. (A-B) Confocal microscopy analysis of 293T cells transfected with CD1d alone (A left) or cotransfected with CD1d and Vpu-EGFP (A right, B). Cells were permeabilized and stained with anti-CD1d antibody. Original magnification of all images, ×630; insets at higher magnification. Scale bars, 15 μm. (C) 293T cells were cotransfected with CD1d and Vpu-EGFP or EGFP alone and lysed in buffer containing 1% Igepal. After immunoprecipitation with anti-GFP microbeads, proteins were separated on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and transferred onto a nitrocellulose membrane. The membrane was first probed with anti-CD1d antibody (clone NOR3.2) and subsequently stripped and reprobed with anti-GFP antibody.
Complex formation of CD1d and Vpu. (A-B) Confocal microscopy analysis of 293T cells transfected with CD1d alone (A left) or cotransfected with CD1d and Vpu-EGFP (A right, B). Cells were permeabilized and stained with anti-CD1d antibody. Original magnification of all images, ×630; insets at higher magnification. Scale bars, 15 μm. (C) 293T cells were cotransfected with CD1d and Vpu-EGFP or EGFP alone and lysed in buffer containing 1% Igepal. After immunoprecipitation with anti-GFP microbeads, proteins were separated on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and transferred onto a nitrocellulose membrane. The membrane was first probed with anti-CD1d antibody (clone NOR3.2) and subsequently stripped and reprobed with anti-GFP antibody.
To identify the compartment where CD1d and Vpu colocalize, we performed costainings with EEA-1 and LAMP-1, marker proteins for early endosomal compartments and late endosomes/lysomes, respectively. Substantial colocalization of CD1d and Vpu was observed in EEA-1–positive compartments (Figure 6A). However, smaller vesicles containing both CD1d and Vpu did not stain positive for EEA-1 and are not yet identified. We could not detect any significant colocalization of CD1d and Vpu in late endosomes or lysosomes (Figure 6B). On the contrary, although CD1d and LAMP-1 colocalized to some degree, there was no overlap between Vpu and LAMP-1, and, accordingly, LAMP-1 seemed to be excluded from vesicles containing both CD1d and Vpu. Taken together, these data show colocalization and complex formation of CD1d and Vpu and suggest that HIV-1 Vpu retains CD1d in early endosomal compartments.
CD1d and HIV-1 Vpu colocalize in early endosomal compartments. (A-B) Costaining of CD1d and EEA-1 (A) or LAMP-1 (B), respectively, in permeabilized 293T cells transiently coexpressing CD1d and Vpu-EGFP. Filled arrowheads (A) indicate vesicles staining positive for CD1d, Vpu, and EEA-1. (B) Filled arrowheads indicate colocalization of CD1d and LAMP-1; open arrowheads indicate colocalization of CD1d and Vpu. Squares indicate the section shown in higher magnification. Original magnification of all images, ×630; all data are representative of at least 3 independent experiments. Scale bars, 15 μm.
CD1d and HIV-1 Vpu colocalize in early endosomal compartments. (A-B) Costaining of CD1d and EEA-1 (A) or LAMP-1 (B), respectively, in permeabilized 293T cells transiently coexpressing CD1d and Vpu-EGFP. Filled arrowheads (A) indicate vesicles staining positive for CD1d, Vpu, and EEA-1. (B) Filled arrowheads indicate colocalization of CD1d and LAMP-1; open arrowheads indicate colocalization of CD1d and Vpu. Squares indicate the section shown in higher magnification. Original magnification of all images, ×630; all data are representative of at least 3 independent experiments. Scale bars, 15 μm.
Vpu and Nef cooperatively inhibit CD1d-mediated lipid antigen presentation in HIV-1–infected DCs
Finally, we wanted to verify the capacity of Vpu to inhibit CD1d surface expression in productively HIV-1–infected DCs. Therefore, we generated a virus mutant deficient in Vpu expression by introducing a deletion mutation in the vpu gene (81AΔVpu). To enhance infectivity of the virus mutant, particles were pseudotyped with the G protein of vesicular stomatitis virus, resulting in infection levels comparable with wt (81A; data not shown). Infection of monocyte-derived DCs with 81AΔVpu showed that abrogation of Vpu expression resulted in reduced capacity of the virus to interfere with CD1d expression (Figure 7A). This confirms that Vpu is a major viral factor interfering with CD1d but also suggests that other viral factors might be involved. Earlier reports about the influence of HIV-1 on the expression of CD1d were contradictory. Whereas 2 studies suggested a Nef-dependent effect on CD1d expression in different cell lines,30,36 a third study failed to show any influence of HIV-1 on CD1d surface expression in human DCs.37 To address the contribution of Nef to down-regulation of CD1d in DCs, we infected DCs with 2 additional virus mutants defective in the expression of Nef (81AΔNef) or defective in both Vpu and Nef (81AΔNefΔVpu). In these experiments, Nef indeed contributed to CD1d down-regulation (Figure 7A). Interestingly, the HIV-1 lacking both Vpu and Nef displayed an almost complete loss of CD1d down-regulatory activity, indicating a cooperative effect of these 2 proteins on CD1d-mediated lipid antigen presentation in DCs. Importantly, DCs infected with the double mutant 81AΔNefΔVpu virus had a similar capacity to induce IFN-γ production in CD1d-restricted NKT cells as MOCK-infected DCs (Figure 7B). This indicates that not HIV-1 infection of DCs, per se, but rather the specific down-regulation of CD1d inhibits NKT-cell activation.
HIV-1 Vpu- and Nef-deletion mutants lose their capacity to inhibit CD1d expression and NKT-cell activation. (A) Human monocyte-derived DCs were infected with VSV-G pseudotyped HIV-1 81Awt or mutants lacking the expression of Vpu (ΔVpu), Nef (ΔNef), or both (ΔNefΔVpu), and CD1d down-regulation was analyzed at day 4 after infection. CD1d down-regulation by 81Awt was set as 100%. Data show experiments with DCs generated from 8 different donors. Error bars represent mean ± SE; **P < .01; ***P < .001. (B) NKT cells were coincubated with αGalCer-loaded DCs infected with the viruses indicated for 2 hours in the presence of brefeldin A and subsequently analyzed for expression of p24 (green) and IFN-γ production (red). The data are representative of 3 independent experiments. Scale bars, 15 μm.
HIV-1 Vpu- and Nef-deletion mutants lose their capacity to inhibit CD1d expression and NKT-cell activation. (A) Human monocyte-derived DCs were infected with VSV-G pseudotyped HIV-1 81Awt or mutants lacking the expression of Vpu (ΔVpu), Nef (ΔNef), or both (ΔNefΔVpu), and CD1d down-regulation was analyzed at day 4 after infection. CD1d down-regulation by 81Awt was set as 100%. Data show experiments with DCs generated from 8 different donors. Error bars represent mean ± SE; **P < .01; ***P < .001. (B) NKT cells were coincubated with αGalCer-loaded DCs infected with the viruses indicated for 2 hours in the presence of brefeldin A and subsequently analyzed for expression of p24 (green) and IFN-γ production (red). The data are representative of 3 independent experiments. Scale bars, 15 μm.
Discussion
The innate-like functions of CD1d-restricted NKT cells and their interaction with DCs, and possibly also other CD1d-expressing cells, are probably targets for viral immune evasion mechanisms. Here, we show that HIV-1 inhibits surface expression of CD1d in productively infected human DCs and identify this as a novel activity of the HIV-1 accessory protein Vpu. Vpu retains internalized CD1d molecules in an early endosomal compartment and inhibits their recycling back to the plasma membrane.
CD1d has an intricate intracellular trafficking pattern that is essential to survey the endocytic system for the presence of foreign lipid antigens. During assembly in the endoplasmic reticulum (ER) and transport to the cell surface, self lipids such as phosphatidylinositol are loaded onto CD1d to facilitate assembly.38 After clathrin-dependent internalization, CD1d is targeted to late endosomal and/or lysosomal compartments where the molecular machinery and biochemical conditions are available for the exchange of these self lipids for other self or foreign-derived lipid and glycolipid antigens.39 When HIV-1 Vpu inhibits the transition of CD1d from early endosomal compartments to late endosomal/lysosomal compartments, it may not only reduce the cell surface expression of CD1d but probably at the same time deprive CD1d of the conditions required for efficient antigen loading. CD1d molecules that eventually escape and recycle to the cell surface directly from EEA-1–positive compartments may not be able to present new antigen content. Hence, keeping CD1d away from the antigen-loading compartment in combination with reducing its surface expression may be an efficient strategy for HIV-1 to avoid recognition of infected DCs by NKT cells. In fact, HIV-1–infected DCs are unable to efficiently induce NKT-cell activation, indicating a limited capacity of NKT cells to recognize HIV-1–infected cells.
Several reports suggest a role for CD1d-restricted immunity in the control of both acute and chronic viral infections. CD1d-deficient mice show an altered course of infection after challenge with respiratory syncytial virus, herpes simplex virus 1, coxsackievirus B3, and lymphocytic choriomeningitis virus compared with wt mice.40-42 In mice infected with influenza A virus, the absence of CD1d-restricted NKT cells results in the expansion of myeloid-derived suppressor cells that suppress the virus-specific immune response, leading to high virus loads and increased mortality.43 Interestingly, this suppressive effect can be abolished by adoptive transfer of NKT cells. Human studies have shown that chronic infection with HIV-1 or hepatitis C virus leads to significantly reduced numbers of circulating CD1d-restricted NKT cells.44,45 The precise role of lipid-antigen presentation by CD1d and recognition by NKT cells in antiviral immune responses is not yet well defined, because virally derived antigens presented by CD1d and recognized by NKT cells have yet to be identified. However, recognition of infected cells by NKT cells might not depend on direct recognition of viral antigens. NKT-cell activation might instead be triggered by recognition of endogenous lipid antigens presented by CD1d in combination with cytokines secreted by virus-infected or virus-exposed DCs.4 A new indirect line of evidence for the potential contribution of the CD1d system to antiviral defenses is the recently discovered ability of certain human viruses to inhibit the cell surface expression of CD1d in infected cells. The Kaposi sarcoma–associated herpesvirus protein MIR2 enhances endocytosis of CD1d, whereas not yet identified factors in herpes simplex virus 1 interfere with CD1d expression by redistributing endocytosed CD1d to the lysosome.31,46 We have identified CD1d down-regulation as an activity of Vpu, both in infected DCs and in a model cell line, and 2 earlier studies have suggested Nef-dependent down-regulation of CD1d in different cell lines.30,36 With the use of a Nef-deleted HIV-1 mutant, we could confirm that Nef interferes with CD1d expression also in infected DCs. Furthermore, whereas singly Vpu- or Nef-deleted viruses had lost approximately 40% of their capacity to down-regulate CD1d, a double knockout resulted in the almost complete loss of this capacity. These data indicate that Vpu and Nef cooperatively inhibit CD1d surface expression in infected DCs. Mechanistically, Nef may enhance internalization of CD1d from the cell surface,30 and subsequently Vpu traps internalized CD1d in the endosomal compartments and inhibits recycling back to the cell surface. The fate of retained CD1d remains so far unclear because no enhanced CD1d degradation could be detected in Vpu-expressing cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and further experimentation will be required. An important aim for future studies will be to describe in detail the contributions of Vpu and Nef to the down-regulation of CD1d in infected DCs. The increasing number of viruses known to interfere with CD1d surface expression, or alternatively, to impair the number and function of NKT cells is an intriguing indication of a significant role for the CD1d system in antiviral immunity. Indeed, virus infection of a cell may be detected via CD1d, and an important task for the future will be to identify the glycolipid antigens presented by CD1d during viral infections.
In experimental settings as well as in naturally occurring infections, Vpu-expressing viruses such as HIV-1 are more pathogenic than viruses that do not express a Vpu protein, such as HIV-2 and the majority of SIV isolates. However, it is largely unclear to what degree differences in pathogenicity can be attributed to the activity of Vpu. Recent studies have corroborated the importance of Vpu in the process of HIV-1 particle release and virus spread,21,22 and it seems clear that Vpu contributes to pathogenesis by raising viral loads. However, this activity might not be unique to HIV-1 Vpu because the HIV-2 envelope protein seems to harbor a similar activity stimulating virus particle release47 and may not explain the differences in pathogenic potential. It remains to be investigated if the less pathogenic Vpu-deficient viruses have the ability to inhibit CD1d surface expression. Vpu-mediated interference with CD1d surface expression and the differences in pathogenic potential between Vpu-containing and -deficient viruses support a role for CD1d-restricted immune responses in HIV-1 immunity.
HIV-1 can interfere with CD1d-restricted immunity at different levels. Throughout infection HIV-1 may directly target CD4+ CD1d-restricted NKT cells, resulting in the loss of these cells in circulation and a significant skewing of the NKT-cell compartment.45 Moreover, NKT cells retained in chronically infected patients display a dysfunctional phenotype and are unable to respond with cytokine production or proliferation on stimulation.48 We speculate that early in infection at mucosal entry sites HIV-1 may evade rapid detection and elimination by innate responses by inhibiting CD1d surface expression in infected DCs. This may facilitate spread of infection by rendering it possible for productively infected DCs to migrate unhindered from mucosal transmission sites to draining lymph nodes where the virus has access to abundant numbers of CD4+ target cells. Interestingly, CD1d expression is not only restricted to professional APCs but can also be found in epithelial cells in both the female and male genital tract.11 Moreover, CD1d is abundantly expressed in the gut, another major HIV-1 transmission site, and gut epithelial cells can activate NKT cells.15 Although controversially discussed, HIV-1 might enter epithelial cells leading to productive infection and spread of infectivity.49 Much remains to be learned about the importance of CD1d-restricted antigen presentation in epithelial cells and corresponding NKT-cell activation pathways, but a role in mucosal immunity is probable. In this respect it is important to note that the ability of HIV-1 to interfere with CD1d expression is clearly not restricted to DCs. Vpu efficiently inhibits expression of CD1d in the epithelial cell line 293T used in this study, indicating that inhibition is not strictly cell type dependent and that CD1d-restricted antigen presentation pathways may be similar in APCs and epithelial cells. Taken together, emerging evidence suggests a role for NKT cells in the control of HIV-1 in both early and the chronic phases of infection and that efficient recognition of HIV-1–infected cells may depend at least in part on CD1d.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank H. G. Ljunggren and M. A. Eller for critical reading and discussion and M. Kroll for technical assistance. 81A and 81AΔnef were provided by B. Chesebro (Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases). The plasmid pEGFP-IRES2-nef-ΔXbaI was kindly provided by A. Brown (Johns Hopkins University School of Medicine).
This work was supported by the Swedish Research Council (M.M. and J.K.S.), the Swedish International Development Agency (M.M. and J.K.S.), the Swedish Foundation for Strategic Research (M.M. and J.K.S.), the Swedish Physicians Against AIDS Foundation (M.M.), the Jeanssons Foundation (M.M), the Åke Wiberg Foundation (M.M.), the Clas Groschinsky Foundation (M.M.), and the National Institutes of Health (grant AI52731; J.K.S.).
National Institutes of Health
Authorship
Contribution: M.M., S.K.A., and A.S.-S. designed, performed, and analyzed experiments; and M.M. and J.K.S. supervised research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of A.S.-S. is Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, Stockholm, Sweden.
Correspondence: Markus Moll, Center for Infectious Medicine, Department of Medicine, Karolinska Institutet, Karolinska University Hospital Huddinge F59, 14186 Stockholm, Sweden; e-mail: markus.moll@ki.se.
References
Author notes
M.M. and S.K.A. contributed equally to this study.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal